Diffuse large B-cell lymphoma (DLBCL) in adults is a heterogeneous disease. Biologic subgroups of DLBCL with a favorable prognosis (germinal center B-cell–like, GCB) and with a poor prognosis (activated B-cell–like, ABC) have been defined by gene expression profiling and can be distinguished by immunohistochemistry. In contrast to their adult counterparts, children with DLBCL have an excellent prognosis. We analyzed 63 cases of DLBCL in pediatric patients by immunohistochemistry and fluorescence in situ hybridization (FISH) and found a striking predominance of a GCB subtype, which might explain the good clinical outcome in these lymphomas. Interestingly, FISH applied to 50 of these cases, as well as conventional cytogenetics available in 3 cases, revealed absence of the translocation t(14;18) involving the BCL2 gene, which is present in about 15% of adult GCB subtype DLBCL. Our data indicate that pediatric DLBCL differs from adult DLBCL and might comprise a biologically unique subgroup of DLBCL from which important insights into the pathogenesis and biology of this disease might be gained.
Introduction
In Western countries malignant non-Hodgkin lymphomas (NHLs) represent the fourth most common childhood cancer, accounting for about 6% of pediatric malignancies (German Childhood Cancer Registry yearly report 2004, http://info.imsd.uni-mainz.de/K_Krebsregister/english/). The majority of pediatric NHLs derive from B cells (B-NHL) and represent primary high-grade lymphomas, the 3 most prevalent entities being Burkitt lymphoma (43%), B-lymphoblastic lymphoma (7%), and diffuse large B-cell lymphoma (13%).1
The response to treatment differs significantly between pediatric and adult DLBCL. Childhood DLBCL responds very well to therapy and has an event-free survival (EFS) of about 90%.2-4 In adults, the overall clinical outcome of DLBCL is much poorer and very heterogeneous. Attempts have been undertaken to classify adult DLBCL into clinically relevant subtypes by gene expression profiling.5-7 One of these approaches defined 3 subgroups of DLBCL whose gene expression profiles display similarities to normal, nonmalignant B-cell counterparts.5,6 The germinal center B-cell type (GCB) is characterized by expression of genes also found in normal germinal center B cells and shows favorable outcome compared to the activated B-cell type (ABC) and type 3 DLBCL. Approximately 50% of the adult DLBCLs are of the GCB subtype.5 Interestingly, the translocation t(14;18)(q32;q21), involving the immunoglobulin heavy (IGH) chain gene and the BCL2 gene, is found almost exclusively in the GCB type of DLBCL.8,9 Since pediatric DLBCL represents a group of DLBCLs with a favorable outcome, it would be interesting to determine whether these lymphomas resemble one of the described DLBCL subgroups or differ from their adult counterparts. The few reports about the immunophenotype and cytogenetic characteristics of a very limited number of cases suggest that there might indeed be differences between childhood and adulthood DLBCL.10,11
We present a clinical, immunohistochemical, and cytogenetic characterization of the largest series of pediatric patients with DLBCL described so far. Our results provide evidence that in contrast to the situation in adults, pediatric DLBCL has a favorable prognosis and is predominantly of GC origin but lacks t(14;18).
Patients, materials, and methods
Patients
All pediatric patients (up to 18 years of age) from Germany, Austria, and part of Switzerland with a diagnosis of diffuse large B-cell lymphoma included in the 2 consecutive Berlin-Frankfurt-Münster Group Multicenter Trials NHL-BFM 90 and 95 during the period from April 1990 to December 1998 (n = 134) were identified.12 From 63 patients, sufficient paraffin-embedded tumor biopsy specimens taken at diagnosis were available for the present study in the central pathology reference laboratory of the trial at the Lymph Node Registry Kiel. Most of the paraffin blocks were sent to the reference center in Kiel from other pathology laboratories. Thus, the size and quality of the biopsy specimens, the tissue processing protocols, and the paraffin blocks were heterogeneous. All cases were reviewed by at least 2 expert pathologists and classified according to the World Health Organization (WHO) classification.13
The analyzed patients included 2 patients with an underlying immunodeficiency syndrome (one centroblastic lymphoma and one T-cell–rich B-cell lymphoma). The study was carried out according to the local ethical guidelines and in accordance with the ethical guidelines of the studies in which the patients were treated. The scientific studies were done with informed consent of all parents.
Immunohistochemistry
Three- to 5-μm sections were cut and dried overnight, dewaxed in xylene, and rehydrated using serial concentrations of ethanol. The paraffin sections were then immunostained with monoclonal antibodies to CD20 (clone generated in the Department of Hematopathology Kiel), CD10 (Novocastra, Newcastle, United Kingdom), MUM-1/IRF4, BCL2, and BCL6 (Dako Diagnostica GmbH, Hamburg, Germany). Heat-mediated antigen retrieval was required. For this, the sections were placed in a pressure cooker with citrate buffer (0.01 M, pH 6) for 2 minutes after reaching operating temperature and pressure. For bcl-6, an EDTA (ethylenediaminetetraacetic acid) buffer (1 mM EDTA, pH 8) was used. Primary antibodies were incubated for 1 hour. Detection of the primary antibody was performed by the APAAP (alkaline-phosphatase antialkaline phosphatase) method. Tonsils with reactive lymphoid hyperplasia served as an external control tissue. Neutrophil granulocytes served as additional internal control for the CD10 staining, reactive T lymphocytes for the BCL2 staining, plasma cells for MUM-1, and germinal center residues for BCL6. Immunostaining for the 4 antibodies was assessed by three of the authors (I.O., W.K., H.H.W.).
Cases were scored as positive for BCL2 if more than 50% of the tumor cells showed cytoplasmic staining.14 For BCL6 and MUM-1, only nuclear staining of more than 30% of the tumor cells was interpreted as positive reaction.15 A germinal center (GCB) or non-GCB immunophenotype was defined according to the decision tree suggested by Hans and coworkers.15
Detection of t(14;18)(q32;q21) by interphase cytogenetics and metaphase analyses
Interphase FISH for the detection of t(14;18)(q32;q21) was carried out on paraffin sections of tumor tissues using the commercially available LSI IGH/BCL2 probe set (Vysis, Downers Grove, IL), which has been thoroughly evaluated previously.16,17 FISH was performed according to standard methods. Briefly, 5-μm-thick tissue sections of tumor tissues mounted on adhesive glass slides were dried overnight, deparaffinized, rehydrated in increasing concentrations of ethanol, and pretreated in proteinase K (10 μg/mL) at 37°C for 30 minutes. Ten μL LSI IGH/BCL2 probe was applied to the specimen, followed by denaturation for 5 minutes at 85°C. Hybridization and subsequent washing were done according to the recommendations of the manufacturer. Fluorescence microscopy was performed with a Zeiss photomicroscope equipped with appropriate filter sets, and evaluation was performed following recently described principles.16,17
Aberrant karyotypes were available from tumor samples of 3 patients included in the present study. Karyotyping was performed at the cytogenetic reference laboratory in Kiel using fluorescent R banding on metaphase preparations from short-time culture of tumor tissue as described recently.18
Statistical analysis
The duration of event-free survival (EFS) is defined as the time from diagnosis until the date of the first adverse event (tumor failure, death of any cause, or the development of a second malignancy), or if no such event occurred, until the date of latest contact. Probabilities of EFS were estimated by the method of Kaplan and Meier, with standard errors according to Greenwood, and were compared using the log-rank test.19 Differences in the distribution of individual parameters among patient subsets were analyzed using the χ2 test or Fisher exact test. The statistical analysis was carried out using SAS (SAS-PC, Version 9.1; SAS Institute, Cary, NC).
Results
Histomorphologic diagnosis and clinical characteristics
The majority of pediatric patients suffering from a malignant lymphoma are treated in Germany within a multicenter trial. The study population can thus be considered representative for all nonlymphoblastic, non-Burkitt B-cell non-Hodgkin lymphoma occurring in children in Germany. A total of 134 patients with DLBCL were treated in the NHL-BFM (Berlin-Frankfurt-Münster) Multicenter Trial during the period from April 1990 to December 1998. Table 1 shows the clinical characteristics of the 63 patients included in the present study compared to the rest of the clinical trial population not analyzed. We detected a higher proportion of cases with elevated lactate dehydrogenase (LDH) levels in the analyzed as compared to the not analyzed patients (23.0% vs 7.1%). However, it is unlikely that this difference significantly influences our results since all other important clinical parameters were comparable in both groups (Table 1). The DLBCL patients were morphologically subclassified as DLBCL, centroblastic variant (CB) in 52 cases; DLBCL, immunoblastic variant (IB) in 6 cases; and DLBCL, T-cell/histiocyte–rich B-cell variant (TCRB) in 5 cases. The pediatric DLBCLs included in our study that were classified as the centroblastic variant consisted of sheets of rather monomorphic centroblasts. The content of immunoblasts was usually low, leading most cases to be classified as “monomorphic centroblastic variant” (56%) and only a minority as the “polymorphic centroblastic variant” (29%) according to the Kiel classification.20,21 The “multilobulated centroblastic” subtype was diagnosed in only 4% of cases. In 6 cases (12%) the centroblastic lymphomas were not further classified. The immunoblastic subtype of DLBCL is extremely rare in children; it accounts for only 10% (6 cases) of the DLBCL in our cohort. While a plasmacytoid differentiation of the tumor cells was seen in 3 cases of immunoblastic lymphomas, we did not find a single case with a clear mature plasma cell component in our series.
Clinical and laboratory characteristics
Characteristic . | Patients analyzed, no. (%) . | Patients not analyzed, no. (%) . | P . |
|---|---|---|---|
| Sex | NS | ||
| Male | 37 (58.7) | 49 (69) | |
| Female | 26 (41.3) | 22 (31) | |
| Age | NS | ||
| 1-9 years | 25 (39.7) | 30 (42.3) | |
| At least 10 years | 38 (60.3) | 41 (57.7) | |
| CNS* | NS | ||
| Positive | 3 (4.9) | 2 (2.9) | |
| Negative | 58 (95.1) | 68 (97.1) | |
| BM | NS | ||
| Positive | 0 (0) | 0 (0) | |
| Negative | 63 (100) | 71 (100) | |
| Bone | NS | ||
| Positive | 5 (7.9) | 2 (2.8) | |
| Negative | 58 (92.1) | 69 (97.2) | |
| Stage | NS | ||
| 1 | 12 (19.0) | 20 (28.2) | |
| 2 | 20 (31.7) | 24 (33.8) | |
| 3 | 29 (46.0) | 25 (35.2) | |
| 4 | 2 (3.2) | 2 (2.8) | |
| LDH* | .01 | ||
| Less than 500 U/L | 47 (77.0) | 65 (92.9) | |
| At least 500 U/L | 14 (23.0) | 5 (7.1) | |
| Extranodal involvement | NS | ||
| No | 10 (15.9) | 20 (28.2) | |
| Yes | 53 (84.1) | 51 (71.8) |
Characteristic . | Patients analyzed, no. (%) . | Patients not analyzed, no. (%) . | P . |
|---|---|---|---|
| Sex | NS | ||
| Male | 37 (58.7) | 49 (69) | |
| Female | 26 (41.3) | 22 (31) | |
| Age | NS | ||
| 1-9 years | 25 (39.7) | 30 (42.3) | |
| At least 10 years | 38 (60.3) | 41 (57.7) | |
| CNS* | NS | ||
| Positive | 3 (4.9) | 2 (2.9) | |
| Negative | 58 (95.1) | 68 (97.1) | |
| BM | NS | ||
| Positive | 0 (0) | 0 (0) | |
| Negative | 63 (100) | 71 (100) | |
| Bone | NS | ||
| Positive | 5 (7.9) | 2 (2.8) | |
| Negative | 58 (92.1) | 69 (97.2) | |
| Stage | NS | ||
| 1 | 12 (19.0) | 20 (28.2) | |
| 2 | 20 (31.7) | 24 (33.8) | |
| 3 | 29 (46.0) | 25 (35.2) | |
| 4 | 2 (3.2) | 2 (2.8) | |
| LDH* | .01 | ||
| Less than 500 U/L | 47 (77.0) | 65 (92.9) | |
| At least 500 U/L | 14 (23.0) | 5 (7.1) | |
| Extranodal involvement | NS | ||
| No | 10 (15.9) | 20 (28.2) | |
| Yes | 53 (84.1) | 51 (71.8) |
The clinical characteristics of the patients analyzed in this study were compared to the rest of patients with the diagnosis DLBCL from the 2 consecutive Berlin-Frankfurt-Münster Group Multicenter Trials NHL-BFM 90 and 95 that were not included in this study (Fisher exact or χ2 test). The number of patients for which the clinical information was available and the percentage is shown.
CNS indicates central nervous system; NS indicates not significant.
Data not available for all patients.
Immunohistochemical results and classification in the GCB or non-GCB subgroup
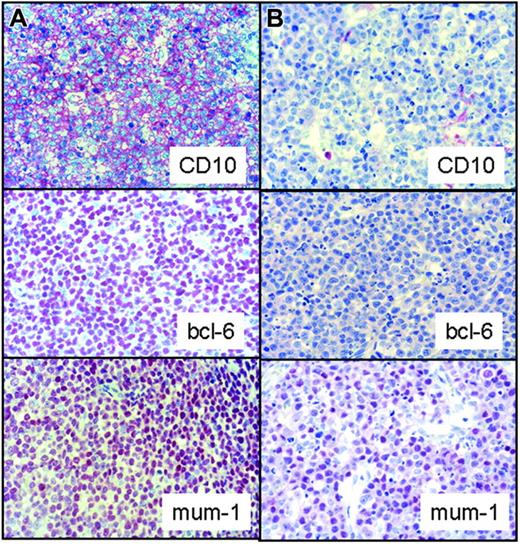
The expression of CD20, CD10, BCL2, BCL6, and MUM-1 was evaluated immunohistochemically in 63 pediatric DLBCL patients. As described in the “Patients, materials, and methods” section, we only evaluated stainings if appropriate controls were available. Table 2 summarizes the results of the immunohistochemical analysis indicating the number of cases with positive staining in relation to the number of evaluable cases. All cases except for one immunoblastic DLBCL expressed CD20. The B-cell origin of the CD20-negative case was demonstrated by staining for CD79a and immunoglobulin rearrangement analysis. The majority of DLBCL cases under study expressed CD10 (39 positive cases of 57 evaluable cases) and BCL6 (47 of 52). Only 22 of 55 and 21 of 35 DLBCL cases expressed bcl-2 and MUM-1, respectively (Table 2). Pediatric DLBCL cases of CB subtype were significantly more often CD10 and bcl-6 positive than IB or TCRB variants (P ≤ .001 and P = .029, respectively, χ2 test). No significant difference was found between the DLBCL subtypes in terms of bcl-2 or MUM-1 expression (Table 2, χ2 test for MUM-1 and Fisher exact test for bcl-2). Based on the expression of CD10, bcl-6, and MUM-1, we classified the cases as GCB type and non-GCB type according to the decision tree suggested by Hans and coworkers.15 Thirty-nine cases were classified as GCB due to expression of CD10, and 4 cases because of the immunophenotype CD10–/BCL6+/Mum-1–. Since in adults the ABC type and the type 3 DLBCL show a similar clinical outcome, and both types cannot be reliably distinguished by immunohistochemistry, these groups are combined in the non-GCB subtype.15 The GCB subtype was defined as CD10+ or CD10–/BCL6+/MUM-1– (Figure 1). All other lymphomas not fulfilling these immunohistochemical criteria for the GCB subtype were classified as non-GCB subtype. Of all 52 pediatric DLBCL evaluable for this classification, only 9 cases displayed a non-GCB immunophenotype. The majority of pediatric DLBCL cases are thus of the GCB subtype. Of the 9 non-GCB subtype DLBCLs in our series, 4 were classified as CB, 3 as IB, and 2 as TCRB variant. The non-GCB subtype of DLBCLs included 4 females and 5 males. There was no significant difference in the mean age between the GCB (10.8 years) and the non-GCB (11.7 years) groups of patients (P = .3, χ2 test). All clinical data for the GCB and non-GCB cases are shown in Table 3. A comparison of our results with published data on adult DLBCL and unpublished data from our own group (W.K., R.S., manuscript in preparation) revealed that pediatric DLBCLs are significantly more often CD10+, BCL6+ (P ≤ .001 and P = .002, respectively, χ2 test), and of GCB type (P ≤ .001, χ2 test) than their adult counterparts (Table 4). There was no significant difference in bcl-2 or MUM-1 expression between pediatric and adult DLBCL (Table 4).
Results of the immunohistochemical analysis of DLBCL
. | CB, no. (%) . | IB, no. (%) . | TCRB, no. (%) . | All pediatric DLBCL, no. (%) . | P . |
|---|---|---|---|---|---|
| CD10 | 37 of 46 (80) | 2 of 6 (33) | 0 of 5 (0) | 39 of 57 (68) | ≤ .001 |
| BCL2 | 18 of 49 (37) | 4 of 6 (67) | ND | 22 of 55 (40) | NS |
| BCL6 | 40 of 42 (95) | 3 of 5 (60) | 4 of 5 (80) | 47 of 52 (90) | .029 |
| Mum-1 | 17 of 29 (59) | 3 of 3 (100) | 1 of 3 (33) | 21 of 35 (60) | NS |
| GCB | 39 of 44 (87) | 2 of 5 (40) | 2 of 3 (67) | 43 of 52 (83) | .02 |
. | CB, no. (%) . | IB, no. (%) . | TCRB, no. (%) . | All pediatric DLBCL, no. (%) . | P . |
|---|---|---|---|---|---|
| CD10 | 37 of 46 (80) | 2 of 6 (33) | 0 of 5 (0) | 39 of 57 (68) | ≤ .001 |
| BCL2 | 18 of 49 (37) | 4 of 6 (67) | ND | 22 of 55 (40) | NS |
| BCL6 | 40 of 42 (95) | 3 of 5 (60) | 4 of 5 (80) | 47 of 52 (90) | .029 |
| Mum-1 | 17 of 29 (59) | 3 of 3 (100) | 1 of 3 (33) | 21 of 35 (60) | NS |
| GCB | 39 of 44 (87) | 2 of 5 (40) | 2 of 3 (67) | 43 of 52 (83) | .02 |
The immunohistochemical results and the classification as GCB subtype are shown in number of positive cases of the evaluable cases (percentage of positive cases) in the morphologic subgroups as well as all DLBCL (χ2 test or Fisher exact test).
ND indicates not done.
Clinical and laboratory characteristics of pediatric GCB and non-GCB DLBCL
Characteristic . | GCB, no. (%) . | non-GCB, no. (%) . | P . |
|---|---|---|---|
| Sex | NS | ||
| Male | 25 (58.1) | 5 (55.6) | |
| Female | 18 (41.9) | 4 (44.4) | |
| Age | NS | ||
| 1-9 years | 18 (41.9) | 2 (22.2) | |
| At least 10 years | 25 (58.1) | 7 (77.8) | |
| CNS* | NS | ||
| Positive | 1 (2.3) | 1 (11.1) | |
| Negative | 40 (93.0) | 8 (88.9) | |
| BM | NS | ||
| Positive | 0 (0) | 0 (0) | |
| Negative | 43 (100) | 9 (100) | |
| Bone | NS | ||
| Positive | 4 (9.3) | 0 (0) | |
| Negative | 39 (90.7) | 9 (100) | |
| Stage | NS | ||
| 1 | 9 (20.9) | 2 (22.2) | |
| 2 | 12 (27.9) | 2 (22.2) | |
| 3 | 21 (48.8) | 5 (55.6) | |
| 4 | 1 (2.3) | 0 (0) | |
| LDH* | NS | ||
| Less than 500 U/L | 33 (76.7) | 5 (55.6) | |
| At least 500 U/L | 9 (20.9) | 3 (33.3) | |
| Extranodal involvement | NS | ||
| No | 37 (86.0) | 6 (14.0) | |
| Yes | 9 (100) | 0 (0) |
Characteristic . | GCB, no. (%) . | non-GCB, no. (%) . | P . |
|---|---|---|---|
| Sex | NS | ||
| Male | 25 (58.1) | 5 (55.6) | |
| Female | 18 (41.9) | 4 (44.4) | |
| Age | NS | ||
| 1-9 years | 18 (41.9) | 2 (22.2) | |
| At least 10 years | 25 (58.1) | 7 (77.8) | |
| CNS* | NS | ||
| Positive | 1 (2.3) | 1 (11.1) | |
| Negative | 40 (93.0) | 8 (88.9) | |
| BM | NS | ||
| Positive | 0 (0) | 0 (0) | |
| Negative | 43 (100) | 9 (100) | |
| Bone | NS | ||
| Positive | 4 (9.3) | 0 (0) | |
| Negative | 39 (90.7) | 9 (100) | |
| Stage | NS | ||
| 1 | 9 (20.9) | 2 (22.2) | |
| 2 | 12 (27.9) | 2 (22.2) | |
| 3 | 21 (48.8) | 5 (55.6) | |
| 4 | 1 (2.3) | 0 (0) | |
| LDH* | NS | ||
| Less than 500 U/L | 33 (76.7) | 5 (55.6) | |
| At least 500 U/L | 9 (20.9) | 3 (33.3) | |
| Extranodal involvement | NS | ||
| No | 37 (86.0) | 6 (14.0) | |
| Yes | 9 (100) | 0 (0) |
The clinical characteristics were compared between the GCB and non-GCB subgroups. The number of patients of which the clinical information was available and the percentage is shown (Fisher exact or χ2 test).
Data not available for all patients.
Comparison of immunohistochemical findings in pediatric and adult DLBCL
. | Pediatric DLBCL, no. (%) . | Adult DLBCL, no. (%) . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Hans et al15 . | Chang et al24 . | Colomo et al25 . | Klapper et al . | Total adult DLBCL . | P . | ||||
| CD10 | 39 of 57 (68) | 42 of 152 (28) | 16 of 42 (38) | 27 of 128 (21) | 76 of 207 (37) | 161 of 529 (30) | < .001 | ||||
| BCL2 | 22 of 55 (40) | 76 of 152 (50) | ND | 74 of 126 (59) | 137 of 199 (69) | 287 of 477 (60) | NS | ||||
| BCL6 | 47 of 52 (90) | 85 of 152 (56) | 21 of 41 (51) | 91 of 127 (72) | 188 of 221 (85) | 385 of 541 (71) | .002 | ||||
| Mum-1 | 21 of 35 (60) | 71 of 152 (47) | 22 of 42 (52) | 68 of 128 (53) | 146 of 210 (70) | 307 of 532 (58) | NS | ||||
| GCB | 43 of 52 (83) | 64 of 152 (42) | 15 of 38 (40) | 57 of 119 (48) | 97 of 207 (47) | 233 of 516 (45) | < .001 | ||||
. | Pediatric DLBCL, no. (%) . | Adult DLBCL, no. (%) . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | Hans et al15 . | Chang et al24 . | Colomo et al25 . | Klapper et al . | Total adult DLBCL . | P . | ||||
| CD10 | 39 of 57 (68) | 42 of 152 (28) | 16 of 42 (38) | 27 of 128 (21) | 76 of 207 (37) | 161 of 529 (30) | < .001 | ||||
| BCL2 | 22 of 55 (40) | 76 of 152 (50) | ND | 74 of 126 (59) | 137 of 199 (69) | 287 of 477 (60) | NS | ||||
| BCL6 | 47 of 52 (90) | 85 of 152 (56) | 21 of 41 (51) | 91 of 127 (72) | 188 of 221 (85) | 385 of 541 (71) | .002 | ||||
| Mum-1 | 21 of 35 (60) | 71 of 152 (47) | 22 of 42 (52) | 68 of 128 (53) | 146 of 210 (70) | 307 of 532 (58) | NS | ||||
| GCB | 43 of 52 (83) | 64 of 152 (42) | 15 of 38 (40) | 57 of 119 (48) | 97 of 207 (47) | 233 of 516 (45) | < .001 | ||||
The immunohistochemical results are shown in number of positive cases of evaluable cases (percentage of positive cases). The data presented in this study on pediatric DLBCL are compared to data on adult DLBCL combined from published studies by Hans et al,15 Chang et al,24 and Colomo et al,25 as well as an unpublished study from our own group (W.K., R.S., unpublished observations, August 2005) by the χ2 test.
ND indicates not done; NS, not significant.
Absence of t(14;18)(q32;q21)
FISH analyses for the detection of the t(14;18)(q32;q21)/IGH-BCL2 fusion was successfully performed in 50 of the DLBCL cases (44 CB, 6 IB, 0 TCRB) under study, including 31 GCB and 6 non-GCB tumors. None of the cases contained a significant number of cells displaying a signal constellation indicative for this translocation. In line with these molecular cytogenetic analyses, conventional metaphase studies in 2 GCB and 1 non-GCB case revealed complex karyotypes lacking t(14;18).
Immunohistochemical example of aGCB subtype of DLBCL that is positive for the germinal center markers CD10 and bcl-6 and co-expresses MUM-1. (A) A rare case of pediatric DLBCL with a non-GCB immunophenotype with negative staining for CD10 and bcl-6 and positivity for MUM-1 (B). Images were captured with a Zeiss Axioskop 40 microscope (Carl Zeiss, Jena, Germany) equipped with a 40×/0.75 objective lens and an RT slider camera (Diagnostic Instruments, Sterling Heights, MI). Images were processed with MetaVue software (Diagnostic Instruments) and Adobe Photoshop (Adobe Systems, San Jose, CA).
Immunohistochemical example of aGCB subtype of DLBCL that is positive for the germinal center markers CD10 and bcl-6 and co-expresses MUM-1. (A) A rare case of pediatric DLBCL with a non-GCB immunophenotype with negative staining for CD10 and bcl-6 and positivity for MUM-1 (B). Images were captured with a Zeiss Axioskop 40 microscope (Carl Zeiss, Jena, Germany) equipped with a 40×/0.75 objective lens and an RT slider camera (Diagnostic Instruments, Sterling Heights, MI). Images were processed with MetaVue software (Diagnostic Instruments) and Adobe Photoshop (Adobe Systems, San Jose, CA).
Clinical biologic correlation
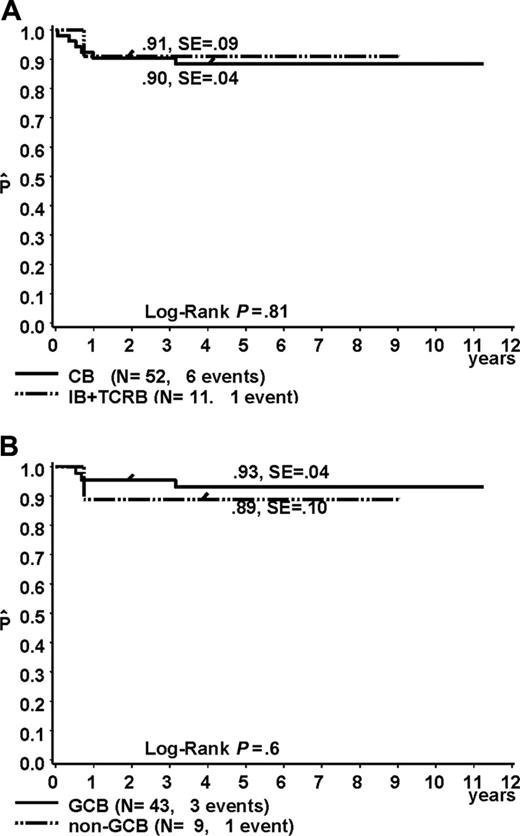
The EFS at 3 years was 90% for the CB variant and 91% for the IB and TCRB variants (Figure 2A, P = .81), reflecting the excellent overall prognosis of pediatric DLBCL compared to the adult counterpart.22,23 It is interesting to note that the immunophenotypical markers of a poor prognosis described for DLBCL in adults, such as a non-GCB immunophenotype15,24,25 or expression of BCL2,14 did not reach significance in pediatric patients (Figure 2B, P = .6 and .08, respectively). The number of pediatric DLBCL cases in our series that expressed CD10 (39 of 57) or BCL6 (47 of 55) was very high. Nevertheless, in contrast to reports on DLBCL in adults, CD10-positive or BCL6-positive pediatric cases did not show a significantly higher EFS at 3 years (P = .93 and P = .07, respectively).
EFS was estimated by the method of Kaplan and Meier and was compared using the log-rank test. Due to the small sample size of the morphologic subtypes IB and TCRB, both were combined and compared to CB subtype (A). The survival curve for GCB and non-GCB subtype is shown in panel B.
EFS was estimated by the method of Kaplan and Meier and was compared using the log-rank test. Due to the small sample size of the morphologic subtypes IB and TCRB, both were combined and compared to CB subtype (A). The survival curve for GCB and non-GCB subtype is shown in panel B.
Discussion
According to the WHO classification, DLBCLs are defined as diffuse proliferations of large neoplastic mature B cells, and it is, however, recognized that this definition comprises a group of morphologically, immunohistochemically, and clinically heterogeneous tumors rather than one single entity.13 In adults several attempts have been undertaken to classify DLBCL into biologically and clinically relevant subgroups. By comparing normal B-cell subsets to lymphoma samples, gene expression profiling studies helped to assign the lymphoma more precisely to a possible normal cell of origin.5,6 DLBCL cases whose gene expression profiles resemble germinal center cells (GCB) showed a much better prognosis than non-GCB–type DLBCL. These data strongly support the idea that the developmental stage of the B cell that transforms into a DLBCL influences the biologic and thus clinical behavior of the lymphoma over a long period of time. While considerable progress has been made in our understanding of the pathogenesis and biology of adult DLBCL cases, data on pediatric DLBCL cases are limited. Here we present the first comprehensive clinical, morphological, immunohistochemical, and genetic study of a large cohort of pediatric DLBCL cases, making special reference to prognostic factors and markers that have been reported to define biologic subgroups of DLBCL in adults. Contrasting the situation in many adult lymphoma studies, this patient cohort comprises half of all patients uniformly treated within 2 prospective clinical trials, which, moreover, recruits the vast majority of all pediatric DLBCL cases in Germany. Thus, the results can be considered highly representative regarding both epidemiologic and clinical features.
The morphologic spectrum of the pediatric DLBCL cases seems to differ from the adult cases. The majority of cases are of the centroblastic variant (83%), compared to 75% reported in adults.20 The “monomorphic centroblastic variant” occurred more often in our series (63%) compared to adults (21%).20 Immunoblastic variants are rare in children and comprise only 7% of our cases compared to 15% in adults.20 We classified the pediatric lymphomas in our series as GCB or non-GCB type following the approach of Hans et al.15 The majority of pediatric DLBCL cases are CD10- and BCL6-positive, indicating a germinal center origin of these lymphomas. Co-expression of the germinal center markers CD10 or BCL6 and the postgerminal center marker MUM-1 like in several cases in our series has been reported by other authors.15,24,25 According to the published criteria, a lymphoma is assigned to the GCB subtype on the basis of CD10 positivity, independent of MUM-1 expression.15 Although non-GCB type DLBCL, according to Hans et al, occurred in our series (9%), they were significantly more rare than in adults (approximately 50%).5 Our data are in line with the recently presented abstract, which also showed a predominance of the GCB subtype and lack of t(14;18) in pediatric DLBCL.26 The association of the GCB subtype and the monomorphic centroblastic morphology also was described in adults.5 The classification of DLBCL as GCB or non-GCB most likely reflects biologic differences, which might influence therapeutic decisions in the future. For example non-GCB DLBCL can be specifically targeted by interfering with the NF kappaB pathway, a therapeutic approach that will be ineffective in GCB subtype DLBCL.27
Since low-grade B-cell lymphomas rarely occur in children, pediatric DLBCL will rarely be transformed into low-grade lymphomas and can definitely be regarded as primary high-grade lymphomas, whereas in adults an unknown number of DLBCLs are considered to occur as secondary high-grade lymphomas progressing from a preceding, clinically often unrecognized low-grade B-cell lymphoma. Especially indolent (grade 1 or 2) follicular lymphoma transforms to DLBCL in a considerable number of cases.28 Thus, in contrast to adults, pediatric DLBCL represents a more uniform group of true primary high-grade lymphomas that prove useful for studying the biology and pathogenesis of DLBCL. The translocation t(14;18) involving the immunoglobulin heavy chain gene and the BCL2 gene can be detected in the majority of follicular lymphomas. In addition, a large number of adult DLBCLs harbor t(14;18). Although the number of t(14;18)-positive DLBCL in adults varies from study to study, the translocation is found almost exclusively in the GCB subtype.9,25 One explanation for the presence of t(14;18) in DLBCL might be that a follicular lymphoma had transformed into a DLBCL.9,29 However, it is most likely that t(14;18) also occurs in de novo DLBCL. A recent study on the presence of t(14;18) in pediatric and adolescent DLBCLs detected the translocation in only 1 of 7 cases.11 However, the patient carrying t(14;18) was 20 years old at diagnosis and can be considered an adult patient.11 In our series no pediatric DLBCL carried the t(14;18), although most pediatric DLBCLs are of the GCB subtype. Pediatric DLBCL thus represent a subgroup of DLBCLs with an excellent prognosis, a GCB immunophenotype, and lack of t(14;18).
We did not detect a difference in EFS between the pediatric GCB and non-GCB DLBCLs. Four female and 5 male patients displayed an ABC subtype, indicating that the ABC immunophenotype is not related to the group of adolescent female patients described as showing an unfavorable outcome.1 Similarly, we found that other indicators of a poor (BCL2-positive) or good prognosis (CD10-BCL6-positive) described in adults failed to predict EFS in pediatric patients.14,15,30 Although one of the largest series published so far, our study might still be too small to detect a slight difference in clinical outcome. Our findings suggest that pediatric DLBCLs are, in contrast to their adult counterparts, a very homogeneous group, indicated by the strong predominance of monomorphic centroblastic variants and the GCB immunophenotype. Due to this homogeneity, it might not be surprising that the identification of histologic and immunophenotypical markers to distinguish groups of different outcome among pediatric patients turns out to be difficult. The absence of bio-markers in pediatric patients that are prognostic in adults might again suggest that pediatric DLBCLs are a distinct and homogenous subgroup of DLBCL. We cannot rule out that the difference in outcome between pediatric and adult DLBCL might as well be influenced by the current therapy and host factors such as the immune response. Proving this will be difficult or almost impossible because children and adults would have to be treated in the same study.
Recently, primary central nervous system lymphomas, which are classified as DLBCL according to the WHO classification, were reported to be predominantly of non-GCB subtype.31 This finding was discussed as an explanation for the poor outcome of the disease. Pediatric DLBCL might represent the other extreme in the spectrum of DLBCL, that with a very good prognosis. One might speculate that the good clinical outcome of pediatric DLBCL is linked to the GCB subtype and the lack of t(14;18).
The WHO classification does not distinguish between pediatric and adult DLBCL. However, our data indicate that pediatric DLBCL comprises a biologically distinct subgroup of germinal center B-cell lymphomas that differ from the morphological, identical disease found in adults. In contrast to adult DLBCL, pediatric DLBCL is predominantly of germinal center origin. It differs from the adult DLBCL GCB subtype in that it lacks t(14;18). These data might explain the differences in clinical outcome of DLBCL between children and adults and suggest that pediatric DLBCL can be a model for studying the biology of DLBCL.
I.O. and W.K. contributed equally to this work.
Prepublished online as Blood First Edition Paper, January 19, 2006; DOI 10.1182/blood-2005-10-4213.
Supported by the Kinderkrebsinitiative Bucholz-Holm-Seppensen and the Deutsche Krebshilfe Verbundprojekt “Molekulare Mechnismen bei malignen Lymphomen,” no. 70-3173-Tr 3.
Reza Parwaresch died during the preparation of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to O. Batic, A. Juhl, and M. Weiss for their excellent technical assistance; and to K. Dege and D. Wright for critical comments on the manuscript.
This manuscript is dedicated to Reza Parwaresch, who passed away during its preparation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal