Although anemia is common in older adults, its prognostic significance is uncertain. A total of 17 030 community-dwelling subjects 66 years and older were identified between July 1 and December 31, 2001, and followed until December 31, 2004. Cox proportional hazards analyses were performed to determine the associations between anemia (defined as hemoglobin < 110 g/L) and hemoglobin and all-cause mortality, all-cause hospitalization, and cardiovascular-specific hospitalization. Overall, there were 1983 deaths and 7278 first hospitalizations. In patients with normal kidney function, adjusting for age, sex, diabetes mellitus, and comorbidity, anemia was associated with an increased risk for death (hazard ratio [HR], 4.29; 95% confidence interval [CI], 3.55-5.12), first all-cause hospitalization (HR, 2.16; 95% CI, 1.88-2.48), and first cardiovascular-specific hospitalization (HR, 2.49; 95% CI, 1.99-3.12). An inverse J-shaped relationship between hemoglobin and all-cause mortality was observed; the lowest risk for mortality occurred at hemoglobin values between 130 to 150 g/L for women and 140 to 170 g/L for men. Anemia is associated with an increased risk for hospitalization and death in community-dwelling older adults. Consideration should be given to redefine “normal” hemoglobin values in the elderly. Clinical trials are also necessary to determine whether anemia correction improves quality or quantity of life in this population.
Introduction
In patients with chronic kidney disease (CKD), anemia is associated with abnormalities in left ventricular geometry, cardiovascular disease events, and all-cause mortality.1,2 Until recently, clinical outcomes associated with anemia in non-CKD elderly cohorts were less well described and restricted to select populations. In a report from the Netherlands, community-dwelling subjects older than 85 years with anemia had higher 5-year mortality rates than subjects with normal hemoglobin levels.3 In a cohort of older women with mild-to-moderate physical disability, Chaves et al also noted an increase in mortality associated with hemoglobin levels less than 110 g/L.4 In addition to its association with mortality, anemia also has been shown to have a negative impact on quality of life and physical functioning in older persons.5-7
Recently, in an analysis of 5888 community-dwelling older adults enrolled in the Cardiovascular Health Study, a reverse J-shaped relationship between hemoglobin and survival was observed.8 This relationship persisted after adjustment of relevant demographics, comorbidity, and cause of anemia. Although the data collection and analyses in the Cardiovascular Health Study were valid and robust, the authors noted that participants within the trial may not be representative of a true cross section of the elderly population, and therefore the results may not be generalizable to all community-based elderly subjects who seek medical care.
Zakai et al8 also raised a concern around the definitions for anemia in older adults. This uncertainty was emphasized in an accompanying editorial9 and has also been questioned by several other investigators.4,10,11 The current World Health Organization definitions for anemia were derived from small reference samples of nonelderly subjects.12 This is worrisome given that hemoglobin levels in men fall with age due to declining testosterone levels,13 and, with the cessation of menses, a physiologic explanation for anemia in women no longer exists. Therefore, the current level of hemoglobin that is considered normal in older individuals may not be appropriate.
Given the limited data linking anemia with clinically relevant outcomes, and the uncertainty around the threshold for the diagnosis of anemia in the elderly, we sought to determine the relationship between anemia and incident hospitalizations and all-cause mortality in a large cohort of community-dwelling older adults. We also sought to examine the sex-specific relations between hemoglobin and mortality risk to help refine the current definition of anemia in older adults.
Patients, materials, and methods
Study population
The conjoint ethics review board at the University of Calgary approved the study. A cohort of elderly subjects 66 years or older was identified from the computerized Calgary Laboratory Services (CLS) database in Calgary, AB. CLS provides laboratory testing for the entire Calgary Health Region (catchment population, 1.1 million; 80 567 subjects 66 years or older in 2001) using a single regional laboratory and standardized methods that are recalibrated routinely against reference samples. To be eligible for inclusion in this study, participants required at least one outpatient serum creatinine and one outpatient hemoglobin measurement during the baseline study period of July 1, 2001, to December 31, 2001 (n = 18 076). Subjects were excluded if they initiated dialysis during the baseline study period (n = 17), if they were residents of a long-term care or assisted-living facility (n = 949), or if the baseline estimate of kidney function was clinically implausible (mean glomerular filtration rate > 150 mL/min, n = 80).
Measurement of hemoglobin, kidney function, and definition of outcomes
Hemoglobin was measured with the Beckman Coulter MAXM/HMX analyzer (Beckman Coulter, Miami, FL) using the manufacturer's reagents and methods. The first hemoglobin measurement during the baseline study period served as the index hemoglobin. Mean glomerular filtration rate (GFR), determined during the baseline study period, was used to estimate kidney function. GFR was estimated using the abbreviated modification of diet in renal disease equation (MDRD-GFR), which includes variables for age, sex, and serum creatinine.14 Because of concerns about the validity of the MDRD equation for subjects with higher levels of kidney function,15 subjects with baseline GFR values exceeding 150 mL/min per 1.73 m2 were excluded (n = 80). Hemoglobin and serum creatinine measurements were analyzed in a single laboratory, thus eliminating the potential for interlaboratory measurement variation.
Outcomes for this study were included only if they occurred more than 30 days after the index hemoglobin. The primary outcome chosen was all-cause mortality. Secondary outcomes included first hospitalizations (all-cause) and first hospitalizations for cardiovascular causes (the composite of acute myocardial infarction [MI], congestive heart failure [CHF], cerebrovascular accident [CVA], or transient ischemic attack [TIA]). For subjects with multiple hospitalizations, only the first hospitalization was included. Using the unique provincial health care number for each subject, incident hospitalizations and date of death were determined with linkages to the Calgary Health Region corporate database and the Alberta Health and Wellness Vitals Statistics registry, respectively. These data linkages have been used in the past.16,17 Cause-specific hospitalizations were determined using hospitalization discharge coding performed by trained individuals in accordance with the International Classification of Diseases, Ninth and Tenth Revisions (ICD-9 and ICD-10). The following validated ICD codes were used to identify cause-specific hospitalizations: acute MI,18,19 410, 411.1, I21, or I22; CHF,17,20 428 or I50; and CVA/TIA,21 362.3, 433.x1, 434.x1, 436, 431.x, 430.x, 435.x, H34.1, I63.x, I64.x, I61.x, I60.x, or G45.x. Linkages with the provincial administrative Alberta Blue Cross database were also performed to obtain information on prescription drug use for the 12 months prior to the baseline study period. Prescription medication use data were available for all subjects. All patients were followed until death or up to December 31, 2004.
Measurement of covariates
To control for the impact of other important predictor variables, we collected information on patient age and sex, baseline kidney function, diabetes, and comorbidity status. Subjects were identified as having diabetes if they received at least one prescription for insulin or an oral hypoglycemic agent in the year prior to entry into the study. A measurement of comorbidity status based on the use of prescription drugs over the 6 months prior to the index hemoglobin was calculated using the chronic disease score (CDS).22 The CDS is a validated weighted index on patterns of drug use, with higher scores reflecting increased comorbidity. The CDS does not include agents used to treat anemia such as iron preparations, B12, and erythropoietin-stimulating proteins.
Statistical methods
Baseline characteristics by the presence or absence of anemia are presented as means and standard deviations for normally distributed continuous variables and percent prevalence for dichotomous variables. Given the skewed nature of the comorbidity score, these data are presented as median with interquartile range. A priori, anemia was defined as a hemoglobin less than 110 g/L, as this threshold is often used to guide prescribing of erythropoietin-stimulating proteins.23,24 The association between the presence of anemia and the primary and secondary outcomes was assessed using Cox proportional hazards analyses, adjusting for age, sex, diabetes mellitus, and comorbidity score (quartiles of the CDS). The interaction between kidney function and anemia, as shown in prior reports,25,26 was tested formally by including interaction terms and testing their statistical significance in the fully adjusted Cox models. Rates for hospitalization and mortality were determined by Poisson regression. Finally, we performed an analysis to determine the functional form of the relationship between hemoglobin and mortality across the range of hemoglobin. For these sex-specific analyses, we set the median hemoglobin plus or minus 5 g/L as the reference range. In fully adjusted Cox models, we then determined the risk for death for each 10 g/L hemoglobin above and below the reference. Assumptions for the Cox and Poisson regression models were tested and met. Analyses were conducted using SAS (version 8.01; SAS Institute, Cary, NC) and STATA (version 8; STATA, College Station, TX).
Results
A total of 17 030 subjects met the inclusion and exclusion criteria and were followed for a median of 3.2 years (52 468 person-years of follow-up). Baseline subject characteristics by anemia status are shown in Table 1. Of the subjects, 4.2% had hemoglobin levels less than 110 g/L; 13% had anemia using the World Health Organization definition. Using the World Health Organization's definition, anemia prevalence increased with age, affecting more than 20% of subjects 80 years or older. The proportion of subjects with anemia was also higher in subjects with diabetes mellitus and subjects with more advanced kidney disease. The use of erythropoietin-stimulating proteins was uncommon (0.4%) in the 12 months preceding the index hemoglobin, even among those subjects with hemoglobin levels less than 110 g/L.
Baseline demographics by the presence or absence of anemia
. | . | Anemia, Hb less than 110 g/L . | . | Anemia, WHO criteria* . | . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | Total¶ . | Absent† . | Present‡ . | Absent§ . | Present∥ . | ||
| Mean age, y (SD) | 74.9 (6.3) | 74.8 (6.4) | 77.7 (7.1) | 74.6 (6.3) | 77.3 (7.0) | ||
| 66 to 70, % | 5100 (30.0) | 30.3 | 19.5 | 31.5 | 19.9 | ||
| 71 to 75, % | 4903 (28.8) | 29.2 | 20.6 | 29.5 | 24.0 | ||
| 76 to 80, % | 3618 (21.2) | 21.2 | 24.2 | 21.0 | 23.1 | ||
| 81 to 85, % | 2148 (12.6) | 12.2 | 22.5 | 11.5 | 19.8 | ||
| Older than 85, % | 1261 (7.4) | 7.2 | 13.2 | 6.5 | 13.1 | ||
| Female, % | 9471 (55.6) | 55.2 | 64.0 | 56.3 | 51.4 | ||
| Male, % | 7559 (44.4) | 44.8 | 36.0 | 43.7 | 48.6 | ||
| Estimated GFR, % | |||||||
| Normal, GFR of 60 | 12 871 (75.6) | 77.0 | 43.7 | 79.3 | 50.5 | ||
| Stage 3, GFR 30 to 59 | 3694 (21.7) | 21.0 | 37.4 | 19.4 | 37.2 | ||
| Stages 4 and 5, GFR less than 30 | 465 (2.7) | 2.0 | 18.9 | 1.3 | 12.4 | ||
| Diabetes, % | 2278 (13.4) | 12.9 | 23.2 | 11.8 | 24.2 | ||
| Comorbidity score, median (IQR) | NA | 2134 (1496-3044) | 3299 (2293-4473) | 2075 (1460-2933) | 3057 (2152-4139) | ||
| Erythropoietin-stimulating protein use, % | 69 (0.4) | 0.3 | 2.6 | 0.2 | 1.7 | ||
. | . | Anemia, Hb less than 110 g/L . | . | Anemia, WHO criteria* . | . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | Total¶ . | Absent† . | Present‡ . | Absent§ . | Present∥ . | ||
| Mean age, y (SD) | 74.9 (6.3) | 74.8 (6.4) | 77.7 (7.1) | 74.6 (6.3) | 77.3 (7.0) | ||
| 66 to 70, % | 5100 (30.0) | 30.3 | 19.5 | 31.5 | 19.9 | ||
| 71 to 75, % | 4903 (28.8) | 29.2 | 20.6 | 29.5 | 24.0 | ||
| 76 to 80, % | 3618 (21.2) | 21.2 | 24.2 | 21.0 | 23.1 | ||
| 81 to 85, % | 2148 (12.6) | 12.2 | 22.5 | 11.5 | 19.8 | ||
| Older than 85, % | 1261 (7.4) | 7.2 | 13.2 | 6.5 | 13.1 | ||
| Female, % | 9471 (55.6) | 55.2 | 64.0 | 56.3 | 51.4 | ||
| Male, % | 7559 (44.4) | 44.8 | 36.0 | 43.7 | 48.6 | ||
| Estimated GFR, % | |||||||
| Normal, GFR of 60 | 12 871 (75.6) | 77.0 | 43.7 | 79.3 | 50.5 | ||
| Stage 3, GFR 30 to 59 | 3694 (21.7) | 21.0 | 37.4 | 19.4 | 37.2 | ||
| Stages 4 and 5, GFR less than 30 | 465 (2.7) | 2.0 | 18.9 | 1.3 | 12.4 | ||
| Diabetes, % | 2278 (13.4) | 12.9 | 23.2 | 11.8 | 24.2 | ||
| Comorbidity score, median (IQR) | NA | 2134 (1496-3044) | 3299 (2293-4473) | 2075 (1460-2933) | 3057 (2152-4139) | ||
| Erythropoietin-stimulating protein use, % | 69 (0.4) | 0.3 | 2.6 | 0.2 | 1.7 | ||
NA indicates not applicable.
Hemoglobin than 130 g/L in males and less than 120 g/L in females
n = 16 311, 95.8% of the total.
n = 719, 4.2% of the total.
n = 14 812, 87.0% of the total.
n = 2218, 13% of the total.
Numbers in parentheses in this column are percentages; those outside parentheses represent total n.
All-cause mortality
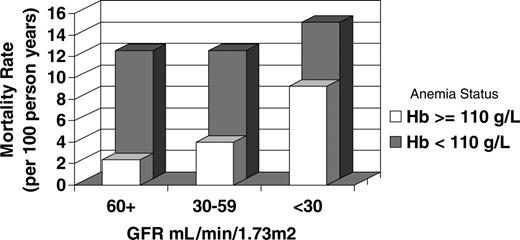
A total of 1983 subjects experienced the primary outcome for an overall mortality rate of 37.8 per 1000 person-years. Anemia (hemoglobin < 110 g/L) was associated with a 5-fold increase in risk for all-cause mortality in unadjusted analyses (hazard ratio [HR], 5.01; 95% confidence intervals [CI], 4.43-5.66). In the fully adjusted model, there was a significant interaction between mean GFR and anemia (P < .001). Therefore, results are presented stratified by mean baseline GFR (Table 2). Among subjects with a normal GFR, anemia was associated with a 4-fold increase in risk for mortality (HR, 4.29; 95% CI, 3.55-5.12). The mortality risk imposed by anemia was lower in subjects with a GFR of 30 to 59 mL/min per 1.73 m2 (HR, 2.80; 95% CI, 2.28-3.43) and decreased even further in subjects with a GFR less than 30 mL/min per 1.73 m2 (HR, 1.53; 95% CI, 1.12-2.07). Similar analyses were performed for subjects older than 80 years. Given the presence of an interaction between mean GFR and anemia (P = .02), these results are once again stratified by category of baseline GFR. Among subjects with a normal GFR, anemia was associated with a 3-fold increase in risk for mortality (HR, 3.34; 95% CI, 2.47-4.51). The mortality HR associated with anemia in subjects with a GFR of 30 to 59 mL/min per 1.73 m2 was 2.52 (95% CI, 1.91-3.33) and in subjects with a GFR less than 30 mL/min per 1.73 m2 the HR was 1.58 (95% CI, 1.06-2.34).
Primary and secondary outcomes by anemia status and CKD category
. | No anemia . | . | Anemia . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Outcome . | No. . | Age-*and sex-adjusted rate per 100 person-years (95% CI) . | No. . | Age-*and sex-adjusted rate per 100 person-years (95% CI) . | Hazard ratios†(95% CI) . | |||
| GFR, 60 mL/min per 1.73 m2 | ||||||||
| All-cause mortality | 999 | 2.39 (2.24-2.54) | 122 | 11.92 (9.95-14.28) | 4.29 (3.55-5.12) | |||
| All-cause hospitalization | 4770 | 15.24 (14.81-15.67) | 220 | 38.80 (33.98-44.36) | 2.16 (1.88-2.48) | |||
| CVD-specific hospitalization | 1318 | 4.18 (3.96-4.41) | 83 | 13.44 (10.82-16.69) | 2.49 (1.99-3.12) | |||
| GFR, 30 to 59 mL/min per 1.73 m2 | ||||||||
| All-cause mortality | 565 | 4.00 (3.65-4.38) | 114 | 11.95 (9.90-14.42) | 2.80 (2.28-3.43) | |||
| All-cause hospitalization | 1747 | 20.05 (19.10-21.06) | 202 | 42.46 (36.94-48.80) | 1.87 (1.61-2.12) | |||
| CVD-specific hospitalization | 717 | 7.38 (6.82-7.99) | 102 | 18.38 (15.08-22.39) | 2.15 (1.74-2.65) | |||
| GFR, less than 30 mL/min per 1.73 m2 | ||||||||
| All-cause mortality | 117 | 9.20 (7.64-11.07) | 66 | 14.61 (11.43-18.67) | 1.53 (1.12-2.07) | |||
| All-cause hospitalization | 230 | 34.72 (30.48-39.55) | 109 | 46.33 (38.36-55.97) | 1.23 (0.97-1.56) | |||
| CVD-specific hospitalization | 127 | 17.24 (14.44-20.59) | 60 | 21.40 (16.56-27.65) | 1.15 (0.84-1.59) | |||
. | No anemia . | . | Anemia . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Outcome . | No. . | Age-*and sex-adjusted rate per 100 person-years (95% CI) . | No. . | Age-*and sex-adjusted rate per 100 person-years (95% CI) . | Hazard ratios†(95% CI) . | |||
| GFR, 60 mL/min per 1.73 m2 | ||||||||
| All-cause mortality | 999 | 2.39 (2.24-2.54) | 122 | 11.92 (9.95-14.28) | 4.29 (3.55-5.12) | |||
| All-cause hospitalization | 4770 | 15.24 (14.81-15.67) | 220 | 38.80 (33.98-44.36) | 2.16 (1.88-2.48) | |||
| CVD-specific hospitalization | 1318 | 4.18 (3.96-4.41) | 83 | 13.44 (10.82-16.69) | 2.49 (1.99-3.12) | |||
| GFR, 30 to 59 mL/min per 1.73 m2 | ||||||||
| All-cause mortality | 565 | 4.00 (3.65-4.38) | 114 | 11.95 (9.90-14.42) | 2.80 (2.28-3.43) | |||
| All-cause hospitalization | 1747 | 20.05 (19.10-21.06) | 202 | 42.46 (36.94-48.80) | 1.87 (1.61-2.12) | |||
| CVD-specific hospitalization | 717 | 7.38 (6.82-7.99) | 102 | 18.38 (15.08-22.39) | 2.15 (1.74-2.65) | |||
| GFR, less than 30 mL/min per 1.73 m2 | ||||||||
| All-cause mortality | 117 | 9.20 (7.64-11.07) | 66 | 14.61 (11.43-18.67) | 1.53 (1.12-2.07) | |||
| All-cause hospitalization | 230 | 34.72 (30.48-39.55) | 109 | 46.33 (38.36-55.97) | 1.23 (0.97-1.56) | |||
| CVD-specific hospitalization | 127 | 17.24 (14.44-20.59) | 60 | 21.40 (16.56-27.65) | 1.15 (0.84-1.59) | |||
Absence of anemia is defined as Hb at or above 110 g/L; anemia was defined as Hb below 110 g/L.
Age adjusted to the sample mean using Poisson regression.
Adjusted for age, sex, diabetes mellitus, and comorbidity.
Mortality rates stratified by anemia status are also presented in Table 2. Although absolute mortality rates are the greatest in subjects with anemia and a GFR less than 30 mL/min per 1.73 m2, the greatest relative increase in mortality associated with anemia is seen in subjects with normal kidney function (age- and sex-adjusted rates: 11.92 versus 2.39 deaths per 100 patient-years in subjects with and without anemia, respectively). Conversely, the greatest relative increase in mortality by level of kidney function occurs in subjects without anemia (age- and sex-adjusted rates: 9.20 versus 2.39 deaths per 100 patient-years in subjects with GFR values < 30 mL/min per 1.73 m2 versus subjects with GFR values > 60 mL/min per 1.73 m2). The relative increase in mortality by level of kidney function is modest in patients with anemia (age- and sex-adjusted rates: 14.61 versus 11.92 deaths per 100 patient-years in subjects with GFR values < 30 mL/min per 1.73 m2 versus subjects with GFR values > 60 mL/min per 1.73 m2), reflecting the high underlying mortality rate in anemic, non-CKD patients. These relationships are depicted in Figure 1.
Hospitalizations
Overall, 7278 subjects experienced at least one hospitalization; 2407 subjects experienced at least one hospitalization for the composite outcome of acute MI/CHF/CVA/TIA. Similar to the associations between anemia and mortality, anemia was also associated with an increased risk of all-cause hospitalization (unadjusted HR, 2.69; 95% CI, 2.46-2.94) and hospitalization for the composite outcome (unadjusted HR, 3.75; 95% CI, 3.28-4.28). These risks were the greatest in subjects with anemia and normal kidney function compared with nonanemic patients with normal kidney function. Similar to the mortality results, absolute rates for hospitalization were the greatest in anemic subjects with GFR less than 30 mL/min per 1.73 m2, and the greatest relative increase in hospitalization rates occurred with the presence of anemia in subjects with normal kidney function (Table 2). For subjects with normal baseline kidney function, compared with subjects without anemia, the presence of anemia was also associated with increased length of hospital stay (median increase in length of stay, 8.0 days [95% CI, 4.7-11.3 days], data not shown).
Mortality rate (per 100 person-years) by stage of kidney function and the presence or absence of anemia (adjusted for age and sex).
Mortality rate (per 100 person-years) by stage of kidney function and the presence or absence of anemia (adjusted for age and sex).
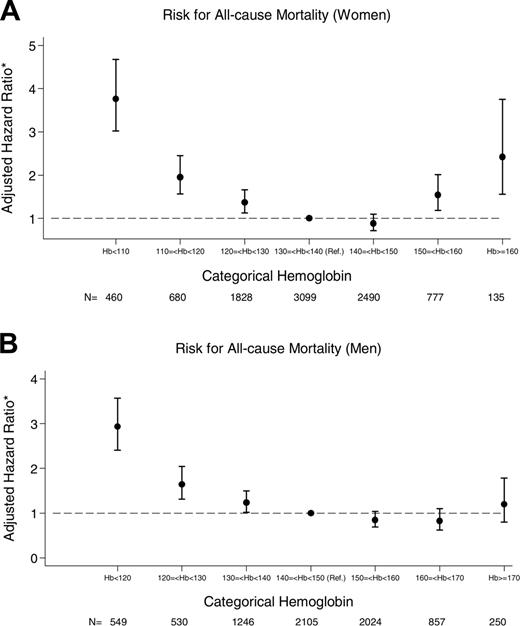
Association between hemoglobin and all-cause mortality
The adjusted probability of death as a function of baseline hemoglobin for women and men is shown in Figure 2A and B, respectively. A reverse J-shaped relationship between hemoglobin and all-cause mortality is seen for both sexes, although an increased mortality risk associated with higher hemoglobin values reached statistical significance in women only.
Discussion
In this community-based study of more than 17 000 older adults, anemia was common and increased with age, diabetes, and advanced kidney disease. A significant association was found between anemia and risk for all-cause hospitalization, hospitalization secondary to cardiovascular disease, and all-cause mortality. Furthermore, the association between hemoglobin and mortality was not linear; risk for death increased at both extremes of hemoglobin.
Other investigators have reported adverse outcomes associated with anemia in select populations, including patients with congestive heart failure,27-29 patients with chronic kidney disease,25,26 older women with physical disabilities,4 and community-dwelling subjects older than 85 years.3 To our knowledge, the only other study to examine the association between anemia and clinically relevant outcomes across a wide age range of older community-dwelling adults was the Cardiovascular Health Study.8 Of importance, in a different population, our findings confirm the results from this recent report. Specifically, both studies found an increased mortality risk associated with anemia and a reverse J-shaped relationship between hemoglobin and survival. Although U-shaped or J-shaped relationships between hematocrit and mortality have been reported in other community-based studies, including the Framingham Heart Study,30 the Puerto Rico Health Program,31 and the Honolulu Heart Study,32 these studies are limited by the exclusion of females31,32 and a relative paucity of older adults.30-32
The interrelations described herein with respect to kidney function, anemia, and mortality are also novel. Consistent with prior reports,26,33,34 subjects at greatest risk for death had both anemia and advanced CKD. However, to our surprise, the relative impact of anemia on hospitalization and mortality was greatest in subjects with normal kidney function. This is an important finding on a population level given that the majority of adults have GFR levels more than 60 mL/min per 1.73 m2.
The sheer magnitude of the associations between anemia and clinical outcomes in this study is notable and is supported by biologic plausibility. Hemodynamic changes associated with anemia include systemic arterial vasodilatation and a resultant decrease in systemic vascular resistance.35 Anemia also activates the sympathetic nervous system, which results in an increase in heart rate.36 Both these adaptations may aggravate myocardial ischemia37,38 and potentially lead to altered left-ventricular (LV) structure and function.39 Alternatively, anemia may be simply a proxy for other factors including comorbidities and underlying chronic inflammation.40 In the NHANES analysis, anemia of chronic disease was not unexpectedly associated with elevated C-reactive protein and rheumatoid factor.11
There are 2 major potential implications of this study. First, the presence of anemia in patients with normal kidney function should be regarded as a marker for subsequent adverse outcomes. These data, combined with the Cardiovascular Health Study report,8 should be a stimulus for clinical trials to determine if the correction of anemia in older adults impacts quality and/or duration of life. Using data from the Baltimore Longitudinal Study of Aging, Ershler et al recently suggested that, with advanced age, the regular erythropoietin compensatory mechanism associated with blood loss or erythropoietin resistance becomes inadequate, resulting in anemia.41 This inadequate response may occur even in patients without measurable kidney disease,42 or in patients with occult interstitial kidney disease and a normal GFR.43 As it is uncertain whether treatment with erythropoietin-stimulating proteins in these patients would improve outcomes, controlled trials are necessary before this is advocated for routine clinical practice. Second, our data suggest that the optimal hemoglobin in older adults is approximately 130 to 150 g/L in women and 140 to 170 g/L in men. Taking these data into consideration with the Cardiovascular Health Study report,8 one could argue that cut points for the diagnosis of anemia in the elderly should be redefined. The current World Health Organization definitions for anemia are based upon statistical distribution considerations corresponding to minus 2 standard deviations below the mean in a reference nonelderly population.12 Our current findings suggest the need to define biologic variables, such as anemia, using associations with relevant clinical outcomes as opposed to statistical normality. Such a process has occurred with other risk factors, including blood pressure and serum cholesterol. While changing the definition for anemia seems warranted, more data are clearly needed before one could advocate correcting hemoglobin levels into the “normal” range with therapies other than treatment of nutritional abnormalities.
Association between hemoglobin and risk for all-cause mortality. (A) Women. (B) Men. Each • indicates a point estimate for this risk, and the vertical lines represent 95% confidence intervals around these estimates. *Adjusted for age, diabetes mellitus, GFR, and comorbidity.
Association between hemoglobin and risk for all-cause mortality. (A) Women. (B) Men. Each • indicates a point estimate for this risk, and the vertical lines represent 95% confidence intervals around these estimates. *Adjusted for age, diabetes mellitus, GFR, and comorbidity.
Although the current study provides important information on the relationship between anemia and clinically relevant outcomes in older adults, the results should be interpreted within the context of the study's limitations. Specifically, GFR was not measured directly but was estimated using a serum creatinine measurement, which in itself may not be a good indicator of kidney function in older adults who are at risk for substantial muscle wasting. Although more accurate than serum creatinine alone, there are well-known limitations of the MDRD estimation equation, and potential misclassification of subjects with regard to the diagnosis of CKD may have occurred. To ensure that the adverse risks seen with anemia in non-CKD subjects were not secondary to misclassification, we also performed analyses using 70 mL/min per 1.73 m2 as the cut point for CKD diagnosis. This sensitivity analysis did not appreciably change the results.
There are also limitations as a result of the study design. First, hospitalization outcomes were determined using ICD coding by trained personnel. Misclassification may have occurred with respect to cardiovascular disease–related hospitalizations. Such misclassification would be nondifferential and bias the association between anemia and this outcome toward the null. Second, the use of laboratory data to define the study cohort limited the study to subjects who sought medical care and had a hemoglobin measurement obtained. As the study sample was based on the elderly, who are more likely to access the healthcare system and have laboratory testing performed, this is unlikely to substantially bias the study results. Given that the prevalence rates of anemia and CKD are also similar to prior population-based reports,11,44-46 and the overall mortality rate is similar to the Cardiovascular Health Study report, selection bias is likely minimal. Data from a cohort identified by laboratory-based case finding are also easily generalized to primary care practice. Third, as with all observational studies, the possibility of residual confounding cannot be excluded. Given the nature of the data collection, we were unable to directly adjust for established cardiovascular risk factors such as blood pressure, smoking habits, and dyslipidemia. Nevertheless, we were able to account for comorbidity using a validated technique. The hazard ratios associated with anemia and the clinical outcomes chosen for this study are substantial and are unlikely to be completely negated by further covariate adjustment. Finally, the cause of anemia could not be determined in this study. Prior population-based studies have established that approximately one third of anemia cases in older adults are caused by reversible conditions such as folate, B12, or iron deficiency; one third can be ascribed to chronic inflammation or chronic illnesses such as CKD; and one third are unexplained.11 Future studies should examine whether the prognostic significance of anemia differs by anemia etiology.
Despite these limitations, the current study has several strengths. The validity of our study is reinforced by the consistency of our results with previous knowledge. This is evident with the prevalence estimates for anemia and CKD, the effect modification by CKD, and the recognition of nonlinear relations between hemoglobin and mortality—results similar to prior reports. Also, the size of the cohort (more than 17 000 elderly subjects), its community-based setting, and the wide age distribution of the study subjects increase the generalizability of the study results to community-dwelling elderly individuals. This is particularly relevant given the prevalence of anemia and CKD in this demographic. This strength cannot be understated as a substantial number of subjects were older than 80 years (n = 3420) or had GFR levels less than 30 mL/min per 1.73 m2 (n = 465). Few studies have reported these numbers, allowing this study the power to report outcomes in pertinent subgroups.
In conclusion, anemia is associated with increased risk for hospitalization and mortality in older adults. As this risk occurs at hemoglobin levels that are currently considered normal, consideration should be given to refining the current definition of anemia in older adults to reflect this continuum of risk. Furthermore, these results should provide an impetus for future interventional trials of anemia correction in the elderly.
Prepublished online as Blood First Edition Paper, January 10, 2006; DOI 10.1182/blood-2005-10-4308.
B.F.C. and B.R.H. participated in study conception and design, acquisition of data, analysis and interpretation of data, funding acquisition, and drafting and critical revision of the paper for important intellectual content. B.J.M. participated in study conception and design and critical revision of the paper for important intellectual content. J.Z. participated in data analysis. M.T. and S.K. participated in interpretation of the data and critical revision of the paper for important intellectual content. All authors have seen and approved the final version of the paper. All authors accept responsibility for the scientific content of the paper. No author has a potential conflict of interest.
Supported by The Kidney Foundation of Canada and Amgen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal