The association of fibroblast growth factor receptor 3 (FGFR3) expression with t(4;14) multiple myeloma (MM) and the demonstration of the transforming potential of this receptor tyrosine kinase (RTK) make it a particularly attractive target for drug development. We report here a novel and highly specific anti-FGFR3–neutralizing antibody (PRO-001). PRO-001 binds to FGFR3 expressed on transformed cells and inhibits FGFR3 autophosphorylation and downstream signaling. The antibody inhibited the growth of FGFR3-expressing FDCP cells (IC50 of 0.5 μg/mL) but not that of cells expressing FGFR1 or FGFR2, and potently inhibited FGFR3-dependent solid tumor growth in a mouse xenograft model. Furthermore, PRO-001 inhibited the growth of the FGFR3-expressing, human myeloma cell line, UTMC2. Inhibition of viability was still observed when cells were cocultured with stroma or in the presence of IL-6 or IGF-1. PRO-001 did not inhibit constitutive activation of K650E, G384D, and Y373C FGFR3 in myeloma cell lines and failed to inhibit the growth of these cells. Most importantly, however, PRO-001 induced cytotoxic responses in primary t(4;14)+ MM samples with an increase in apoptotic index of 20% to 80% as determined by annexin V staining. The data demonstrate that PRO-001 is a potent and specific inhibitor of FGFR3 and deserves further study for the treatment of FGFR3-expressing myeloma.
Introduction
Treatment of multiple myeloma (MM) has advanced over the past decade, resulting in prolongation of median survival from 3 to 5 years. Despite the improved outcome with treatment regimens that include dose intensification, patients invariably relapse, and MM remains a universally fatal disease.1-3 Because the limits of current chemotherapy have been reached, new approaches to therapy are urgently required. Various studies have delineated fundamental genetic lesions in MM that affect well-defined oncogenic pathways, growth, and survival signaling cascades.4,5 These key cellular and genetic pathogenic processes provide a framework to identify novel therapeutic targets.
The t(4;14)(p16.3;q32) translocation, which occurs in approximately 15% to 20% of MM tumors,6,7 results in the dysregulated expression of 2 putative oncogenes, MMSET and fibroblast growth factor receptor 3 (FGFR3).8 FGFR3 belongs to a family of 5 receptors, FGFR1-5, 4 of which harbor a functional tyrosine kinase. The FGFRs are characterized by 2 to 3 immunoglobulin (Ig)–like extracellular domains that bind ligand, a hydrophobic transmembrane domain, and a cytoplasmic region that contains a split tyrosine kinase domain.9 Binding of fibroblast growth factor (FGF) ligand and heparin promotes receptor dimerization and activation of the kinase domain, resulting in autophosphorylation of specific tyrosines. Activation of FGFRs transduces signals through mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3K) pathways, among others that regulate multiple cellular processes, including cell growth, differentiation, migration, and survival depending on the cellular context.9,10
Studies indicate that FGFR3 may play a significant, albeit not a singular, role in myeloma oncogenesis, thus making this receptor tyrosine kinase (RTK) an attractive target for therapeutic intervention. Activation of wild-type (WT) FGFR3 promotes proliferation of myeloma cells and is weakly transforming in a hematopoietic mouse model.11,12 Subsequent acquisition of activating mutations of FGFR3 in some MMs is associated with disease progression and is strongly transforming in several experimental models.12,13 In vitro studies suggest that FGFR3 can impart chemoresistance14 consistent with clinical data that demonstrate poor responses to conventional chemotherapy15,16 and shortened median survival of t(4;14) MM patients.15,17,18 The data have spurred the development of selective FGFR3 tyrosine kinase inhibitors for the potential treatment of MM. To date, several small-molecule inhibitors have been reported to induce cytotoxic responses of FGFR3-expressing myeloma cells.19-23 However, limitations of small-molecule inhibitors include substantial cross-inhibition of other kinases as well as acquired drug resistance based on selection of clones that no longer bind the drug as seen with imatinib for the treatment of chronic myelogenous leukemia (CML).24 The inherent lack of specificity of these drugs might limit their dose to a level below that required to achieve maximal target inhibition. Neutralizing antibodies, on the other hand, are by their nature highly specific and have high binding affinity and thus present an attractive alternative for the development of FGFR3 inhibitors. We describe here a fully human anti-FGFR3–neutralizing antibody (PRO-001) that selectively inhibits the proliferation of FGFR3-transformed cells and induces apoptosis of FGFR3-expressing human myeloma cells. These findings provide the framework for potential clinical application of anti-FGFR3 antibodies in t(4;14) MM.
Materials and methods
Reagents
FGF9 was prepared by ProChon Biotech. Recombinant IL-3 was supplied by PeproTech (London, United Kingdom). Rabbit anti-FGFR3 (H100 and C15) and rabbit anti–P-JNK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), mouse anti–P-ERK1/2 was from Cell Signaling Technology (Beverly, MA), and 4G10 was from R&D Systems (Minneapolis, MN). Purified goat anti–human F(ab′)2-phycoerythrin (PE) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). PD173074 was obtained from Pfizer (Ann Arbor, MI).
PRO-001
The phage display HuCAL-Fab 1 (Human Combinatorial Antibody Library) described in Rauchenberger et al25 was challenged against FGFR3 recombinant protein immobilized to 96-well plates (Maxisorp; Nunc, Rochester, NY) or on cells expressing FGFR3. The pool of phagemid from this alternate selection was amplified in Escherichia coli and subsequently purified. Fab-encoding fragments were excised as pool and cloned into the expression vector pMORPHX9_Fab_FS25 for transformation of TG1-F– (TG-1 depleted for the F pilus). Single-clone expression and preparation of periplasmic extract containing HuCAL-Fabs was described previously.26
Cell lines
Non–transformed rat chondrocyte cell line expressing inducible FGFR3 (RCJ-FGFR3) has been described previously.25 Cells were maintained in α-minimum essential media supplemented with 15% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 600 μg/mL G418 (Gibco Life Technologies, Burlington, ON, Canada), 2 μg/mL tetracycline (Sigma-Aldrich, Oakville, ON, Canada), and 50 μg/mL hygromycin B (Gibco BRL). FGFR3 expression was induced by removal of tetracycline. Serum starvation preceded ligand stimulation. The mouse myeloid progenitor cell line (FDCP-1) was transfected with full-length FGFR1 (FDCP-FGFR1), FGFR2 (FDCP-FGFR2), FGFR3 (FDCP-FGFR3), or FGFR3 mutant S249C (FDCP-FGFR35249C) cDNAs and cultured in Iscove medium (Gibco BRL) with 10% FCS, 100 μg/mL penicillin, 100 μg/mL streptomycin, 10 ng/mL FGF (FGF9 for FDCP-FGFR1 and FDCP-FGFR3 cells and FGF1 for FDCP-FGFR2 cells), and 5 μg/mL heparin (Sigma). Human myeloma cell lines were maintained in Iscove modified Dulbecco medium (IMDM) supplemented with 2.5% FCS and penicillin-streptomycin (Hyclone, Logan, UT). B9 cells expressing WT FGFR3 (B9-FGFR3WT), FGFR3-K650E (B9-FGFR3K650E), FGFR3-F394L (B9-FGFR3F394L), FGFR3-Y373C (B9-FGFR3Y373C), FGFR3-807C (B9-FGFR3807C), FGFR3-G384D (B9-FGFR3G384D), or empty retrovirus (B9-MINV) have been described previously.11,22 These were maintained in IMDM supplemented with 5% FCS, penicillin-streptomycin, and 1% IL-6–conditioned medium. Bone marrow stroma cells (BMSCs) were derived from bone marrow (BM) specimens obtained from patients with MM and prepared as previously described.27 BMSCs were grown on 6-well plates until confluent and were then irradiated with 20 Gy for the apoptosis studies (see “Apoptosis analysis”).
Immunoprecipitation and immunoblotting
Cells were lysed in lysis buffer (50 mM Tris/HCl, pH 8.0, 150 mM NaCl2, 0.1 mM ZnCl2, 0.5% Nonidet NP-40, 1 mg/mL, complete protease inhibitor mix [Roche Molecular Biochemicals, Mannheim, Germany]), and clarified by centrifugation at 12 000g for 15 minutes. The lysates were subjected to immunoprecipitation for 16 hours at 4°C with anti-FGFR3 (C15) and analyzed by 7.5% sodium dodecyl-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. Protein bands were visualized using secondary antibodies coupled to horseradish peroxidase and the enhanced chemiluminescence (ECL) kit from Pierce (Rockford, IL) according to the manufacturer's instructions.
Viability assay
Cell viability was assessed by 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium (MTT) or (2,3-bis (2-methoxy-4-nitro-5-sulphophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) dye absorbance where indicated. Cells were seeded in 96-well plates at a density of 20 000 (FDCP-1 cells), 5000 (B9 cells), or 25 000 cells (MM cell lines) per well in culture medium. Cells were incubated in the absence or presence of one or more of the following cytokines: 10 ng/mL FGF9 and 5 μg/mL heparin, 1% murine IL-6, 50 ng/mL IGF-1, or 50 ng/mL human IL-6 where indicated and increasing concentrations of PRO-001, control antibody (purified human Fab), or 100 nM PD173074. Plates were incubated for 48 or 72 hours at 37°C, 5% CO2. MTT and XTT assays were performed according to the manufacturer's instruction (Boehringer Mannheim, Mannheim, Germany and Roche Applied Science, Mannheim, Germany). Each experimental condition was performed in duplicate or triplicate.
Flow cytometric analysis
Cells (5 × 105) were washed in cold phosphate-buffered saline (PBS) and then incubated for 30 minutes with one of the following: PRO-001 Fab or human Fab control antibody, rabbit anti-FGFR3 (H100) or rabbit preimmune serum in PBS. The cells were then stained with PE-conjugated goat anti–human F(ab′)2 secondary antibody or goat anti–rabbit IgG-PE for 30 minutes on ice. Flow cytometry was performed on a fluorescence-activated cell-sorting flow cytometer (FACSCaliber, BD Biosciences, San Jose, CA) and analyzed using CellQuest software (BD Biosciences). To assess the ability of FGF ligand to compete for binding, cells were incubated in the presence or absence of 30 ng/mL FGF and 5 μg/mL heparin for 30 minutes and then stained with PRO-001 Fab as described.
Intracellular phospho-protein staining
Determination of ERK1/2 phosphorylation by flow cytometry has been described previously.28 Cells were serum starved overnight and then stimulated with 10 ng/mL FGF9 and 5 μg/mL heparin for 10 minutes at 37°C. The cells were immediately fixed by adding 10% formaldehyde directly into the culture medium to obtain a final concentration of 2%. Cells were incubated in fixative for 10 minutes at 37°C then on ice for an additional 2 minutes. The cells were permeabilized by adding ice-cold methanol (to a final concentration of 90%) while vortexing and incubated on ice for 30 minutes. Cells were washed with PBS plus 4% FCS, stained with anti–P-ERK1/2 for 15 minutes, and then labeled with fluorescein isothiocyanate (FITC)–conjugated goat anti–rabbit and anti–CD138-PE (PharMingen, San Diego, CA) where indicated. Malignant cells were identified as cells that expressed high levels of CD138. Flow cytometry was performed on a FACSCaliber flow cytometer (BD Biosciences) and analyzed using CellQuest software (BD Biosciences).
Apoptosis analysis
For studies of apoptosis, cells were seeded at an initial density of 2.5 × 105/mL in 6-well plates coated with BMSCs and supplemented with control antibody or 5 μg/mL PRO-001 and cultured for 48 hours in IMDM containing 2.5% FCS. Apoptosis of suspension cells that were almost exclusively CD38++/CD45dim was determined by annexin V staining (Boehringer Mannheim, Indianapolis, IN) and analyzed by flow cytometry.
Primary patient samples
Patients identified for the study were determined to possess a t(4;14) translocation by fluorescence in situ hybridization (FISH). Expression of FGFR3 was confirmed by flow cytometry as previously described.13 All t(4;14)+ samples were further analyzed for the presence of FGFR3 mutations. Four pairs of primers were designed to amplify the regions of FGFR3 containing codons of the extracellular domain, transmembrane domain, tyrosine kinase domain, and stop codon, known hot spots for activating mutations. A first polymerase chain reaction (PCR) was performed on gDNA extracted from CD138 purified myeloma cells and amplicons were used for analysis by denaturing high-performance liquid chromatography (DHPLC). Results were confirmed by sequence analysis of the PCR products.
For cell-death analysis, mononuclear cells freshly isolated from BM aspirates were separated by Ficoll-Hypaque gradient sedimentation and plated at a cell density of 5 × 105 cells/mL in IMDM supplemented with 20% FCS, 1% glutamine, penicillin-streptomycin, and 10 ng/mL FGF9 and 5 μg/mL heparin. Cells were cultured in the presence of control or 5 μg/mL PRO-001 for up to 12 days. The medium, FGF/heparin, and drug were replenished every 3 days. After 3, 7, and 12 days, cells were triple stained with anti–CD38-PE, anti–CD45-CyChrome (PharMingen), and FITC-conjugated annexin V or labeled with anti–CD138-PE and FITC-conjugated annexin V. Controls included unstained cells, isotype control stained cells, and single stained cells. Malignant plasma cells were defined as cells that expressed CD138 or high levels of CD38 and no or low levels of CD45 (CD38++/CD45–). Samples were analyzed by FACScan analysis using CellQuest software. BM aspirates were obtained by consent under approved protocol by the University Health Network Research Ethics Board (Toronto, ON, Canada).
Xenograft mouse model
FDCP-FGFR3S249C cells were washed 3 times in PBS then resuspended at 2 × 106 cells/200 μL PBS. The cells were injected subcutaneously into CD1 nude adult females (Harlan Laboratories, Jerusalem, Israel) with a 25-gauge needle at one or both mouse flanks. Treatment was initiated 1 week after tumor inoculation at which time mice were randomized to receive PRO-001 or an equal volume of PBS alone. Dosing was performed twice weekly by intraperitoneal injection for 3 weeks. Three mice were randomized per treatment group. Mice were followed every 2 to 4 days and developing tumors were measured at 3 dimensions using a caliper. Tumor volume was estimated by multiplying these 3 values.
Results
Blocking activity of PRO-001 and selectivity for FGFR3
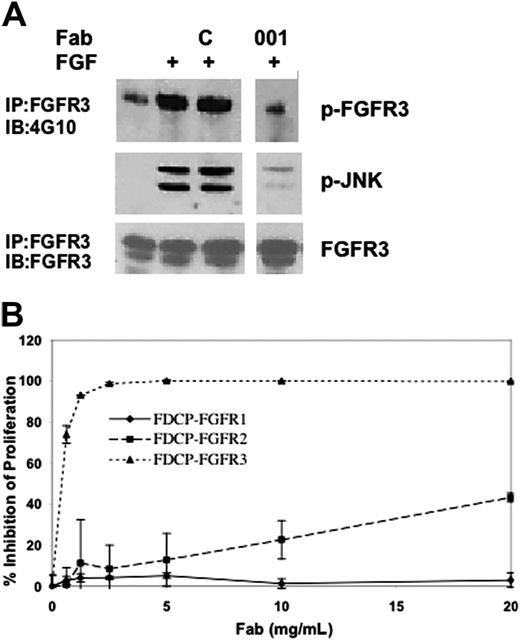
The human anti-FGFR3 Fab PRO-001 was isolated from the Hu-CAL-Fab-1 human combinatorial library using a differential whole-cell panning approach.25 To confirm that PRO-001 inhibits the kinase activity of FGFR3, we tested the effect of PRO-001 on ligand-stimulated receptor phosphorylation in RCJ cells transfected with WT FGFR3 (RCJ-FGFR3). Antiphosphotyrosine immunoblots revealed enhanced autophosphorylation of FGFR3 on ligand stimulation that was inhibited by PRO-001 but not by the control Fab (Figure 1A). Inhibition of FGFR3 activation was associated with reduction in downstream JNK phosphorylation. To confirm the specificity and blocking activity of PRO-001 in a cell-based assay, we tested the activity of PRO-001 against FGFR1-3–expressing FDCP-1 cell lines. Cell growth of FDCP-1 is normally dependent on the presence of IL-3. However, IL-3 can be substituted by FGF ligand in cells expressing the cognate RTK. FGF-stimulated proliferation of FDCP-FGFR3 cells was potently inhibited by PRO-001, with a concentration that inhibits 50% (IC50) of 0.5 μg/mL (Figure 1B). In contrast, the proliferation of FDCP-1 cells expressing FGFR1 or FGFR2 was unaffected at up to 40- or 10-fold higher concentrations, respectively. Thus, PRO-001 is a highly specific and potent inhibitor of FGFR3.
PRO-001 inhibits FGF-induced phosphorylation of WT-FGFR3 and proliferation of FGFR3-expressing FDCP cells. (A) RCJ cells overexpressing FGFR3 (RCJ-FGFR3) were serum starved, and then stimulated (+) with 20 ng/mL FGF9 for 5 minutes alone or following preincubation with a control (C) or a neutralizing anti-FGFR3 Fab (001). Total cell lysates were probed by Western blot with anti–P-JNK. In parallel, lysates were immunoprecipitated with anti-FGFR3 antibody and immunocomplexes were analyzed by Western with antiphosphotyrosine (4G10) and anti-FGFR3. (B) FDCP cells that stably express FGFR1, FGFR2, or FGFR3 were cultured in the presence of increasing amounts of PRO-001. Two days later, cell proliferation was determined by XTT analysis. Data are the average of duplicate cultures.
PRO-001 inhibits FGF-induced phosphorylation of WT-FGFR3 and proliferation of FGFR3-expressing FDCP cells. (A) RCJ cells overexpressing FGFR3 (RCJ-FGFR3) were serum starved, and then stimulated (+) with 20 ng/mL FGF9 for 5 minutes alone or following preincubation with a control (C) or a neutralizing anti-FGFR3 Fab (001). Total cell lysates were probed by Western blot with anti–P-JNK. In parallel, lysates were immunoprecipitated with anti-FGFR3 antibody and immunocomplexes were analyzed by Western with antiphosphotyrosine (4G10) and anti-FGFR3. (B) FDCP cells that stably express FGFR1, FGFR2, or FGFR3 were cultured in the presence of increasing amounts of PRO-001. Two days later, cell proliferation was determined by XTT analysis. Data are the average of duplicate cultures.
PRO-001 demonstrates variable activity against FGFR3 mutants
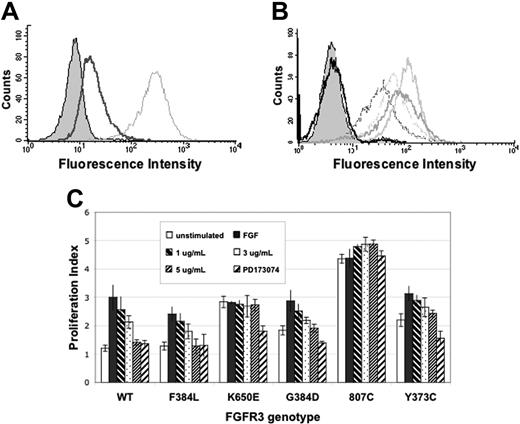
We next tested the ability of PRO-001 to bind to and inhibit constitutively activated FGFR3 mutants identified in MM cell lines (Y373C, G384D, K650E, 807C).13,29 Stable expression of these cDNAs confers IL-6–independent growth to B9 cells and provides a platform for testing potential FGFR3 inhibitors against the various classes of FGFR3 mutations. FACS analysis reveals that PRO-001 IgG binds to WT and FGFR3-F394L (a non–transforming polymorphism) and that the binding to B9-WT cells can be reduced by addition of FGF ligand, further supporting the notion that PRO-001 and FGF share a common epitope (Figure 2A-B). PRO-001 also recognized Y373C-, K650E-, and G384D-FGFR3 mutants but failed to bind to 807C-FGFR3 and did not bind to B9-MINV control cells. The 807C mutation occurs within the stop codon and results in an elongated protein that has transforming potential in experimental models.30 To rule out the possibility that this modified form of FGFR3 is not expressed on the cell surface, B9807C cells were labeled with H100, a commercially available nonfunctional antibody to FGFR3. In contrast to PRO-001, H100 recognized all FGFR3 mutants expressed in B9 cells (data not shown).
To further determine the effect of PRO-001 on WT and mutant FGFR3-mediated cell growth, B9 cells expressing FGFR3-WT and FGFR3-F394L and the FGFR3-activated mutants were grown in the presence of increasing concentrations of inhibitor for 48 hours following which viability was determined by MTT assay (Figure 2C). As predicted, B9-WT and B9-F394L failed to proliferate in the absence of FGF9 (proliferation index of 1.2 ± 0.2 and 1.3 ± 0.2, respectively) and responded to FGF9 with a 3.0- and 2.8-fold increase in optical density (OD) reading, respectively. PRO-001 inhibited the FGF-stimulated growth of B9WT and B9F394L cells in a dose-dependent manner with an IC50 of approximately 3 μg/mL. In contrast, B9 cells expressing constitutively activated FGFR3 mutants proliferated in the absence of FGF9 with PIs ranging from 1.8 to 4.4, depending on the FGFR3 mutant. Addition of FGF9 further enhanced the growth of B9 cells expressing G384D and Y373C-FGFR3, an effect that was blocked by PRO-001 consistent with its ability to inhibit FGF ligand binding. Of note, however, and in contrast to PD173074, PRO-001 failed to completely inhibit ligand-independent growth of these cells. Further, PRO-001 did not inhibit the growth of B9K650E and B9807C cells that appear to be maximally activated in the absence of ligand. In comparison, PD173074, a small-molecule inhibitor of FGFR3, nearly completely inhibited the growth of all FGFR3-expressing B9 cells with the exception of B9807C.
PRO-001 binds to WT and mutant FGFR3, competes with FGF for FGFR3 binding, and inhibits the growth of B9WT cells. PRO-001 binding to FGFR3 was determined by flow cytometric analysis using a PE-conjugated anti–human secondary antibody. (A) Competition of PRO-001 binding to cell-surface FGFR3 on B9WT cells. The filled histogram indicates parental B9 cells; dotted gray line, B9WT without FGF9; solid line, B9WT in the presence of FGF9. (B) PRO-001 binding to cell-surface WT and mutant FGFR3 on B9 cells. The filled histogram indicates parental cells; thick light gray line, B9Y373C; thick dark gray line, B9K650E; dotted gray line, B9G384D; dotted black line, B9WT; solid black line, B9807C. (C) B9 cells were stimulated with FGF and incubated with increasing concentrations of PRO-001 or 100 nM PD173074 for 48 hours, and cell viability was assessed by MTT-based assay. □ indicates unstimulated; ▪, FGF stimulated; ▧, FGF plus 1 μg/mL PRO-001;  , FGF plus 3 μg/mL PRO-001; ▨, FGF plus 5 μg/mL PRO-001; ▨, FGF9 plus 100 nM PD173074. Proliferation index (PI) = (ODd2)/(ODd0), where ODd2 and ODd0 are the optical density (OD) at 48 hours and day 0, respectively. When PI = 1, cells did not proliferate after 48 hours in culture. Values represent mean ± SD of 4 independent experiments.
, FGF plus 3 μg/mL PRO-001; ▨, FGF plus 5 μg/mL PRO-001; ▨, FGF9 plus 100 nM PD173074. Proliferation index (PI) = (ODd2)/(ODd0), where ODd2 and ODd0 are the optical density (OD) at 48 hours and day 0, respectively. When PI = 1, cells did not proliferate after 48 hours in culture. Values represent mean ± SD of 4 independent experiments.
PRO-001 binds to WT and mutant FGFR3, competes with FGF for FGFR3 binding, and inhibits the growth of B9WT cells. PRO-001 binding to FGFR3 was determined by flow cytometric analysis using a PE-conjugated anti–human secondary antibody. (A) Competition of PRO-001 binding to cell-surface FGFR3 on B9WT cells. The filled histogram indicates parental B9 cells; dotted gray line, B9WT without FGF9; solid line, B9WT in the presence of FGF9. (B) PRO-001 binding to cell-surface WT and mutant FGFR3 on B9 cells. The filled histogram indicates parental cells; thick light gray line, B9Y373C; thick dark gray line, B9K650E; dotted gray line, B9G384D; dotted black line, B9WT; solid black line, B9807C. (C) B9 cells were stimulated with FGF and incubated with increasing concentrations of PRO-001 or 100 nM PD173074 for 48 hours, and cell viability was assessed by MTT-based assay. □ indicates unstimulated; ▪, FGF stimulated; ▧, FGF plus 1 μg/mL PRO-001;  , FGF plus 3 μg/mL PRO-001; ▨, FGF plus 5 μg/mL PRO-001; ▨, FGF9 plus 100 nM PD173074. Proliferation index (PI) = (ODd2)/(ODd0), where ODd2 and ODd0 are the optical density (OD) at 48 hours and day 0, respectively. When PI = 1, cells did not proliferate after 48 hours in culture. Values represent mean ± SD of 4 independent experiments.
, FGF plus 3 μg/mL PRO-001; ▨, FGF plus 5 μg/mL PRO-001; ▨, FGF9 plus 100 nM PD173074. Proliferation index (PI) = (ODd2)/(ODd0), where ODd2 and ODd0 are the optical density (OD) at 48 hours and day 0, respectively. When PI = 1, cells did not proliferate after 48 hours in culture. Values represent mean ± SD of 4 independent experiments.
PRO-001 inhibits growth and is cytotoxic to UTMC2 MM cells
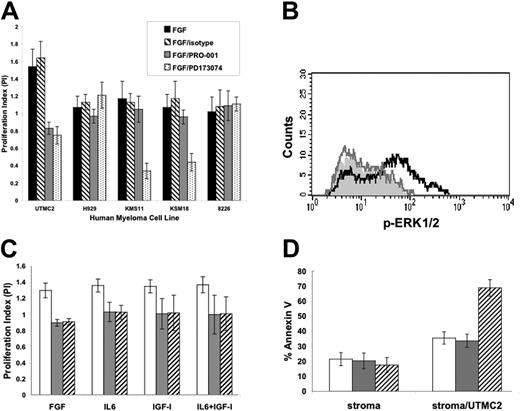
PRO-001 was tested against t(4;14) myeloma cell lines that express WT-FGFR3 (UTMC2, H929) and mutant FGFR3 (KMS11, KMS18). Cell growth in the presence of FGF and PRO-001, control antibody, or PD173074 was determined by MTT assay. Consistent with the results in B9 cells, PRO-001 failed to inhibit the viability of KMS11 (FGFR3-Y373C) and KMS18 (FGFR3-G384D), cells that express mutant FGFR3 and grow independent of FGF ligand (Figure 3A). Similarly, H929 cells that express WT-FGFR3 but in addition harbor a downstream activating mutation of N-Ras and 8226 cells that lack FGFR3 do not demonstrate enhanced proliferation in response to FGF when grown in the presence of 2.5% serum and are not sensitive to PRO-001 or PD173074. On the other hand, stimulation of UTMC2 (WT-FGFR3) cells with FGF in the presence of 2.5% FCS induced ERK phosphorylation and proliferation was significantly inhibited by PRO-001 (Figure 3A-B). Inhibition of FGF-stimulated growth of UTMC2 by PRO-001 was comparable to that induced by PD173074. Of note, we have previously demonstrated that FGFR3-expressing HMCLs (UTMC2, H929, KMS11, and KMS18) when grown in serum-free conditions display FGF-stimulated growth responses that are inhibited by PD173074.19 Under these culture conditions PRO-001 similarly inhibits FGF-mediated growth (data not shown) consistent with the ability of PRO-001 to inhibit FGF-ligand binding to HMCLs and in agreement with results in B9 cells.
Inhibition of viability with PRO-001 was still observed when UTMC2 cells were grown in the presence of 50 ng/mL IL-6 or 50 ng/mL IGF-1 or both, and was comparable to that of cells cultured in the presence of FGF alone (Figure 3C), demonstrating that paracrine factors that signal via overlapping signaling pathways and are known to confer drug resistance fail to overcome the antitumor effects of FGFR3 inhibition. Further, PRO-001 induced apoptosis of UTMC2 cells cocultured on BMSCs but had no direct toxicity on BMSCs when compared to control antibody (Figure 3D). These data are consistent with previous studies of FGFR3 and insulin-like growth factor receptor 1 (IGF-1R) small-molecule inhibitors in myeloma.22,31 The mechanisms by which inhibition of a selective growth factor overrides the protective effects of IL-6, IGF-1, or stroma are not clear; however, they suggest a critical role for FGFR3 in sensitive t(4;14) myeloma cells.
PRO-001 inhibits FGF-mediated ERK1/2 phosphorylation and inhibits the viability of UTMC2 in the presence of FGF, IL-6, IGF-1, and BMSCs. (A) Human myeloma cell lines (HMCLs) expressing FGFR3 (UTMC2, H929, KMS11, KMS18) or not (8226) were stimulated with FGF and incubated with 5 μg/mL PRO-001, control antibody, or 100 nM PD173074. Growth at 48 hours was assessed by MTT assay and is reported as PI = (OD+FGF±DRUG)/(OD-FGF), where OD+FGF±DRUG is the OD in the presence of FGF with or without inhibitor and OD-FGF is the OD in the absence of FGF. When PI = 1, this indicates no enhanced growth with the addition of FGF. ▪ indicates stimulated with FGF; ▧, FGF plus control antibody;  , FGF plus 5 μg/mL PRO-001;, FGF plus 100 nM PD173074. (B) Flow cytometric analyses of ERK1/2 phosphorylation. UTMC2 cells were stimulated with aFGF (black line) or pretreated with 5 μg/mL PRO-001 and then stimulated with FGF9 (gray line). The filled histogram indicates unstimulated cells. (C) UTMC2 cells were cultured in media containing 2.5% FCS and 10 ng/mL FGF9 with control antibody (□), 5 μg/mL PRO-001 (
, FGF plus 5 μg/mL PRO-001;, FGF plus 100 nM PD173074. (B) Flow cytometric analyses of ERK1/2 phosphorylation. UTMC2 cells were stimulated with aFGF (black line) or pretreated with 5 μg/mL PRO-001 and then stimulated with FGF9 (gray line). The filled histogram indicates unstimulated cells. (C) UTMC2 cells were cultured in media containing 2.5% FCS and 10 ng/mL FGF9 with control antibody (□), 5 μg/mL PRO-001 ( ), or 100 nM PD173074 (▧) in the presence or absence of 50 ng/ml IL-6 or 50 ng/ml IGF-I or both. Cell viability after 48 hours was assessed by MTT assay and is reported as PI = (OD+CYTOKINES)/(OD-FGF), where OD+CYTOKINES is the OD in the presence of FGF with or without IL-6 or IGF-I or both and OD-FGF is the OD in the absence of FGF. When PI = 1, this indicates no enhanced growth with the addition of cytokines. (D) BMSCs alone or BMSCs together with UTMC2 cells (□) were cultured with control antibody (
), or 100 nM PD173074 (▧) in the presence or absence of 50 ng/ml IL-6 or 50 ng/ml IGF-I or both. Cell viability after 48 hours was assessed by MTT assay and is reported as PI = (OD+CYTOKINES)/(OD-FGF), where OD+CYTOKINES is the OD in the presence of FGF with or without IL-6 or IGF-I or both and OD-FGF is the OD in the absence of FGF. When PI = 1, this indicates no enhanced growth with the addition of cytokines. (D) BMSCs alone or BMSCs together with UTMC2 cells (□) were cultured with control antibody ( ) or 5 μg/mL PRO-001 (▧) for 72 hours and apoptosis was assessed by means of flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent means of quadruplicate cultures ± SD.
) or 5 μg/mL PRO-001 (▧) for 72 hours and apoptosis was assessed by means of flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent means of quadruplicate cultures ± SD.
PRO-001 inhibits FGF-mediated ERK1/2 phosphorylation and inhibits the viability of UTMC2 in the presence of FGF, IL-6, IGF-1, and BMSCs. (A) Human myeloma cell lines (HMCLs) expressing FGFR3 (UTMC2, H929, KMS11, KMS18) or not (8226) were stimulated with FGF and incubated with 5 μg/mL PRO-001, control antibody, or 100 nM PD173074. Growth at 48 hours was assessed by MTT assay and is reported as PI = (OD+FGF±DRUG)/(OD-FGF), where OD+FGF±DRUG is the OD in the presence of FGF with or without inhibitor and OD-FGF is the OD in the absence of FGF. When PI = 1, this indicates no enhanced growth with the addition of FGF. ▪ indicates stimulated with FGF; ▧, FGF plus control antibody;  , FGF plus 5 μg/mL PRO-001;, FGF plus 100 nM PD173074. (B) Flow cytometric analyses of ERK1/2 phosphorylation. UTMC2 cells were stimulated with aFGF (black line) or pretreated with 5 μg/mL PRO-001 and then stimulated with FGF9 (gray line). The filled histogram indicates unstimulated cells. (C) UTMC2 cells were cultured in media containing 2.5% FCS and 10 ng/mL FGF9 with control antibody (□), 5 μg/mL PRO-001 (
, FGF plus 5 μg/mL PRO-001;, FGF plus 100 nM PD173074. (B) Flow cytometric analyses of ERK1/2 phosphorylation. UTMC2 cells were stimulated with aFGF (black line) or pretreated with 5 μg/mL PRO-001 and then stimulated with FGF9 (gray line). The filled histogram indicates unstimulated cells. (C) UTMC2 cells were cultured in media containing 2.5% FCS and 10 ng/mL FGF9 with control antibody (□), 5 μg/mL PRO-001 ( ), or 100 nM PD173074 (▧) in the presence or absence of 50 ng/ml IL-6 or 50 ng/ml IGF-I or both. Cell viability after 48 hours was assessed by MTT assay and is reported as PI = (OD+CYTOKINES)/(OD-FGF), where OD+CYTOKINES is the OD in the presence of FGF with or without IL-6 or IGF-I or both and OD-FGF is the OD in the absence of FGF. When PI = 1, this indicates no enhanced growth with the addition of cytokines. (D) BMSCs alone or BMSCs together with UTMC2 cells (□) were cultured with control antibody (
), or 100 nM PD173074 (▧) in the presence or absence of 50 ng/ml IL-6 or 50 ng/ml IGF-I or both. Cell viability after 48 hours was assessed by MTT assay and is reported as PI = (OD+CYTOKINES)/(OD-FGF), where OD+CYTOKINES is the OD in the presence of FGF with or without IL-6 or IGF-I or both and OD-FGF is the OD in the absence of FGF. When PI = 1, this indicates no enhanced growth with the addition of cytokines. (D) BMSCs alone or BMSCs together with UTMC2 cells (□) were cultured with control antibody ( ) or 5 μg/mL PRO-001 (▧) for 72 hours and apoptosis was assessed by means of flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent means of quadruplicate cultures ± SD.
) or 5 μg/mL PRO-001 (▧) for 72 hours and apoptosis was assessed by means of flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent means of quadruplicate cultures ± SD.
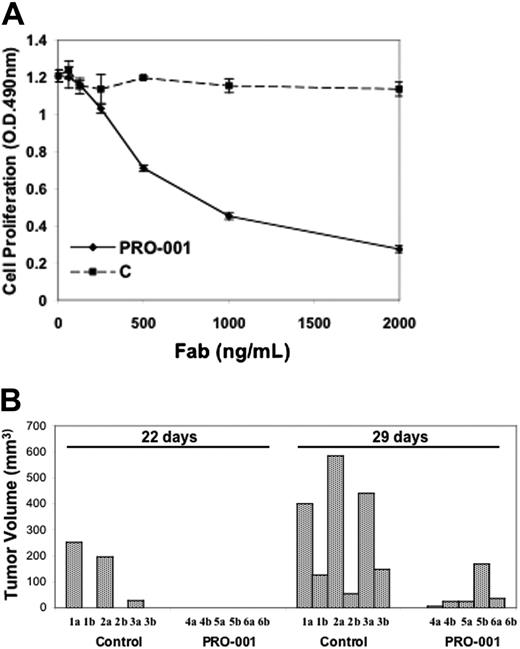
PRO-001 demonstrates in vivo antitumor activity in an FGFR3-driven xenograft tumor model. (A) FDCP-FGFR3S249C cells were cultured in the presence of increasing amounts of PRO-001 or control antibody (C). Two days later, cell proliferation was determined by XTT analysis. Data are the average of duplicate cultures. (B) Nude mice (3 in each group) were injected subcutaneously at 2 locations, one on each flank (a, right flank; b, left flank), with 2 × 106 FDCP-FGFR3S249C cells each. A week later, mice were randomized to receive PRO-001 or PBS control by intraperitoneal injection, according to the schedule described in Table 1. Tumor volume was estimated from measurements in 3 dimensions at 22 or 29 days after cell injection.
PRO-001 demonstrates in vivo antitumor activity in an FGFR3-driven xenograft tumor model. (A) FDCP-FGFR3S249C cells were cultured in the presence of increasing amounts of PRO-001 or control antibody (C). Two days later, cell proliferation was determined by XTT analysis. Data are the average of duplicate cultures. (B) Nude mice (3 in each group) were injected subcutaneously at 2 locations, one on each flank (a, right flank; b, left flank), with 2 × 106 FDCP-FGFR3S249C cells each. A week later, mice were randomized to receive PRO-001 or PBS control by intraperitoneal injection, according to the schedule described in Table 1. Tumor volume was estimated from measurements in 3 dimensions at 22 or 29 days after cell injection.
Evaluation of PRO-001 in an FGFR3-driven mouse tumor model
We next asked if PRO-001 could prove effective against an in vivo model of FGFR3-mediated tumor growth. UTMC2 cells, when injected into immunodeficient mice, grow with low penetrance and at a variable rate and therefore do not represent an informative model for the study of tumor progression. FDCP cells that express the constitutive mutant FGFR3S249C (FDCP-FGFR3S249C) proliferate in the absence of IL-3 and FGF and rapidly (within 2-3 weeks) form tumors on injection to nude mice. We showed that PRO-001 efficiently blocked this ligand-independent proliferation in vitro (Figure 4A). We therefore set out to determine the effectiveness of the antibody in vivo. Nude mice were injected subcutaneously at 2 locations, one on each flank, with 2 × 106 FDCP-FGFR3S249C cells each. A week after cell injection, mice were treated with PRO-001 Fab. During the first week of treatment, mice received a relatively high dose of about 1 mg Fab per mouse to saturate FGFR3. This was followed by slightly reduced doses during the following 12 days of Fab delivery (Table 1). Mice were treated every 3 days on average because we found no significant difference in efficacy of this schedule in comparison to daily injections (not shown). Four weeks after cell injection, PRO-001 dramatically reduced tumor growth to 10% on average of that in the control mice (Figure 4B). No major toxicities or significant weight loss were observed over the treatment period.
Schedule and dosing of PRO-001 Fab or PBS administration
. | Days after FDCP-FGFR3S249C cell injection . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 7 . | 10 . | 13 . | 16 . | 20 . | 23 . | 25 . | ||||||
| PRO-001, μg | 400 | 400 | 275 | 275 | 275 | 275 | 275 | ||||||
. | Days after FDCP-FGFR3S249C cell injection . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 7 . | 10 . | 13 . | 16 . | 20 . | 23 . | 25 . | ||||||
| PRO-001, μg | 400 | 400 | 275 | 275 | 275 | 275 | 275 | ||||||
Effect of PRO-001 on primary MM samples
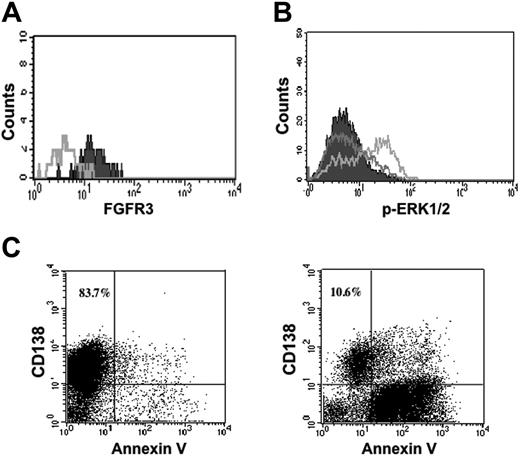
Given the limitation of the mouse model for the study of myeloma, the next experiments were designed to examine the effect of PRO-001 on primary human MM cells. We tested BM samples from 10 patients, 5 of whom were t(4;14)+ by FISH. The characteristics of the samples including measurement of FGFR3 expression by flow cytometry and FGFR3 genotype have been reported previously and are summarized in Table 2.22 Of the t(4;14)+ samples tested, all demonstrated surface expression of FGFR3 on CD138 myeloma cells and no mutations of FGFR3 were identified. In the t(4;14)+ primary myeloma cells, adding FGF increased downstream ERK phosphorylation, demonstrating that these cells express a functional receptor. PRO-001 bound to FGFR3 on the cell surface and blocked FGF-induced ERK phosphorylation in myeloma cells (Figure 5A-B). The mononuclear cell fractions isolated from fresh bone marrow samples were incubated with 5 μg/mL PRO-001 or isotype control, and apoptosis was determined by annexin V staining of CD38++/ CD45– cells and loss of surface CD138 expression (Figure 5C). All FGFR3-expressing myeloma samples were sensitive to treatment, demonstrating potent apoptotic responses to PRO-001 when compared to the control antibody. Further, the cytotoxic effect was selective in that none of the t(4;14)– samples demonstrated increased apoptosis in response to PRO-001.
Summary of expression of FGFR3 on primary MM cells in relation to sensitivity to PRO-001
Patient no. . | FGFR3 by flow cytometry . | FGFR3 genotype . | % annexin V control . | % annexin V PRO-001, 5 μg/mL . | % increase annexin V . |
|---|---|---|---|---|---|
| 1 | ND | WT | 8.0 | 47.6 | 36.5 |
| 2 | + | WT | 12.4 | 35.7 | 20.4 |
| 3 | ++ | WT | 35.3 | 67.5 | 32.2 |
| 4 | ++ | WT | 18.2 | 98.3 | 80.0 |
| 5 | +++ | WT | 10.0 | 28.3 | 18.3 |
| 6 | - | ND | 5.0 | 10.6 | 5.6 |
| 7 | - | ND | 12.9 | 9.9 | -3.0 |
| 8 | - | ND | 23.0 | 30.0 | 7.0 |
| 9 | - | ND | 10.8 | 9.5 | -1.3 |
| 10 | - | ND | 22.3 | 24.3 | 2.0 |
Patient no. . | FGFR3 by flow cytometry . | FGFR3 genotype . | % annexin V control . | % annexin V PRO-001, 5 μg/mL . | % increase annexin V . |
|---|---|---|---|---|---|
| 1 | ND | WT | 8.0 | 47.6 | 36.5 |
| 2 | + | WT | 12.4 | 35.7 | 20.4 |
| 3 | ++ | WT | 35.3 | 67.5 | 32.2 |
| 4 | ++ | WT | 18.2 | 98.3 | 80.0 |
| 5 | +++ | WT | 10.0 | 28.3 | 18.3 |
| 6 | - | ND | 5.0 | 10.6 | 5.6 |
| 7 | - | ND | 12.9 | 9.9 | -3.0 |
| 8 | - | ND | 23.0 | 30.0 | 7.0 |
| 9 | - | ND | 10.8 | 9.5 | -1.3 |
| 10 | - | ND | 22.3 | 24.3 | 2.0 |
FGFR3 expression on CD138 primary MM cells was analyzed by flow cytometry and the fluorescence was expressed as follows: +, weak; ++ intermediate; +++ strong; -, absent. CD138 selected cells were screened for the FGFR3 mutations.
ND indicates not determined; WT, wild-type status.
Discussion
Protein tyrosine kinases and particularly RTKs are key players in oncogenic pathways leading to a variety of human malignancies. The recent discovery and clinical success of several tyrosine kinase inhibitors, including imatinib as a prominent example, marked kinase inhibition as a legitimate therapeutic modality.32-35 An unexpected but important concept that has emerged is the notion of “kinase dependency” and “oncogene addiction.”36 These terms relate to the observations that tumors come to require activated oncogenes such as mutated protein tyrosine kinases (eg, BCR-ABL in CML) such that their inhibition, even in the presence of other oncogenic lesions, can inhibit tumor growth and survival. It is not surprising therefore that protein kinases have emerged as a most highly pursued class of new drug targets in oncology and other disease areas.
PRO-001 binds to FGFR3, inhibits downstream ERK1/2 phosphorylation, and induces apoptosis of primary t(4;14)+ MM cells. (A) Freshly isolated BMNCs were stained with PRO-001 (filled) or control antibody (open) and then stained with PE-conjugated anti–human secondary antibody. Myeloma cells were identified by CD138 labeling. (B) Primary myeloma cells were incubated in the absence (filled) or presence of FGF (gray line) or preincubated with 5 μg/mL PRO-001 (black line) for 2 hours and then stimulated with FGF. ERK1/2 phosphorylation was assessed by flow cytometric analysis. (C) Primary myeloma cells were cultured in the presence of control Fab (left panel) or 5 μg/mL PRO-001 (right panel). Cells were harvested after 7 days and stained with annexin V-FITC and analyzed by flow cytometry. Myelomas cells were identified as CD138+. The total percentage of CD138+ cells is shown in the top right quadrant. Shown is a representative experiment.
PRO-001 binds to FGFR3, inhibits downstream ERK1/2 phosphorylation, and induces apoptosis of primary t(4;14)+ MM cells. (A) Freshly isolated BMNCs were stained with PRO-001 (filled) or control antibody (open) and then stained with PE-conjugated anti–human secondary antibody. Myeloma cells were identified by CD138 labeling. (B) Primary myeloma cells were incubated in the absence (filled) or presence of FGF (gray line) or preincubated with 5 μg/mL PRO-001 (black line) for 2 hours and then stimulated with FGF. ERK1/2 phosphorylation was assessed by flow cytometric analysis. (C) Primary myeloma cells were cultured in the presence of control Fab (left panel) or 5 μg/mL PRO-001 (right panel). Cells were harvested after 7 days and stained with annexin V-FITC and analyzed by flow cytometry. Myelomas cells were identified as CD138+. The total percentage of CD138+ cells is shown in the top right quadrant. Shown is a representative experiment.
The t(4;14) translocation that occurs in approximately 15% of MM6,7 results in the aberrant expression of FGFR3, which in some instances also contains activating mutations.13,32 The therapeutic potential of targeting FGFR3 in t(4;14) myeloma has been demonstrated in several model systems. Inhibition of FGFR3 expression by RNA interference in t(4;14) myeloma cell lines results in decreased levels of BCL2 and MCL1 proteins and is associated with apoptosis.37 Similarly, mRNA cleavage by ribozymes that target FGFR3 results in reduction of FGFR3 expression and decreased viability of t(4;14) myeloma cell lines.38 The novel ginseng saponin metabolite, IH-901, was also found to downregulate FGFR3 expression and signaling in KMS11 cells and to induce apoptosis.39 To date, several FGFR3 small-molecule inhibitors have demonstrated selective inhibition of t(4;14) myeloma cell growth and antimyeloma activity in animal models.19-23 One of these, CHIR-258, is currently being tested in patients with relapsed and refractory myeloma. All of these inhibitors, however, are not FGFR3 specific because they also target other class III, IV, and V RTKs. It is therefore difficult to assess to what extent inhibiting FGFR3 is contributing to the antitumor activity of these molecule inhibitors. However, taken together these results validate FGFR3 as a therapeutic target in t(4;14) MM and encourage the clinical development of FGFR3 inhibitors for the treatment of patients who fall within this poor-prognosis group.
Accordingly, high-affinity human antibodies that block FGFR3 could be of therapeutic value for the treatment of FGFR3-expressing MM. Landmark clinical studies demonstrating improved chemotherapy response and prolonged survival of patients with breast cancer who were treated with trastuzumab, a monoclonal antibody targeting the ERBB-2 receptor, have illustrated the concept of therapeutic intervention with antibodies that neutralize single molecular targets that are overexpressed in tumor cells.40 Function-blocking antibodies offer the advantage of being highly specific and therefore potentially less toxic than small-molecule inhibitors that as a rule are multitargeted and nonspecific. Further, resistance to small-molecule RTK inhibitors has emerged as a significant clinical problem, not only limited to imatinib, but also described for inhibitors of FLT3 and epidermal growth factor receptor (EGFR).41,42 Through continuous and prolonged drug exposure, resistance can arise and in some cases has been associated with acquired mutations that hinder drug binding. The predicament of acquired resistance has motivated the development of alternative methods of RTK inhibition. Monoclonal antibodies thus have the potential to overcome some of the shortcomings of small-molecule inhibitors.
In this report we describe the characterization of PRO-001, a high-affinity fully human, anti-FGFR3–neutralizing antibody. The antibody is highly selective, inhibiting FGF-stimulated proliferation of FGFR3-expressing FDCP cells but not the growth of FDCP cells expressing FGFR1 or FGFR2 within the effective range of antibody concentration. PRO-001 binds to FGFR3 expressed on the surface of t(4;14)+ myeloma cells and inhibits FGF ligand-mediated activation of FGFR3 signaling in cell lines and primary myeloma cells. The data show that PRO-001 can selectively induce apoptosis of UTMC2 cells and can overcome the protective effects of myeloma-BMSC interactions and IL-6 and IGF-1. Most importantly, primary myeloma cells expressing WT FGFR3 were also susceptible to PRO-001–induced cell death. The effect on primary t(4;14) myeloma cells was particularly impressive with all patients demonstrating sensitivity to PRO-001 and one sample with up to 80% induction of apoptosis. The potential clinical application of PRO-001 was further evaluated in a xenograft mouse model in which PRO-001 treatment inhibited FGFR3-mediated tumor growth demonstrating in vivo efficacy with a favorable therapeutic window. The findings recapitulate the biologic effects of the small molecule inhibitors, PD173074 and CHIR-258, in mutant FGFR3-expressing MM cells.19,22
Studies in B9- or FDCP-transformed cell lines suggest that PRO-001 can inhibit not only WT-FGFR3 but also constitutively activated extracellular FGFR3 S249C mutant (Figure 4A) and juxtamembrane and transmembrane 370C, 371C, 375C, and 380R mutants (data not shown). Interestingly, despite its ability to bind, the antibody fails to inhibit proliferation induced by FGFR3Y373C and FGFR3G384D mutants, suggesting that the mechanism underlying the ability of PRO-001 to inhibit specific FGFR3 mutants is complex and is not likely to be as simple as antibody-mediated internalization and down-regulation of cell-surface FGFR3 receptor. Rather, we have previously shown that the differential activation of FGFR3 extracellular mutants results from differences in receptor orientation on dimerization that allows for maximal phosphorylation.43 This model may offer a potential explanation for the differential effect of PRO-001 on FGFR3 extracellular mutants. The antibody binding to the receptor may interfere with the receptor being able to establish the correct spatial orientation required for effective signal transduction. Clearly additional studies are required to elucidate the mechanisms of PRO-001 activity against these FGFR3 mutants. However, because PRO-001 was selected for its ability to recognize the extracellular portion of FGFR3, it is not surprising that it does not inhibit FGFR3 activated by mutations of the catalytic kinase domain (K650E). In addition, PRO-001 does not recognize and therefore inhibit proliferation induced by FGFR3807C. Mutations of codon 807 that occur within the stop codon produce an elongated protein that is recognized by H-100 but not PRO-001. It is likely that the mutation induces FGFR3 conformational changes that specifically block the PRO-001–binding epitope. Interestingly, B9 cells expressing FGFR3-807C were also resistant to PD173074, suggesting that the conformational changes induced by the elongated protein similarly hinder binding of this small-molecule inhibitor. In contrast, we have previously shown that CHIR-258 potently inhibits the growth of B9-807C, implying that CHIR-258 has less stringent conformation requirements for binding.
Consistent with results in B9-transformed cells, and in contrast to PD173074, PRO-001 failed to inhibit the viability of KMS11 (FGFR3Y373C) and KMS18 (FGFR3G384D) cells. The low frequency of FGFR3 mutations in MM has made it difficult to accurately assess the spectrum of mutations implicated in this disease; however, it appears that mutations of the extracellular domain occur rarely.44 On the other hand, these mutations are associated with 35% of cases of bladder cancer and 25% of cases of cervical carcinoma.9,10,45,46 Furthermore, a recent report by Logie et al suggests that a large proportion of seborrheic keratosis also harbors somatic-activating mutations of FGFR3 including S249C, 372C, and 375C.47 PRO-001 may therefore be of therapeutic benefit for these tumor types, although the contribution of activated FGFR3 to tumorigenesis in these disease sites has yet to be defined.
In summary, PRO-001 is a full human neutralizing antibody that specifically targets FGFR3 and represents a novel, potential therapeutic strategy for the treatment of tumor types for which FGFR3 activation is required for tumor maintenance. One such tumor type is likely to be myeloma overexpressing WT-FGFR3, which represents 90% of FGFR3-expressing MM, and FGFR mutants for which sensitivity to PRO-001 has been demonstrated in vitro. Additional testing of these antibodies in combination with other therapies, such as cytotoxic chemotherapy, and novel agents that have shown single-agent activity in MM, such as bortezomib and lenalidomide, and development of highly specific cytotoxic-conjugated antibodies are warranted to further optimize anti-FGFR3 antibody–based treatments.
Prepublished online as Blood First Edition Paper, February 7, 2006; DOI 10.1182/blood-2005-10-4179.
Supported by grants from the Multiple Myeloma Research Foundation (S.T.), the ASH Scholar Award (S.T.), the Eli Lilly/CCO/CIHR Hollenberg Award (S.T.), and the Ontario Cancer Research Network (S.T.) through funding provided by the Province of Ontario.
E.R., I.C., Y.S., S.K., and A.Y. are employed by a company (ProChon Biotech Ltd) whose potential product (PRO-001) was studied in the present work.
A.Y. has declared financial interest in a company (ProChon Biotech Ltd) whose potential product (PRO-001) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mehrdad Yazdanpanah for his assistance with the FGFR3 and Ras mutation analysis. We are grateful to Christine Chen, Donna Reece, Wilfred Jaksic, and Joseph Mikhael for their assistance with obtaining BM samples. We would like to thank Young Trieu for his thoughtful review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal