Leukocyte adhesion deficiency II (LAD II) is a rare congenital disease caused by defective fucosylation leading to immuno-deficiency and psychomotor retardation. We have previously identified the genetic defect of LAD II in a patient whose Golgi GDP-fucose transporter (GFTP) bears a single amino acid exchange that renders this protein nonfunctional but correctly localized to the Golgi. We now report a novel dual defect by which a truncated GFTP causes the disease in a new LAD II patient. We show that the truncation renders this GFTP unable to localize to the Golgi, the compartment where it is required. Furthermore, the missing part of the GFTP can be dissected into 2 regions, one that is needed for Golgi localization and one that is additionally required for the function of the GFTP. We investigated the subcellular localization of all known defective GFTPs allowing us to divide all genetically analyzed LAD II patients into 2 groups, one in which single amino acid exchanges in the GFTP impair its function but not its subcellular localization, and another group with a dual defect in function and Golgi expression of the GFTP due to the absence of 2 important molecular regions.
Introduction
Leukocyte adhesion deficiency II (LAD II) is a congenital disorder of glycosylation (CDG-IIc) that affects leukocyte interactions with blood-vessel endothelium. These interactions are required for the extravasation of leukocytes to secondary lymphoid organs and sites of infection and involve a cascade of binding events that are initiated by selectin-dependent leukocyte tethering and rolling.1,2 Selectins are a group of C-type lectin adhesion molecules that bind to ligands that are decorated with fucosylated glycostructures similar or identical to sialyl Lewis X.3,4 Patients with LAD II display a generalized defect in fucosylation that affects the selectin ligands, strongly impairing leukocyte–endothelial-cell interactions. This causes reduced extravasation of neutrophils and immunodeficiency. The fucosylation defect also leads to prominent neutrophilia as well as mental and growth retardation.5-8
We and others have identified mutations in the gene that codes for the GDP-fucose transporter (GFTP) as the genetic defect causing the disease.9,10 The GFTP resides in the Golgi membrane, where it serves to transport the nucleotide sugar GDP-fucose into the Golgi lumen.9,11 GDP-fucose is synthesized in the cytosol mainly from mannose and glucose via a de novo pathway and, to a minor extent, via a salvage pathway that uses either exogenous l-fucose or fucose derived from degraded glycoconjugates.12,13 Following transport into the Golgi by the GFTP, the nucleotide sugar serves as a substrate for fucosylation reactions carried out by several fucosyltransferases. The human GFTP is a protein of 364 amino acids with 10 transmembrane (TM) domains and the amino and carboxy termini exposed to the cytosol9,10 (Figure 2). The protein is expressed as a homodimer and translocates GDP-fucose into the Golgi lumen in exchange for GMP.
So far, 6 patients with LAD II have been identified, 5 of whom were analyzed for the genetic defect. These patients all show mutations in the GFTP gene. The patient in whom we previously identified the genetic cause of LAD II is of Turkish origin and displays a single amino acid exchange (R147C) in the fourth TM domain of the GFTP due to a point mutation in the gene.9 Of 4 Israeli patients of Arab origin, 3 were analyzed for the genetic defect and were found to display a different single amino acid exchange (T308R) in the ninth TM domain.10,14 Recently, the sixth LAD II patient, who is of Brazilian origin, was described.15 The GFTP gene of this patient showed a nucleotide deletion (ΔG588) causing a reading frame shift that leads to a premature termination codon. The resulting protein was predicted to be severely truncated, lacking the last 5 TM domains and the cytoplasmic C-terminus.
A therapy for LAD II was established when we orally administered l-fucose to the Turkish patient and found that this treatment caused reappearance of functional selectin ligands and a complete abrogation of the patient's immunodeficiency and neutrophilia.16,17 Using identical or similar protocols, 3 more patients with LAD II were treated with varying success. Whereas fucose supplementation apparently had no effect on 2 of the Israeli patients,18,19 the therapy caused the same beneficial effects in the Brazilian patient as in the Turkish patient, albeit accompanied by a controllable autoimmune reaction against refucosylated antigens.15 How GDP-fucose is transported into the Golgi in LAD II patients under conditions of therapy is not understood.
The mechanisms by which defective GFTPs cause reduced or absent transport of GDP-fucose in LAD II patients are ill defined. In particular, it is not established if and where the defective transport molecules are expressed. In this respect 3 alternatives are possible. First, the defective GFTP is expressed and resides in the Golgi but is deficient in transport function due to its mutation. Second, the transporter may be expressed, but not in the Golgi, or third, the mutated protein may not be expressed at all. So far, the expression and localization of the GFTP has been analyzed only for the Turkish patient. We found that the defective GFTP of this patient is correctly localized in the Golgi but is nonfunctional.8
Here we analyze a new LAD II patient and find that her GFTP is retained in the endoplasmic reticulum (ER) due to a missing TM domain. In addition, this patient's GFTP lacks a region in the C-terminal cytoplasmic domain rendering the protein inactive. We show that all other LAD II patients can be subdivided by the 2 mechanisms that we detected in the Turkish and the new LAD II patient, respectively. Interestingly, despite the dual defect of the GFTP, fucosylation of cells of the new LAD II patient can be restored by exogenous fucose, indicating that fucose supplementation therapy of LAD II is based on an alternative GDP-fucose transport mechanism.
Patient, materials, and methods
Patient
Patient G (G indicates Glasgow, the area in which this patient was first identified; the indication is not related to the patient's name) was born at term. At birth, she was in good condition and showed no obvious dysmorphism. Her birth weight was 2.81 kg (9th-25th centile) and her occipitofrontal circumference (OFC) was 33.8 cm (25th-50th centile). She remained in the maternity hospital for a week because of slow feeding. At 4 months of age she was referred to the hospital because of poor feeding, failure to thrive, and hypotonia. Her head circumference was noted to have fallen to the third centile and weight was below third centile. She was also noted to have spiky, brittle hair, frontal bossing, hypertelorism, a cardiac murmur, and short proximal limb segments. She has had few significant infections, although she had widely disseminated infection of the skin with herpes simplex virus at the age of 4, which required hospital admission. She had delayed eruption of teeth and has mild periodontitis. She had an episode of pustular dermatitis of her hands and feet at the age of 1 year. She is allergic to egg. At the age of 2 years she was diagnosed with epileptic seizures and treated with sodium valproate. Blood levels of factor XI, protein C, free and total protein S, and antithrombin activity are normal.
Her main problems have been poor weight gain and global developmental delay. She required overnight nasogastric feeds for a period of time at the age of 2 years. She has continued to have poor oral intake and a gastrostomy tube is being considered. She is significantly delayed in her development. Formal assessment at the age of 3.5 years showed her to be performing at the 12- to 15-month-old level in most areas, with locomotor skills scoring slightly higher than visual/cognitive areas. She speaks 2 words, communicating her needs only by vowel sounds and cries. A magnetic resonance imaging (MRI) brain scan at 4 months of age was normal, with no evidence of a neuronal migration disorder or other structural abnormality. She suffers from bilateral myopic astigmatism. Blood samples, cultured fibroblasts, and DNA were used in accordance with institutional guidelines of the Yorkhill NHS Trust, Royal Hospital for Sick Children (Glasgow, United Kingdom); written informed consent of the patient's parents was obtained in accordance with the Declaration of Helsinki.
Cells
Fibroblasts derived from LAD II patient G, the Turkish LAD II patient,7,9 and healthy individuals as well as COS-7 cells were cultured at 37°C with 5% CO2 in Dulbecco modified Eagle medium (DMEM; Invitrogen, Karlsruhe, Germany) containing 15% (fibroblasts) or 10% (COS-7) fetal calf serum (PAN Biotech, Aidenbach, Germany). Peripheral-blood neutrophils were obtained from anticoagulated blood in a Histopaque 1077/Histopaque 1119 double gradient (Sigma-Aldrich, Deisenhofen, Germany) and used for flow cytometry after 2 washes with PBS/0.5% BSA. Erythrocytes were obtained from the pellet of the double gradient.
Sequence analysis and DNA cloning
gDNA was prepared from whole blood using a kit (Qiagen, Hilden, Germany). Both exons of the GFTP gene (AF323970) were sequenced using standard procedures20 using the following primers (in 5′-3′ direction): TGGACTCCAGGGAATCAGAGTTC, ACTGCTTCAGTCCCCATGACC, CTTCTTCCTCCTCGTCCTCATCC, ACCGCCTTCACTACTGTGCTGG. The polymerase chain reaction (PCR) products were purified and sequenced using an automated DNA sequencer (Applied Biosystems, Weiterstadt, Germany).
Cloning of GFTP cDNAs without GFP tag. The GFTP cDNA from patient G was cloned by reverse-transcription (RT)–PCR (2 independent RT reactions) using MMuLV reverse transcriptase (Stratagene, La Jolla, CA) and total RNA isolated with the RNeasy Mini Kit (Qiagen) from the patient's fibroblasts. RT-PCRs were performed with the following primers (sense primer followed by antisense primer, in 5′-3′ direction): CCACCATGGCGCTGACCGGGGCCTCAGACCCCTC, TCACACCCCCATGGCGCT.
For sequencing and some of the complementation studies the PCR product (with a stop codon at the 3′ end, ie, expressed without GFP tag) was cloned into pcDNA3.1/CT-GFP TOPO (Invitrogen). Sequencing was done using the Big Dye 3.1 sequencing kit (Applied Biosystems, Foster City, CA). GFTP cDNAs (without tag) from healthy donors and the Turkish LAD II patient in pcDNA3.1/CT-GFP TOPO were published before.9
Cloning of GFTP cDNAs with N-terminal GFP tag. For targeting and complementation experiments the GFTP cDNAs from patient G, the Turkish patient, and a healthy donor in pcDNA3.1/CT-GFP TOPO were used in PCRs to create fusion proteins with an N-terminal GFP tag. For the deletion constructs Δ25C and Δ22C the GFTP cDNA of a healthy donor was used as a template. In the PCRs the sense primer ATGGCGCTGACCGGGGCCTCAGACCCCT was used. Antisense primers were: for full-length constructs from patients and healthy control: TCACACCCCCATGGGCGCT; for construct Δ25C: TCACCAGGTGTAGGCGGAGGAG; for construct Δ22C: TCAGCCCCTGACCCAGGTGTA. The PCR products were cloned into pcDNA3.1/NT-GFP TOPO (Invitrogen) and sequenced.
Cloning of GFTP cDNAs with C-terminal GFP tag. To create deletion construct Δ37N with a C-terminal GFP tag a PCR was performed using the GFTP cDNA (without tag) from a healthy donor in pcDNA3.1/CT-GFP TOPO as template using sense primer CCACCATGGCATTGCAGATCGCGCTGGT and antisense primer GCACCCCCATGGCGCTCTT. The PCR product was cloned into pcDNA3.1/CT-GFP TOPO (Invitrogen) and sequenced. C-terminally tagged GFTPs from the Turkish patient and healthy donors were described before.8,9
Site-directed mutagenesis
GFTPs bearing the nucleotide deletion ΔG588 of the Brazilian LAD II patient15 and the C923G transversion of LAD II patients of Arab origin10,14 were generated by site-directed mutagenesis. Using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) PCRs were performed according to the manufacturer's protocols with wild-type GFTP cDNA in pcDNA3.1/NT-GFP TOPO (see “Cloning of GFTP cDNAs with N-terminal GFP tag”) as template and the following primers (sense, antisense): for introduction of the ΔG588 deletion: GGCACCCTGTCGTGCTGGGCACCGTCTTC, GAAGACGGTGCCCAGCACGACAGGGTGCC; for introduction of the C923G transversion: CCAAGGCCTGTGCCCAGAGAGTGCTGGCCGTGCTC, GAGCACGGCCAGCACTCTCTGGGCACAG GCCTTGG. For each mutated GFTP, PCR products from 2 independent PCRs were produced, sequenced, and used in transfection experiments.
Flow cytometry
Flow cytometry was performed according to standard protocols.16 Biotinylated Aleuria aurantia lectin (AAL; Vector Laboratories, Burlingame, CA), anti-sLex antibody CSLEX-1 (American Type Culture Collection, Manassas, VA), anti-Lex antibody (BD PharMingen, Heidelberg, Germany), anti–H-antigen antibody (Mast Diagnostica, Reinfeld, Germany), and negative control IgM monoclonal antibody (mAb; BD Pharmingen) were used at 10 μg/mL. AAL was detected with phycoerythrin (PE)–conjugated streptavidin. Streptavidin-PE alone served as negative control. E- and P-selectin–IgG and VE-cadherin–IgG constructs were published before.21,22
Complementation and targeting experiments
LAD II fibroblasts and COS-7 cells were seeded on slides in 90-mm dishes, transfected with 4 μg of the indicated DNAs using the GeneJammer transfection reagent (Stratagene) as described9 and cultured for 48 hours (in some experiments in the presence of 0.03-10 mM l-fucose [Sigma-Aldrich]). Subsequently, the cells were fixed and permeabilized as described23 and stained. Biotin-conjugated AAL (10 μg/mL; Vector Laboratories) and streptavidin-Cy3 (Jackson ImmunoResearch, West Grove, PA) were used for specific detection of α1,3- and α1,6-linked fucose. GFP was detected with a polyclonal anti-GFP antibody (1:500, Abcam, Cambridge, United Kingdom) and Alexa Fluor 488–conjugated anti–rabbit IgG (Molecular Probes-Invitrogen, Karlsruhe, Germany). The Golgi bodies were stained with anti–golgin-97 antibody (mouse IgG, Molecular Probes-Invitrogen) and Alexa Fluor 568–conjugated anti–mouse IgG (Molecular Probes-Invitrogen). For detection of the ER, COS-7 cells were cotransfected with 2 μg subcellular localization vector, pDsRed-ER (red autofluorescence; BD Pharmingen) and 3 μg various GFTP cDNAs. For analysis, a 20 ×/0.75 objective on a Zeiss Axioskope 50 microscope (Zeiss, Oberkochen, Germany) and a SPOT imaging system (Diagnostic Instruments, Sterling Heights, MI) were used, except for cells transfected with pDsRed-ER, which were analyzed with a 100 ×/1.40 oil-immersion objective. For complementation experiments that were analyzed by flow cytometry, LAD II fibroblasts were transfected by nucleofection using the “Human Dermal Fibroblast Nucleofactor” kit (Amaxa Biosystems, Cologne, Germany) and a Nucleofactor I transfection device (Amaxa Biosystems) according to the manufacturer's neonatal fibroblast transfection instructions and grown for 48 hours in FGM-2 Bulletkit medium (Cambrex BioScience, Verviers, Belgium).
Results
Clinical presentation of a new LAD II patient
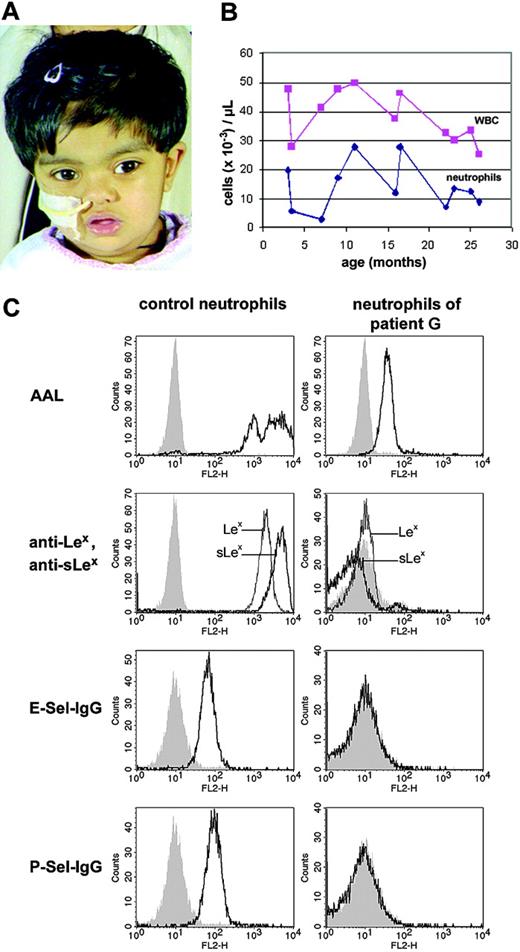
The girl (named patient G in this report) is the child of parents who are British Asians of Pakistani origin and are first cousins. The patient suffers from global developmental delay and poor weight gain combined with poor oral intake. The patient was noted to have short proximal limb segments, hypotonia, microcephaly, a depressed nasal bridge, hypertelorism, and bilateral myopic astigmatism (Figure 1A). At the age of 2 years, the girl developed epileptic seizures and was treated with sodium valproate. In addition, the patient shows a significant delay in psychomotor development (see “Patient and methods”). She has had few significant infections including pustular dermatitis of her hands and feet and widely disseminated herpes simplex virus infection of the skin. The patient had delayed eruption of teeth and has mild periodontitis. Total white blood cell counts as well as neutrophil counts were constantly elevated in the patient (Figure 1B).
Clinical phenotype, blood-cell counts, and fucosylation in neutrophils of patient G. (A) Patient G at the age of 26 months. A broad and depressed nasal bridge and divergent strabismus are present. Gastric tube feeding is necessary. (B) Peripheral total white blood cell (WBC) and neutrophil counts of the patient during the first 2 years of life. Total WBC counts were permanently elevated. (C) Fucosylation and selectin ligand expression in neutrophils of the patient. Healthy and patient neutrophils were incubated with the fucose-specific Aleuria aurantia lectin (AAL), anti-Lex, or anti-sLex antibodies, and selectin-IgG (Sel-IgG) chimeric proteins, respectively, before they were analyzed by flow cytometry. Negative controls were secondary antibody only (for AAL), control IgM antibody (for anti-Lex and anti-sLex antibodies) and VE-cadherin-IgG (for selectin-IgG), respectively.
Clinical phenotype, blood-cell counts, and fucosylation in neutrophils of patient G. (A) Patient G at the age of 26 months. A broad and depressed nasal bridge and divergent strabismus are present. Gastric tube feeding is necessary. (B) Peripheral total white blood cell (WBC) and neutrophil counts of the patient during the first 2 years of life. Total WBC counts were permanently elevated. (C) Fucosylation and selectin ligand expression in neutrophils of the patient. Healthy and patient neutrophils were incubated with the fucose-specific Aleuria aurantia lectin (AAL), anti-Lex, or anti-sLex antibodies, and selectin-IgG (Sel-IgG) chimeric proteins, respectively, before they were analyzed by flow cytometry. Negative controls were secondary antibody only (for AAL), control IgM antibody (for anti-Lex and anti-sLex antibodies) and VE-cadherin-IgG (for selectin-IgG), respectively.
Neutrophils of the new LAD II patient show strong hypofucosylation and fail to express selectin ligands
We analyzed the fucosylation of the new patient's neutrophils using AAL, a lectin which is specific for α1,3- and α1,6-fucosylated glycoconjugates. We found that binding of this lectin to patient cells was strongly reduced with a residual staining of only 1.5% of healthy levels indicating a strong hypofucosylation (Figure 1C). AAL binding to the patient's erythrocytes was equivalently reduced and was accompanied by a lack of A- and B- blood group antigens including the fucosylated blood group precursor, the H antigen (not shown). Moreover, the fucosylated Lewis X antigen (Lex) was absent from the patient's neutrophils as was its sialylated form, sialyl Lewis X (sLex), which is the prototype glycostructure required for binding of the selectins (Figure 1C). Absence of binding of E-selectin– and P-selectin–IgG chimeric proteins to the patient's neutrophils showed that the hypofucosylation in the patient leads to a defect in expression of functional selectin ligands (Figure 1).
Taken together, the clinical and molecular data show that patient G suffers from a strong hypofucosylation that causes typical LAD II manifestations. Thus, patient G is the seventh LAD II patient who has been reported.
The GFTP gene of the new LAD II patient displays a premature translational termination codon
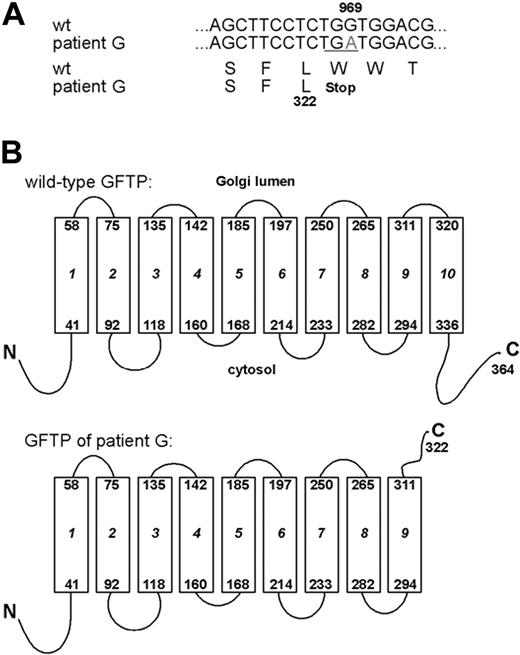
Analysis of the genomic sequence of the patient's GFTP gene revealed a homozygous G969A transition in exon 2 resulting in a premature TGA termination codon in the reading frame. Sequencing of cDNAs of the respective transcripts revealed the same mutation (Figure 2A). Both parents and an apparently healthy sibling of the patient were heterozygous for the mutation. Due to the premature termination codon the patient's GFTP molecule is predicted to be truncated after amino acid L322 and to lack the last of its 10 TM domains as well as the C-terminal cytoplasmic domain (Figure 2B).
The GFTP of the new LAD II patient is nonfunctional
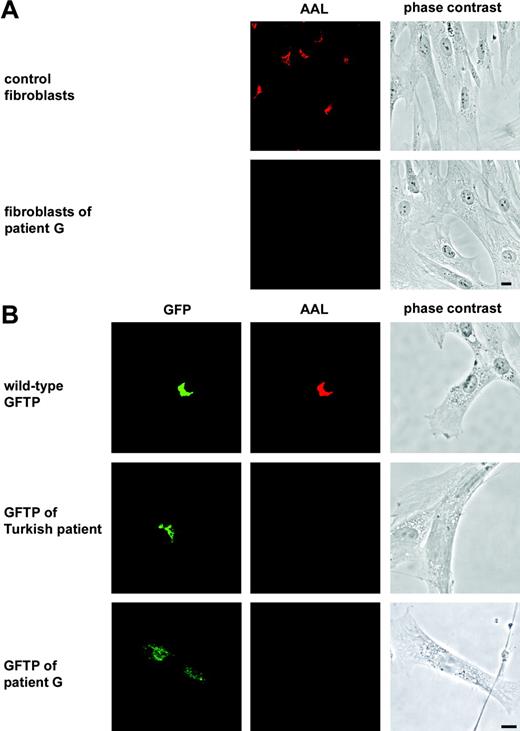
The function of the new patient's GFTP was tested in transfection experiments in comparison to the wild-type GFTP and the GFTP of the Turkish LAD II patient bearing the single amino acid substitution R147C.9,10 To detect expression of the transporters they were N-terminally tagged with GFP. The molecules were expressed in LAD II fibroblasts derived from patient G, which show strong hypofucosylation as detected by lack of AAL binding (Figure 3A). We found that transient transfection of the wild-type transporter into these LAD II cells restored fucosylation in all cells that expressed the GFP-tagged molecule (Figure 3B). In contrast, neither the GFTP of the Turkish patient nor the GFTP of patient G were able to restore fucosylation in the fibroblasts showing that the GFTP of the new LAD II patient is nonfunctional (Figure 3B). Identical results were obtained when fibroblasts of the Turkish patient were transfected with the GFTPs (not shown). The functional defect of the new patient's GFTP was also seen in complementation experiments with untagged molecules (data not shown).
LAD II patient G displays a premature translational termination codon in the GFTP gene. (A) The genomic sequence coding for the GFTP and the respective transcripts obtained from 2 independent RT-PCRs were sequenced and found to bear a G969A nucleotide substitution resulting in a premature stop codon. (B) Predicted topology of wild-type and mutated GFTP. Boxes represent transmembrane domains. The GFTP of LAD II patient G is predicted to be truncated after the ninth transmembrane domain.
LAD II patient G displays a premature translational termination codon in the GFTP gene. (A) The genomic sequence coding for the GFTP and the respective transcripts obtained from 2 independent RT-PCRs were sequenced and found to bear a G969A nucleotide substitution resulting in a premature stop codon. (B) Predicted topology of wild-type and mutated GFTP. Boxes represent transmembrane domains. The GFTP of LAD II patient G is predicted to be truncated after the ninth transmembrane domain.
The GFTP of the new LAD II patient is nonfunctional. (A) Fibroblasts derived from a healthy control donor and from patient G were stained with AAL. Perinuclear fucoslyation in the Golgi is seen in healthy but not in patient cells. (B) Complementation of LAD II fibroblasts with green fluorescent protein (GFP)–tagged GFTPs of a healthy donor (wild-type), the Turkish LAD II patient, and patient G, respectively. Cells were double-stained with anti-GFP and AAL. One representative experiment of 6 is shown. Bars represent 10 μm.
The GFTP of the new LAD II patient is nonfunctional. (A) Fibroblasts derived from a healthy control donor and from patient G were stained with AAL. Perinuclear fucoslyation in the Golgi is seen in healthy but not in patient cells. (B) Complementation of LAD II fibroblasts with green fluorescent protein (GFP)–tagged GFTPs of a healthy donor (wild-type), the Turkish LAD II patient, and patient G, respectively. Cells were double-stained with anti-GFP and AAL. One representative experiment of 6 is shown. Bars represent 10 μm.
The GFTP of the new LAD II patient fails to localize to the Golgi
The GFTP of healthy individuals resides in the Golgi membrane9,11 (Figure 4) where it serves to provide GDP-fucose for the action of several fucosyltransferases. We have previously noted that the defective GFTP of the Turkish patient with the R147C amino acid exchange was correctly expressed in the Golgi as shown by colocalization with a Golgi marker8 (Figure 4). We now studied the subcellular distribution of another defective GFTP that was found in 3 LAD II patients of Arab origin and bears a different single amino acid exchange (T308R).10,14 We found that the defective GFTP of these patients was also correctly located in the Golgi (Figure 4). However, in contrast to these defective transporters bearing single amino acid exchanges, the truncated GFTP of patient G did not localize to the Golgi. Instead, we found the molecule to be distributed virtually over the entire cell body (Figure 4). This was not the consequence of overexpression of the defective GFTP because (1) in the same transfection experiments the wild-type GFTP was well confined to the Golgi, (2) we used a transfection method that resulted in very low autofluorescence of the GFP so that anti-GFP antibodies had to be used for detection, and (3) cells displaying even very faint staining with anti-GFP showed the same staining pattern (not shown). We conclude that the new patient's GFTP has lost its ability to localize to the Golgi, the compartment in which its function is required.
The GFTP of patient G does not localize to the Golgi. Fibroblasts derived from LAD II patient G were transiently transfected with GFP-tagged GFTPs from a healthy donor, the Turkish LAD II patient, 3 Arab LAD II patients, and patient G, respectively. Cells were double-stained with anti-GFP antibodies and antibodies against the Golgi protein golgin-97. One representative experiment of 3 is shown. Bar represents 10 μm.
The GFTP of patient G does not localize to the Golgi. Fibroblasts derived from LAD II patient G were transiently transfected with GFP-tagged GFTPs from a healthy donor, the Turkish LAD II patient, 3 Arab LAD II patients, and patient G, respectively. Cells were double-stained with anti-GFP antibodies and antibodies against the Golgi protein golgin-97. One representative experiment of 3 is shown. Bar represents 10 μm.
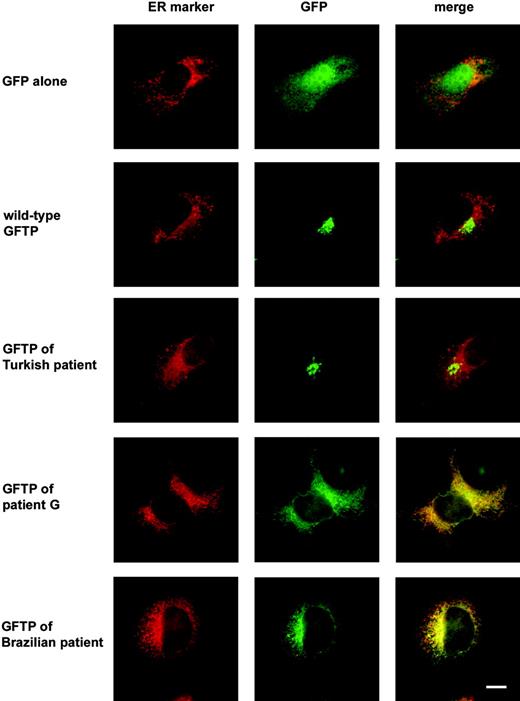
We suspected a localization of the new patient's GFTP in the ER. We therefore used COS-7 cells in which the reticular staining pattern of the ER, including the nuclear envelope, is more clearly visible than in fibroblasts and cotransfected them with GFP-tagged GFTPs and a vector coding for a fluorescent protein that localizes to the ER due to an ER retention signal (amino acids KDEL). We found that neither GFP alone nor the GFP-tagged wild-type GFTP nor the GFTP of the Turkish patient colocalized with the ER marker (Figure 5). In contrast, the transporter of patient G gave a GFP label that considerably overlapped with the ER marker (Figure 5). Localization of the patient's GFTP in the ER could also be observed in fibroblasts, although in these cells the ER was more difficult to distinguish from the cytoplasm than in COS-7 cells (not shown). These data suggest that the truncated GFTP is not able to leave the ER to enter the Golgi.
Recently, Hidalgo et al15 described an LAD II patient of Brazilian origin who expresses a nonfunctional GFTP with an even more severe truncation. This defective protein was predicted to lack the C-terminal 5 TM domains plus the C-terminal cytoplasmic domain due to a frame shift in its gene. The subcellular localization of this GFTP had not been determined. Because the deletion in this GFTP includes the deletion in patient G, we expected the GFTP of the Brazilian patient to be unable to localize to the Golgi. Indeed, when a tagged GFTP with the mutation of the Brazilian patient was expressed in COS-7 cells it did not localize to the Golgi but was found mainly in the ER resembling the subcellular localization of the GFTP of patient G (Figure 5). Thus, in both known LAD II patients with truncated GFTPs the defective molecule is mislocalized.
Absence of the last transmembrane domain causes mislocalization of the GFTP
We next investigated which of the lacking regions in the GFTP of patient G, the 10th TM domain or the cytoplasmic domain, is responsible for the mislocalization and defective function of the transporter. We first asked whether reconstituting the 10th TM domain while still omitting the cytoplasmic C-terminus would result in an active GFTP. We predicted the 10th TM domain of the GFTP to end with amino acid A336 using 2 structure prediction programs.9 To compensate for uncertainties that are immanent to such predictions we generated a C-terminal deletion construct (Δ25C) that terminates after amino acid W339, making sure that the entire 10th TM domain was included. We found that this construct was correctly located in the Golgi (Figure 6A). This shows that the lack of the 10th TM domain in the GFTP of patient G is responsible for the mislocalization of the transport molecule. This result also implies that the lack of the last TM domain in the Brazilian patient's GFTP is sufficient to cause its retention in the ER.
The truncated GFTPs of 2 LAD II patients localize preferentially to the ER. COS-7 cells were transfected with GFP-tagged GFTPs of a healthy individual and of the indicated LAD II patients together with vector pDsRed-ER coding for an ER-resident fluorescent protein. Cells were stained with anti-GFP antibodies. One representative experiment of 3 is shown. Bar represents 10 μm.
The truncated GFTPs of 2 LAD II patients localize preferentially to the ER. COS-7 cells were transfected with GFP-tagged GFTPs of a healthy individual and of the indicated LAD II patients together with vector pDsRed-ER coding for an ER-resident fluorescent protein. Cells were stained with anti-GFP antibodies. One representative experiment of 3 is shown. Bar represents 10 μm.
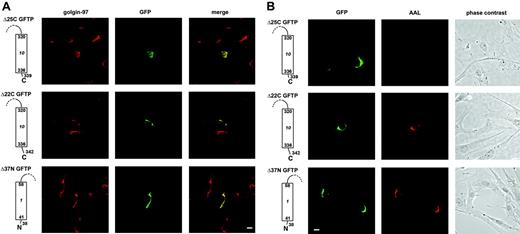
Two missing regions in the patient's GFTP are required for Golgi localization and transport function. The C-terminal deletion mutants Δ25C and Δ22C lack the last 25 and 22 amino acids, respectively. These constructs bear an N-terminal GFP-tag. The N-terminal deletion mutant Δ37N lacks the first 37 amino acids and bears a C-terminal GFP-tag. Only one of 10 transmembrane domains is shown. Fibroblasts of patient G were transfected with the deletion constructs and analyzed for the GFP-tag and golgin-97 (A) or GFP and AAL (B). Construct Δ37N was compared with a C-terminally tagged wild-type GFTP that was functional and located in the Golgi (not shown). One representative experiment of 3 is shown. Bars represent 10 μm.
Two missing regions in the patient's GFTP are required for Golgi localization and transport function. The C-terminal deletion mutants Δ25C and Δ22C lack the last 25 and 22 amino acids, respectively. These constructs bear an N-terminal GFP-tag. The N-terminal deletion mutant Δ37N lacks the first 37 amino acids and bears a C-terminal GFP-tag. Only one of 10 transmembrane domains is shown. Fibroblasts of patient G were transfected with the deletion constructs and analyzed for the GFP-tag and golgin-97 (A) or GFP and AAL (B). Construct Δ37N was compared with a C-terminally tagged wild-type GFTP that was functional and located in the Golgi (not shown). One representative experiment of 3 is shown. Bars represent 10 μm.
Absence of a membrane-proximal C-terminal cytoplasmic region renders the GFTP inactive
Interestingly, complementation of LAD II fibroblasts with construct Δ25C did not restore fucosylation (Figure 6B). This shows that Golgi localization of Δ25C was not sufficient to make this truncated construct functional and that a region within the C-terminal cytoplasmic domain is required for transport function. We next sought to identify this functionally important cytoplasmic region. We found that an elongation of construct Δ25C by only 3 more amino acids resulted in a transporter molecule (construct Δ22C) that did not only localize to the Golgi but also restored AAL staining in all transfected LAD II fibroblasts in a manner indistinguishable from the wild-type GFTP (Figure 6A-B). Cell-surface AAL staining below saturation was also compared and quantified and was found to be very similar following transfection with wild-type GFTP and construct Δ22C, respectively (Supplemental Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). This shows that one or more of the membrane-proximal cytoplasmic amino acids 340-342 (VRG) are required for the function of the GFTP molecule. Interestingly, in contrast to the C-terminal domain described, deletion of the entire N-terminal cytoplasmic domain in construct Δ37N neither influenced the subcellular localization nor the function of the GFTP (Figure 6A-B).
Thus, the known LAD II patients with truncated GFTPs suffer from a dual defect of the transport molecule, a deficiency in Golgi localization due to the lack of one or more TM domains, and an additional defect in transport function due to the absence of a functionally important region within the C-terminal cytoplasmic domain.
Effect of exogenous fucose on the fucosylation of the new patient's fibroblasts
It is still an open question whether the successful fucose-based therapy of LAD II patients that we first tested in the Turkish patient16,17 is based on a residual function of the GFTP or on another transport system that allows GDP-fucose transport into the Golgi as soon as exogenous fucose is given. We tested the effect of culturing fibroblasts of patient G in the presence of l-fucose and found that the fucose supplementation with 0.1 to 10 mM l-fucose caused refucosylation of the cells (Figure 7A and Figure S2), whereas it did not alter the aberrant localization of the transport molecule (Figure 7B). This is in line with results obtained with the Brazilian patient who was successfully treated with l-fucose15 despite the mislocalization of his GFTP (Figure 5).
Our stainings do not fully exclude that a small percentage of the truncated GFTPs is localized in the Golgi. We therefore tested the function of the truncated GFTP of patient G under conditions of fucose supplementation. We transfected LAD II fibroblasts with the wild-type GFTP and the truncated GFTP of patient G, respectively, and cultured the cells in medium containing a suboptimal concentration of l-fucose at which the corrective transport system is active causing a restricted refucosylation. Under these conditions the overexpression of a transporter that is active during fucose supplementation is expected to further increase fucosylation. We find that, whereas the wild-type GFTP is able to perform in such a way, the GFTP of patient G remains inactive (Figure S3). Even construct Δ25C, which fully locates to the Golgi but lacks amino acids 340-342, is unable to induce fucosylation under these conditions (Figure S3). This shows that even if some of the truncated GFTP molecules are located in the Golgi they cannot account for the therapeutic effect of exogenous l-fucose.
Exogenous fucose can restore fucosylation in cells of the new patient. (A) Fibroblasts derived from patient G were cultured without or with 10 mM l-fucose for 48 hours before they were analyzed for AAL binding. (B) Fibroblasts of patient G were transfected with the GFP-tagged GFTP of this patient and cultured without or with 10 mM l-fucose for 48 hours. Staining of GFP and golgin-97 is shown. One representative experiment of 3 is shown. Bars represent 10 μm.
Exogenous fucose can restore fucosylation in cells of the new patient. (A) Fibroblasts derived from patient G were cultured without or with 10 mM l-fucose for 48 hours before they were analyzed for AAL binding. (B) Fibroblasts of patient G were transfected with the GFP-tagged GFTP of this patient and cultured without or with 10 mM l-fucose for 48 hours. Staining of GFP and golgin-97 is shown. One representative experiment of 3 is shown. Bars represent 10 μm.
Because it is difficult to explain how a nonfunctional transport molecule that, in addition, is not able to localize to the Golgi can support fucosylation events in the Golgi, these data strongly support—although they do not formally prove—the notion that an alternative transport system is responsible for the successful treatment of LAD II patients with l-fucose.
Discussion
In this report we define a group of patients with LAD II who display a novel dual defect of the GFTP including, first, truncations of one or more transmembrane domains causing mislocalization of the transport protein and, second, the absence of a region in the C-terminal cytoplasmic domain, which is required for the function of the transporter. In addition, we show that exogenous fucose restores fucosylation in cells of such a patient, which suggests the presence of an alternative GDP-fucose transport system.
The mislocalized truncated GFTPs, when ectopically expressed, are located mainly in the ER presumably because their truncation leads to recognition by the ER-resident protein quality control system similarly to truncated CMP-sialic acid transporters that have recently been detected in a sialylation-deficient patient.24 Expression of the GFTP, however, is required in the Golgi because the fucosyltransferases that catalyze fucosylation of N- and O-linked carbohydrate chains reside in that compartment. Thus, it is very likely that the mislocalization of truncated GFTPs contributes to the fucosylation defect in the affected LAD II patients.
Staining with ER markers (Figure 5) visualizes a very dense network that makes it difficult to tell whether an ER-resident protein is entirely excluded from other compartments like the Golgi. We therefore cannot exclude from these stainings that a small percentage of the truncated GFTPs may still be targeted to the Golgi. However, our double stainings for GFP-tagged transporters and the Golgi marker golgin-97 show no colocalization (Figure 4). In addition, even if some of the truncated GFTP was expressed in the Golgi, the transporter would not be functional due to the lack of one or more of the C-terminal amino acids V340-R341-G342. Thus, the LAD II patients with truncated GFTPs suffer from a dual defect of mislocalization and nonfunction of the transport molecule.
We have previously noted that 2 splicing variants of the human GFTP exist, one which is full-length, and another in which the first 13 amino acids are missing. We and others showed that mRNAs for both variants are expressed and that the corresponding proteins are fully functional.9,10 Now we show that the entire N-terminal cytoplasmic domain of 24 amino acids is dispensable for Golgi localization and function. More interestingly, the cytoplasmic C-terminus bears a stretch of 3 amino acids that appears to be required for transport function of the GFTP. Data on the role of the C-terminal cytoplasmic domain in other nucleotide sugar transporters are inconsistent. Deletion of this domain in the mouse UDP-galactose transporter resulted in a construct that could not be expressed,25 whereas replacement of the C-terminal cytoplasmic domain of the human UDP-galactose transporter by the corresponding domain of the human CMP-sialic acid transporter had no effect.26,27 Deletion of the C-terminal cytoplasmic domain of the yeast GDP-mannose transporter was reported to cause loss of function.28 However, in another study these data could not be reproduced.29 Future studies will have to address the exact role of the 3 cytoplasmic amino acids 340-342 in the GFTP, whether it be to support the assembly of the transporter protein dimer or the binding and translocation of the nucleotide sugar.
Several years ago we treated the first LAD II patient (the patient of Turkish origin) with oral l-fucose and found that this treatment induced refucosylation of glycostructures including selectin ligands and that it cured the patient's immunodeficiency.16,17 This child is still immunologically healthy after 7 years of continuing treatment (own unpublished results, August 2005). It is clear that the salvage pathway is responsible for the cytoplasmic biosynthesis of GDP-fucose from exogenous l-fucose. However, it is not known how sufficient amounts of GDP-fucose are transported into the Golgi under conditions of therapy. It is possible that with elevated GDP-fucose levels in the cytoplasm the defective GFTP in the Turkish patient has residual transport activity or that an alternative unknown transport molecule translocates GDP-fucose into the Golgi.
The fucose-based therapy was repeated in 2 Arab LAD II patients without success.18,19 One of these patients was analyzed for the genetic defect and was found to bear the T308R mutation.14 Because this mutation differed from that in the Turkish patient (R147C), it was assumed that the different outcome of the therapy was due to the different GFTP mutations in the patients.14 This notion implied that an alternative GDP-fucose transport mechanism is not relevant and was underscored by studies on one Arab patient whose Golgi vesicles were shown to display a decreased maximal rate of transport,19 whereas this feature was not seen in the Turkish patient.30
In contradiction to the notion mentioned, we now show that LAD II cells can be refucosylated with exogenous fucose even if the GFTP is mislocalized and remains mistargeted during fucose supplementation. In addition, we show that the severely truncated GFTP of the Brazilian patient who was successfully treated with oral l-fucose15 is also mislocalized. Our immunofluorescence stainings do not fully exclude that a small percentage of the truncated GFTPs is localized in the Golgi. However, it is very unlikely that this can account for the refucosylation by exogenous l-fucose for the following reasons: (1) Even if some of the truncated GFTPs are located in the Golgi the lack of amino acids 340-342 would keep them inactivated. (2) Overexpression of the truncated GFTP of patient G and of the Golgi-located construct Δ25C under conditions of suboptimal supplementation with l-fucose did not further increase fucosylation.
These findings are difficult to reconcile with the notion that the defective GFTP itself is responsible for the therapeutic effect of fucose supplementation and rather strongly support the assumption that an alternative transport system is responsible for the success of oral l-fucose treatment.
The nature of the putative alternative transport system is unknown. No other transport molecule that is able to transport GDP-fucose has been described and database searches reveal no protein with high homology to the GFTP (own unpublished results, July 2005). Recently, it was found that O-fucosylation, a process that directly attaches fucose to the protein core and that requires GDP-fucose, occurs in the ER.31 Thus, an ER-based GFTP system can be postulated, which appears to be active in at least one LAD II patient because no defect of O-fucosylation of notch could be detected in this individual.32 Chen et al recently suggested that a multi–transmembrane protein termed SLC35C2 (gene CGI-15/Ovcov1) might transport GDP-fucose into the ER but expression of this protein reduced the Golgi-based fucosylation of Lex arguing against SLC35C2 to be the alternative transporter.33 Thus, the transporter that is active during fucose supplementation remains elusive. We speculate that one of the remaining genes of the SLC35 family34 coding for nucleotide sugar transporters of unknown function is responsible for the therapeutic effect of l-fucose.
In conclusion, this study identifies a dual defect in subcellular localization and function by which a truncated GFTP causes LAD II and strongly supports the notion that the fucose-based therapy of LAD II is dependent on an alternative GDP-fucose transport system.
Prepublished online as Blood First Edition Paper, February 2, 2006; DOI 10.1182/blood-2005-08-3334.
Supported by the Max-Planck Society and by the SFB 293 of the Deutsche Forschungsgemeinschaft (D.V., M.K.W.) and by a fellowship of the International Graduate Research School “Molecular Basis of Dynamic Cell Processes,” GRK 1050 (S.Y.).
Y.H., J.D., and S.Y. performed the research; P.R., D.L.W., and P.J.M. described and attended the patient; K.L. contributed vital new reagents; D.V. designed the research; T.M. designed the research and analyzed data; and M.K.W. designed the research, analyzed data, and wrote the paper.
Y.H. and J.D. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Carlos B. Hirschberg (Boston University, MA) and Andrés Hidalgo (Mount Sinai School of Medicine, New York, NY) for helpful discussions. We thank Ute Ipe for excellent technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal