Cellular prion protein (PrPC) is a glycophosphatidylinositol (GPI)–anchored protein, of unknown function, found in a number of tissues throughout the body, including several blood components of which platelets constitute the largest reservoir in humans. It is widely believed that a misfolded, protease-resistant form of PrPC, PrPSc, is responsible for the transmissible spongiform encephalopathy (TSE) group of fatal neurodegenerative diseases. Although the pathogenesis of TSEs is poorly understood, it is known that PrPC must be present in order for the disease to progress; thus, it is important to determine the physiologic function of PrPC. Resolving the location of PrPC in blood will provide valuable clues as to its function. PrPC was previously shown to be on the alpha granule membrane of resting platelets. In the current study platelet activation led to the transient expression of PrPC on the platelet surface and its subsequent release on both microvesicles and exosomes. The presence of PrPC on platelet-derived exosomes suggests a possible mechanism for PrPC transport in blood and for cell-to-cell transmission.
Introduction
Cellular prion protein (PrPC) is a membrane-bound, glycophosphatidylinositol (GPI)–anchored protein1 found primarily in lipid rafts on the cell membrane of neuronal and non-neuronal cells, including tonsils, spleen, and of the secretory granules of epithelial cells in the stomach, as well as in cultured cell lines.2,3 Although PrPC has been shown to be present on the surface of a number of peripheral blood cells,4 the relative levels on individual cell types have been contentious. Individual studies have reported that the majority of PrPC is associated with both platelets5,6 and red blood cells.7 In the former case the surface expression of PrPC is increased following stimulation, suggesting an additional internal membrane source of the protein,8 recently shown to be alpha granule membranes.9 Furthermore, platelet activation is associated with the accumulation of PrPC in releasates,10 and in platelet concentrates, stored for up to 10 days, there is an increase in initially the microsomal, then plasma levels of PrPC.11
Transmissible spongiform encephalopathies (TSEs) are a family of neurodegenerative disorders, including Creutzfeldt-Jakob disease (CJD), Gerstmann-Straussler-Schienker syndrome, and fatal familial insomnia in humans; scrapie in sheep; and bovine spongiform encephalopathy (BSE) in cattle.12,13 They are all characterized by the accumulation of a protease-resistant isomer (PrPSc) of PrPC in the brain of affected individuals. It is generally considered that PrPSc acts as a template inducing the same structural changes within other normally folded PrPC molecules on contact, thus propagating the misfolded state of the protein.14
The CNS is the site at which TSE pathology is apparent in prion infections; however, the agent must first replicate and be transported to the CNS after peripheral infection. The spread of PrPSc has been tracked from the gastrointestinal tract and the spleen to the CNS.15 The lymphoreticular system (LRS) is believed to be an important site of prion replication, and an accumulation of PrPSc is apparent in spleen and lymph nodes after peripheral infection. Indeed, neuroinvasion is delayed without a functional LRS.16
A new variant of CJD (vCJD) identified in humans in the United Kingdom is almost certainly the result of infection with the BSE agent.17 Patients infected with vCJD, in contrast to those with classic CJD, have been shown to have widespread deposition of PrPSc in the LRS.18,19 Immune cells, in particular B cells and follicular dendritic cells, have been identified as harboring infectious PrPSc in the LRS. However PrPSc, the standard biochemical marker used for diagnosis of TSEs, cannot be detected by current technology in circulating lymphocytes or whole blood. Bioassays are a more sensitive assay for infectivity, and a number of studies have demonstrated that the infectious agent is present in blood and blood components, buffy coats, plasma, and platelets in animal models.20-23 The removal of all white cells by standard leukoreduction reduced infectivity by only 42%, suggesting that other blood components carry PrPSc.24 There is therefore a significant concern that blood transfusions may represent a portal for the transmission of TSEs. Indeed since 2004, 3 apparent cases of transmission of vCJD by transfusion have been reported in the United Kingdom.25,26
To understand the transmission of the disease by PrPSc, it is important to determine the physiologic behavior and function of normal PrPC in blood cells and plasma. In the current study the cellular localization, and stimulus-induced redistribution, of PrPC in platelets has been examined.
Materials and methods
The protocols of this study were approved by the Human Research Ethics Board of the University of Manitoba.
Antibodies and reagents
Monoclonal antibody (Ab) 308, raised against amino acids 106 to 126 of human PrPC, was purchased from Cayman Chemical, AnnArbor, MI, and the polyclonal Ab FL253, raised against the full-length PrPC, was purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Anti-CD62P Ab (D541) was a generous gift from Dr Sara Israels, Manitoba Institute of Cell Biology, Winnipeg, MB.
Polyclonal Ab to human fibrinogen was purchased from Calbiochem-Novabiochem, San Diego, CA. Horseradish peroxidase– and FITC-conjugated Abs were purchased from DakoCytomation, Mississauga, ON. Secondary Abs conjugated to gold, along with bovine thrombin, protease inhibitors, and all other reagents were purchased from Sigma-Aldrich Canada, Oakville, ON, and were of the highest grade available.
Preparation of washed platelets
Blood was collected following informed consent, by venipuncture of human volunteers who had denied taking medication known to interfere with platelet function within the previous 2 weeks. The blood was drawn into syringes containing acid citrate dextrose anticoagulant (ACD; 3.8 mM citric acid, 7.5 mM trisodium citrate, 125 mM dextrose; 1.8 mL anticoagulant/8.1 mL whole blood). Washed platelets were obtained as previously described.27,28
Flow cytometry
Plasma-free platelet suspensions were prepared and incubated with agonist, or saline control, for the times indicated. The samples were fixed by the addition of an equal amount of 4% paraformaldehyde, then incubated with anti-PrPC Ab 308 diluted in PBS/0.1% BSA. Following washing 3 times in PBS/0.1% BSA, the samples were incubated in a FITC-conjugated secondary Ab. The samples were finally washed 3 times in PBS and resuspended in PBS. Flow cytometry was carried out on a Becton Dickinson (Mississauga, ON, Canada) FacsCalibur flow cytometer with forward and side scatter set to a logarithmic scale.
An area was drawn around the unstimulated platelet sample according to the forward and side scatter properties and labeled platelet region (P). A second region was drawn to gate on smaller particles and labeled microvesicle (MVS) region.29 Fluorescence backgating was used to determine the number of PrPC-positive events in each region.
Microvesicle and exosome preparation
Microvesicles and exosomes were prepared according to the method of Heijnen et al.29 Briefly, plasma-free platelet suspensions were prepared and incubated with agonist or saline control for the times indicated, and the reaction was stopped by the addition of an equal amount of ice-cold ACD. Samples were centrifuged (Beckman GH-3 swing bucket rotor [Beckman Coulter Canada, Mississauga, ON, Canada]; 800g; 15 minutes) to obtain the platelet pellet fraction (P). The supernatant was further centrifuged (Beckman F3602 fixed angle rotor [Beckman Coulter Canada, Mississauga, ON, Canada]; 10 000g; 30 minutes) to obtain the microvesicle fraction (MV), and the supernatant from the MV fraction was centrifuged (Beckman MLS-50 swing bucket rotor; 100 000g; 60 minutes) to obtain the exosome pellet (EX). All pellets were resuspended in PBS.
To ensure exosome isolation, the supernatants from MV preparations were mixed with 2 M sucrose, and a 0.8 to 0.25 M sucrose gradient was layered on top. The gradient was centrifuged (Beckman MLS-50 swing bucket rotor; 65 000g; 16 hours) and 500-μL fractions were collected. The fractions were diluted in 4.5 mL PBS and centrifuged (100 000g; 60 minutes; 4°C), and the resultant pellets were resuspended in PBS.
In some studies, fractions from the sucrose gradient were vacuum-transferred onto a nitrocellulose membrane using a Schleicher and Schuell (Dassel, Germany) slot blot apparatus and immunoblotted with anti-PrPC Ab. Briefly, following blocking in 5% nonfat milk, the membrane was incubated in Ab 308, washed in TBS with 0.01% Tween 20 (TBS-T) followed by incubation in a horseradish peroxidase–conjugated secondary Ab. After washing in TBS-T 6 times, the membrane was incubated in Super Signal West Pico chemiluminescence substrate (Pierce Biotechnology, Rockford, IL), and protein bands were visualized on a Bio-Rad Fluoro S Max imaging system. Densitometry was performed using Quantity One software (Bio-Rad, Mississauga, ON).
Electron microscopy and immunocytochemistry
Exosomes for double immunolabeling studies were prepared from the sucrose gradient fractions. Briefly, the exosomes were resuspended in PBS and adsorbed onto 300 mesh carbon-coated formvar nickel grids for 10 minutes. Excess PBS was blotted with filter paper, and the grids were fixed with 2% paraformaldehyde in PBS for 10 minutes. After fixation, the grids were incubated in PBS with 1% BSA for 15 minutes, incubated with the anti-PrPC Ab for 30 minutes, washed 3 times in PBS with 0.1% BSA, and incubated in a secondary Ab conjugated to 5 nm gold for 30 minutes. The grids were rewashed 3 times in PBS, followed by 3 changes of PBS with 1% BSA, incubated in the anti-CD62 Ab for 30 minutes, washed (3 × 5 minute) with PBS with 0.1% BSA, and incubated in a secondary Ab conjugated to 10 nm gold for 30 minutes. Finally, the grids were washed (3 × 5 minutes) with PBS, followed by deionized water (3 × 5 minutes), embedded in uranyl acetate methyl cellulose. All specimens were examined in a Tecnai 20 transmission electron microscope (FEI Systems, Toronto, ON, Canada) operating at 200 kV at magnifications ranging from 25 500 × to 135 000 ×. Digital images (1024 × 1024-pixel) were acquired from the TEM via an AMT Advantage XR-12 TEM camera (AMT, Danvers, MA). Digital images were arranged in final figure format using the Canvas software package (ACD Systems of America, Miami, FL).
Frozen sections of platelets were prepared according to a method by Tokuyasu,30 as previously described.31 Cryo sections were cut with an Ultracut UCT ultramicrotome (Leica Microsystems Canada, Richmondhill. ON), equipped with an electron microscope (EM) FCS-sectioning chamber. Immunolabeling was carried out as described for exosome preparations with gold-conjugated protein G replacing the secondary Ab. Following gold labeling, the sections were stained and embedded in a uranyl acetate/methyl cellulose mixture.30,31
Results
PrPC is present on platelet alpha granule, but not dense granule, membranes
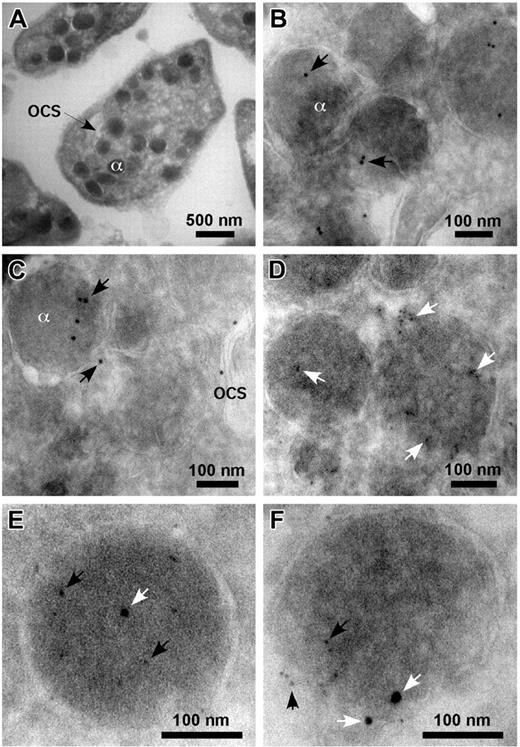
Frozen sections of quiescent human platelets showed the characteristic platelet intracellular architecture (Figure 1A). Immunoelectron microscopy studies of these platelets using an anti-PrPC Ab, FL253, followed by gold-conjugated Protein G, confirmed the association of PrPC with intracellular granules (Figure 1B-C) and the membranes of the open canalicular system (Figure 1C). A polyclonal Ab to fibrinogen was used to identify alpha granules (Figure 1D), and double staining of these sections using Abs to PrPC and fibrinogen was consistent with both proteins localizing to the same organelle (Figure 1E-F).
PrPC translocates to and is released from the platelet surface following activation
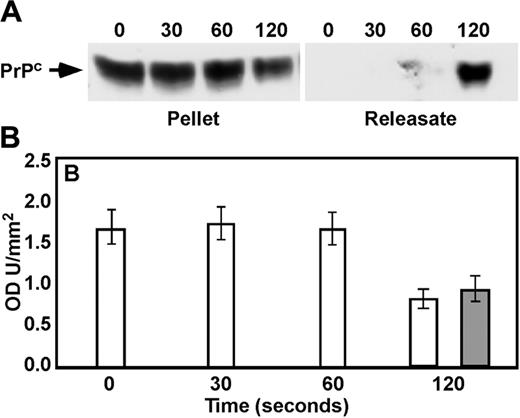
Lysates from thrombin-stimulated platelets (1 U/mL; 0-120 seconds) were immunoblotted with anti-PrPC Ab 308. There was a decrease in PrPC levels associated with the platelet pellet over time (Figure 2A). Although there was significant donor variability in the time course of the loss of PrPC from the pellet, densitometric analysis indicated that by 120 seconds of stimulation the intensity of the PrPC band from the platelet pellet had diminished to about 50% of control (Figure 2B). This decrease in platelet-associated PrPC from activated platelets was accompanied by a corresponding accumulation of PrPC in the releasate (Figure 2A-B).
Immunoelectron microscopy of resting platelets. Frozen sections of resting platelets were incubated with Abs to PrPC (FL253) and fibrinogen, followed by incubation in protein G conjugated to 5 or 10 nm gold. Normal resting platelets showing alpha granule (α) and open canalicular system (OCS) (A). PrPC (10 nm gold, arrows) is seen in alpha granule and the open canalicular system (B-C). Alpha granules are identified by the presence of fibrinogen (5 nm gold, arrows; D). Double labeling with Abs to PrPC (5 nm gold, black arrows) and fibrinogen (10 nm gold, white arrows) localized both proteins to the same granules (E-F).
Immunoelectron microscopy of resting platelets. Frozen sections of resting platelets were incubated with Abs to PrPC (FL253) and fibrinogen, followed by incubation in protein G conjugated to 5 or 10 nm gold. Normal resting platelets showing alpha granule (α) and open canalicular system (OCS) (A). PrPC (10 nm gold, arrows) is seen in alpha granule and the open canalicular system (B-C). Alpha granules are identified by the presence of fibrinogen (5 nm gold, arrows; D). Double labeling with Abs to PrPC (5 nm gold, black arrows) and fibrinogen (10 nm gold, white arrows) localized both proteins to the same granules (E-F).
PrPC is present on platelet microvesicles
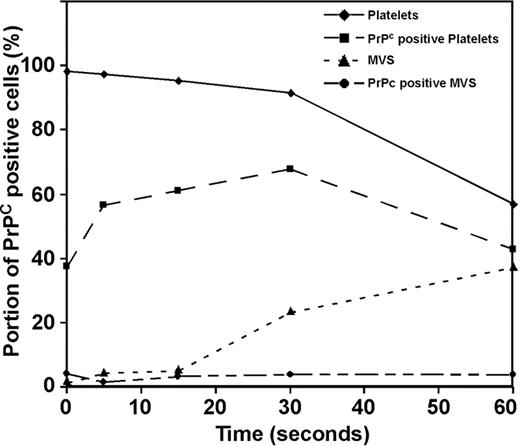
Flow cytometry, using anti-PrPC Ab 308, demonstrated the presence of PrPC on the surface of unstimulated platelets (Figure 3). Following thrombin stimulation (1 U/mL) there was a transient increase in the expression of PrPC on the platelet surface, followed by its release into the supernatant. There was a corresponding shedding of microvesicles from the platelet surface in response to thrombin; however, the level of PrPC on the surface of the microvesicles was low (< 10% of total released PrPC) and remained constant with time (Figure 3). Platelet activation with A23187 (20 μM) was accompanied by increased levels of microvesicle production when compared with thrombin; however, the level of PrPC associated with the microvesicles remained low (data not shown).
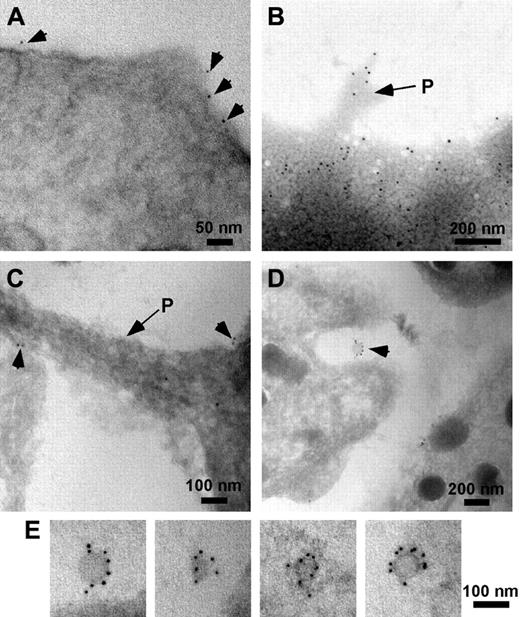
Immunogold labeling demonstrated the presence of PrPC on the surface of unstimulated platelets (Figure 4A). Following activation with thrombin, PrPC was observed around the periphery of the platelet and at the tips of pseudopods (Figure 4B-C). In addition, PrPC was associated with small (< 100 nm) membranous vesicles released from the platelets (Figure 4D-E).
Immunoblotting of pellets and releasates in activated platelets. Platelets were incubated with 1 U/mL thrombin for 0, 30, 60, or 120 seconds. Following termination, platelet pellet and releasates were prepared, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted with anti-PrPC Ab 308 (A), and densitometry was performed (B) on pellet (□) and releasates (▦) (n = 3). Error bars indicate standard error of the mean.
Immunoblotting of pellets and releasates in activated platelets. Platelets were incubated with 1 U/mL thrombin for 0, 30, 60, or 120 seconds. Following termination, platelet pellet and releasates were prepared, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted with anti-PrPC Ab 308 (A), and densitometry was performed (B) on pellet (□) and releasates (▦) (n = 3). Error bars indicate standard error of the mean.
PrPC is present on platelet exosomes
Previous studies have shown that, in addition to microvesicles, thrombin stimulates the release of exosomes from platelets.29 Given the relatively low levels of PrPC on the surface of released microvesicles (Figure 3) and its presence on the surface of smaller membrane fractions (Figure 4D-E), the possible association of PrPC with exosomes was examined.
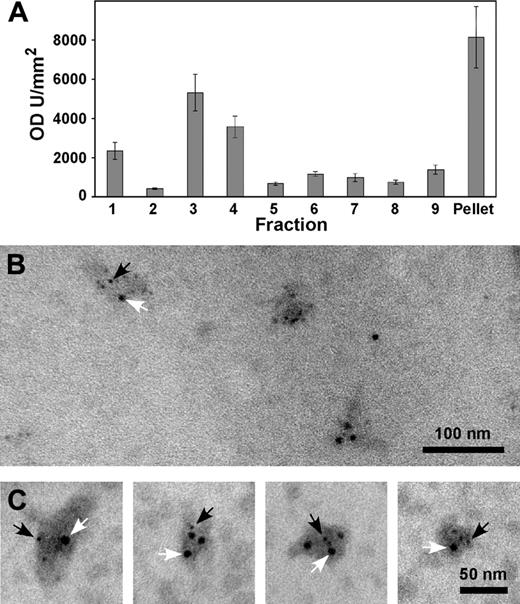
Exosomes were prepared by differential centrifugation, and separation through a sucrose gradient, of the releasate of thrombin-stimulated platelets. Immunoblotting using anti-PrPC Ab 308 was consistent with the presence of PrPC in these exosome fractions (Figure 5A).
Immunoelectron microscopy of fractions 3 and 4 using anti-PrPC Abs (308 or FL253) demonstrated the presence of PrPC on vesicles ranging from 40 to 100 nm (Figure 5B), the size being consistent with previous reports of platelet-derived exosomes.29 Double-labeling these fractions with anti-CD62 Ab D541 confirmed that these exosomes were derived from alpha granules (Figure 5C).
Flow cytometry of activated platelets. Platelets were incubated with 1 U/mL thrombin for 0, 5, 15, 30, or 60 seconds and labeled with anti-PrPC Ab 308. Flow cytometry was used to distinguish platelets from microvesicles on the basis of their forward-scatter (FSC-H) and side-scatter (SSC-H) profiles (platelets, solid line ♦, microvesicles, broken line ▴). Fluorescence backgating determined the percentage of PrPC-positive cells in each region. (Percentage of platelet region positive for PrPC, ▪; percentage of microvesicle region positive for PrPC, •).
Flow cytometry of activated platelets. Platelets were incubated with 1 U/mL thrombin for 0, 5, 15, 30, or 60 seconds and labeled with anti-PrPC Ab 308. Flow cytometry was used to distinguish platelets from microvesicles on the basis of their forward-scatter (FSC-H) and side-scatter (SSC-H) profiles (platelets, solid line ♦, microvesicles, broken line ▴). Fluorescence backgating determined the percentage of PrPC-positive cells in each region. (Percentage of platelet region positive for PrPC, ▪; percentage of microvesicle region positive for PrPC, •).
Immunogold labeling of resting and activated platelets. Resting and activated platelets were incubated with Abs to PrPC (308 or FL253), followed by protein G or secondary antibodies conjugated to 10 nm gold (arrowheads). In resting platelets preincubated with Ab 308 before embedding, PrPC is seen around the periphery of the cell (A). In activated platelets PrPC is found at the periphery of the cell and is associated with pseudopods (P, arrows) (B, whole mount; C, frozen section). In frozen sections of activated platelets labeled with Ab FL253, PrPC was also localized to released exosomes (seen between cells, D; and at higher magnification, E).
Immunogold labeling of resting and activated platelets. Resting and activated platelets were incubated with Abs to PrPC (308 or FL253), followed by protein G or secondary antibodies conjugated to 10 nm gold (arrowheads). In resting platelets preincubated with Ab 308 before embedding, PrPC is seen around the periphery of the cell (A). In activated platelets PrPC is found at the periphery of the cell and is associated with pseudopods (P, arrows) (B, whole mount; C, frozen section). In frozen sections of activated platelets labeled with Ab FL253, PrPC was also localized to released exosomes (seen between cells, D; and at higher magnification, E).
Discussion
The current study localizes PrPC to platelet alpha granule, but not dense granule, membranes, confirming a recent study by Starke et al.9 Thus, PrPC is present with proteins such as the αIIb/β3 integrin, CD62 (P-selectin), CD36, and the GPIb/V/IX complex32-35 inherent in the alpha granule membrane, and, in common with these other proteins, there is an activation-mediated increase in expression of PrPC on the external platelet surface. The function of PrPC in platelets is unknown; preincubation with anti-PrPC Abs has a limited effect on platelet adhesion to a variety of matrices but no effect on agonist-induced aggregation (Robertson et al, unpublished); therefore, it is unlikely that PrPC plays a significant role in either of these platelet functions. In contrast to the expression of other activation-associated proteins, the thrombin-induced expression of PrPC on the platelet surface was transient and was followed by its release. Previous studies have shown that PrPC is present in platelet releasates10 ; however, the current study demonstrates that the released PrPC is associated with membranes, initially in small quantities on microvesicles and subsequently in higher levels on exosomes.
Exosomes are small (40-100 nm), membrane-bounded vesicles which are released from a variety of cells following exocytosis36 and are present in human plasma.37 Denzer et al38 reviewed a large number of proteins and lipids that are associated with exosomes, which include members of the tetraspanin protein family, the immunoglobulin supergene family, as well as GPI-anchored proteins and cytosolic proteins. Exosomes have been implicated in cell-to-cell communication mechanisms by transferal of proteins directly from the exosomes to target cells, in a manner similar to the movement of GPI-anchored proteins from the plasma membrane of red blood cells to endothelial cells.39,40 Furthermore, exosomes have been implicated in the activation of the immune system, including the stimulation of T lymphocytes and a potential interaction with follicular dendritic cells.38 Reticulocyte-derived exosomes may participate in complement regulation.41 Interestingly, Whiteside42 has recently proposed that exosomes play a role in the evasion of tumor cells from the immune system.
Studies in platelets have shown the release of alpha granule membrane–derived exosomes following exocytosis.29 Therefore, the presence of PrPC on exosomes is entirely consistent with the alpha granule membrane source of these vesicles. The function of platelet-derived exosomes is unknown, although the low binding of factor X, prothrombin, and annexin V to their surface suggests that they do not have the same procoagulant activity as platelet-derived microvesicles.29 The expression of CD62 on the surface of platelet-derived exosomes points to a possible role in adhesion, or cell-to-cell transfer of adhesive properties, because CD62 is known to mediate adhesion between leukocytes and endothelial cells.43
The presence of prion protein on exosomes has recently been highlighted by Fevrier et al,44 who reported the presence of infectious PrPSc in exosomes derived from cultured epithelial and neuroglial cell lines after infection with scrapie. They subsequently proposed that exosomes may provide a vehicle for transport of PrPSc from cell to cell, thus providing a mechanism for transmission of infectious proteins in the body.45,46 The current finding that PrPC is present on platelet-derived exosomes strengthens the hypothesis that exosome release is a general mechanism for transport of proteins and inferentially pathogen transmission, including prions, between cells. Platelets contain a large proportion of circulating PrPC 5,6; therefore, platelet-derived exosomes could potentially act as an important source of protein for prion replication. In addition, the transferral of exosomes containing PrPC to cell types in which it is normally absent may confer susceptibility to infection with prions. To date, this has not been addressed.
Immunoblotting and immunoelectron microscopy of isolated exosomes. Platelets were incubated with 1 U/mL thrombin for 120 seconds. Following termination, platelets were removed by centrifugation at 800g. Further centrifugation of the supernatant removed the microvesicles. Exosomes were isolated by differential centrifugation through a sucrose gradient. Fractions were collected from the top, and immunoblotting was carried out in each fraction using anti-PrPC Ab 308. The blots were subjected to densitometry and are expressed as mean plus or minus standard error of the mean; n = 3 (A). Fractions 3 and 4 from the sucrose gradient were adsorbed onto formvar-coated grids and double labeled with anti-PrPC Ab 308 followed by an anti-CD62 Ab (D541). The respective secondary Abs were conjugated to 5 nm (anti-PrPC; black arrows) and 10 nm (anti-CD62; white arrows) gold (B-C).
Immunoblotting and immunoelectron microscopy of isolated exosomes. Platelets were incubated with 1 U/mL thrombin for 120 seconds. Following termination, platelets were removed by centrifugation at 800g. Further centrifugation of the supernatant removed the microvesicles. Exosomes were isolated by differential centrifugation through a sucrose gradient. Fractions were collected from the top, and immunoblotting was carried out in each fraction using anti-PrPC Ab 308. The blots were subjected to densitometry and are expressed as mean plus or minus standard error of the mean; n = 3 (A). Fractions 3 and 4 from the sucrose gradient were adsorbed onto formvar-coated grids and double labeled with anti-PrPC Ab 308 followed by an anti-CD62 Ab (D541). The respective secondary Abs were conjugated to 5 nm (anti-PrPC; black arrows) and 10 nm (anti-CD62; white arrows) gold (B-C).
Although there is no biochemical evidence for the presence of PrPSc on platelets, a recent study by Cervenakova et al23 identified prion infectivity in the platelet and plasma fractions of murine blood from mice infected with mouse-adapted vCJD. The present finding that PrPC is released on exosomes from activated platelets therefore raises the possibility that PrPSc is similarly released from platelets. Although this has not been addressed in the current study, it is clearly plausible that the generation of PrPSc-containing platelet exosomes during preparation of blood products could account for the transmission of variant CJD by blood transfusion. Leukoreduction of plasma, a process which would not remove exosomes, reduced infectivity by only 42%24 and, when taken in concert with the current study, suggests that further investigation into the possible role of platelet-derived exosomes as vehicles for prion transmission is clearly warranted.
Prepublished online as Blood First Edition Paper, January 24, 2006; DOI 10.1182/blood-2005-02-0802.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Debra Godal for technical assistance and Dr Sara Israels for providing the monoclonal Ab D541 and for helpful discussions and critical reading of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal