Terminal erythropoiesis is accompanied by extreme demand for iron to ensure proper hemoglobinization. Thus, erythroblasts must modify the “standard” post-transcriptional feedback regulation, balancing expression of ferritin (Fer; iron storage) versus transferrin receptor (TfR1; iron uptake) via specific mRNA binding of iron regulatory proteins (IRPs). Although erythroid differentiation involves high levels of incoming iron, TfR1 mRNA stability must be sustained and Fer mRNA translation must not be activated because iron storage would counteract hemoglobinization. Furthermore, translation of the erythroid-specific form of aminolevulinic acid synthase (ALAS-E) mRNA, catalyzing the first step of heme biosynthesis and regulated similarly as Fer mRNA by IRPs, must be ensured. We addressed these questions using mass cultures of primary murine erythroid progenitors from fetal liver, either undergoing sustained proliferation or highly synchronous differentiation. We indeed observed strong inhibition of Fer mRNA translation and efficient ALAS-E mRNA translation in differentiating erythroblasts. Moreover, in contrast to self-renewing cells, TfR1 stability and IRP mRNA binding were no longer modulated by iron supply. These and additional data stemming from inhibition of heme synthesis with succinylacetone or from iron overload suggest that highly efficient utilization of iron in mitochondrial heme synthesis during normal erythropoiesis alters the regulation of iron metabolism via the IRE/IRP system.
Introduction
Maturing erythroid progenitors require large amounts of iron to enable high rates of heme synthesis. The daily generation of about 20 g erythrocytes in adult humans requires the delivery of 20 mg iron via iron-loaded transferrin (Fe2-Tf) to the bone marrow. The corresponding transferrin receptor (TfR1) is highly expressed on the cell surface of erythroblasts. Recently, a second variant, TfR2, was characterized, which among other cells is also found on the surface of immature erythroid cells,1,2 but seems to play a minor role during terminal maturation.2,3 Tf-TfR1 complexes are internalized into endosomes via receptor-mediated endocytosis, and endosome acidification leads to release of iron4,5 and, subsequently, to its export into the cytoplasm via the transporter DMT-1.6,7 Finally, iron is delivered to its sites of utilization; any excess gets stored in ferritin (Fer). This sequestration is important to avoid oxidative damage via this highly reactive metal. Therefore, the balance between iron uptake, utilization, and storage has to be tightly regulated.
Cis-acting elements involved in this control are the stem-loop structures called iron regulatory elements (IREs) in several mRNAs (for a review, see Pantopoulos8 ). Such IREs are found in the 5′-untranslated region (UTR) of ferritin light (FerL) and heavy chain (FerH) mRNA, and in 5 copies within the 3′-UTR of TfR1 mRNA. These elements are recognized by trans-acting factors, that is, iron regulatory proteins 1 and 2 (IRP1, IRP2), which bind with high affinity to IREs in their cognate mRNAs. One difference between IRP1 and IRP2 lies in their mode of regulation. IRP1 is a bifunctional protein, which under high iron releases the IRE, incorporates a cubane 4Fe-4S cluster, and gains enzymatic activity as cytosolic aconitase.9 In contrast, IRP2 mRNA-binding activity is turned off by proteasomal degradation.10-12
In a generally accepted “standard” model, cytosolic iron concentrations regulate the mRNA-binding activity of IRP1/2 to IREs, which are localized in the UTRs of a growing number of transcripts.8 IRPs bind to IREs under low iron concentrations, which, for example, inhibits translation of Fer messages13,14 and stabilizes TfR1 mRNA.15-17 Consequently, cellular iron uptake is stimulated,18,19 whereas storage is inhibited. At high iron concentrations, IRP1 incorporates iron-sulfur clusters and thus does not bind target mRNAs while IRP2 gets degraded.10-12 This leads to an increase in Fer synthesis and TfR1 mRNA degradation via a specific endonuclease pathway,15-17 which in turn reduces cellular iron uptake rates. These posttranscriptional feedback mechanisms allow cells to balance cellular iron homeostasis.
There are, however, growing numbers of reports on specialized cell types and tissues that at least under certain instances bypass the IRE/IRP system,20-23 one prominent example being erythroid cells.24,25 Apart from their especially high iron needs, they express an erythroid-specific isoform of aminolevulinic acid synthase (ALAS-E), a key enzyme in erythroid heme synthesis. ALAS-E is essential for erythroid differentiation.26 Like Fer mRNAs it contains a functional IRE in its 5′-UTR (for a review, see Sadlon et al27 ). Thus iron-dependent regulation of ALAS-E should allow cells to coordinate the production of protoporphyrin IX with cellular iron levels. The resulting coregulation of TfR1, Fer subunits, and ALAS-E may be meaningful in erythroid cells undergoing self-renewal (ie, sustained proliferation without differentiation), but once induced for terminal differentiation, these cells need saturating doses of Fe2-Tf for proper maturation.28-30 Consequently, committed erythroblasts have to maintain high expression of TfR1 despite increasing intracellular iron concentrations. Furthermore, synthesis of Fer must not be activated by incoming iron, because this would lead to counterproductive storage in a phase of high iron demand.31 Thus, the regulation of TfR1, Fer, and ALAS-E expression during erythroid differentiation is difficult to reconcile mechanistically with the principles outlined.
In this paper, we therefore addressed iron metabolism during terminal erythropoiesis of primary mouse erythroid progenitors, using a unique hematopoietic culture system. This, for the first time, permitted us to analyze the complexity of posttranscriptional regulation of IRE-containing mRNAs by iron during sustained proliferation versus terminal differentiation of immature erythroid progenitors under in vivo–like conditions. This cell model allows (1) proliferation and differentiation of erythroblasts derived from mouse fetal liver in serum-free medium, (2) mass cultures with up to 107-fold expansion of cells, yielding enough material for studies requiring a high amount of sample material, such as polysome gradient analyses, and (3) production of enucleated, fully hemoglobinized cells.32,33
Analyses of self-renewing or differentiating mouse erythroblasts demonstrated that the coordinate regulation of Fer, ALAS-E, and TfR1 mRNAs via iron is abolished during terminal differentiation. Instead, both proteins involved in iron homeostasis (Fer and TfR1) are insensitive toward changes in physiologic concentrations of Fe2-Tf. Moreover, translation of Fer mRNA is almost entirely blocked, whereas ALAS-E mRNA is used to a significant extent. Artificially boosting cytosolic iron levels by addition of ferric ammonium citrate (FAC), succinylacetone (SA), an inhibitor of heme biosynthesis, or unphysiologically high concentrations of Fe2-Tf could, however, reinduce expression of Fer in maturing erythrocytes.
Materials and methods
Culture of primary mouse erythroblasts
Erythroid cells were isolated and cultivated as described.32,33 Briefly, cells were grown from fetal livers from E12.5 embryos (wild-type, MF1 background) and resuspended in serum-free StemPro-34 medium plus Nutrient Supplement (Invitrogen-Gibco, Carlsbad, CA) plus 2 U/mL human recombinant erythropoietin (Epo; 100 ng/mL), murine recombinant stem cell factor (SCF; 100 ng/mL), the synthetic glucocorticoid dexamethasone (Dex; 10–6 M), and insulin-like growth factor 1 (IGF-1; 40 ng/mL). Cell number and size distribution of cell populations were monitored daily in an electronic cell counter (CASY-1, Schärfe-System, Reutlingen, Germany). Dead or differentiating cells were removed by Ficoll purification.
To induce terminal differentiation, continuously self-renewing erythroblasts were washed twice in PBS and seeded in StemPro-34, containing 10 U/mL Epo, insulin (4 × 10–4 IU/mL), the Dex antagonist ZK-112993 (3 × 10–6M)34 and iron-saturated human transferrin (Fe2-Tf; 1 mg/mL = 12.5 μM = 25 μM Fe = physiologic levels; Sigma, St Louis, MO). Where indicated, heme synthesis was inhibited by 0.2 mM SA (Sigma).35
To induce iron starvation, cells were incubated with 50 μM of the iron chelator desferrioxamine (Des); iron overload was induced by adding Fe2-Tf up to 0.1 mM or FAC (20 μg/mL, 17% saturation = 63 μM iron) 24 hours before harvest.
Cell morphology, histologic staining, and determination of hemoglobin content
Changes in cell morphology during differentiation were monitored by phase-contrast microscopy. For histologic analysis, erythroblasts were cytocentrifuged36 at various stages of maturation onto glass slides and stained with histologic dyes and neutral benzidine for hemoglobin as described.36 Hemoglobin content was analyzed by removing 50-μL aliquots from the cultures and by undergoing photometric determination as described.37 Values obtained from triplicate determinations were averaged and normalized to cell number and cell volume.
Flow cytometry
Self-renewing or differentiating erythroblasts (1 × 106) were washed twice with PBS/2% fetal calf serum (FCS) and stained with fluorescently labeled antibodies against transferrin receptor (FITC; PharMingen, San Diego, CA; no. 01595) and Ter119 (PE; PharMingen, no. 09085).38 Surface marker expression was analyzed by flow cytometry (LSR-I; Becton Dickinson, Franklin Lakes, NJ).
Northern blot analysis
Total RNA was prepared from 2 to 4 × 107 cells using TRIzol (Invitrogen). Then, 10 μg total or polysomal RNA/sample was separated in denaturing 1% formaldehyde-agarose gels. Equal loading was controlled by ethidium bromide staining. RNA was transferred to nylon membranes (Gene Screen; DuPont, Wilmington, DE), and fixed by UV irradiation (1200 mJ; UV-Crosslinker; Stratagene, LaJolla, CA). Membranes were sequentially hybridized with [32P]-labeled cDNA probes generated by random-primed labeling (Prime-it-II; Stratagene) specific for mouse FerH (0.85-kb AatII-NdeI fragment), FerL (0.3 kb, AatII-NdeI), ALAS-E (1.4 kb, AatII-NotI), TfR1 (0.75 kb, EcoRI-HindIII), and α-globin mRNAs. Probes for IRP1 and IRP2 mRNA were obtained from the RZPD library of the German Resource Center for Genome Research (#IMAGp998J182131, Eco-HindIII; IRP2 #IMAGp998I191339, SacII-MluI, respectively). Signals were quantified by phospho-imaging.
Polysome gradients
The extent of mRNA association with polysomes was determined by sucrose-gradient analysis39 with RNA prepared from 2 to 4 × 107 cells. After removal of nuclei and cell debris by centrifugation, lysates were laid onto 15% to 40% sucrose gradients and separated by ultracentrifugation. RNA was harvested from 18 fractions, separated on denaturing agarose gels, and transferred onto nylon membranes as described.40 Distribution of 18S and 28S rRNA was visualized by staining of filters with methylene blue.
Electrophoretic mobility shift assays
RNA-protein complexes were resolved essentially as described.39,41 Briefly, for electrophoretic mobility shift assays (EMSAs), cytoplasmic extracts were incubated with [32P]-labeled transcripts produced by T7 RNA-polymerase after linearization of the plasmid pGEM-3Zf(+)-mouse FerH-IRE (clone42)42 with BamHI. Protein (2 μg) and 1.3 × 106 disintegrations per minute of labeled IRE-containing in vitro transcript were incubated for 20 minutes at room temperature. The total amount of IRP1 was assessed by in vitro reduction with 2% β-mercaptoethanol (2-ME)43 prior to the binding reaction. After treatment with RNAse T1 and heparin, RNA-protein complexes were resolved on 6% non–denaturing polyacrylamide gels at 4°C. Bands corresponding to IRE/IRP complexes were quantified by phospho-imaging (Molecular Dynamics, Sunnyvale, CA).
Western blot analysis
Cell pellets were lysed in sample buffer as described38 and 10 to 20 μg protein was separated on sodium dodecyl sulfate-polyacrylamide gels. Protein transfer and loading were visualized by staining with acidic Ponceau-S solution. Thereafter, membranes were blocked 1 hour at room temperature with 1% low-fat dry milk in TBS and probed overnight with anti–horse spleen ferritin (Sigma; no. F-6136) or anti–rat IRP1,44 rat monoclonal anti–mouse TfR1 (BioSource, Nivelles, Belgium; no. AMS7102) or, for normalization, with anti-Erk1/2 (Sigma; no. 5670). After washing, filters were incubated with second antibody (horseradish peroxidase–coupled anti–rabbit IgG antibody (Jackson Laboratories, West Grove, PA; no. 111-035-008) for Fer, IRP1, Erk1/2, eIF4E, and anti–rat IgG (Jackson Laboratories; no. 112-035-008) for TfR1. After washing, immunoreactive signals were detected by enhanced chemoluminescence (Amersham, Buckinghamshire, United Kingdom).
ALAS-E immunoprecipitation and enzyme activity
Protein levels of ALAS-E were determined by immunoprecipitation of [35S]-methionine pulse-labeled cell extracts using a polyclonal antiserum developed in rabbit (a kind gift from M. Hentze and B. Galy, European Molecular Biology Laboratory, Heidelberg, Germany EMBL) and visualized by phospho-imaging. Enzyme activity was determined by fluorometric high-performance liquid chromatography (HPLC) of the reaction product δ-aminolevulinic acid45 in cell extracts after removal of nuclei.
Results
Extended self-renewal and synchronous differentiation of primary, fetal liver–derived erythroblasts
Most studies on iron metabolism in erythroid cells so far were done with erythroleukemic cell lines or reticulocytes. Murine erythroleukemia cells have provided insights into mechanisms controlling erythroid differentiation46 but have severe drawbacks. Importantly, they are unresponsive toward physiologic maturation stimuli, including Epo. Thus, differentiation is induced artificially by nonphysiologic agents such as dimethyl sulfoxide, hexamethylenebis-actamid and the like. In addition, poor hemoglobinization and abnormal morphologic changes during differentiation originate from patterns of gene expression different from those in normal erythropoiesis.47 Reticulocytes represent a more physiologic system, but poorly represent the proliferative and early differentiation aspects of erythropoiesis.48
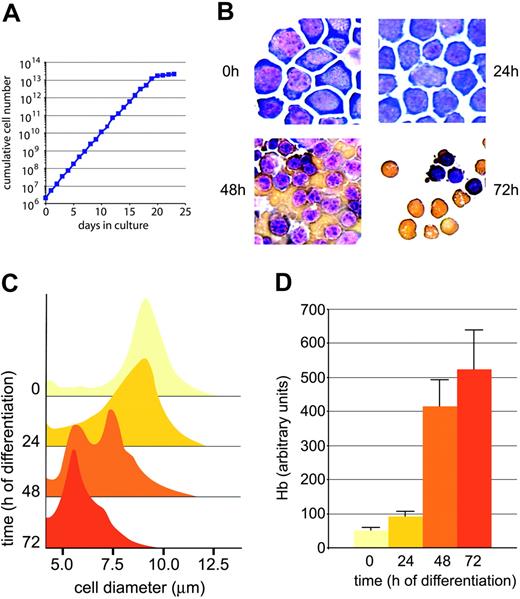
In this study, primary mouse erythroid cells were used, which closely recapitulate several aspects of terminal maturation in vivo, including size decrease, full hemoglobinization, and enucleation.33,49 These cells, from fetal livers of E12.5 mouse embryos, can be expanded under self-renewing conditions (sustained proliferation without differentiation) for 15 to 20 days before undergoing senescence (Figure 1A). Substitution of SCF plus Dex with insulin and the glucocorticoid antagonist ZK34 plus increase of erythropoietin are sufficient to induce highly synchronous terminal maturation, largely completed within 72 hours (Figure 1B). Noteworthy, the most substantial increase in hemoglobin content occurs between 24 and 48 hours after induction of differentiation (Figure 1D), accompanied by cell size decrease to half the original volume (Figure 1C). At this stage, the erythroblasts up-regulate differentiation markers like Ter119, whereas markers typical for immature cells disappear.32 Thus, with respect to regulation of iron metabolism, special focus was put on this interval. This period is also characterized by sufficient transcriptional activity allowing interference with gene expression, whereas at later stages chromatin condensation (prior to enucleation) shuts down nuclear activity.
Translational repression of Fer mRNA and efficient utilization of ALAS-E mRNA in differentiating mouse erythroblasts
Following the “standard” model of IRP/IRE-mediated translational regulation, increase in cellular iron uptake in hemoglobinizing cells should not only activate more efficient translation of ALAS-E mRNA (to ensure high levels of protoporphyrin IX synthesis) but also favor (futile) synthesis of iron storage proteins.
Extended self-renewal and synchronous differentiation of primary, fetal liver–derived mouse erythroblasts. (A) Cells from fetal livers of E12.5 mouse embryos were cultivated in serum-free StemPro (Life Technologies) medium plus NutriMix supplement in the presence of stem cell factor (SCF), erythropoietin (Epo; 2 U/mL), and the synthetic glucocorticoid dexamethasone (Dex). Proliferation kinetics of outgrowing erythroblasts were determined by daily measurements of aliquots in an electronic cell counter (CASY) and cumulative cell numbers calculated as described.50 (B) Terminal differentiation was induced by replacing proliferation factors with insulin, the glucocorticoid antagonist ZK11299334 plus high levels of Epo (10 U/mL) and Fe2-Tf (1 mg/mL = 12.5 μM). To monitor morphologic changes in maturing cells, aliquots were withdrawn at daily intervals, cytocentrifuged onto slides, and stained with neutral benzidine (to detect hemoglobin; brownish stain) and histologic dyes.36 Note size decrease and enucleation of mature cells (72 hours, bottom right panel). Photomicrographs were taken using an Axiovert 10 microscope (Zeiss, Oberkochen, Germany) equipped with a 63 × oil-immersion objective lens (numerical aperture 44-07-61; Zeiss). Images are presented at original magnification, × 630. Images were captured with a Sony 3CCD color video camera (Sony, Tokyo, Japan) and prepared for publication with IP Lab Spectrum P software 3.1.1 (Signal Analytics, Vienna, VA). (C) Measurements of the decline in cell volume during differentiation were performed with an electronic multichannel cell analyzer. Appearance of 5-μm peak indicates mature cells with volumes close to that of peripheral blood erythrocytes. (D) Hemoglobin levels during differentiation were quantitated using a photometric assay previously described, and normalized to both cell numbers and cell volume from 50-μL aliquots in triplicate32,37 ; error bars, SD of mean, n = 4.
Extended self-renewal and synchronous differentiation of primary, fetal liver–derived mouse erythroblasts. (A) Cells from fetal livers of E12.5 mouse embryos were cultivated in serum-free StemPro (Life Technologies) medium plus NutriMix supplement in the presence of stem cell factor (SCF), erythropoietin (Epo; 2 U/mL), and the synthetic glucocorticoid dexamethasone (Dex). Proliferation kinetics of outgrowing erythroblasts were determined by daily measurements of aliquots in an electronic cell counter (CASY) and cumulative cell numbers calculated as described.50 (B) Terminal differentiation was induced by replacing proliferation factors with insulin, the glucocorticoid antagonist ZK11299334 plus high levels of Epo (10 U/mL) and Fe2-Tf (1 mg/mL = 12.5 μM). To monitor morphologic changes in maturing cells, aliquots were withdrawn at daily intervals, cytocentrifuged onto slides, and stained with neutral benzidine (to detect hemoglobin; brownish stain) and histologic dyes.36 Note size decrease and enucleation of mature cells (72 hours, bottom right panel). Photomicrographs were taken using an Axiovert 10 microscope (Zeiss, Oberkochen, Germany) equipped with a 63 × oil-immersion objective lens (numerical aperture 44-07-61; Zeiss). Images are presented at original magnification, × 630. Images were captured with a Sony 3CCD color video camera (Sony, Tokyo, Japan) and prepared for publication with IP Lab Spectrum P software 3.1.1 (Signal Analytics, Vienna, VA). (C) Measurements of the decline in cell volume during differentiation were performed with an electronic multichannel cell analyzer. Appearance of 5-μm peak indicates mature cells with volumes close to that of peripheral blood erythrocytes. (D) Hemoglobin levels during differentiation were quantitated using a photometric assay previously described, and normalized to both cell numbers and cell volume from 50-μL aliquots in triplicate32,37 ; error bars, SD of mean, n = 4.
To address this question, translation of both Fer and ALAS-E mRNAs was monitored by polysome gradient analysis of erythroblasts kept under proliferation or differentiation conditions, either iron-depleted by addition of the iron chelator Des or fully iron-loaded by incubation with additional Fe2-Tf (1 mg/mL). Polysome-associated mRNAs were fractionated from untranslated mRNPs by linear sucrose gradients (see “Materials and methods”). After RNA isolation and separation, blots were hybridized with probes specific for FerH, FerL, ALAS-E, and α-globin mRNAs. In self-renewing erythroblasts, translation of FerH, FerL, and ALAS-E mRNAs was still modulated by availability of iron (Figure 2A). Under high iron conditions, there was an increase of mRNA in polysome-bound fractions from 2% to 9% for FerH, from 2% to 19% for FerL, and from 9% to 25% for ALAS-E (Figure 2B, left panels). The small percentages of polysome-bound FerL/H mRNA in proliferating erythroblasts even under high iron conditions are comparable to the earlier observations.51,52 When erythroblasts differentiating for 48 hours were subjected to the same analysis, however, iron-induced translational activation of FerH or FerL (or both) was abolished (Figure 2A-B). Control α-globin mRNA was translated with high efficiency under both low and high iron conditions. Thus, Fer mRNA translation can be activated by physiologic concentrations of Fe2-Tf under self-renewal conditions but not in maturing erythroblasts that are accumulating hemoglobin. In contrast, ALAS-E mRNA translation remained regulated by iron levels after the onset of differentiation (16% polysome-bound mRNA under iron starvation versus 41%; Figure 2B, right panels). Therefore, under all conditions tested, ALAS-E mRNA was translated more efficiently than Fer transcripts in proliferating as well as differentiating cells.
Translational repression of Fer mRNA and efficient utilization of ALAS-E mRNA in differentiating mouse erythroblasts. Self-renewing (designated “proliferation” in this and the following panels) or differentiating (labeled “48h diff”) primary mouse erythroblasts were incubated with the iron chelator desferrioxamine (Des, 50 μM) or physiologic concentrations of iron-loaded human transferrin (Tf, 12.5 μM) for 24 hours prior to harvesting. (A) Polysome gradient analysis. Cytoplasmic extracts were separated in linear 15% to 40% sucrose gradients39 and the RNA isolated from 18 fractions analyzed by Northern blotting. Fraction 1, top, fraction 18, bottom of the gradient. Filters were sequentially hybridized with [32P]-labeled probes specific for mouse FerH, FerL, ALAS-E, and (in the case of differentiating cells) α-globin mRNA as control. Bottom panel, loading control; methylene blue stain of total RNA. The constant molar ratio between 28S and 18S RNA (top and bottom band, respectively) around fraction 9 indicates the assembly of 80S initiation complexes and marks the approximate boundary between the ribosome-free, untranslated, and polyribosome-bound, translated mRNA compartment, as schematically depicted at the bottom. (B) Quantification of polysome-bound, translated mRNA. Bar diagrams depict the sum of the percentage of mRNA in fractions 9-18 as determined by PhosphoImage analysis. (C) Fer protein expression in proliferating and differentiating cells. The antibody used (see “Materials and methods”) recognizes both FerH and FerL. (D) ALAS-E expression as determined by immunoprecipitation of cell extracts (normalized to equal number of counts per sample) pulse labeled for 20 minutes with [35S]-methionine; to visualize the ALAS-E band in proliferating cells, this signal was amplified electronically 5 times. (E) Total mRNA levels for ALAS-E, FerH, and FerL mRNAs. Loading and quality control, 28S rRNA stained with methylene blue.
Translational repression of Fer mRNA and efficient utilization of ALAS-E mRNA in differentiating mouse erythroblasts. Self-renewing (designated “proliferation” in this and the following panels) or differentiating (labeled “48h diff”) primary mouse erythroblasts were incubated with the iron chelator desferrioxamine (Des, 50 μM) or physiologic concentrations of iron-loaded human transferrin (Tf, 12.5 μM) for 24 hours prior to harvesting. (A) Polysome gradient analysis. Cytoplasmic extracts were separated in linear 15% to 40% sucrose gradients39 and the RNA isolated from 18 fractions analyzed by Northern blotting. Fraction 1, top, fraction 18, bottom of the gradient. Filters were sequentially hybridized with [32P]-labeled probes specific for mouse FerH, FerL, ALAS-E, and (in the case of differentiating cells) α-globin mRNA as control. Bottom panel, loading control; methylene blue stain of total RNA. The constant molar ratio between 28S and 18S RNA (top and bottom band, respectively) around fraction 9 indicates the assembly of 80S initiation complexes and marks the approximate boundary between the ribosome-free, untranslated, and polyribosome-bound, translated mRNA compartment, as schematically depicted at the bottom. (B) Quantification of polysome-bound, translated mRNA. Bar diagrams depict the sum of the percentage of mRNA in fractions 9-18 as determined by PhosphoImage analysis. (C) Fer protein expression in proliferating and differentiating cells. The antibody used (see “Materials and methods”) recognizes both FerH and FerL. (D) ALAS-E expression as determined by immunoprecipitation of cell extracts (normalized to equal number of counts per sample) pulse labeled for 20 minutes with [35S]-methionine; to visualize the ALAS-E band in proliferating cells, this signal was amplified electronically 5 times. (E) Total mRNA levels for ALAS-E, FerH, and FerL mRNAs. Loading and quality control, 28S rRNA stained with methylene blue.
In maturing erythroid progenitors, efficient ALAS-E mRNA translation was accompanied by a massive increase in transcript levels of more than 20-fold. Interestingly, also transcription of FerL augmented significantly (3-fold; Figure 2E)53 but unlike ALAS-E mRNA, this increase was not accompanied by an increase of transcripts engaged to polysomes (Figure 2B), as reported earlier for differentiating murine erythroleukemia (MEL) cells.54
The data on the mRNA level were corroborated by analyses at the protein level. In differentiating mouse erythroblasts Fer expression stayed at low levels, irrespective of cell preincubation with Des or Fe2-Tf (Figure 2C), whereas ALAS-E protein synthesis rates as measured by immunoprecipitation indicated a 2.4-fold increase (Figure 2D). The low levels of ALAS-E protein synthesis in proliferating cells were in accordance with the much lower abundance of the corresponding mRNA (Figure 2E). Moreover, quantitation of the ALAS-E reaction product, δ-aminolevulinic acid by HPLC produced similar results (data not shown).
TfR1 expression in differentiating primary mouse erythroblasts is independent of iron
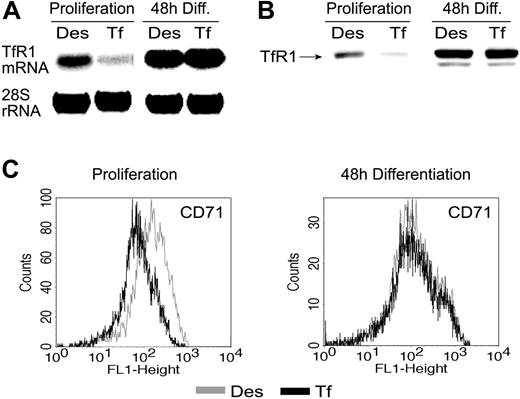
It is well documented that during definitive erythropoiesis uptake of iron by mouse erythroid cells is mediated predominantly via Fe2-Tf/TfR1 endocytosis.30 Studies with mouse erythroid cell lines24,25 have emphasized the importance of this pathway to supply maturing erythroid cells with sufficient iron. After initial work with primary chicken erythroid progenitors,24,55 here we extended these observations to primary mouse erythroblasts, using physiologic concentrations of Fe2-Tf as iron source and Des to induce iron deprivation. Whereas in self-renewing cells TfR1 mRNA levels were regulated by iron, erythroblasts induced to differentiate maintained high expression of TfR1 mRNA under both conditions (Figure 3A). Furthermore, TfR transcript levels in maturing cells were elevated, even in comparison to self-renewing cells supplemented with Des. The corresponding total cellular TfR1 protein levels followed the same pattern (Figure 3B). Additionally, cell-surface TfR1 expression was determined by flow cytometry. In the absence of iron chelator, self-renewing erythroblasts decreased the number of TfR1 molecules on the cell surface, whereas during differentiation, in the phase of high iron demand, no iron-dependent changes could be observed (Figure 3C). Interestingly, although terminal erythropoiesis led to a significant elevation of TfR1 mRNA and total protein levels, cell-surface expression was even somewhat reduced compared with self-renewing cells, reminiscent of the situation in chicken erythroblasts55 and arguing for redistribution of TfR1 toward later endosomal compartments in the cell during maturation.56
Transferrin receptor expression is independent of iron in differentiating mouse erythroblasts. Fetal liver–derived mouse erythroid progenitors pretreated as described in the legend to Figure 2 were analyzed for transferrin receptor (TfR1) expression. (A) TfR1 mRNA determined by Northern blotting (28S rRNA hybridization as control); (B) total TfR1 protein determined by Western blotting; Erk1/2, loading control. (C) TfR1 cell-surface expression was determined by flow cytometry.
Transferrin receptor expression is independent of iron in differentiating mouse erythroblasts. Fetal liver–derived mouse erythroid progenitors pretreated as described in the legend to Figure 2 were analyzed for transferrin receptor (TfR1) expression. (A) TfR1 mRNA determined by Northern blotting (28S rRNA hybridization as control); (B) total TfR1 protein determined by Western blotting; Erk1/2, loading control. (C) TfR1 cell-surface expression was determined by flow cytometry.
In differentiating erythroblasts, IRP1 and IRP2 are not regulated by iron
Next we sought to address the role of IRPs during self-renewal and late stage of erythroid differentiation. For this, we performed EMSAs between IRP1 and IRP2 and radiolabeled mouse FerH-IRE RNA probes transcribed in vitro, using the IRE probe C42, which was shown to exhibit an equal binding affinity for IRP1 and IRP2.42
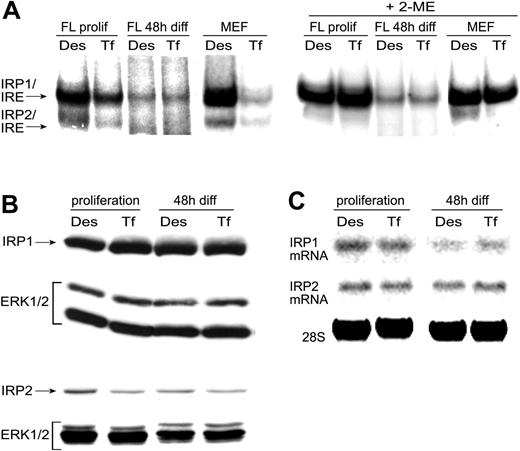
In line with the regulation observed for FerH/L, ALAS-E, and TfR1 mRNAs, self-renewing erythroblasts still showed an iron-dependent regulation of IRP1 mRNA-binding activity, even though this difference was clearly weaker compared with extracts prepared from control mouse embryo fibroblasts (Figure 4A). In differentiating erythroblasts, however, mRNA-binding activity of IRP1 declined strongly and remained totally indifferent toward iron sequestration. To determine the total amount of potentially available IRP1 mRNA-binding capacity, 2-mercaptoethanol (2-ME)43,57 was added to the binding reaction prior to addition of labeled IRE probes. Whereas this treatment strongly activated binding activity in self-renewing erythroblasts as well as control cells, the level of activation in differentiating cells was weaker, although comparison of the differences in IRP1 expression revealed that total IRP1 protein and mRNA levels from self-renewing versus differentiating cells remained almost constant under all conditions tested (Figure 4B-C). One reason for this apparent discrepancy (see “Discussion”) lies in the technical principle of EMSAs. This assay for native IRE/IRP complexes using radiolabeled IRE transcripts detects free IRP not already stably associated with endogenous unlabeled IREs.58 Therefore, in committed erythroid cells, a higher proportion of IRPs may be associated with an increased number of IRE-bearing transcripts.
mRNA-binding activity of IRP is independent of iron in differentiating primary erythroblasts. (A) Determination of apparent (left panels) and total IRP mRNA-binding activities (right panels; +2-ME43 ) in extracts of mouse erythroblasts (designated “FL,” for fetal liver–derived cells), pretreated as described in Figures 2 and 3. Electrophoretic mobility shift assays (EMSAs) of complexes between IRP and radiolabeled in vitro–transcribed RNAs containing the IREs of mouse FerH mRNA (clone 42)42 were performed as described in “Materials and methods.” Control extracts, demonstrating the full regulatory potential of IRP, were prepared from mouse embryo fibroblasts (MEFs). Total IRP1 and IRP2 protein (B) and mRNA levels (C) were determined by Western (Erk1/2 used as loading control) and Northern blotting (28S rRNA signal as RNA quality and loading control), respectively.
mRNA-binding activity of IRP is independent of iron in differentiating primary erythroblasts. (A) Determination of apparent (left panels) and total IRP mRNA-binding activities (right panels; +2-ME43 ) in extracts of mouse erythroblasts (designated “FL,” for fetal liver–derived cells), pretreated as described in Figures 2 and 3. Electrophoretic mobility shift assays (EMSAs) of complexes between IRP and radiolabeled in vitro–transcribed RNAs containing the IREs of mouse FerH mRNA (clone 42)42 were performed as described in “Materials and methods.” Control extracts, demonstrating the full regulatory potential of IRP, were prepared from mouse embryo fibroblasts (MEFs). Total IRP1 and IRP2 protein (B) and mRNA levels (C) were determined by Western (Erk1/2 used as loading control) and Northern blotting (28S rRNA signal as RNA quality and loading control), respectively.
We also assessed the regulation of IRP2 expression during terminal erythropoiesis by Northern and Western blot analyses. Similar to IRP1, no significant increase in total IRP2 mRNA or protein levels was detected during differentiation; thus the decline in IRP1 mRNA-binding activity is apparently not compensated by an increase of IRP2 expression. More importantly, whereas pretreatment with Des in self-renewing cells was able to increase IRP2 protein abundance (and thus mRNA-binding activity as measured in EMSAs; Figure 4A), no iron-dependent response was detectable in differentiating erythroblasts (Figure 4C).
Iron overload and inhibition of heme synthesis restore up-regulation of Fer expression in differentiating mouse erythroblasts
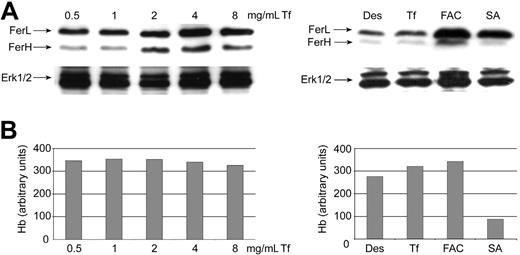
Finally, we tried to gain some mechanistic insight into the question why high iron levels fail to induce Fer mRNA translation during normal differentiation of mouse erythroid progenitors. For this we used (1) FAC to test whether iron overload via this reagent would result in a cellular response, (2) unphysiologically high concentrations of Fe2-Tf, and (3) the heme synthesis inhibitor SA. All 3 types of intervention led to a substantial increase in the protein level of FerL and FerH (Figure 5A), arguing against an iron-independent mechanism specifically inhibiting Fer mRNA translation. None of these treatments, not even addition of high Fe2-Tf levels, resulted in significantly elevated rates of heme synthesis, but, as expected, was reduced by 70% on treatment with SA (Figure 5B). Judging by the amount of Fer synthesized, Fe2-Tf endocytosis rates may reach a saturation at about 4 mg/mL, 4-fold higher than the in vivo serum concentration. Apparently the endocytosis machinery for TfR1 internalization is not the limiting factor for hemoglobin production but rather the synthesis capacity for heme/hemoglobin itself. At present we cannot distinguish between 2 alternative explanations for our results. First, the reagents used may directly increase the so called “labile iron pool”59,60 in the cytosol. This is likely to occur in the case of FAC, which might be taken up directly via non–TfR-based pathways.20-25 Second, cytoplasmic iron levels might increase secondarily after efflux of excess iron from mitochondria, a plausible event in response to SA and high Fe2-Tf treatment.
In either case our data strongly support the view that the IRE/IRP system in differentiating erythroid cells is sensing a “low-iron” state despite increasing cellular iron levels, but remains fully functional. Furthermore, the results obtained from inhibition of heme synthesis support the hypothesis that the cytoplasmic iron levels sensed by IRP may actually be kept low during erythropoiesis by a vectorial transport of iron into mitochondria,29,61-63 the site of iron insertion into protoporphyrin, thus bypassing the cytoplasmic “labile iron pool.” As detailed (Figure 6), all our observations can be integrated into a comprehensive working model of how the flow of iron might occur during the phase of massive hemoglobinization.
Iron overload and inhibition of heme synthesis restore iron-dependent Fer expression in differentiating mouse erythroblasts. Fer expression as determined by Western blotting in erythroid progenitors differentiating for 48 hours. (A) Cells were either incubated for 24 hours with Fe2-Tf (6.3-100 μM; highest concentration corresponds to 8 times the physiologic level; top left panel), or Des (50 μM), Fe2-Tf (12.5 μM), FAC (63 μM Fe) and SA (0.2 μM; inhibition of heme synthesis) (top right panel). FAC probably can enter the cells directly, bypassing Tf/TfR-mediated endocytosis and the assumed vectorial iron transport from endosomes into mitochondria, and may thus lead to direct cytosolic iron overload ERK1/2 (bottom panels), loading control; membranes restained with corresponding antibody. (B) Hemoglobin synthesis in the cells described in panel A.
Iron overload and inhibition of heme synthesis restore iron-dependent Fer expression in differentiating mouse erythroblasts. Fer expression as determined by Western blotting in erythroid progenitors differentiating for 48 hours. (A) Cells were either incubated for 24 hours with Fe2-Tf (6.3-100 μM; highest concentration corresponds to 8 times the physiologic level; top left panel), or Des (50 μM), Fe2-Tf (12.5 μM), FAC (63 μM Fe) and SA (0.2 μM; inhibition of heme synthesis) (top right panel). FAC probably can enter the cells directly, bypassing Tf/TfR-mediated endocytosis and the assumed vectorial iron transport from endosomes into mitochondria, and may thus lead to direct cytosolic iron overload ERK1/2 (bottom panels), loading control; membranes restained with corresponding antibody. (B) Hemoglobin synthesis in the cells described in panel A.
Discussion
Here we demonstrate that during terminal differentiation of primary mouse erythroid progenitors, Fer mRNA translation is massively impaired, whereas translation of ALAS-E mRNA, presumed to be regulated coordinately with Fer mRNA via the IRE/IRP system, proceeds unimpeded. Furthermore, maturing erythroblasts express very high levels of TfR1, again independent of varying iron supply or IRP activity levels. These observations contrast the “standard” mode for regulation of intracellular iron metabolism in most other cell lineages, which includes (1) up-regulation of IRP mRNA-binding activity on iron depletion, which in turn (2) increases Fe2-Tf import via stabilization of TfR1 mRNA and (3) represses iron storage via translational inhibition of Fer mRNA. Interestingly, this “standard” mode also applies to committed, self-renewing mouse erythroid progenitors, which do not yet accumulate hemoglobin and are thus independent of mechanisms to ensure high iron uptake. In contrast, terminal differentiation into erythrocytes uncouples the coordinate regulation of Fer and ALAS-E mRNA translation, and elevated expression of TfR1 persists despite the presence of high (physiologic) levels of Fe2-Tf. This type of regulation is perfectly suited to ensure maximum hemoglobin accumulation but difficult to reconcile mechanistically with the “standard” model.
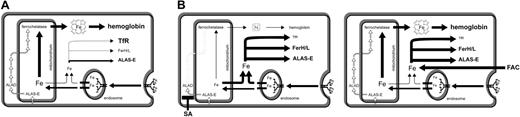
Working model for the regulation of iron metabolism in differentiating primary erythroblasts. (A) The model shown in this scheme is essentially based on the “kiss-and-run” hypothesis62 of vectorial iron transport toward mitochondria. It depicts the distribution of iron (cytosolic versus mitochondrial) in differentiating erythroid cells as well as how the expression levels of TfR1, Fer, and ALAS-E are regulated via IRP. Thick and thin black arrows symbolize high and low rates of iron flow, respectively; open white arrows depict heme synthesis; lettering size for hemoglobin, TfR1, Fer, ALAS-E and “Fe” (iron-loaded heme) indicates the expression level of the corresponding protein or compound. (B) Predicted and in part experimentally verified consequences of (1) perturbation of mitochondrial iron uptake/flow by adding inhibitors of heme biosynthesis like succinylacetone (SA; left panel) or (2) of direct cytoplasmic iron overload with low-molecular-weight iron salts (ie, addition of ferric ammonium citrate, FAC; right panel).
Working model for the regulation of iron metabolism in differentiating primary erythroblasts. (A) The model shown in this scheme is essentially based on the “kiss-and-run” hypothesis62 of vectorial iron transport toward mitochondria. It depicts the distribution of iron (cytosolic versus mitochondrial) in differentiating erythroid cells as well as how the expression levels of TfR1, Fer, and ALAS-E are regulated via IRP. Thick and thin black arrows symbolize high and low rates of iron flow, respectively; open white arrows depict heme synthesis; lettering size for hemoglobin, TfR1, Fer, ALAS-E and “Fe” (iron-loaded heme) indicates the expression level of the corresponding protein or compound. (B) Predicted and in part experimentally verified consequences of (1) perturbation of mitochondrial iron uptake/flow by adding inhibitors of heme biosynthesis like succinylacetone (SA; left panel) or (2) of direct cytoplasmic iron overload with low-molecular-weight iron salts (ie, addition of ferric ammonium citrate, FAC; right panel).
Previously we reported related findings for committed or differentiating chicken erythroid progenitors.55 There, (1) TfR1 levels were very high, even under saturating doses of Fe2-Tf, involving transcriptional and posttranscriptional mechanisms15,25,55,64,65 ; (2) Fer mRNA translation was massively impaired and could not be modulated by iron,40 whereas (3) ALAS-E mRNA was translated efficiently.40 Nevertheless, avian erythroblasts differ in several aspects from those in mammals, for example, in their lack of enucleation. Furthermore, apparently missing expression of FerL40 and deviation in the hexa-loop consensus sequence (5′-CAGUGN-3′→5′-CAGCGN-3′) of the ALAS-E-IRE40 could result in differences of iron metabolism during avian versus mammalian erythropoiesis.
Obviously, based on both chicken and mouse data, the regulation model of iron metabolism needed extension to account for specific requirements of maturing erythroblasts, which have to establish exceedingly high rates of iron uptake for successful hemoglobinization without activating iron storage. We addressed this problem using primary mouse erythroblasts,32,33 which expand more than 107-fold in serum-free media and undergo terminal differentiation in response to Epo plus insulin (Figure 1). In polysome gradient analyses, we compared iron-dependent translational regulation of FerH, FerL, and ALAS-E mRNAs. “Low” iron conditions were simulated by addition of the iron chelator Des, “high” iron conditions were achieved by supplementing the medium with physiologic levels of Fe2-Tf (1 mg/mL). In immature, self-renewing erythroblasts, translation of all 3 transcripts was still coordinately regulated by the availability of iron. During advanced stages of erythroid maturation, however, translation of both Fer transcripts was almost completely blocked, irrespective of iron (Figure 2A-B). In contrast, the fraction of polysome-bound ALAS-E mRNA almost doubled during differentiation. Furthermore, there was a more than 10-fold increase in total ALAS-E mRNA (Figure 2D). Interestingly, the 2.5-fold difference in the amount of polysome-bound ALAS-E transcripts (2.4-fold in protein expression) between samples treated with or without iron chelator only slightly affected hemoglobin formation (15% ± 2%; not shown). This suggests that ALAS-E alone is not limiting for hemoglobinization under physiologic conditions (12.5 μMFe2-Tf), in line with our observation that in ALA assays the factor of regulation by iron was smaller (1.6-fold ± 0.1) than on the level of ALAS-E-protein or -mRNA. These and related findings by others are most likely due to compensatory mechanisms.66,67 A potential contribution of the non–IRE-containing isoform of ALAS68 is unlikely, because ALAS-E–/– mouse embryos have no hemoglobinized cells and die at day E11.5, at the onset of fetal liver erythropoiesis.26
Why then should ALAS-E mRNA expression be regulated by iron at all? Translational repression in self-renewing cells, together with other mechanisms, might help to avoid premature onset of heme synthesis. One might further assume that, together with high expression of TfR1 mRNA, massive transcriptional activation of the ALAS-E gene leads to an excess of IRE sites over available IRP molecules. Thus abundant de novo synthesized ALAS-E transcripts would escape this inhibitory interaction due to the limiting amount of “free” IRPs available.
Reduced availability of IRPs and increase in IREs may be, however, insufficient to fully explain the observed uncoupling of translation efficiency between Fer and ALAS-E mRNAs during erythropoiesis. A second important factor in the translational activation of ALAS-E mRNA may be the potentially different role of IRP1 versus IRP2 in erythropoiesis. As recently reported, IRP2 knockout mice exhibit microcytic anemia.69,70 Although there are discrepancies regarding the regulation of Fer and ALAS-E in erythroid cells from these animals, which may arise from the use of total bone marrow cells versus sorted erythroid progenitors, both reports describe down-regulation of TfR1 in the erythroid compartment. On the other hand, mice lacking IRP1 did not show an erythroid phenotype. Thus, in mice, IRP2 can compensate for the loss of IRP1 but not vice versa.71 This, however, does not rule out an important function for IRP1 during erythropoiesis because heme synthesis in zebrafish is strongly connected to regulation of IRP1 binding activity.72 Moreover, the microcytic anemia observed in IRP2–/– mice is more severe in an IRP1+/– background, again arguing for a contribution of IRP1 to iron regulation in erythroid cells. These in vivo data and in vitro experiments showing a higher affinity of IRP2 for bulge/loop hairpins as in Fer mRNA73,74 plus high affinity of IRP2 for the multiple IREs in the context of TfR mRNA75 could explain the difference in IRE-mediated translational inhibition via IRPs in erythroid cells; IRP2 might be mainly responsible for regulation of TfR1, whereas IRP1 would preferentially modulate expression of ALAS-E. At present this scenario is speculative, especially because the existing knockout data on the roles of IRPs in the hematopoietic lineage are somewhat contradictory.
The observations described in this paper would fit to the so called “kiss-and-run” hypothesis.29,62 It suggests that during terminal erythropoiesis endosomes come into close vicinity/physical contact with mitochondria to directly shuttle iron into this organelle for heme synthesis.61,63 Thus, intermediary release of iron from endosomes into the cytosol would be avoided, rendering the metal concentration “low” for the IRE/IRP system. Alternatively, and to the same consequence, increased activity of mitochondrial iron importers may prevent metal accumulation in the cytosol. Increased iron influx into mitochondria also might explain why ALAS-E but not TfR1 or Fer mRNA remains subject to translational control during erythroid differentiation. Excess iron not incorporated into heme can be used for Fe-S cluster synthesis, which on export from mitochondria will reduce IRP1 mRNA-binding activity. Consequently, as described, this might activate mainly ALAS-E mRNA translation, as detected in polysome gradients and immunoprecipitations.
Building on the “kiss-and-run” hypothesis, we put our data into a working model of iron utilization in late-stage erythropoiesis (Figure 6). During differentiation, ALAS-E mRNA abundance increases. Because total IRP1 and IRP2 protein levels do not rise in parallel, IRP may become limiting. This might primarily affect ALAS-E mRNA due to the lower affinity of IRP1 for its IRE,73,74 resulting in enhanced ALAS-E synthesis. The increase in TfR1 expression can be reconciled with potentially limited availability of IRPs by a decline in the activity of the endonuclease involved in TfR mRNA turnover, as in chicken.55 At the same time, endosomes might increasingly shuttle their iron load directly toward mitochondria, requiring coordinated and directed vesicle flow (Figure 6A),61-63 most likely involving the iron transporter DMT1.76-78 In differentiating erythroblasts endosomes become increasingly acidified, favoring release of Fe from Fe2-Tf and its export.56 Furthermore, endosomes are redistributed from the cell periphery toward the deeper intracellular space, confirmed by confocal laser and electron microscopy (Lioba Lobmayr and Iris Killisch, unpublished data, June 2004). Thus, the cytosol may again be recognized as “low iron” by the IRE/IRP system, despite a massive net increase of iron import into the cell. Within mitochondria, iron will be efficiently incorporated in protoporphyrin IX by ferrochelatase to form heme, which is assembled into hemoglobin immediately after mitochondrial export.
To test the idea of a “low iron” cytosol, we artificially increased the cytosolic iron pool. For this we either inhibited heme biosynthesis or overloaded the cells with iron (Figure 6B). A block of heme synthesis by SA, an inhibitor of ALA-D,62 resulted in a significant increase in Fer protein. Most likely, this was due to an efflux of non–heme-bound iron via the mitochondrial iron export machinery,77 inducing secondary cytosolic iron overload (as confirmed by others26 ). In turn, this should reduce IRP mRNA-binding activity and actually increased Fer protein expression. Fer protein levels were similarly up-regulated on iron overload with FAC, which enters many cell types directly, bypassing TfR-mediated endocytosis.20-23 However, TfR-independent FAC uptake has not been rigorously confirmed for mouse erythroid progenitors so far.24,25,79
At present, we cannot address whether or not mitochondrial Fer80,81 may influence iron efflux versus storage in mitochondria. Although mitochondrial Fer has high homology to cytosolic Fer and is abundant in mitochondria of human patients with sideroblastic anemia, its role in healthy individuals is unclear.81,82
Here we present a comprehensive analysis of key players regulating iron metabolism in self-renewing versus differentiating primary mouse erythroblasts. Terminal erythropoiesis caused a switch of regulation to a mode where the IRP/IRE system sensed a low-iron state despite massively increased iron uptake, intracellular transport, and utilization for hemoglobin synthesis. The altered iron metabolism in differentiating erythroblasts may have evolved to ensure maintenance of high levels of TfR1 mRNA for rapid uptake of large amounts of Fe2-Tf. This mode of regulation should be important to ensure efficient heme production by synthesizing high levels of ALAS-E without favoring Fer mRNA translation, thus avoiding futile iron storage during a phase of high iron demand, a situation known to disturb hemoglobinization.31
Supported by the Austrian “Fonds zur Förderung der wissenschaftlichen Forschung (FWF)” (H.B. and E.W.M.) and the Herzfelder Family Foundation (E.W.M.), Vienna, Austria.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 19, 2006; DOI 10.1182/blood-2005-05-1809.
We thank R. S. Eisenstein for IRP1 antibody, T. Rouault and E. Meyron-Holtz for IRP2 antibody, M. Hentze and B. Galy for ALAS-E antiserum, L. C. Kühn for the FerH-IRE clone42, P. Ponka for many helpful discussions and suggestions, and W. Mikulits and C. Leberbauer for critically reading the manuscript.

![Figure 2. Translational repression of Fer mRNA and efficient utilization of ALAS-E mRNA in differentiating mouse erythroblasts. Self-renewing (designated “proliferation” in this and the following panels) or differentiating (labeled “48h diff”) primary mouse erythroblasts were incubated with the iron chelator desferrioxamine (Des, 50 μM) or physiologic concentrations of iron-loaded human transferrin (Tf, 12.5 μM) for 24 hours prior to harvesting. (A) Polysome gradient analysis. Cytoplasmic extracts were separated in linear 15% to 40% sucrose gradients39 and the RNA isolated from 18 fractions analyzed by Northern blotting. Fraction 1, top, fraction 18, bottom of the gradient. Filters were sequentially hybridized with [32P]-labeled probes specific for mouse FerH, FerL, ALAS-E, and (in the case of differentiating cells) α-globin mRNA as control. Bottom panel, loading control; methylene blue stain of total RNA. The constant molar ratio between 28S and 18S RNA (top and bottom band, respectively) around fraction 9 indicates the assembly of 80S initiation complexes and marks the approximate boundary between the ribosome-free, untranslated, and polyribosome-bound, translated mRNA compartment, as schematically depicted at the bottom. (B) Quantification of polysome-bound, translated mRNA. Bar diagrams depict the sum of the percentage of mRNA in fractions 9-18 as determined by PhosphoImage analysis. (C) Fer protein expression in proliferating and differentiating cells. The antibody used (see “Materials and methods”) recognizes both FerH and FerL. (D) ALAS-E expression as determined by immunoprecipitation of cell extracts (normalized to equal number of counts per sample) pulse labeled for 20 minutes with [35S]-methionine; to visualize the ALAS-E band in proliferating cells, this signal was amplified electronically 5 times. (E) Total mRNA levels for ALAS-E, FerH, and FerL mRNAs. Loading and quality control, 28S rRNA stained with methylene blue.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/10/10.1182_blood-2005-05-1809/2/m_zh80100695730002.jpeg?Expires=1769081417&Signature=MbeRa7d7loDENO4Kqx9e6vwBnwHrIEUdV-2c28dinmeoUKjuDaxFOtSa~GbPGn-vGyBllwXQgVP4htWSRKBbvAb2MElFNPdZlATi~5gavK4MUQY3ryXTDGNQTv8jrrdn5Q9tCOg5PLfvoxk42ywszFYipBAUpTXxgdeZdmDhGggyLV7IM~0D-96Xy6DJNsnYXHFSMkqEOauL3gXAmo5cBnDBYbKlhcxNwZMNQ47RMjJuHnDSY3~2gcHER8zi086oIYLuSRjURZ0H1Hft4FBrIvlr8BvH8nG3yHmZTajep1UuyA22NFY1DhPUsvab7b~6r9vU1RBjEZS4uiI4PaU6gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal