The role of the collagen receptor glycoprotein VI (GPVI) in arteriolar thrombus formation was studied in FcRγ-null mice (FcRγ–/–) lacking platelet surface GPVI. Thrombi were induced with severe or mild FeCl3 injury. Collagen exposure was significantly delayed and diminished in mild compared with severe FeCl3 injury. Times to initial thrombus formation and vessel occlusion were delayed in FcRγ–/– compared with wild-type mice after severe injury. Platelet accumulation in wild-type mice was decreased after mild compared with severe injury. However, there was little difference between platelet accumulation after severe or mild injury in FcRγ–/–. These data indicate a significant role for GPVI in FeCl3-induced thrombus formation. Pretreatment of wild-type mice with lepirudin further impaired mild FeCl3-induced thrombus formation, demonstrating a role for thrombin. Laser-induced thrombus formation in wild-type and FcRγ–/– was comparable. Collagen exposure to circulating blood was undetectable after laser injury. Normalized for thrombus size, thrombus-associated tissue factor was 5-fold higher in laser-induced thrombi than in severe FeCl3-induced thrombi. Thus, platelet activation by thrombin appears to be more important after laser injury than platelet activation by GPVI-collagen. It may thus be important when considering targets for antithrombotic therapy to use multiple animal models with diverse pathways to thrombus formation.
Introduction
Platelet adhesion and activation and blood coagulation are critical for cessation of blood loss at sites of vascular injury in the high-pressure circulatory system. Thrombosis, or pathologic clot formation, is a key component of arterial thromboembolic disease such as myocardial infarction and stroke. Collagen mediates platelet adhesion when subendothelial extracellular matrix proteins are exposed to blood,1,2 and may play a role in atherothrombosis as well. Platelet adhesion to collagen is primarily mediated by 4 interactions: direct binding to α2β1 and glycoprotein VI (GPVI) and indirect binding via collagen-bound von Willebrand Factor (vWF) bound to αIIbβ3 and to glycoprotein Ib-IX-V.3-5 Platelets deficient in GPVI are defective for platelet adhesion and thrombus growth.6,7 In addition, several studies have emphasized the potential for antithrombotic therapy targeting collagen receptors.8-11
Surface expression of GPVI requires concomitant expression of the γ-subunit of the FcR receptor (FcRγ).12,13 Their association is functionally relevant, as collagen binding to GPVI results in platelet signaling via FcRγ.14,15 Treatment of mice with the anti-GPVI monoclonal antibody, JAQ1, induces internalization and proteolytic degradation of GPVI in circulating platelets and a prolonged platelet deficiency of GPVI.8
We examined the role of GPVI in platelet adhesion, activation, and thrombus formation in vivo in several models of vessel wall injury. In agreement with earlier studies in JAQ1-treated mice or FcRγ-null mice, our results in a severe ferric chloride injury model (10% FeCl3 for 5 minutes) indicate that platelet recruitment and thrombus formation are diminished at sites of vascular injury induced by disruption of the endothelium.8-10 After mild ferric chloride injury (8% FeCl3 for 2 minutes) where collagen exposure is delayed and diminished, thrombus formation in wild-type mice is markedly decreased but thrombus formation in FcRγ-null animals is similar after severe or mild FeCl3 injury. Absence of platelet GPVI or decreased exposure of its ligand leads to decreased thrombus formation after arterial insult with FeCl3 indicating a significant role for this receptor in thrombus formation in this model. Platelet thrombus formation is further diminished in wild-type mice when the animals are treated with lepirudin prior to mild FeCl3 injury, indicating that thrombin activation of platelets contributes to thrombus formation as well. In contrast, thrombus formation in FcRγ-null mice is comparable with that in wild-type mice after laser-induced injury and collagen exposed to circulating blood is undetectable. Platelet activation by thrombin appears to predominate after laser injury. The definition of the mechanisms initiating thrombus formation in these models has implications for evaluation of antithrombotic therapies in animal models.
Materials and methods
Reagents
Anti-mouse tissue factor antibody has been previously described.16 Other antibodies used were: rat anti-CD41 (BD Biosciences, San Jose, CA), rabbit anti–mouse collagen type I (AB765P; Chemicon, Temecula, CA), Alexa 488–conjugated goat anti–rabbit immunoglobulin G (IgG; Molecular Probes, Eugene, OR), and mouse anti-GPVI (clone 6.E10) and anti-α2 antibodies (clone Sam.G4) (Emfret Analytics, Würzburg, Germany). Wild-type C57Bl/6J mice were from Jackson Laboratory (Bar Harbor, ME) and FcRγ-null mice were from Taconic Laboratories (Hudson, NY).
In vivo depletion of GPVI
Intravital microscopy
Intravital videomicroscopy was performed as previously described.19 For the FeCl3 experiments mice were treated as for the cremaster muscle preparation except that the mesentery was isolated and pinned across the intravital microscope stage. Procedures were approved by the Animal Care and Use Committee, Beth Israel Deaconess Medical Center.
Laser injury and data analysis
Laser injury was created with a nitrogen dye laser (Coumarin 440 nm; Photonics, St Charles, IL) adjusted between 55% and 65% depending on the thickness of the tissue preparation and depth of the vessel to be injured.19 Several images of the site to be injured are captured prior to injury. Image analysis was performed using Slidebook (Intelligent Imaging Innovations, Santa Monica, CA). For each thrombus, a rectangular mask is defined in an uninjured portion of vessel. A background value is calculated independently within the mask for each fluorescence channel used and subtracted from the fluorescence signal of the thrombus in that channel to determine the integrated fluorescence intensity for the thrombus in each frame. The value of the fluorescence signal plotted versus time provides the kinetics of thrombus formation. Data from multiple thrombi are used to determine the median value of integrated fluorescence intensity (arbitrary units) at each time point and plotted versus time to account for the variability in thrombus formation at any given set of experimental conditions. See text of Supplemental Materials for more information (see the Supplemental Figures link at the top of the online article, at the Blood website).
FeCl3 injury
A paper filter saturated with 8% or 10% FeCl3 was applied to the mouse mesentery or the cremaster muscle for 2 or 5 minutes, respectively.20 The filter paper was removed, the tissue washed using bicarbonate-buffered saline solution, and the platelet accumulation recorded. Time to initial thrombus formation and time to occlusion were calculated from the time the FeCl3 saturated filter paper was removed from the tissue. Data analysis was as for laser surgery.
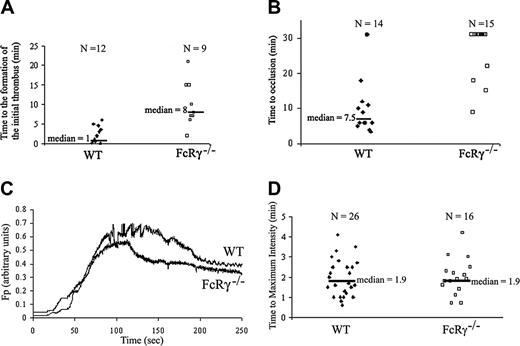
Thrombus formation in FcRγ-null mice and wild-type mice in the FeCl3 and laser-induced thrombosis models. Platelets were labeled by infusion of Alexa 660–conjugated rat anti–mouse CD41 antibody (250 ng/g body weight). (A-B) Injuries were induced with 10% FeCl3 for 5 minutes in wild-type and FcRγ-null mice. (A) The time to the formation of an initial thrombus (WT, n = 12; FcRγ–/–, n = 9). (B) The time to vessel occlusion (WT mice, n = 14; FcRγ–/–, n = 15) are reported and the median times calculated. (C-D) Laser-induced injuries in cremaster arterioles of wild-type and FcRγ-null mice. (C) Median integrated platelet fluorescence intensity for multiple thrombi of each genotype: WT (26 thrombi in 3 mice); FcRγ–/– (16 thrombi in 3 mice). Fluorescence of platelets in arbitrary units is presented as a function of time. (D) For each thrombus formed, the time to the maximum platelet accumulation into the growing thrombus is reported and the calculated median time indicated.
Thrombus formation in FcRγ-null mice and wild-type mice in the FeCl3 and laser-induced thrombosis models. Platelets were labeled by infusion of Alexa 660–conjugated rat anti–mouse CD41 antibody (250 ng/g body weight). (A-B) Injuries were induced with 10% FeCl3 for 5 minutes in wild-type and FcRγ-null mice. (A) The time to the formation of an initial thrombus (WT, n = 12; FcRγ–/–, n = 9). (B) The time to vessel occlusion (WT mice, n = 14; FcRγ–/–, n = 15) are reported and the median times calculated. (C-D) Laser-induced injuries in cremaster arterioles of wild-type and FcRγ-null mice. (C) Median integrated platelet fluorescence intensity for multiple thrombi of each genotype: WT (26 thrombi in 3 mice); FcRγ–/– (16 thrombi in 3 mice). Fluorescence of platelets in arbitrary units is presented as a function of time. (D) For each thrombus formed, the time to the maximum platelet accumulation into the growing thrombus is reported and the calculated median time indicated.
Confocal microscopy
Injured cremaster arterioles were isolated and frozen in Optimal Cutting Temperature (OCT) Compound (Sakura Finetek, Torrance, CA). Sections (5 μm) were stained with hematoxylin and eosin to localize the thrombi. After staining with fluorescently labeled antibodies, confocal images of the tissue section were acquired at 0.5-μm intervals across the vessel diameter perpendicular to the long axis of the vessel by incrementally altering the working distance between the microscope objective and the specimen with a piezo-electric driver. The microscope, camera, and image capture and analysis software were the same as those used for intravital microscopy. The image in Figure 2A was visualized with an Olympus UMP Plan FI 20 ×/0.50 objective lens (Olympus, Melville, NY); in 2B and 2C, with an Olympus UPlanApo 100 ×/1.35 oil-immersion objective lens.
Results
Exposure of blood vessels to ferric chloride is a well established mode of thrombus generation21 that results in denudation of the endothelium and exposure of subendothelial matrix components to flowing blood.22 We examined thrombus formation induced by exposure of the adventitial surface of the mouse mesentery to 10% FeCl3 for 5 minutes (severe FeCl3 injury) in vivo using digital high-speed intravital microscopy to determine the role of GPVI in platelet adhesion.20 In wild-type mice, platelet accumulation in injured arterioles was observed between 1 and 5 minutes after FeCl3 exposure (Figure S1A). The median time to initial thrombus formation was 1 minute (Figure 1A). The median time to occlusion of the arteriole was 7.5 minutes (Figure 1B). In FcRγ-null mice the median time to initial platelet accumulation was 8 minutes (Figure 1A). Only 4 of 15 FcRγ-null mice studied formed occlusive thrombi during the 30-minute experiment (Figure 1B). These results indicate a role for GPVI in platelet accumulation after FeCl3 injury in arterioles.
We have previously described the course of thrombus formation in vivo after laser-induced injury to the arteriolar wall in the mouse cremaster microcirculation.19,23,24 When we examined the course of thrombus formation in wild-type and FcRγ-null mice in this model we found that platelet accumulation was comparable in wild-type and FcRγ-null mice (Figure S1B). The median platelet accumulation curves in wild-type and FcRγnull mice were similar (Figure 1C). There was no difference in the median time to maximum platelet accumulation after laser-induced injury in wild-type compared with FcRγ-null mice (Figure 1D). We confirmed that the normal platelet response observed in thrombus formation in FcRγ-null mice after laser-induced injury is due to differences in response to the 2 methods of injury and not to differences in the microcirculation of the mesentery and cremaster muscle. The median time to maximum platelet accumulation into a growing thrombus after laser-induced injury in mesentery arterioles was the same in wild-type and FcRγ-null mice (Figure S1C), demonstrating that FcRγ-null mice form thrombi normally in response to laser-induced injury irrespective of the vascular bed.
FcRγ is associated with and can induce intracellular signaling by glycoprotein Ib-IX-V.25,26 We repeated experiments using an alternate model of GPVI deficiency to demonstrate that the results obtained after laser-induced vessel wall injury were not influenced by alteration in FcRγ-null platelets other than loss of GPVI. We specifically depleted GPVI from the platelet surface by treating wild type mice with a monoclonal anti-GPVI antibody.8 After 10 days, platelet counts were normal but flow cytometric analyses demonstrated that GPVI was depleted from the surface of platelets in mice treated with anti-GPVI antibody but not in platelets from mice treated with anti-α2 antibody (Figure S2A). Platelet accumulation at sites of laser-induced injury was similar in mice depleted of GPVI and wild-type mice, confirming the results obtained in FcRγ-null mice (Figure S2B). Since GPVI does not play a role in thrombus formation after laser-induced injury we looked for collagen type I exposure after this injury.
Collagen type 1 exposure to circulating blood was below levels of detection on mesenteric arteriolar walls in vivo after laser-induced injury (Figure 2A). In contrast, type 1 collagen was exposed in both arterioles and venules of the mouse mesentery in vivo after severe FeCl3 injury (Figure 2A). Confocal images of frozen sections of cremaster muscles injured with 10% FeCl3 for 5 minutes or with laser confirmed the in vivo results. After severe FeCl3 injury, collagen was distributed widely in the vessel wall but was also observed colocalized with platelet thrombi (Figure 2B). However, after laser injury, collagen was present in the vessel wall but was not detectable colocalized with the platelet thrombus in the lumen of the arteriole (Figure 2B), indicating that after laser-induced injury, collagen exposure to the blood circulation is absent or is present below the level of detection.
Detection of collagen type I and tissue factor in thrombi after FeCl3 or laser-induced injury. (A) Injuries in wild-type or FcRγ-null mice were induced by application of 10% FeCl3 for 5 minutes or by laser. Platelets were labeled by injection of Alexa 660–conjugated rat anti-CD41 antibody into the circulation prior to injury (red pseudocolor). Collagen was labeled by injection of a rabbit anti–mouse collagen type I antibody (1 μg/g body weight) plus an Alexa 488–conjugated goat anti–rabbit antibody (1 μg/g body weight) prior to injury (green pseudocolor). Merge of platelets and collagen, yellow pseudocolor. (B-C) Cremaster muscles injured by FeCl3 or by laser were isolated and sectioned. Image sections of a thrombus were acquired at 0.5-μm intervals across the vessel diameter perpendicular to the long axis of the vessel by incrementally altering the working distance between the microscope objective and the speciment with a piezo-electric driver.23 (B) Tissue sections were stained with Alexa 660 anti–mouse CD41 antibody, rabbit anti–mouse collagen type I antibody, and an Alexa 488–conjugated goat antirabbit antibody. Platelets are indicated by red pseudocolor; collagen, green pseudocolor; and the merge, yellow pseudocolor. Pictures shown are representative of 4 independent experiments. (C) Tissue sections were stained with Alexa 660–conjugated anti–mouse CD41 antibody, rabbit anti–mouse tissue factor (TF) antibody, and Alexa 488–conjugated goat antirabbit antibody. Platelets are indicated by red pseudocolor; tissue factor, green pseudocolor; and the merge, yellow pseudocolor. Pictures shown are representative of 4 independent experiments.
Detection of collagen type I and tissue factor in thrombi after FeCl3 or laser-induced injury. (A) Injuries in wild-type or FcRγ-null mice were induced by application of 10% FeCl3 for 5 minutes or by laser. Platelets were labeled by injection of Alexa 660–conjugated rat anti-CD41 antibody into the circulation prior to injury (red pseudocolor). Collagen was labeled by injection of a rabbit anti–mouse collagen type I antibody (1 μg/g body weight) plus an Alexa 488–conjugated goat anti–rabbit antibody (1 μg/g body weight) prior to injury (green pseudocolor). Merge of platelets and collagen, yellow pseudocolor. (B-C) Cremaster muscles injured by FeCl3 or by laser were isolated and sectioned. Image sections of a thrombus were acquired at 0.5-μm intervals across the vessel diameter perpendicular to the long axis of the vessel by incrementally altering the working distance between the microscope objective and the speciment with a piezo-electric driver.23 (B) Tissue sections were stained with Alexa 660 anti–mouse CD41 antibody, rabbit anti–mouse collagen type I antibody, and an Alexa 488–conjugated goat antirabbit antibody. Platelets are indicated by red pseudocolor; collagen, green pseudocolor; and the merge, yellow pseudocolor. Pictures shown are representative of 4 independent experiments. (C) Tissue sections were stained with Alexa 660–conjugated anti–mouse CD41 antibody, rabbit anti–mouse tissue factor (TF) antibody, and Alexa 488–conjugated goat antirabbit antibody. Platelets are indicated by red pseudocolor; tissue factor, green pseudocolor; and the merge, yellow pseudocolor. Pictures shown are representative of 4 independent experiments.
To further examine the influence of the extent of collagen exposure on GPVI participation in thrombus formation, we examined platelet accumulation and arteriolar occlusion after a milder FeCl3-induced injury.27 After a mild FeCl3 injury the appearance of collagen was substantially delayed and reduced in comparison with severe FeCl3 injury (Figure 3A). The median time to initial platelet accumulation (7.3 minutes) and the percent of arterioles patent at 30 minutes (70%) for wild-type mice after mild FeCl3 injury were similar to the time to initial platelet accumulation (8 minutes) and percentage of arterioles patent at 30 minutes (73%) observed for FcRγ-null mice after severe FeCl3 injury (compare Figure 3B-C to Figure 1A-B). While the percent of patent arterioles at 30 minutes in wild-type mice increased from 14% after severe ferric chloride injury to 70% after mild ferric chloride injury, the percent of patent arterioles at 30 minutes in FcRγ-null mice went from 73% after severe ferric chloride injury to 88% after mild ferric chloride injury. Alteration in the severity of the FeCl3 injury leads to a more marked decrease in formation of occlusive thrombi in wild-type mice compared with FcRγ-null mice, suggesting that GPVI is the primary collagen receptor promoting thrombus formation in this model. The absence of platelet GPVI or the decreased exposure of its ligand, collagen, leads to decreased thrombus formation after arterial insult with FeCl3, indicating a significant role for this receptor in thrombus formation in this model. When wild-type mice were treated with lepirudin prior to induction of a mild ferric chloride injury, the time to initial thrombus formation was increased and no occlusive thrombi formed in the 7 animals tested, indicating that thrombin activation of platelets contributed to thrombus formation in this model (Figure 3B-C).
Collagen exposure after injury at varying FeCl3 concentrations and effect of lepirudin on thrombus formation in wild-type mice. (A) Injuries in wild-type mice (WT) were induced by application of 10% or 8% FeCl3 for 5 or 2 minutes, respectively. Collagen exposed to the blood circulation was labeled by injection of a rabbit anti–mouse collagen type I antibody (1 μg/g body weight) plus Alexa 488–conjugated goat antirabbit antibody (1μg/g body weight). Integrated fluorescence intensity of collagen at several time points after injury is indicated for individual thrombi under the 2 conditions (n = 5 for each condition). (B) Local administration of an 8% solution of FeCl3 for 2 minutes to the adventitial surface of the mesentery was used to induce vascular injury in wild-type mice. Mice were treated with 5 μg/g body weight of lepirudin prior to vessel injury where indicated. For each thrombus the time to the formation of an initial thrombus is reported and the median time indicated (n = 10). (C) For each thrombus in panel B the time to vessel occlusion is reported. Most vessels did not occlude during the 30 minutes of the experiment.
Collagen exposure after injury at varying FeCl3 concentrations and effect of lepirudin on thrombus formation in wild-type mice. (A) Injuries in wild-type mice (WT) were induced by application of 10% or 8% FeCl3 for 5 or 2 minutes, respectively. Collagen exposed to the blood circulation was labeled by injection of a rabbit anti–mouse collagen type I antibody (1 μg/g body weight) plus Alexa 488–conjugated goat antirabbit antibody (1μg/g body weight). Integrated fluorescence intensity of collagen at several time points after injury is indicated for individual thrombi under the 2 conditions (n = 5 for each condition). (B) Local administration of an 8% solution of FeCl3 for 2 minutes to the adventitial surface of the mesentery was used to induce vascular injury in wild-type mice. Mice were treated with 5 μg/g body weight of lepirudin prior to vessel injury where indicated. For each thrombus the time to the formation of an initial thrombus is reported and the median time indicated (n = 10). (C) For each thrombus in panel B the time to vessel occlusion is reported. Most vessels did not occlude during the 30 minutes of the experiment.
P-selectin and P-selectin glycoprotein ligand 1 (PSGL-1) mediate tissue factor accumulation in the growing thrombus in vivo after laser injury.23 Tissue factor from the vessel wall and microparticle-borne tissue factor are important for thrombus formation in this injury model.19 Here we compared the intensity of tissue factor staining at sites of arteriolar injury induced by laser or by severe FeCl3 conditions. Tissue factor accumulation, normalized to platelet thrombus size, was 5-fold higher in thrombi generated in response to laser injury than thrombi formed after severe FeCl3 injury (Figure 2C). This increased level of tissue factor suggests that thrombin plays a more important role in thrombus formation after laser-induced injury than after FeCl3 injury. These data may also indicate a role of endothelial cell P-selectin in recruitment of tissue factor bearing microparticles, as this source of P-selectin is not available at sites of severe FeCl3 injury where endothelial cells are stripped away. Alternatively, laser injury may activate the endothelium leading to surface exposure of tissue factor as well as exposure of phosphatidylserine, which would provide a surface for assembly of the tenase and prothrombinase complexes that lead to thrombin generation. After severe FeCl3 injury with loss of the endothelial cells lining the arteriolar wall and concomitant exposure of subendothelial collagen, interaction of GPVI with collagen may play a more important role in early thrombus formation. Nevertheless, thrombin plays a role in thrombus formation after severe FeCl3 injury as hirudin inhibits thrombus formation after a ferric chloride injury that denudes the endothelium.22
Discussion
Although to date only a few studies of thrombus formation after laser-induced injury have been reported, we anticipate that differences will also appear within injury model with respect to methodology and magnitude of injury, potentially leading to mechanistic differences in thrombus formation in response to this injury. Indeed, a recent study by Nonne et al reports that treatment with the anticoagulant hirudin does not affect arterial thrombus formation following “superficial” laser-induced injury of mesenteric arterioles defined by biphasic platelet accumulation,28 but does effect a more “severe” injury in which platelet accumulation does not appear to be biphasic. While the injuries resulting from “superficial” and “severe” conditions have not been characterized with regard to the nature of vascular injury, these results suggest that thrombus formation is independent of the generation of thrombin under at least 1 of these experimental conditions. To date we have chosen in all of our studies to adjust the intensity of the laser-induced injury to generate a platelet thrombus that forms with kinetics defined by a rapid net positive gain of platelets, followed by a net loss of platelets, and finally a steady state in which thrombus size is stabilized. Maximal thrombus size under these conditions occurs at about 90 to 120 seconds after injury. We suspect that the severe injury model described by Nonne et al is most similar to our model, and a net loss of platelets and stabilization of thrombus size would be observed in their system at later time points. These authors find that thrombus formation in their severe laser injury model is markedly decreased in animals treated with hirudin. Under the conditions of our laser injury model thrombus formation was markedly impaired in PAR-4–null mice, indicating a critical role for thrombin in platelet activation in our model as well.29 Use of greater laser power can lead to occlusive thrombi, and ultimately, with continued increases in laser power, to rupture of the vessel wall with frank hemorrhage from the injured vessel. We have not explored conditions of milder laser injury that might lead to thrombus formation with the kinetics of the superficial injury described by Nonne et al.28
As a hemostatic response, the initiation of thrombus formation in humans is associated with tissue injury, disruption of vascular integrity, and the exposure of flowing blood to the injured vasculature and to extravascular tissue. Atherothrombosis is caused by acute plaque rupture, leading to the exposure of tissue factor and active lipids to flowing blood. Inflammatory reactions, associated with the blood vessel as in vasculitides or systemic inflammation as in sepsis, also lead to thrombus formation. In the current study, we have compared 2 animal models of thrombosis, one initiated by denudation of the endothelium by FeCl3 and exposure of the subendothelial matrix, and the other a laser-induced heat injury involving biochemical activation of the endothelium. The results presented here suggest that the initiating event can determine the relative importance of GPVI-mediated versus thrombin-mediated platelet activation in thrombus formation. Although animal models of thrombosis can only be loosely linked to the human disorders of medical interest, they provide insight into the mechanisms involved in thrombus formation. However, when considering targets for antithrombotic therapy it is important to consider that there are multiple pathways to thrombus formation. Thus, blockade of the GPVI-collagen interaction, as proposed previously as a promising antithrombotic strategy,8-10 should be evaluated in different injury models for antithrombotic potential.
Prepublished online as Blood First Edition Paper, February 2, 2006; DOI 10.1182/blood-2005-09-3687.
Supported by grants from the National Institutes of Health, Bethesda, MD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal