We aimed to compare AIDS risk–adapted intensive chemotherapy in AIDS-related lymphoma (ARL) patients before and after the advent of highly active antiretroviral therapy (HAART). A total of 485 patients aged from 18 to 67 years were randomly assigned to chemotherapy after stratification according to an HIV score based on performance status, prior AIDS, and CD4+ cell counts below 0.10 × 109/L (100/mm3). A total of 218 good-risk patients (HIV score 0) received ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisolone) or CHOP (doxorubicin, cyclophosphamide, vincristine, and prednisolone); 177 intermediate-risk patients (HIV score 1), CHOP or low-dose CHOP (Ld-CHOP); and 90 poor-risk patients (HIV score 2-3), Ld-CHOP or VS (vincristine and steroid). The 5-year overall survival (OS) in the good-risk group was 51% for ACVBP versus 47% for CHOP (P = .85); in the intermediate-risk group, 28% for CHOP versus 24% for Ld-CHOP (P = .19); and in the poor-risk group, 11% for Ld-CHOP versus 3% for VS (P = .14). The time-dependent Cox model demonstrated that the only significant factors for OS were HAART (relative risk [RR] 1.6, P < .001), HIV score (RR 1.7, P < .001), and the International Prognostic Index (IPI) score (RR 1.5, P < .001) but not chemotherapy regimen. Our findings indicate that in ARL patients, HIV score, IPI score, and HAART affect survival but not the intensity of the CHOP-based chemotherapy.
Introduction
Lymphomas are increasingly frequent in patients with congenital or acquired immunodeficiency.1 The incidence of non-Hodgkin lymphoma (NHL) has increased about 100-fold in individuals infected with HIV.2 Previous studies have indicated that patients with AIDS-related lymphoma (ARL) have a poor prognosis. Features contributing to this prognosis include lymphoma-specific factors (eg, aggressive histology or extranodal disease) and HIV-specific factors such as poor bone marrow reserve, CD4 lymphopenia, or opportunistic infection.3,4 Since 1996, the use of combination antiretroviral therapy consisting of protease inhibitors and nucleoside analogs to achieve maximal viral burden reduction, a treatment strategy known as highly active antiretroviral therapy (HAART),5,6 together with improved management and prophylaxis of opportunistic infections, has been found to delay progression of HIV infection to acquired immunodeficiency syndrome (AIDS).7 This finding was followed by a substantial reduction in morbidity and mortality secondary to HIV infection.8-10 In addition, other studies have indicated that HAART is associated with a decreased risk of developing ARL.11,12 Recently, the use of nonnucleoside reverse transcriptase inhibitor–based HAART has been shown to be at least as effective as protease inhibitor–based HAART in the management of HIV infection and indeed in preventing the development of ARL.13
Different chemotherapy regimens have been tested,4,14-18 but there is still disagreement regarding the optimal treatment for patients with ARL even in the post-HAART era. Overall improvement has been observed by some investigators but not by others.19-22 The 4-drug CHOP (doxorubicin, cyclophosphamide, vincristine, and prednisolone) regimen seems to confer some benefit as regards the duration of remission and is one of the standard treatments for non–HIV-infected patients with high-grade NHL.23,24 The aggressive presentation of ARL suggests the need for more intensive treatment regimens, which have resulted in high complete response and survival rates for patients with non–HIV-associated NHL.14,15,17,25-27 On the other hand, the poor tolerability associated with chemotherapy has prompted investigators to test reduced-dose regimens.16,28 Consequently, the Groupe d'Etude des Lymphomes de l'Adulte (GELA) and the Gruppo Italiano Cooperativo AIDS e Tumori (GICAT) together initiated the NHL-HIV-93 trial of risk-adapted CHOP-based intensive chemotherapy in ARL patients. When the study started in May 1993, concurrent antiretroviral therapy with the nucleoside analog didanosine was not stipulated by the protocol, because previous studies had demonstrated that its combination with intensive chemotherapy was not feasible.14,29 Because of the change in practice patterns and the improving prognosis for patients with HIV infection, the study was continued after 1996 and the protocol was modified to allow HAART. We present here the final analysis of the NHL-HIV-93 trial. Special attention was paid to the impact of the pre- and post-HAART eras on clinical outcome.
Patients, materials, and methods
Study design
NHL-HIV-93 was a phase 3, multicentric randomized trial designed to evaluate the efficacy and safety of a risk-adapted chemotherapy in adult HIV-infected patients. Patients received CHOP-based chemotherapy after a pretreatment stratification of dose intensity according to their HIV score. This score was defined by the results of multivariate regression analysis in our previous trials.14,30 It is based on 3 independent risk factors (Eastern Cooperative Oncology Group [ECOG] performance status of 2 to 4, prior AIDS, and a CD4+ cell count below 0.10 × 109/L [100/mm3]) and leads to patient classification according to 3 degrees of risk: good (HIV score, 0 factors), intermediate (HIV score, 1 factor), and poor (HIV score, 2 to 3 factors). The primary end point was overall survival (OS). The secondary end points were event-free survival (EFS), response rate, and toxicity. Calculation of sample size was based on primary OS end point. To detect a change at 2 years of 15% (null hypothesis: 30%; alternative hypothesis: 45%) with an intensified chemotherapy regimen, we calculated that 400 patients would be required to provide the study with 80% power at an overall 5% significance level. This stratified approach 15% difference between intensive and less intensive regimens was applied to all the patients without specific goal within stratum.
No interim analysis was planned. The study was monitored by the GELA coordinating center, which issued treatment allocation by fax after confirmation of the patient eligibility. Case report forms collected at participating centers were sent to the GELA coordinating center and keyed in twice for verification. Outliers and erroneous values were checked routinely. Queries and on-site monitoring were used for final validation.
The trial was approved by the local committee on human investigations and was conducted in accordance with a written assurance approved by the local department of health and human services. All the patients included had to give informed consent to participate.
Patients
Inclusion criteria included diagnosis of untreated, biopsy-confirmed aggressive NHL in HIV-infected patients over 18 years old. There was no upper age limit, but participants had to have no contraindication for doxorubicin or cyclophosphamide. All high-grade histologic subtypes except leukemic forms of Burkitt lymphoma were eligible. Patients with primary cerebral lymphoma were excluded, but those with only meningeal or secondary brain involvement were eligible. Histologic subtypes were reviewed by independent pathologists, and lymphomas were reclassified according to the World Health Organization–Revised European-American Lymphoma (WHO-REAL) classification.31,32 Seventy percent of pathologic slides were reviewed (for the remaining 30% with no material available for central review, the diagnosis was performed by the local experienced hematopathologist; clinical outcomes were well balanced). For discordant cases (less than 5%), agreement was reached using a 2-headed microscope. Routine staging procedures used before treatment and to assess responses included physical examination, bone marrow biopsy, and computed tomography (CT) of the brain, chest, abdomen, and pelvis. Patients were staged according to the Ann Arbor system. Involvement of the extracerebral central nervous system (CNS) was defined here as the presence of lymphoma cells in cerebrospinal fluid (CSF) or cranial nerve paralysis. HIV disease status was assessed on the basis of clinical and biologic parameters. The parameters used to define the age-adjusted International Prognostic Index (aa-IPI) and the Straus index were also measured. The aa-IPI included 3 risk factors: stage 3-4, performance status 2-4, and elevated LDH.33 The Straus index included 4 risk factors: age above 35, intravenous drug use, NHL stage 3-4, and a CD4+ cell count below 0.10 × 109/L (100/mm3).3
Treatment regimen
Patients were centrally randomized after stratification according to center and HIV score (block randomization within strata); good-risk patients (HIV score 0) received either ACVBP (3 courses every 2 weeks of doxorubicin 75 mg/m2 on day 1, cyclophosphamide 1200 mg/m2 on day 1, vindesine 2 mg/m2 on days 1 and 5, bleomycin 10 mg on days 1 and 5, and prednisolone 60 mg/m2 on days 1 to 5) or standard CHOP (4 cycles every 3 weeks of doxorubicin 50 mg/m2, cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2, and prednisolone 60 mg/m2); intermediate-risk patients (HIV score 1) received CHOP or low-dose CHOP (Ld-CHOP) consisting of 4 cycles every 3 weeks of doxorubicin 25 mg/m2, cyclophosphamide 400 mg/m2, vincristine 1.4 mg/m2, and prednisolone 60 mg/m2; and poor-risk patients (HIV score 2-3) received Ld-CHOP or VS consisting of 12 cycles every 2 weeks of vincristine 2 mg on day 1 and prednisolone 60 mg/m2 on days 1 to 5).
In the absence of CSF involvement, CNS prophylaxis consisted of intrathecal injection of methotrexate 12 mg before each chemotherapy course (maximum, 4 injections). In the presence of CSF involvement, intrathecal chemotherapy was administered at least twice weekly until the disappearance of NHL cells (maximum 9 injections).
Patient management
Granulocyte colony-stimulating factor (G-CSF, filgrastim 300 μg; Amgen, Thousand Oaks, CA) was administered on day 6 after each chemotherapy cycle of ACVBP or CHOP until neutrophil counts exceeded 0.5 × 109/L (0.5 g/L) for 2 consecutive days. For Ld-CHOP and VS, only patients with febrile neutropenia after any cycle of chemotherapy were given G-CSF. Blood cells were counted twice weekly. CD4+ cells were counted before chemotherapy. For patients with grade 4 neutropenia or thrombocytopenia after any cycle of chemotherapy, the next cycle was delayed for a week, and if these disorders persisted, the doses of cyclophosphamide and doxorubicin were reduced by 25% at count recovery. Chemotherapy was stopped if lymphoma progressed, if the patient refused to continue, or at the discretion of the investigator in cases of intercurrent illness or adverse events.
Pneumocystis carinii prophylaxis was given routinely (either with monthly pentamidine aerosols or with cotrimoxazole). Zidovudine was not given in the NHL-HIV-93 trial because of the excessive hematologic toxicity observed in a previous trial despite the use of G-CSF but was given at the end of chemotherapy, before HAART become available. Other nonhematotoxic antiretroviral drugs were permitted. HAART became available in France and Italy in June 1996. The antiretroviral regimens that NHL-HIV-93 trial patients were receiving at diagnosis were not modified during chemotherapy if they did not include zidovudine. For patients who were not receiving HAART at diagnosis, a 3-agent regimen was initiated, typically consisting of stavudine, lamivudine and indinavir. Therefore, from June 1996, all patients, either before or at the start of chemotherapy, were receiving HAART.
Response assessment
Tumor responses were assessed after 4 cycles and were classified as complete response (CR), unconfirmed complete response (CRu), partial response (PR), stable disease, or progressive disease, according to the International Workshop criteria.34 These classifications were defined as follows. CR: disappearance of all the lesions and radiologic or biologic abnormalities observed at diagnosis and absence of new lesions. CRu: persistence for 4 months of a palpable node or mass on CT that had regressed in size by at least 75% but not disappeared, normal bone marrow, normal performance status, no symptoms, and disappearance of initial biologic abnormalities. PR: the regression of all measurable lesions by more than 50%, the disappearance of nonmeasurable lesions, and the absence of new lesions. Stable disease: the regression of measurable lesions by 50% or less, or no change in nonmeasurable lesions, and no growth of existing lesions or appearance of new lesions. Progressive disease: the appearance of new lesions, growth of the initial lesions by more than 25%, or growth of measurable lesions that had regressed during treatment by half of their smallest dimensions. Follow-up procedures included physical examination every 3 months for the first 2 years, then every 6 months for 3 years, and then annually. Thoracic, abdominal, and pelvic CT scans were performed every 6 months during the first 2 years and then at the discretion of the treating physician. Dynamic imaging such as gallium or positron emission tomography (PET) scanning was not used.
Statistical analysis
All analyses were performed on an intention-to-treat basis. Patient characteristics and complete remission rates were compared by the χ2 and Fisher exact tests. OS was measured from the date of randomization to death from any cause. EFS was measured from the date of randomization to that of disease progression, relapse, or death from any cause. Survival functions were estimated by the Kaplan-Meier method and compared by log-rank test. Differences between the results of comparative tests were considered significant if the 2-sided P value was less than .05. Because the NHL-HIV-93 trial was not stratified on HAART, we checked for effects of prognostic factors on outcome due to sampling fluctuation in the treatment groups using multivariate analysis of survival. The potential prognostic factors for survival were the risk factors of the aa-IPI, HIV score, and Straus index. The Cox regression model was stratified on HIV score and included aa-IPI, Straus index, HAART, and treatment as explanatory variables. However, to receive HAART, a patient had to survive until June 1996, when HAART became available. In considering the standard multivariate approach with time-fixed treatment of the HAART covariate, the beginning of HAART is erroneously projected back to baseline, leading to artificially classified groups of pre- and post-HAART era patients at time 0, t = 0, irrespective of the duration of HAART. To limit this type of bias in favor of the post-HAART era, we used the time-dependent Cox model with switch variables (ie, before June 1996, HAART was coded at baseline as “pre-HAART era” for all patients, some of whom were switched to the “post-HAART era” after June 1996).35 All other covariates were fixed. All statistical analyses were performed using SAS 9.1 (SAS Institute, Carry, NC) software.
Results
From May 1, 1993, to June 1, 1999, 485 patients from 55 centers (60% GELA and 40% GICAT) were eligible for inclusion in the trial reported here.
Patient characteristics
A total of 414 men (85%) and 71 women (15%) were included (median age, 37 years). HIV infection was transmitted by sexual contact (52%, homosexual in 37% of cases), intravenous drug use (23%), or blood transfusion (1%). In the remaining cases, the cause of infection was unknown or multifactorial. The median CD4+ lymphocyte count at lymphoma diagnosis was 29 × 109/L (129/mm3) (range, 0.001 × 109/L to 1.584 × 109/L [1/mm3 to 1584/mm3]). A total of 189 patients (39%) had CD4+ cell counts below 0.10 × 109/L (100/mm3). The distribution of the Straus index was 0-1 for 27% of patients, 2 for 43%, and 3-4 for 30%. Thirty-seven patients (8%) had meningeal involvement at diagnosis. Table 1 shows the lymphoma and HIV patient characteristics according to risk (good, intermediate, poor). The largest group was the low-risk group, with 218 patients (45%). Three histologic types of NHL were represented in the study population: diffuse large cell (n = 263; 54%), Burkitt (n = 98; 20%), and immunoblastic (n = 85; 18%). The remaining 8% of the population (n = 39) had various unclassified types of large cell NHL. Less than 5% needed a 2-headed microscope agreement.
Clinical characteristics of HIV patients with lymphoma
. | Study population . | HIV score 0 . | HIV score 1 . | HIV score 2-3 . |
|---|---|---|---|---|
| Sex, no. male (%) | 414 (85) | 188 (86) | 148 (84) | 78 (86) |
| Median age, y (range) | 37 (18-67) | 37 (18-67) | 39 (23-67) | 36 (24-61) |
| Lymphoma characteristics, no. (%) | ||||
| Stage III—IV | 303 (62) | 143 (66) | 103 (58) | 57 (63) |
| B symptoms | 252 (52) | 89 (41) | 102 (58) | 61 (67) |
| Tumor size at least 10 cm | 76 (16) | 36 (17) | 28 (16) | 12 (13) |
| Performance status at least 2 | 82 (17) | 1 (0) | 31 (18) | 50 (55) |
| At least 2 extranodal sites | 167 (34) | 75 (34) | 64 (36) | 28 (31) |
| Bone marrow involvement | 76 (16) | 36 (17) | 26 (15) | 14 (16) |
| CNS involvement | 37 (8) | 25 (11) | 9 (5) | 3 (3) |
| LDH level above normal | 268 (55) | 113 (52) | 104 (59) | 51 (57) |
| Albumin level less than 35 g/L | 182 (38) | 70 (32) | 63 (36) | 49 (54) |
| aaIPI score at least 2 | 227 (47) | 92 (47) | 84 (47) | 51 (56) |
| HIV characteristics | ||||
| Sexual transmission, no. (%) | 212 (52) | 109 (50) | 69 (39) | 34 (38) |
| Drug use, no. (%) | 111 (23) | 44 (20) | 44 (25) | 23 (26) |
| Time between HIV and lymphoma, mo (range) | 52 (0-170) | 40 (0-170) | 58 (0-162) | 72 (0-157) |
| AIDS prior to NHL, no. (%) | 99 (20) | 0 (0) | 30 (17) | 69 (77) |
| CD4+ cells | ||||
| Median count per mm3 (range) | 129 (1-1584) | 239 (100-1584) | 72 (2-652) | 21 (1-924) |
| Count less than 100/mm3, no. (%) | 189 (39) | 6 (3) | 106 (60) | 77 (86) |
| Infection before NHL, no. (%) | 103 (21) | 9 (4) | 34 (19) | 60 (66) |
. | Study population . | HIV score 0 . | HIV score 1 . | HIV score 2-3 . |
|---|---|---|---|---|
| Sex, no. male (%) | 414 (85) | 188 (86) | 148 (84) | 78 (86) |
| Median age, y (range) | 37 (18-67) | 37 (18-67) | 39 (23-67) | 36 (24-61) |
| Lymphoma characteristics, no. (%) | ||||
| Stage III—IV | 303 (62) | 143 (66) | 103 (58) | 57 (63) |
| B symptoms | 252 (52) | 89 (41) | 102 (58) | 61 (67) |
| Tumor size at least 10 cm | 76 (16) | 36 (17) | 28 (16) | 12 (13) |
| Performance status at least 2 | 82 (17) | 1 (0) | 31 (18) | 50 (55) |
| At least 2 extranodal sites | 167 (34) | 75 (34) | 64 (36) | 28 (31) |
| Bone marrow involvement | 76 (16) | 36 (17) | 26 (15) | 14 (16) |
| CNS involvement | 37 (8) | 25 (11) | 9 (5) | 3 (3) |
| LDH level above normal | 268 (55) | 113 (52) | 104 (59) | 51 (57) |
| Albumin level less than 35 g/L | 182 (38) | 70 (32) | 63 (36) | 49 (54) |
| aaIPI score at least 2 | 227 (47) | 92 (47) | 84 (47) | 51 (56) |
| HIV characteristics | ||||
| Sexual transmission, no. (%) | 212 (52) | 109 (50) | 69 (39) | 34 (38) |
| Drug use, no. (%) | 111 (23) | 44 (20) | 44 (25) | 23 (26) |
| Time between HIV and lymphoma, mo (range) | 52 (0-170) | 40 (0-170) | 58 (0-162) | 72 (0-157) |
| AIDS prior to NHL, no. (%) | 99 (20) | 0 (0) | 30 (17) | 69 (77) |
| CD4+ cells | ||||
| Median count per mm3 (range) | 129 (1-1584) | 239 (100-1584) | 72 (2-652) | 21 (1-924) |
| Count less than 100/mm3, no. (%) | 189 (39) | 6 (3) | 106 (60) | 77 (86) |
| Infection before NHL, no. (%) | 103 (21) | 9 (4) | 34 (19) | 60 (66) |
For the whole study population, n = 485. For those with an HIV score of 0, n = 218; of 1, n = 177; and of 2-3, n = 90.
Administration of chemotherapy
The 218 patients in the good-risk group received at least 1 cycle of the treatment specified in the protocol: ACVBP (n = 109) and CHOP (n = 109); the 177 in the intermediate group were given CHOP (n = 95) and Ld-CHOP (n = 82); and the 90 in the poor-risk group were given Ld-CHOP (n = 49) and VS (n = 41). Table 2 shows the treatment characteristics. Lymphoma and HIV patients' characteristics were well balanced between treatment arms.
Treatment characteristics of HIV patients with lymphoma
. | HIV score 0 . | . | HIV score 1 . | . | HIV score 2-3 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | ACVBP . | CHOP . | CHOP . | Ld-CHOP . | Ld-CHOP . | VS . | |||
| Median anthracycline DI (range) | 0.91* (0.1-1.1) | 0.98* (0.5-1.2) | 0.96 (0.4-1.8) | 0.96 (0.1-1.4) | 0.97 (0.6-1.2) | NA | |||
| Below 0.80, no. (%) | 32 (29)* | 4 (4)* | 9 (9) | 8 (10) | 5 (10) | NA | |||
| Median cyclophosphamide DI (range) | 0.91 (0.1-1.1) | 0.98 (0.5-1.2) | 0.96 (0.2-1.9) | 0.96 (0.1-1.3) | 0.99 (0.7-1.2) | NA | |||
| Below 0.80, no. (%) | 33 (30)* | 5 (5)* | 8 (8) | 8 (10) | 4 (8) | NA | |||
| G-CSF prophylaxis, no (%) | 97 (89) | 95 (87) | 60 (63) | 42 (51) | 25 (51) | NA | |||
| Toxicity grade 3-4, no. (%) | |||||||||
| Platelets | 50 (46)* | 13 (12)* | 23 (24) | 11 (13) | 1 (22) | 5 (12) | |||
| Leukocytes | 82 (75)* | 39 (36)* | 49 (52)* | 25 (30)* | 20 (41)* | 5 (12)* | |||
| Infection | 28 (26)* | 6 (6)* | 15 (16) | 11 (13) | 12 (24) | 7 (17) | |||
| Mucositis | 21 (19)† | 9 (8)† | 2 (2) | 1 (1) | 1 (2) | 0 (0) | |||
| Response to chemotherapy, no. (%) | |||||||||
| CR/CRu | 66 (61) | 56 (51) | 47 (49)† | 26 (32)† | 10 (20)* | 2 (5)† | |||
| PR | 17 (16) | 16 (15) | 9 (9) | 13 (16) | 6 (12) | 6 (15) | |||
| Survival, % (95% CI) | |||||||||
| 5-y OS | 51 (41, 61) | 47 (38, 57) | 28 (18, 37) | 24 (15, 34) | 11 (2, 20) | 3 (0, 8) | |||
| 5-y EFS | 44 (35, 54) | 40 (30, 49) | 26 (16, 35) | 20 (10, 29) | 8 (0, 16) | 0 | |||
| 5-y DFS | 51 (36, 62) | 49 (35, 63) | 41 (26, 56) | 33 (14, 54) | 27 (1, 53) | 0 | |||
. | HIV score 0 . | . | HIV score 1 . | . | HIV score 2-3 . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | ACVBP . | CHOP . | CHOP . | Ld-CHOP . | Ld-CHOP . | VS . | |||
| Median anthracycline DI (range) | 0.91* (0.1-1.1) | 0.98* (0.5-1.2) | 0.96 (0.4-1.8) | 0.96 (0.1-1.4) | 0.97 (0.6-1.2) | NA | |||
| Below 0.80, no. (%) | 32 (29)* | 4 (4)* | 9 (9) | 8 (10) | 5 (10) | NA | |||
| Median cyclophosphamide DI (range) | 0.91 (0.1-1.1) | 0.98 (0.5-1.2) | 0.96 (0.2-1.9) | 0.96 (0.1-1.3) | 0.99 (0.7-1.2) | NA | |||
| Below 0.80, no. (%) | 33 (30)* | 5 (5)* | 8 (8) | 8 (10) | 4 (8) | NA | |||
| G-CSF prophylaxis, no (%) | 97 (89) | 95 (87) | 60 (63) | 42 (51) | 25 (51) | NA | |||
| Toxicity grade 3-4, no. (%) | |||||||||
| Platelets | 50 (46)* | 13 (12)* | 23 (24) | 11 (13) | 1 (22) | 5 (12) | |||
| Leukocytes | 82 (75)* | 39 (36)* | 49 (52)* | 25 (30)* | 20 (41)* | 5 (12)* | |||
| Infection | 28 (26)* | 6 (6)* | 15 (16) | 11 (13) | 12 (24) | 7 (17) | |||
| Mucositis | 21 (19)† | 9 (8)† | 2 (2) | 1 (1) | 1 (2) | 0 (0) | |||
| Response to chemotherapy, no. (%) | |||||||||
| CR/CRu | 66 (61) | 56 (51) | 47 (49)† | 26 (32)† | 10 (20)* | 2 (5)† | |||
| PR | 17 (16) | 16 (15) | 9 (9) | 13 (16) | 6 (12) | 6 (15) | |||
| Survival, % (95% CI) | |||||||||
| 5-y OS | 51 (41, 61) | 47 (38, 57) | 28 (18, 37) | 24 (15, 34) | 11 (2, 20) | 3 (0, 8) | |||
| 5-y EFS | 44 (35, 54) | 40 (30, 49) | 26 (16, 35) | 20 (10, 29) | 8 (0, 16) | 0 | |||
| 5-y DFS | 51 (36, 62) | 49 (35, 63) | 41 (26, 56) | 33 (14, 54) | 27 (1, 53) | 0 | |||
For patients with an HIV score of 0, n = 218 (ACVBP, n = 109; CHOP, n = 109); of 1, n = 177 (CHOP, n = 95; Ld-CHOP, n = 82); of 2-3, n = 90 (Ld-CHOP, n = 49; VS, n = 41).
Ld indicates low dose; DI, dose intensity (administered-planned ratio); CR/CRu, complete response/unconfirmed complete response; CI, confidence interval; DFS, disease-free survival; and NA, not applicable.
Comparison between treatment arms within HIV score strata: P ≤ .01.
Comparison between treatment arms within HIV score strata: P ≤ .05.
In the good- and intermediate-risk groups, we calculated the amounts of chemotherapeutic agents administered, because there is a dose-response relation between doxorubicin or cyclophosphamide administration and NHL prognosis.36 A total of 1471 chemotherapy courses were planned and 1227 courses administered. For the ACVBP regimen, the theoretical dose intensities of doxorubicin and cyclophosphamide were, respectively, 37.5 mg/m2/wk and 600 mg/m2/wk, and the median doses actually received 92% and 93% of the designated doses. For the CHOP regimen, the theoretical dose intensities of doxorubicin and cyclophosphamide were, respectively, 17 mg/m2/wk and 250 mg/m2/wk, and the median doses actually received 97% and 97% of the designated doses.
Treatment-related toxicity
Forty-three patients (9%) died during chemotherapy: 9 in the good-risk group, 17 in the intermediate group, and 17 in the poor-risk group. In 7 cases, death was due to treatment-related toxicity (1 patient died of heart failure, 1 of encephalitis, 3 of severe sepsis, and 2 of multiorgan failure). Hematology-related toxicities are listed in Table 2. A total of 220 patients (45%) experienced at least 1 day of grade 3-4 neutropenia, and 79 (16%) also experienced grade 3-4 infection. Most cases of hematologic toxicity and mucositis occurred in the ACVBP treatment group. The other most serious nonlethal complications observed were reversible acute renal failure (n = 3; grade 4) and liver toxicity (n = 3; grade 4). There were 11 grade 3-4 neurotoxicities and 7 grade 3-4 infections in the 41 patients treated by VS.
Treatment efficacy
A total of 207 patients (43%) achieved CR/CRu and 67 (14%) PR, whereas 201 patients (41%) exhibited no response. Ten patients (2%) were not evaluable. Table 2 shows that the effect of treatment did not differ significantly between CHOP and ACVBP. However, there was significant benefit for CHOP versus Ld-CHOP (49% versus 32%, P = .02) and a trend for Ld-CHOP versus VS (20% versus 5%, P = .05).
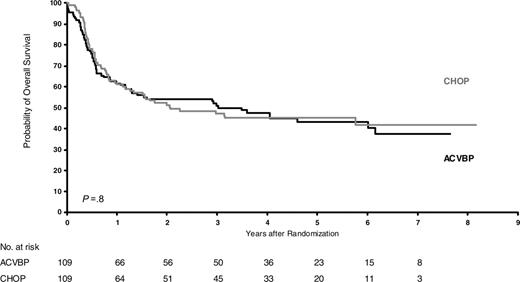
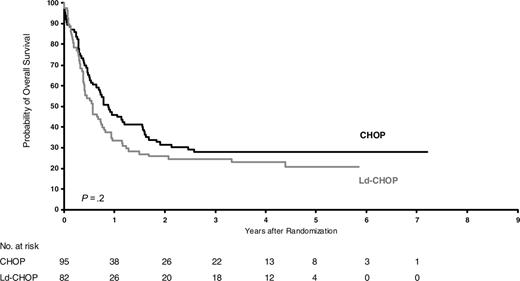
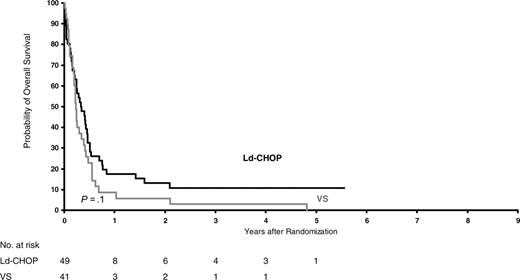
After a median follow-up of 6 years, OS was 30% ± 4%. OS did not differ between intensive and less intensive regimens (29% versus 31%; stratified relative risk, 1.16; P = .17). However, OS differed significantly in the 3 risk groups and was 49%, 26%, and 7% for patients at good, intermediate, and poor risk, respectively (P < .001). EFS also differed significantly, as the corresponding values were, respectively, 42%, 24%, and 5%. As shown in Table 2, the effects of treatments did not differ significantly within risk groups. Figures 1, 2, 3 show the Kaplan-Meier OS curves according to treatment. In all risk groups, the leading cause of death was lymphoma (n = 214; 66%).
Notably, despite the use of intrathecal methotrexate treatment, CNS involvement is still a poor prognostic factor (5-year OS, 19% versus 30%, P = .07) and, despite the use of intrathecal methotrexate prophylaxis, 33 patients developed CNS relapses (but 24 of 33 had no intrathecal methotrexate).
Impact of HAART
The trial began in June 1993 (ie, before HAART) and stopped in June 1999. Therefore, the impact of HAART on the effects of the different treatments had to be assessed. A total of 298 patients were included before June 1996 and 187 thereafter, which led to a median follow-up of 72 months for the former and 49 for the latter. The characteristics of these 2 groups of patients are compared in Table 3. As expected, CD4 counts were higher and HIV scores lower during the post-HAART era, and the immunoblastic lymphoma subtype was less common. Treatment toxicity and efficacy are given in Tables 4, 5, 6.
Comparative patient characteristics in the pre- and post-HAART eras
Characteristic . | Before HAART . | After HAART . | P . |
|---|---|---|---|
| Sex, male (%) | 263 (88) | 151 (81) | .03 |
| Median age, y (range) | 38 (22-67) | 37 (18-66) | .4 |
| Stage 3-4, no. (%) | 192 (64) | 111 (59) | .3 |
| At least 2 extranodal sites, no. (%) | 102 (34) | 65 (34) | .9 |
| Performance status at least 2, no. (%) | 57 (19) | 25 (13) | .1 |
| LDH level above normal, no. (%) | 171 (57) | 97 (52) | .2 |
| aaIPI score at least 2, no. (%) | 145 (49) | 82 (44) | .3 |
| HIV score, no. (%) | |||
| 0 | 132 (44) | 86 (46) | — |
| 1 | 95 (32) | 82 (43) | — |
| 2-3 | 71 (24) | 19 (11) | < .001 |
| Time between HIV and lymphoma, mo (range) | 49 (0-156) | 62 (0-170) | .03 |
| AIDS prior to NHL, no. (%) | 65 (21) | 34 (18) | .3 |
| CD4+ cells | |||
| Median count per mm3 (range) | 114 (1-1005) | 143 (0-1584) | < .001 |
| Count less than 100/mm3, no. (%) | 135 (45) | 54 (29) | < .001 |
| NHL subtypes, no. (%) | |||
| Diffuse large B cell | 153 (51) | 113 (60) | — |
| Immunoblastic | 62 (21) | 23 (12) | — |
| Burkitt | 74 (25) | 24 (13) | — |
| Other large cells | 9 (3) | 30 (16) | .002 |
Characteristic . | Before HAART . | After HAART . | P . |
|---|---|---|---|
| Sex, male (%) | 263 (88) | 151 (81) | .03 |
| Median age, y (range) | 38 (22-67) | 37 (18-66) | .4 |
| Stage 3-4, no. (%) | 192 (64) | 111 (59) | .3 |
| At least 2 extranodal sites, no. (%) | 102 (34) | 65 (34) | .9 |
| Performance status at least 2, no. (%) | 57 (19) | 25 (13) | .1 |
| LDH level above normal, no. (%) | 171 (57) | 97 (52) | .2 |
| aaIPI score at least 2, no. (%) | 145 (49) | 82 (44) | .3 |
| HIV score, no. (%) | |||
| 0 | 132 (44) | 86 (46) | — |
| 1 | 95 (32) | 82 (43) | — |
| 2-3 | 71 (24) | 19 (11) | < .001 |
| Time between HIV and lymphoma, mo (range) | 49 (0-156) | 62 (0-170) | .03 |
| AIDS prior to NHL, no. (%) | 65 (21) | 34 (18) | .3 |
| CD4+ cells | |||
| Median count per mm3 (range) | 114 (1-1005) | 143 (0-1584) | < .001 |
| Count less than 100/mm3, no. (%) | 135 (45) | 54 (29) | < .001 |
| NHL subtypes, no. (%) | |||
| Diffuse large B cell | 153 (51) | 113 (60) | — |
| Immunoblastic | 62 (21) | 23 (12) | — |
| Burkitt | 74 (25) | 24 (13) | — |
| Other large cells | 9 (3) | 30 (16) | .002 |
For pre-HAART patients, n = 298; for post-HAART patients, n = 187.
— indicates not applicable.
Treatment toxicity and efficacy in 218 patients with lymphoma who had an HIV score of 0, according to HAART era
. | Before HAART . | . | After HAART . | . | ||
|---|---|---|---|---|---|---|
| Patients with HIV score of 0 . | ACVBP . | CHOP . | ACVBP . | CHOP . | ||
| Toxicity grade 3-4, no. (%) | ||||||
| Platelets | 25 (37)* | 8 (12)* | 25 (59)* | 5 (11)* | ||
| Leukocytes | 47 (70)* | 20 (31)* | 35 (83)* | 19 (43)* | ||
| Infection | 17 (25)* | 4 (6)* | 11 (26)* | 2 (5)* | ||
| Response to chemotherapy | ||||||
| CR/Cru, no. (%) | 44 (66) | 35 (54) | 22 (52) | 21 (48) | ||
| 3-y OS, % (95% CI) | 46 (34, 59) | 40 (28, 52) | 60 (45, 75) | 57 (43, 72) | ||
| 3-y EFS, % (95% CI) | 40 (28, 52) | 32 (20, 44) | 53 (37, 68) | 48 (33, 63) | ||
| 3-y DFS, % (95% CI) | 50 (35, 65) | 45 (28, 63) | 71 (51, 90) | 55 (32, 78) | ||
. | Before HAART . | . | After HAART . | . | ||
|---|---|---|---|---|---|---|
| Patients with HIV score of 0 . | ACVBP . | CHOP . | ACVBP . | CHOP . | ||
| Toxicity grade 3-4, no. (%) | ||||||
| Platelets | 25 (37)* | 8 (12)* | 25 (59)* | 5 (11)* | ||
| Leukocytes | 47 (70)* | 20 (31)* | 35 (83)* | 19 (43)* | ||
| Infection | 17 (25)* | 4 (6)* | 11 (26)* | 2 (5)* | ||
| Response to chemotherapy | ||||||
| CR/Cru, no. (%) | 44 (66) | 35 (54) | 22 (52) | 21 (48) | ||
| 3-y OS, % (95% CI) | 46 (34, 59) | 40 (28, 52) | 60 (45, 75) | 57 (43, 72) | ||
| 3-y EFS, % (95% CI) | 40 (28, 52) | 32 (20, 44) | 53 (37, 68) | 48 (33, 63) | ||
| 3-y DFS, % (95% CI) | 50 (35, 65) | 45 (28, 63) | 71 (51, 90) | 55 (32, 78) | ||
For pre-HAART patients on ACVBP, n = 67; on CHOP, n = 65. For post-HAART patients on ACVBP, n = 42; on CHOP, n = 44.
Comparison between treatment arms within the HAART era: P ≤ .01.
Treatment toxicity and efficacy in 177 patients with lymphoma who had an HIV score of 1, according to HAART era
Patients with HIV score of 1 . | Before HAART . | . | After HAART . | . | ||
|---|---|---|---|---|---|---|
| . | CHOP . | Ld-CHOP . | CHOP . | Ld-CHOP . | ||
| Toxicity grade 3-4, no. (%) | ||||||
| Platelets | 11 (22) | 6 (14) | 12 (27) | 5 (13) | ||
| Leukocytes | 25 (49) | 13 (30) | 24 (54)* | 12 (32)* | ||
| Infection | 6 (12) | 5 (11) | 9 (20) | 6 (16) | ||
| Response to chemotherapy | ||||||
| CR/Cru, no. (%) | 27 (53)* | 14 (32)* | 20 (45) | 12 (32) | ||
| 3-y OS, % (95% Cl) | 19 (8, 31) | 16 (5, 28) | 38 (23, 53) | 30 (16, 46) | ||
| 3-y EFS, % (95% Cl) | 17 (5, 19) | 17 (6, 28) | 36 (21, 59) | 28 (14, 43) | ||
| 3-y DFS, % (95% Cl) | 23 (7, 39) | 23 (1, 45) | 69 (47, 92) | 50 (19, 81) | ||
Patients with HIV score of 1 . | Before HAART . | . | After HAART . | . | ||
|---|---|---|---|---|---|---|
| . | CHOP . | Ld-CHOP . | CHOP . | Ld-CHOP . | ||
| Toxicity grade 3-4, no. (%) | ||||||
| Platelets | 11 (22) | 6 (14) | 12 (27) | 5 (13) | ||
| Leukocytes | 25 (49) | 13 (30) | 24 (54)* | 12 (32)* | ||
| Infection | 6 (12) | 5 (11) | 9 (20) | 6 (16) | ||
| Response to chemotherapy | ||||||
| CR/Cru, no. (%) | 27 (53)* | 14 (32)* | 20 (45) | 12 (32) | ||
| 3-y OS, % (95% Cl) | 19 (8, 31) | 16 (5, 28) | 38 (23, 53) | 30 (16, 46) | ||
| 3-y EFS, % (95% Cl) | 17 (5, 19) | 17 (6, 28) | 36 (21, 59) | 28 (14, 43) | ||
| 3-y DFS, % (95% Cl) | 23 (7, 39) | 23 (1, 45) | 69 (47, 92) | 50 (19, 81) | ||
For pre-HAART patients on CHOP, n = 51; on Ld-CHOP, n = 44. For post-HAART patients on CHOP, n = 44; on Ld-CHOP, n = 38.
Comparison between treatment arms within the HAART era: P ≤ .05.
Treatment toxicity and efficacy in 90 patients with lymphoma who had an HIV score of 2-3, according to HAART era
Patients with HIV score of 2-3 . | Before HAART . | . | After HAART . | . | ||
|---|---|---|---|---|---|---|
| . | Ld-CHOP . | VS . | Ld-CHOP . | VS . | ||
| Toxicity grade 3-4, no. (%) | ||||||
| Platelets | 9 (23) | 4 (12) | 2 (20) | 1 (11) | ||
| Leukocytes | 17 (44)* | 4 (12)* | 3 (30) | 1 (11) | ||
| Infection | 10 (26) | 3 (9) | 2 (20) | 4 (44) | ||
| Response to chemotherapy | ||||||
| CR/Cru, no. (%) | 8 (21) | 2 (6) | 3 (30) | 1 (11) | ||
| 3-y OS, % (95% Cl) | 3 (0, 7) | 2 (0, 10) | 42 (6, 79) | 0 | ||
| 3-y EFS, % (95% Cl) | 2 (0, 7) | 0 | 28 (0, 62) | 0 | ||
| 3-y DFS, % (95% Cl) | 12 (0, 35) | 0 | 66 (13, 100) | 0 | ||
Patients with HIV score of 2-3 . | Before HAART . | . | After HAART . | . | ||
|---|---|---|---|---|---|---|
| . | Ld-CHOP . | VS . | Ld-CHOP . | VS . | ||
| Toxicity grade 3-4, no. (%) | ||||||
| Platelets | 9 (23) | 4 (12) | 2 (20) | 1 (11) | ||
| Leukocytes | 17 (44)* | 4 (12)* | 3 (30) | 1 (11) | ||
| Infection | 10 (26) | 3 (9) | 2 (20) | 4 (44) | ||
| Response to chemotherapy | ||||||
| CR/Cru, no. (%) | 8 (21) | 2 (6) | 3 (30) | 1 (11) | ||
| 3-y OS, % (95% Cl) | 3 (0, 7) | 2 (0, 10) | 42 (6, 79) | 0 | ||
| 3-y EFS, % (95% Cl) | 2 (0, 7) | 0 | 28 (0, 62) | 0 | ||
| 3-y DFS, % (95% Cl) | 12 (0, 35) | 0 | 66 (13, 100) | 0 | ||
For pre-HAART patients on Ld-CHOP, n = 39; on VS, n = 32. For post-HAART patients on Ld-CHOP, n = 10; on VS, n = 38.
Comparison between treatment arms within the HAART era: P ≤ .01.
Overall survival of good-risk patients (HIV score 0) according to treatment (P = .8).
Overall survival of good-risk patients (HIV score 0) according to treatment (P = .8).
Overall survival of intermediate-risk patients (HIV score 1) according to treatment (P = .2).
Overall survival of intermediate-risk patients (HIV score 1) according to treatment (P = .2).
After adjusting for follow-up, OS was higher in the post-HAART era (21% versus 37% at 3 years, P < .001), but there was still no difference between the therapeutic effect of chemotherapy in each risk group. Because the received dose intensity and toxicity were similar during the pre- and post-HAART eras (Tables 4, 5, 6), the survival benefit due to HAART could be mainly attributable to better immunologic recovery after chemotherapy. In particular, CD4 and CD8 counts were higher during the post-HAART than pre-HAART era, as indicated by measures 3 months after the end of treatment (median CD4: 0.10 × 109/L versus 0.17 × 109/L [100/mm3 versus 170/mm3], P = .01; and CD8: 0.46 × 109/L versus 0.48 × 109/L [460/mm3 versus 480/mm3], P = .45) and 9 months thereafter (median CD4: 0.46 × 109/L versus 0.74 × 109/L [460/mm3 versus 740/mm3], P = .002; CD8: 0.47 × 109/L versus 0.65 × 109/L [470/mm3 versus 650/mm3], P = .30). These higher counts led to lower infection rates at 3 months (53% versus 35%, P < .001).
Risk-adjusted analysis
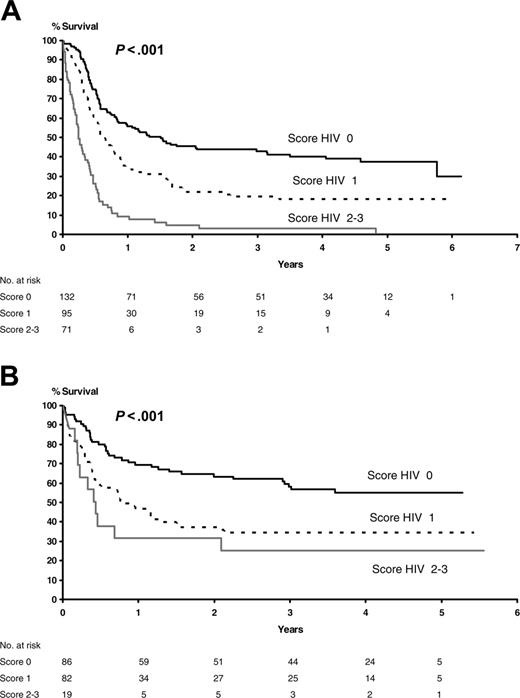
However, because HAART was not randomly assigned, its impact on patient outcome had to be evaluated after checking for the effects of treatment and the main prognostic factors (ie, HAART, HIV score, aa-IPI, and Straus index). As shown in Figure 4, HAART did not invalidate the stratification according to HIV score explored in the present study. In addition, after adjustment for risk factors and stratification on HIV score, the results of multivariate analysis showed that HAART was associated with a significant gain in OS (pre-HAART OS versus post-HAART OS, relative risk [RR] 1.6, P < .001). The other significant factor for OS was the IPI score (score 2-3 versus 0-1, RR 1.7, P < .001). The treatment (intensive versus less intensive) and the Straus index were not significant for OS. There was no significant interaction between treatment and prognostic factors. Notably, the time-dependent Cox model takes into account the 100 patients who received chemotherapy before HAART and survived until June 1996 to begin HAART.
Overall survival of poor-risk patients (HIV score 2-3) according to treatment (P = .1).
Overall survival of poor-risk patients (HIV score 2-3) according to treatment (P = .1).
Prognostic factor analysis
Finally, to assess the impact on OS of the main prognostic factors, we focused on the 335 patients treated by CHOP or Ld-CHOP. The association between prognostic factors and OS calculated by univariate analysis is given in Table 7. It showed that the HIV-related factors such as Straus score and HIV score lose their prognostic values in the post-HAART era. This finding is confirmed by the results of the multivariate analysis in which only the following characteristics retained prognostic value for OS: HIV score (RR 1.86, P < .001) in the pre-HAART era and IPI score in the post-HAART era (RR 2.55, P < .001).
Prognostic factors analysis in 335 ARL lymphoma patients treated by CHOP (n = 204) or Ld-CHOP (n = 131)
. | CHOP/Ld-CHOP population . | . | Before HAART . | . | After HAART . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Prognostic factors . | 3-y OS . | P . | 3-y OS . | P . | 3-y OS . | P . | |||
| aaIPI score, no. (95% CI) | <.001* | <.001 | <.001* | ||||||
| 0-1 | 37 (30, 45) | 27 (18, 35) | 54 (42, 66) | ||||||
| 2-3 | 16 (8, 23) | 12 (3, 20) | 22 (8, 35) | ||||||
| HIV score, no. (95% CI) | <.001* | <.001* | .13 | ||||||
| 0 | 46 (37, 56) | 39 (27, 51) | 56 (41, 71) | ||||||
| 1 | 26 (19, 33) | 19 (11, 27) | 50 (35, 58) | ||||||
| 2-3 | 10 (2, 20) | 2 (0, 7) | 34 (23, 45) | ||||||
| Straus score, no. (95% CI) | <.001 | <.001 | .06 | ||||||
| 0-1 | 44 (32, 56) | 36 (22, 50) | 61 (41, 82) | ||||||
| 2 | 28 (19, 36) | 20 (10, 19) | 40 (25, 54) | ||||||
| 3-4 | 19 (11, 27) | 9 (2, 17) | 34 (19, 51) | ||||||
| NHL subtype, no. (95% CI) | .009* | .21 | .10 | ||||||
| Diffuse large B-cell | 38 (32, 44) | 29 (22, 34) | 49 (41, 57) | ||||||
| Immunoblastic | 22 (14, 30) | 17 (7, 27) | 33 (23, 43) | ||||||
| Burkitt | 28 (21, 36) | 24 (14, 44) | 32 (22, 42) | ||||||
| HAART era, no. (95% CI) | <.001* | NA | NA | ||||||
| Before HAART | 22 (16, 28) | NA | NA | ||||||
| After HAART | 42 (34, 51) | NA | NA | ||||||
. | CHOP/Ld-CHOP population . | . | Before HAART . | . | After HAART . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Prognostic factors . | 3-y OS . | P . | 3-y OS . | P . | 3-y OS . | P . | |||
| aaIPI score, no. (95% CI) | <.001* | <.001 | <.001* | ||||||
| 0-1 | 37 (30, 45) | 27 (18, 35) | 54 (42, 66) | ||||||
| 2-3 | 16 (8, 23) | 12 (3, 20) | 22 (8, 35) | ||||||
| HIV score, no. (95% CI) | <.001* | <.001* | .13 | ||||||
| 0 | 46 (37, 56) | 39 (27, 51) | 56 (41, 71) | ||||||
| 1 | 26 (19, 33) | 19 (11, 27) | 50 (35, 58) | ||||||
| 2-3 | 10 (2, 20) | 2 (0, 7) | 34 (23, 45) | ||||||
| Straus score, no. (95% CI) | <.001 | <.001 | .06 | ||||||
| 0-1 | 44 (32, 56) | 36 (22, 50) | 61 (41, 82) | ||||||
| 2 | 28 (19, 36) | 20 (10, 19) | 40 (25, 54) | ||||||
| 3-4 | 19 (11, 27) | 9 (2, 17) | 34 (19, 51) | ||||||
| NHL subtype, no. (95% CI) | .009* | .21 | .10 | ||||||
| Diffuse large B-cell | 38 (32, 44) | 29 (22, 34) | 49 (41, 57) | ||||||
| Immunoblastic | 22 (14, 30) | 17 (7, 27) | 33 (23, 43) | ||||||
| Burkitt | 28 (21, 36) | 24 (14, 44) | 32 (22, 42) | ||||||
| HAART era, no. (95% CI) | <.001* | NA | NA | ||||||
| Before HAART | 22 (16, 28) | NA | NA | ||||||
| After HAART | 42 (34, 51) | NA | NA | ||||||
For CHOP/Ld-CHOP population, n = 335; for pre-HAART patients, n = 199; for post-HAART patients, n = 136.
NA indicates not applicable.
P ≤ .05 in multivariate analysis.
Discussion
Dose-intensive chemotherapy for aggressive lymphoma remains a matter of debate. Several studies recently confirmed that non–HIV-infected patients with diffuse large cell lymphoma could benefit from more intensive treatment than standard CHOP.25-27,37,38 However, intensive chemotherapy regimens for ARL were associated with high toxicity, and randomized comparison of conventional versus low-dose methotrexate, bleomycin, cyclophosphamide, doxorubicin, and vincristine (m-BACOD) showed no difference for outcome.28 Our first cooperative trial, conducted with ACVBP in ARL patients, demonstrated that such a regimen was not excessively toxic for patients without adverse prognostic factors for HIV, which led to the description of the HIV score.14 Consequently, the present NHL-HIV-93 trial was designed to evaluate different dose-intensive treatments according to HIV score. A total of 485 patients were treated with risk-adapted chemotherapy in the NHL-HIV-93 trial. In the good-risk group, 5-year OS was estimated at 51% for ACVBP versus 47% for CHOP (P = .85). In the intermediate-risk group, it was 28% for CHOP versus 24% for Ld-CHOP (P = .19) and, in the poor-risk group, 11% for Ld-CHOP versus 3% for VS (P = .14). These findings indicate that HIV score, but not the intensity of the CHOP-based regimen, affects survival. Because of tolerance issues based on past experience in 1993, we chose to give only 4 cycles of CHOP or Ld-CHOP or 3 cycles of ACVBP.14 It is possible that the treatment outcome might have been better if more prolonged chemotherapy had been given, as in NHL it is not associated with AIDS (ie, 2 additional cycles beyond complete remission). However, this issue seems to be split between the pre-HAART and post-HAART eras. In the latter, a minimum of 6 cycles of CHOP and up to 8 seems to be the standard. Similarly, in patients with leptomeningeal disease chemotherapy maintenance should be considered after 9 to 12 intrathecal methotrexate treatments to reduce the risk of relapse.
Overall survival according to HIV score. (A) Before HAART (P < .001). (B) After HAART (P < .001).
Overall survival according to HIV score. (A) Before HAART (P < .001). (B) After HAART (P < .001).
During the early period of AIDS, the poor tolerability associated with chemotherapy prompted investigators to test reduced-dose regimens. In the randomized study conducted by Kaplan et al, there was no difference between the CR rates for standard and reduced-dose (42% versus 41%) m-BACOD chemotherapy, but there was less toxicity in the reduced-dose group.28 In the nonrandomized study conducted by Ratner et al,39 the CR rate was 30% in the low-dose CHOP group and 48% in the full-dose group, with comparable toxicity in both groups and a relatively short duration of CR in the low-dose group. Recent trials of CHOP-like regimens in ARL patients resulted in CR rates of 45% to 65%.11,21 In the present randomized study, CHOP and Ld-CHOP were compared in intermediate-risk patients. We confirmed the difference of 17% in CR rates (49% versus 32%, P = .02) but found no benefit in OS or EFS because there it seems to be a trade-off between efficacy and toxicity with more deaths due to infection on CHOP than Ld-CHOP (31% versus 18%). However, this hypothesis needs to be confirmed by competing-risk analysis of the cause of death—that is, if death due to infection on CHOP was early in the course of the trial (while subjects were on therapy and their ANC was depressed), the subject is no longer at risk for death due to lymphoma.
In addition to the poor results for low-dose CHOP, the aggressive presentation of ARL suggests the need for intensive treatment regimens, which result in high complete remission and survival rates for patients with non–HIV-associated NHL. In some cases, autologous hematopoietic stem cell transplantation was successfully performed in ARL patients with relapsed and primary refractory disease.40,41 However, in our trial, the randomized study of ACVBP versus CHOP did not show that intensive ACVBP treatment was at all beneficial for low-risk patients (3-year OS: 51% versus 47%, P = .85; 3-year EFS: 44% versus 40%, P = .73). These results are close to those of Costello et al, who showed with a median follow-up of 40 months that a modified high-dose CHOP regimen, with high-dose cyclophosphamide (2000 mg/m2), gives OS estimates at 43% and EFS at 39%.17 Some of the best results were reported with the EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) regimen in which the dose was individualized based on nadir counts. CR was achieved in 74% of patients, and at 53 months of median follow-up the OS was 60%.18 Notably, drug interactions with HAART may influence the toxicity, and in the later study HAART was suspended until after the chemotherapy.42 Also, intensive chemotherapy regimens used for Burkitt lymphoma outside of the HIV setting (CODOX-M/IVAC [cyclophosphamide, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine]) have had similar success in the HIV setting, probably due to tolerance of treatment with the relatively well-preserved immune function seen in AIDS-related Burkitt lymphoma patients.43
The results of this largest trial of HIV patients with aggressive NHL confirm recently described epidemiologic features of ARL.11,17,44 Advanced and extranodal diseases at diagnosis were common findings, as was severe immunosuppression. These results also confirm reports suggesting that ARL characteristics change over time, with an increase in the CD4+ cell count at diagnosis.19,20 In addition, in the post-HAART era, we and others found that ARL was more likely to be AIDS-defining in the HAART era, indicating that the decrease in ARL is less marked than that seen with other AIDS-defining illnesses.19,20 Thus, ARL remains an important health problem in HIV-infected patients, and the data presented here may have some implications for future treatment strategies. Our results also confirm the strong predictive value of HAART for survival in ARL patients. Three-year OS in these patients in the post-HAART era was 37% even after correction for follow-up duration. Our observations contrast with those of certain other large cohort studies, which reported sobering results in the HAART era.10,20 In the present study, we had a long follow-up of 6 years and analyzed the effects of HAART using the time-dependent Cox model to adjust for potential confounding factors introduced by the effects of baseline characteristics and treatment. The results also confirm that risk factors concerning lymphoma features (ie, IPI score) may be more important in the post-HAART era.45,46 Consequently, ARL treatment strategy should focus not only on the efficacy of chemotherapy but also on the interactions between optimal HAART and preexisting risk factors. Notably, such unplanned subset analyses could lead to false positive conclusions. In addition, in small sample sizes, failure to have a P value less than .05 should not be interpreted to be evidence of lack of a difference (eg, the present trial was not powered to determine real difference in the HIV poor-risk group or toxicity between the pre- and post-HAART eras). However, in other trials, there is also strong evidence that the response to chemotherapy is improved by HAART and maintenance of the immune function is better when chemotherapy is combined with HAART.47,48 In addition, in the National Institutes of Health (NIH) study where HAART was suspended until after the EPOCH, patients with a CD4 count below 0.10 × 109/L (100/mm3) did poorly and thus might benefit from HAART earlier.18
As rituximab was recently proved to have chemosensitization properties,49,50 combination with chemotherapy may be essential to enhance the tolerance of chemotherapy and improve its efficacy. Rituximab was not available throughout the entire period of the present study. However, recent phase 2 trials of CHOP plus rituximab resulted in a high CR rate (80%) with no increase of infection.51 In a report of pooled phase 2 trials with the combination of rituximab and CDE (cyclophosphamide, doxorubicin, and etoposide), the CR rate was 70%, and 2-year EFS and OS rates were 59% and 64%, respectively.52 The point for rituximab in combined chemoimmunotherapy is to eliminate B cells; on the other hand, this could increase immunodeficiency. Intermediate-analysis results for an ongoing phase 3 trial with CHOP chemotherapy failed to demonstrate survival benefit for CHOP plus rituximab.53 However, the improved tumor responses may be offset by an increase in infectious deaths in patients with a CD4 count below 0.05 × 109/L (50/mm3), and it appears possible that rituximab may be beneficial in patients with low HIV comorbidity.
In conclusion, in this randomized study of ARL patients, HAART, HIV score, and IPI score were the strongest predictors of survival but not the intensity of the CHOP-based chemotherapy. However, as in the post-HAART era the IPI score showed the largest predictive value; further evaluation of dose-intensive chemotherapy could be considered, including frontline transplantation in high-IPI-risk patients or immunotherapy as adjuvant therapy or even radioimmunotherapy.
Appendix
The authors thank the following clinicians who actively participated in the NHL-HIV-93 trial: M. Agranat, M. Allard, C. Allegre, L. Amoura, Y. Bastion, C. Batel-Copel, C. Bauduer, A. Baumelou, C. Benothman, P. Biron, M. Blanc, JY. Blay, C. Bonazzi, J. Boscolo, A. Bosly, C. Bottura, P. Boue, C. Bourguet, P. Brice, J. Briere, P. Brottier, D. Brun, P. Cadranel, A. Capmst, G. Caramello, D. Carguel, S. Castaigne, L. Ceccaldi, N. Cheron, G. Chichino, B. Coiffier, C. Corront, D. Cosnard, U. Derienzo, E. Decaudin, E. Degioanni, L. Delaere, M. Delle. Fogli, M. Digregorio, C. Dotissa, C. Dumontet, M. Duong, J. P. Farese, C. Fasan, M. Fathirhiur, D. Filippo, M. Fiorentini, C. Franzetti, A. François, J. Frilay, J. Froger, C. Fruchart, G. Fuzibet, J. Gabarre, A. Gaboriaud, D. Gabriella, C. Gaillat, G. Galliano, J. B. Gauthier, J. Gautier, C. Gisselbrecht, G. Giudici, A. Goujard, A. Guerzider, F. Guilmin, P. Hermans, M. Heuberger, C. Houyau, J. Huart, J. Hunault, P. Jacomet, A. Jaubert, B. Jaureguiberry, A. Jourdan, M. Kargar, L. Kern, Y. Kerneis, F. Kulekci, A. Lacoste, N. Laurent, C. Lavabre, P. Le. Rol, P. Legouffe, P. Lejeune, P. Leloup, A. Leoncini, A. Leroc, L. Lerol, A. Lipani, P. Loury, I. Mahe, M. Malbec, M. Malfitano, I. Marchetti, J. P. Marolleau, C. Martin, I. Mazzotta, A. Mena, B. Minoli, J. M. Molina, T. Montesarchio, P. Morel, J. Morlat, P. Moroni, F. Morvan, I. Moullet, A. Moureau, M. Muron, M. Musi, A. Nezri, A. Nigra, P. Occhini, G. Othman, A. Pagani, M. Palma, T. Panangelo, E. Paty, P. Pautier, J. Pellicelli, O. Perrin, T. Peterelli, A. Petit, C. Piette, I. Prato, A. Pristera, C. Rabian, R. Rapopoet, J. Rapoport, P. Ravel, A. Regazzetti, T. Renoux, V. Ribrag, A. Richard, P. Rienzo, V. Rieux, B. Riou, G. Rizardini, F. Rodon, D. Ross, J. M. Rossi, S. Rovere, P. Rybojad, G. Salles, R. Samani, H. Sarrazin, V. Scalise, P. Schlaifer, C. Schrappe, C. Sebban, F. Sensebe, G. Soranzo, J. J. Sotto, P. Souhami, E. Spina, T. Spittle, Z. Suter, A. Sutton, T. Tertian, A. Thyss, U. Tirelli, V. Torerec, A. Troncy, A. Vallantin, P. Vandergam, A. Veneri, I. Vittori, T. Vivarelli, A. Welker, J. Zerazhi, J. M. Zini, R. Zittoun, A. Zuckerman, and. Y. Zuclman.
Prepublished online as Blood First Edition Paper, January 12, 2006; DOI 10.1182/blood-2005-09-3600.
A complete list of the members of the French-Italian cooperative group appears in “Appendix.”
Supported in part by research funding from the Ensemble Contre le Sida (ECS, formerly named SIDACTION) (France), the Associazione Italiana per la Ricerca sul Cancro (AIRC), and Istituto Superiore di Sanità (Italy).
Presented in part at the 41th annual meeting of the American Society of Clinical Oncology, Orlando, FL, May 15, 2005.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Mathilde Dreyfus for editing the English.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal