Abstract
We investigated the causes of the altered functionality of T cells cultured under conditions designed for cell and gene therapy and the strategies to prevent their defects. We first showed that human T cells cultured for 6 days with anti-CD3 ± anti-CD28 antibodies and interleukin-2 presented a 50% decrease of their proliferative responses to allogeneic or recall antigens. Similarly, day-6 cultured murine T cells completely lost their capacity to reject allogeneic skin grafts and to provoke graft-versus-host disease (GVHD) when infused into irradiated semi-allogeneic mice. Interestingly, injection of higher amounts of cultured T cells restored GVHD induction. Moreover, depletion of CD25+ cells prior to T-cell cultures can prevent these deficiencies both in mice and humans. Therefore, we demonstrated that culture conditions used for T-cell therapy preferentially activated and expanded regulatory T cells (Treg's). Thus, we showed that dividing cells sorted from T-cell cultures strongly suppressed the proliferation of autologous T cells in response to allogeneic stimulation. An increased detection of Foxp3 at mRNA and protein levels in the cultures confirmed the Treg expansion. Overall, we demonstrate that T-cell cultures promote Treg expansion over effector T cells, leading to deleterious immune functions, and that this imbalance can be prevented by an initial depletion of CD25+ cells.
Introduction
Cellular and/or gene therapies based on the use of T lymphocytes or one of their subsets are promising for the treatment of many acquired or inherited immune deficiencies. Most of these therapeutic strategies require ex vivo manipulation, either including or not their genetic modification, of T cells that originate from either the patients themselves or donors. To obtain adequate numbers of therapeutic cells, T cells need to be cultured and expanded for a sufficient period of time. Because most T lymphocytes are quiescent, it is necessary to activate them to trigger their cycling and proliferation. Furthermore, activation and cell division of the target cells are also required for retroviral-based genetic modification, thus allowing for the integration into the host genome and stable and long-term expression of the transgene.1,2 Polyclonal activation of T cells can be achieved using monoclonal antibodies (mAbs) that trigger TCR/CD3 and CD28 signaling, either with the mAbs being immobilized or not on tissue-culture plates. Such in vitro stimulation conditions allow to obtain high levels of transduction using retroviral vectors and efficient cell expansion after 1 to 2 weeks of culture.2,3 Although preservation of T-cell functionality after ex vivo manipulation represents a major key point for further clinical use, several reports have now provided evidence that both murine (m)- and human (h)-activated/expanded T cells display reduced immune responses to allogeneic and recall antigens.4-6 Besides the fact that stimulation of T cells through TCR-CD3 engagement in the absence of costimulation may induce both anergy and cell death,7 the role of natural CD4+CD25+ regulatory/suppressor T cells (Treg) also may be evoked. Indeed, Treg's that represent 1% to 5% of peripheral CD4 T cells and exhibit strong suppressive capacities8,9 are known to play a key role in the control of T-cell homeostasis,10-12 the control of memory T-cell responses,13,14 and the control of effector cells in various T-cell-mediated pathologies such as malaria,15 leishmania,16 cancer,17,18 and HIV infection.19,20 For these reasons, investigating whether CD4+CD25+ Treg's may contribute to the alterations of the immune functions of cultured T cells remains questionable. Thus, after a period of cell expansion, we assayed the functionality of hT cells and mT cells in vitro and in vivo, respectively.
Overall, our results underline that culture conditions commonly used for the expansion of T cells designed for cell/gene therapy favor the expansion of Treg, which in turn suppress further functionality of T cells both in vitro and in vivo. Interestingly, the initial depletion of CD25+ T cells before cell activation and/or expansion proves to be a simple strategy that prevents Treg/effector imbalance and preserves T-cell functionality.
Materials and methods
Experiments with human cells
Approval for these studies was obtained from the institutional review boards of the Université Pierre et Marie Curie (UPMC) and the Centre National de la Recherche Scientifique (CNRS). Written informed consent was provided in accordance with the Declaration of Helsinki.
Purification of hT cells. Blood cells were obtained from peripheral blood or leukapheresis samples freshly collected from healthy donors after informed consent and according to institutional guidelines. Mononuclear cells (MNCs) were separated on Ficoll-Hypaque gradient (density 1.077 g/mL). Total T cells (CD3+) were enriched by positive selection after labeling of MNCs with anti-CD3 monoclonal antibodies (mAbs) directly conjugated to immunomagnetic beads (Miltenyi Biotech, Kohln, Germany). In experiments where T cells and CD25-depleted T cells were compared, MNCs were separated by positive selection for CD8+ T cells and by negative selection for CD4+ T cells (kit II; Miltenyi Biotech). Thereafter, negatively selected CD4+ T cells were either depleted or not of CD25 cells using anti-CD25 conjugated immunomagnetic beads. Prior to being cultured, CD8+ cells were mixed with either CD4+ or CD25-depleted CD4+ cells at the same ratio as that of original MNCs. Purity (> 95%) of the different T-cell purifications were assessed by flow cytometry after direct staining with phycoerythrin (PE)-conjugated anti-CD3, fluorescein isothiocyanate (FITC)-conjugated anti-CD8, or phycoerythrin/cyanin-5 (PC5)-conjugated anti-CD4 mAbs (all from Beckman-Coulter, Villepinte, France), and anti-CD25-PE (Becton Dickinson, Pont de Claix, France).
Culture of hT cells. Purified hT cells were plated onto 6-well tissue culture plates (Costar, Cambridge, MA) previously coated with 5 μg/mL of anti-CD3 mAbs (clone OKT3, Orthoclone, Jansen-Cilag, Paris, France) and/or with 5 μg/mL of anti-CD28 mAbs (Pharmingen, San Diego, CA). Cells were grown at 106 cells/mL in RPMI 1640 medium (GIBCO-BRL, Grand Island, NY) plus 10% heat-inactivated pooled human AB serum (hABS; EFS, Paris, France) either supplemented or not with 600 U/mL recombinant human interleukin-2 (rhIL-2, Chiron, Emeryville, CA). Three different culture conditions were defined: (1) immobilized (i)-anti-CD3 + i-anti-CD28 (CD3/CD28), (2) i-anti-CD3 + rhIL-2 (CD3 + IL-2), and (3) i-anti-CD3 + i-anti-CD28 + rhIL-2 (CD3/CD28 + IL-2). Medium changes were performed every 2 to 3 days, but only once during the first 5 days. Cell proliferation was measured by counting viable cells at different time points based on trypan blue exclusion.
Retroviral infection of hT cells. hT cells were infected with retroviral supernatant obtained from the H293-GalV-Thy1/TK 1704 packaging cells that stably produce retroviral particles harboring a bicistronic Thy1-IRES-TK vector coding for both the human CD90 membrane molecule as a selectable marker and the thymidine kinase activity from herpes virus as already published elsewhere.21 Infections of hT cells were carried out at day 2, 4, or 6 of culture initiated under the different conditions of activation described in “Culture of hT cells.” Two days after infection, T cells were counted and stained with anti-CD90-FITC and anti-CD3-PE mAbs in order to determine the transduction efficiency. Isotype-matched irrelevant mAbs conjugated to FITC or PE were used as negative controls.
Cell-cycle analysis. Cell-cycle analysis of hT cells was performed using Coulter Reagents Kit (Beckman-Coulter) on the basis of DNA staining with propidium iodide (PI; DNA-Prep Stain) as described.2 Cell-cycle analysis was performed on an EPICS Elite cytometer (Beckman-Coulter) on the basis of light scatter properties and fluorescence intensity. The percentage of T cells in the different phases of the cell cycle was analyzed using Multicycle AV software (Phoenix Flow Systems, Seattle, WA).
CFSE staining and cell sorting of dividing and nondividing cells. Freshly purified hT cells were labeled with 5, 6 carboxyfluorescein diacetate succinimidyl ester (CFSE, Sigma, Saint Quentin Falavier, France) as follows: cells at 106/mL were incubated in serum-free phosphate-buffered saline (PBS) containing 5 mM CFSE for 5 minutes. An equivalent volume of fetal calf serum (FCS) was added to stop the reaction. Cells were then washed and cultured as described in “Culture of hT cells.” After 2 to 6 days, CFSE-stained cultured cells were labeled with anti-CD4-APC (allophycocyanin) and anti-CD8-QR (Quantum Red) mAbs (Becton Dickinson). The percentage of dividing cells was determined by taking into account the decrease of the CFSE fluorescence intensity as described.22 Flow cytometric acquisition was performed on a FACScalibur using CellQuest software (Becton Dickinson) and FlowJo software (Tree Star, San Carlos, CA) for analysis. In some experiments, CFSE-labeled cells were purified after 4 days of culture using a FACStar Plus cell sorter (Becton Dickinson) into CFSElow and CFSEhigh cell fractions that correspond to dividing and nondividing cells, respectively.
Intracellular staining of Foxp3. Foxp3 protein expression was detected using the FITC anti-human Foxp3 Staining Set from eBioscience (San Diego, CA). FITC-Rat IgG2a mAbs were used as isotype control. Cells were analyzed by flow cytometry.
Autologous mixed leukocyte reaction (MLR) for recall antigens. Recall antigens all purchased from Pasteur Institute (Paris, France) were tetanus toxin (TT; 100 μg/106 cells), tuberculin (PPD; 20 μg/106 cells), and candidin (10 μg/106 cells). Recall antigens were used to load monocyte-derived dendritic cells (DCs) used as antigen-presenting cells (APCs). Monocyte purification and generation of DCs are described elsewhere.23 In brief, DCs were generated in liquid culture in RPMI medium containing 10% hABS, 20 ng/mL IL-4 (a kind gift from K. Thielemans, ULB, Bruxelles, Belgium), and 100 ng/mL granulocyte-macrophage colony stimulating factor (kindly provided by Schering Plough, Dardilly, France). After 7 days of culture, DCs were loaded either with TT or a mixture of PPD/candidin. After 2 hours, DCs were washed and cultured for another 24 hours under the same conditions, except that trimeric CD40-ligand (Immunex, Seattle, WA) was added to the culture medium at 250 ng/mL to induce activation and maturation of DCs. Loaded or unloaded DCs were plated in triplicates on round-bottom 96-well tissue culture plates (Falcon, 3072; Becton Dickinson) at various concentrations (0-5 × 104 cells/100 μL) in the presence of 105 cells/100 μL autologous cultured T cells or freshly thawed noncultured cells used as control. After 4 days, cell proliferation was determined by incorporation of 1 μCi[0.037 MBq]/well of tritiated thymidine (3H-Tdr, Amersham, Buckinghamshire, United Kingdom) for 16 additional hours and measured using a β-counter (counter-WALLAC). Results expressed in counts per minute represent the mean ± SEM of triplicates.
Allogeneic MLR. Allogeneic MLR for hT cells was performed using either allogeneic Epstein-Barr virus (EBV)-infected B cells or allogeneic DCs monocyte-derived from healthy donors. Freshly thawed noncultured T cells and cultured T cells that have been either depleted or not of Treg were seeded in triplicates in round-bottom 96-well tissue culture plates at 105 cells/100 μL of RPMI medium containing 10% hABS and various numbers (0, 1, or 5 × 104 cell/well) of either 50 Gy-irradiated allogeneic lymphoblastoid cells or 30 Gy-irradiated DCs for 4 days. Then, cell proliferation was determined by 3H-Tdr incorporation. Noncultured T cells stimulated by APCs or by i-anti-CD3 mAbs + rhIL-2 were used as positive controls. Negative controls consisted of T cells alone, irradiated APCs alone, and medium alone. Allogeneic MLR using EBV B cells also were performed on dividing (CFSElow) and nondividing (CFSEhigh) cell fractions sorted from cultured CD3+ T cells.
MLR suppressive T-cell assay. The suppressive activity of dividing (CFSElow) cells sorted from each culture condition was assayed by mixing CFSElow cells at various cell ratios (1:1; 1:2; 1:4; 1:10; 1:20) with 5 × 104 autologous nondividing (CFSEhigh) cells stimulated by 104 50 Gy-irradiated allogeneic EBV B cells. Cocultures were performed in triplicate, and the total number of cells in each well was maintained at a constant by adjusting each coculture with adequate numbers of 15 Gy-irradiated CFSEhigh cells. To evaluate the role of a direct cell-cell contact mechanism, some experiments were performed using 24-well transwell plates (0.4 μm pore size; 6.5 mm in diameter, Costar) in which CFSElow cells were plated in the upper chamber. Cell proliferation was measured by 3H-Tdr incorporation after 4 days of coculture.
RNA isolation and real-time quantitative RT-PCR. Total mRNA was prepared using RNable medium (Eurobio SA, ZA Courtaboeuf, France). First-strand cDNA was synthesized in RT samples, each containing 1.5 μg total RNA isolated from 3 to 7 × 106 cells, 16 U/mL M-MLV reverse transcriptase (Gibco-BRL, Life Technologies, Cergy-Pontoise, France), 4 mM Oligo-(dT) 12-18 (Amersham-Pharmacia Biotech, Saclay, France), and 0.8 mM mixed dNTP (Amersham-Pharmacia Biotech). Quantitative polymerase chain reaction (PCR) was performed in a LightCycler System (Roche Diagnostics, Meylan, France) using a SYBR Green PCR kit from Roche Diagnostics. The sequence of primers are indicated in Table 1. Normalization was achieved by quantification of the expression of the splicing factor 3A1 (SF3A1) gene chosen as a control housekeeping gene (HKG). TGFB1, FOXP3, and SF3A1 mRNA expression were quantified by relative quantification of real-time PCR according to Gibson et al.24 Briefly, cDNA dilution series of calibrator cells were run to set up a standard curve for the 2 target genes and SF3A1, and the Ct values were plotted against the log cDNA concentration added. Purified T lymphocytes of healthy control donors, activated with phorbol ester and ionomycin (5 ng/mL and 1 ng/mL, respectively) for 48 hours, were used as calibrator cells. By using the obtained linear graphs, the differences in Ct values were determined for each sample and were expressed as a relative percentage of mRNA present in the reference dilution of calibrator cells according to the ΔΔCt method after adjustment of PCR efficiency with the RelQuant software (Roche). Each PCR experiment was carried out in duplicate.
Sequences of oligonucleotide primers used for quantifying the expression of TGFB1 and FOXP3 genes
Name . | GenBank accession no. . | Sequences . | Product size (bp) . |
|---|---|---|---|
| SF3A1 | NM_005877 | Forward: TGCAGGATAAGACGGAATGGAAACTGA | 179 |
| Reverse: GTAGTAAGCCAGTGAGTTGGAATCTTTG | |||
| FOXP3 | NM_014009 | Forward: TCCACAACATGCGACCCCCTTTCA | 217 |
| Reverse: ACAGCCCCCTTCTCGCTCTCCA | |||
| TGFB | NM_000660 | Forward: GGAGTTGTGCGGCAGTGGTTG | 259 |
| Reverse: AGCTGAAGCAATAGTTGGTGTCCAGG |
Name . | GenBank accession no. . | Sequences . | Product size (bp) . |
|---|---|---|---|
| SF3A1 | NM_005877 | Forward: TGCAGGATAAGACGGAATGGAAACTGA | 179 |
| Reverse: GTAGTAAGCCAGTGAGTTGGAATCTTTG | |||
| FOXP3 | NM_014009 | Forward: TCCACAACATGCGACCCCCTTTCA | 217 |
| Reverse: ACAGCCCCCTTCTCGCTCTCCA | |||
| TGFB | NM_000660 | Forward: GGAGTTGTGCGGCAGTGGTTG | 259 |
| Reverse: AGCTGAAGCAATAGTTGGTGTCCAGG |
Murine experiments
Mice. C57BL/6 (B6), (C57BL/6 x DBA/2)F1 (B6D2F1), and BALB/c (H-2d) mice were purchased from Charles River (L'arbresle, France).
Donors (B6, H-2b) and recipients (B6D2F1, H-2bxd) were sex-matched and used at 8 to 10 weeks of age at the time of the first experimental procedure. B6 CD3ϵ knocked-out (CD3-KO; CDTA, Orleans, France) and athymic nude (B6, H-2b; Janvier, Le Genest-St-Isle, France) mice were mated in our animal facilities. All animals were kept under specific pathogen-free conditions and manipulated according to European council directive 86/609/EEC of November 24, 1986.
Purification and culture of mT cells. CD4 and CD8 T cells were positively selected from splenocytes of B6 mice by immunomagnetic separation after staining the cells with anti-CD4-biotin or anti-CD8-biotin mAbs (Pharmingen) and then using anti-biotin-conjugated immunomagnetic beads (Miltenyi Biotech). In some experiments, splenocytes were first depleted of CD25+ cells by staining the cells with anti-CD25-biotin mAbs (clone 7D4, Becton Dickinson), followed by an incubation with anti-biotin-conjugated immunomagnetic beads. This CD25- fraction was then treated for CD4 or CD8 T-cell enrichment by immunomagnetic separation. Purity (> 98%) of the different T-cell fractions, and CD25 depletion were assessed by flow cytometry.
CD4- and CD8-purified T cells were plated separately onto 24-well tissue culture plates (Costar) at 106 cells/mL in the presence of anti-CD3 (0.1 μg/mL, clone 145 2C11, Becton Dickinson) and anti-CD28 (0.1 μg/mL, clone 37.51, Becton Dickinson) mAbs that have been either previously coated or not for CD4 T cells and CD8 T cells, respectively. Cells were grown for 2 to 6 days in complete RPMI medium supplemented with 10% heat-inactivated FCS (Hyclone, Perbio, Bezons, France) and 1000 U/mL of rhIL-2. Every 2 days, the cells were seeded at 0.1 × 106 cells/mL in newly coated plates with fresh medium.
Adoptive transfer of mT cells. For reconstitution experiments, 107 fresh CD4- or CD8-purified T cells or 2- to 6-day cultured B6 T cells, which were mixed at a CD4/CD8 cell ratio of 1 prior to inoculation, were injected intravenously into the retro-orbital sinus of B6 CD3-KO or B6 nude mice in 0.2 mL PBS volume. For GVHD experiments, various numbers of either fresh or cultured or initially CD25-depleted T cells from B6 donor mice were injected intravenously into B6D2 mice that were sublethally irradiated (6 Gys Saturne I X-ray source; CGR Mev, Buc sur Yvette, France) 24 hours before. Mice were weighed every 2 to 3 days, and those that either spontaneously died from acute GVHD or were humanely killed for ethical reasons because of an important weight loss (≥ 13%) associated with typical GVHD skin lesions, were represented as a death event in the Kaplan-Meier survival graphs.
Allogeneic MLR of transferred mT cells. Splenocytes from CD3-KO mice killed 5 months after T-cell injection were tested for their ability to respond to the allogeneic stimulation of BALB/c-irradiated splenocytes. 5 × 104 splenocytes from CD3-KO mice and from BALB/c mice were mixed at a 1:1 ratio in triplicate wells and cultured for 4 days and cell proliferation was determined by 3H-Tdr incorporation. The proliferation of noninjected B6 splenocytes tested under the same conditions was taken as a positive control.
Skin graft. Tail skin fragments (1 cm2) from BALB/c mice were grafted onto the back of B6 nude mice under ketamine/xylazine (150 mg/kg and 100 mg/kg, respectively) anesthesia, 2 weeks after adoptive T-cell transfer. Grafts were monitored every 2 to 3 days. Graft rejection was considered when the graft displayed 90% of necrosis. Otherwise, in the absence of rejection healthy hairy skin grafts were observed.
Immunoflow cytometry. One million murine cells were incubated in microtiter plates with mAbs diluted in 50 μL PBS supplemented with 3% newborn calf serum (NCS, Gibco BRL) at 4°C for 20 minutes. Following incubation with anti-CD16/CD32 mAbs (2-4G2, rat IgG2b) to block Fc receptors, specific staining was performed with anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), anti-CD25 (clone 7D4), and anti-B220 (clone RA3-6B2) mAbs directly labeled with various fluorochromes (all from Pharmingen). Acquisition of at least 10 000 gated lymphocytes was performed on a 4-color FACScalibur (Becton Dickinson).
Results
Effects of various culture conditions on the in vitro activation, expansion, and retroviral transduction of human T cells
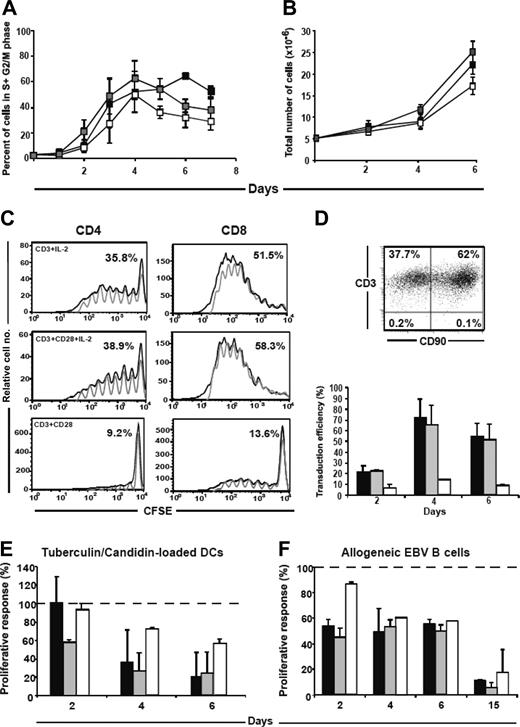
For T-cell therapy, human T cells have to be activated in vitro, and possibly, if necessary, transduced and expanded. Various conditions of cell culture using CD3 ± CD28 costimulation and with either the addition or not of exogenous rhIL-2 were tested to determine the best conditions of activation, expansion, and cell transduction. Whichever the conditions of stimulation, 50% to 60% of the CD3 T cells entered into S+G2/M phase of the cell cycle within 4 days (Figure 1A). In cultures containing IL-2, the activation was sustained, and cell expansion was improved (Figure 1B). The decrease of CFSE staining in T cells cultured for 6 days revealed that at least 35% of CD4 and 51% of CD8 cells had divided since day 0 when stimulated with either CD3 + IL-2 or CD3/CD28 + IL-2, while only 9% of CD4 and 13% of CD8 cells had divided under CD3/CD28 stimulation (Figure 1C). Thus, addition of IL-2 increased by 3-fold the percentage of dividing cells, independently of CD28 co-stimulation. Overall, CD8 cells divided more than CD4 cells, thus leading to a decrease of the CD4/CD8 ratio at day 6 of stimulation. As expected, the transduction efficiency correlated to the fraction of hT cells that divided. Thus, 60% of hT cells were transduced, as assayed by expression of the CD90 reporter gene, after a 4-day period of stimulation in the presence of CD3 + IL-2 or CD3/CD28 + IL-2, while only 10% to 16% of hT cells were transduced using CD3/CD28 stimulation (Figure 1D). Thus, in the absence of exogenous IL-2, cell expansion and transduction remained rather limited.
Culture of human T cells under various conditions: effects on cell cycle, expansion, division, transduction efficiency, and proliferative responses to recall and allogeneic antigens. Purified hT cells were cultured in the presence of CD3 + IL-2 (▪); CD3/CD28 + IL-2 (▦); and CD3/CD28 (□). Except in panel C, results are expressed as mean ± SEM of 3 (A, D, E, F) to 5 separate experiments (B) carried out with hT cells obtained from 3 to 5 different donors, respectively. (A) Cell cycle was analyzed at different time points of culture. Data represent percent of cells into S + G2/M phase. (B) Viable cells were counted at different time points of the cultures. Results represent total number of viable cells. (C) Cell division of cultured hT cells initially stained with CFSE at day 0. The solid line represents CFSE fluorescence intensities in gated CD4+ and CD8+ hT cells analyzed by flow cytometry at day 6 of culture. The gray line represents the modelization of cell division using the FlowJo software, which gives the percentage of cells that have divided since day 0. One representative experiment of 3 is shown. (D) Retroviral transduction of hT cells after 2 to 6 days of culture. The transduction efficiency was determined by expression of CD3+CD90+ 2 days after infection. Top panel: dot plot flow cytometry analysis of transduced hT cells at day 4; bottom panel, percentage of transduced T cells (CD3+CD90+ cells), values from noninfected T cells being subtracted from the indicated percentages. (E-F) After being cultured for 2, 4, 6, and 15 days, hT cells were plated in triplicates at a 10/1 T-cell/APC ratio in the presence of either irradiated autologous DCs loaded with tuberculin/candidin antigens (E) or irradiated EBV B cells used as allogeneic APCs (F). Cell proliferation was measured by 3H-Tdr incorporation 4 days later. Data were normalized comparatively to values from noncultured CD3-purified hT cells (105 cpm), considered as 100% of the response, as represented by the dashed horizontal lines.
Culture of human T cells under various conditions: effects on cell cycle, expansion, division, transduction efficiency, and proliferative responses to recall and allogeneic antigens. Purified hT cells were cultured in the presence of CD3 + IL-2 (▪); CD3/CD28 + IL-2 (▦); and CD3/CD28 (□). Except in panel C, results are expressed as mean ± SEM of 3 (A, D, E, F) to 5 separate experiments (B) carried out with hT cells obtained from 3 to 5 different donors, respectively. (A) Cell cycle was analyzed at different time points of culture. Data represent percent of cells into S + G2/M phase. (B) Viable cells were counted at different time points of the cultures. Results represent total number of viable cells. (C) Cell division of cultured hT cells initially stained with CFSE at day 0. The solid line represents CFSE fluorescence intensities in gated CD4+ and CD8+ hT cells analyzed by flow cytometry at day 6 of culture. The gray line represents the modelization of cell division using the FlowJo software, which gives the percentage of cells that have divided since day 0. One representative experiment of 3 is shown. (D) Retroviral transduction of hT cells after 2 to 6 days of culture. The transduction efficiency was determined by expression of CD3+CD90+ 2 days after infection. Top panel: dot plot flow cytometry analysis of transduced hT cells at day 4; bottom panel, percentage of transduced T cells (CD3+CD90+ cells), values from noninfected T cells being subtracted from the indicated percentages. (E-F) After being cultured for 2, 4, 6, and 15 days, hT cells were plated in triplicates at a 10/1 T-cell/APC ratio in the presence of either irradiated autologous DCs loaded with tuberculin/candidin antigens (E) or irradiated EBV B cells used as allogeneic APCs (F). Cell proliferation was measured by 3H-Tdr incorporation 4 days later. Data were normalized comparatively to values from noncultured CD3-purified hT cells (105 cpm), considered as 100% of the response, as represented by the dashed horizontal lines.
Immune dysfunctions of cultured hT cells
To study whether or not in vitro culture activation and expansion protocols may affect the immune responses of hT cells, their proliferative capacity to recall antigens and/or to alloantigens was tested. In both cases, cultured hT cells exhibited a time-dependent decrease of their proliferative response to tuberculin/candidin presented by autologous DCs and to allogeneic EBV B cells. The proliferative response to tuberculin decreased progressively, and the remaining response represented only approximately 20% of the control (noncultured hT cells) after CD3 + IL2 or CD3/CD28 + IL-2 stimulation at day 6, while the response was less altered (∼ 60% of the control) after CD3/CD28 stimulation (Figure 1E). Comparable results were obtained using TT as recall antigen (data not shown). The proliferative response after stimulation with either allogeneic B-EBV (Figure 1F) or DCs (not shown) decreased to 50% of the control as early as day 2 and up to day 6 of culture. Prolongation of the culture up to 15 days without transduction (Figure 1F) or after transduction on day 4 (not shown) resulted in a dramatic reduction in both cases of the alloreactive responses to 10% to 20% of control values. Although CD3/CD28 stimulation in the absence of exogenous IL-2 appears to delay the dysfunction and partially preserves further immune responses, this culture condition does not seem suitable for achieving desirable levels of T-cell expansion and transduction. Thus, the use of CD3 + IL-2, compatible with clinical-grade protocols, represents one of the best culture conditions for achieving optimal cell expansion and high transduction efficiency of hT cells in spite of a loss of 40% to 60% of their functional immune responses, which still remains a major drawback.
Immune dysfunctions induced during culture of murine T cells can be prevented by injecting increased cell numbers
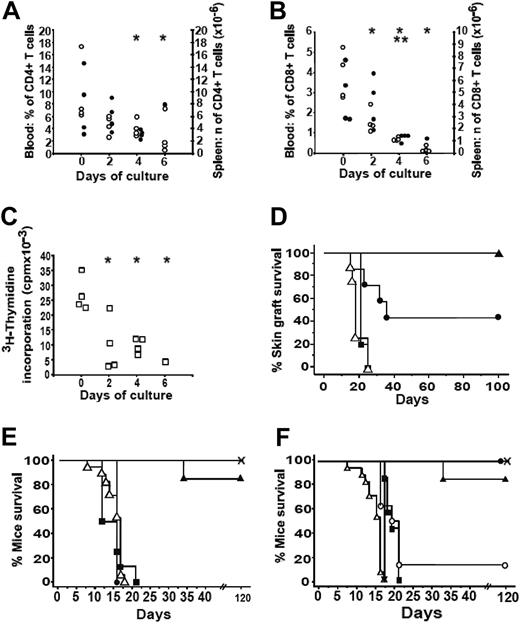
To study whether the in vitro culture and expansion of T cells designed for cell therapy could alter their engraftment capacities and in vivo T-cell functionalities, we established a murine model where the functionality of similarly cultured mT cells was assayed in vivo. Hence, we analyzed the T-cell growth in immunodeficient mice infused with mT cells cultured for 0 to 6 days. Three and a half months after cell transfer, the percentages of CD4+ and CD8+ T cells detected in the blood of CD3-KO mice were significantly lower (P < .05, Fisher PLSD test) when injected cells were cultured for 4 or 6 days as compared to noncultured cells (Figure 2A-B). Five months after cell transfer, the number of CD8+ spleen cells was significantly decreased (P < .05, Fisher PLSD test) after transfer of day-4 cultured T cells (Figure 2B). Of 4 mice that were infused with day-6 cultured cells, 3 died 4 months later from cachexia linked to severe immunodeficiency as shown by the absence of T cells in the blood. Moreover, splenocytes from CD3-KO mice that had been infused with cultured T cells were assayed in vitro for their ability to proliferate in allogeneic MLR. Thus, the functionality of the splenocytes was significantly reduced (P < .05 Fisher PLSD test) as early as when day-2 cultured cells were infused (Figure 2C).
In vivo development and functions of murine T cells after various period of culture. (A, B) T-cell development in CD3-KO mice after injection of 107 fresh (day 0) or cultured mT cells (days 2-6). Each point represents percentages (○) and numbers (•) of CD4 (A) and CD8 (B) T cells in the blood and spleen from individual mice (n = 4 per group) 3.5 and 5 months after adoptive transfer, respectively. Of the 4 mice injected with day 6 cultured cells, 3 died 4 months after transfer. *Indicates blood and **spleen: significant values as compared to those of day 0 (P < .05 Fisher PLSD test). (C) Allogeneic MLR of mT cells from reconstituted CD3-KO mice: the allogeneic proliferative response of splenocytes from CD3-KO mice against BALB/c-irradiated splenocytes was measured 5 months after injection of cultured T cells. Cell proliferation was determined by 3H-Tdr incorporation after 4 days of coculture. □ indicates an individual mouse. *Significant values as compared to those of day 0 (P < .05 Fisher PLSD test). (D) 107 fresh day 0 (▵, n = 8), day 2 (▪, n = 5), day 4 (•, n = 7), or day 6 (▴, n = 6) cultured mT cells were injected into B6 nude mice that were grafted with allogeneic skin tail fragments from BALB/c mice 2 weeks later. Skin graft survival was monitored over time. Results are presented as Kaplan-Meier skin survival graph. (E-F) Ability of cultured donor mT cells to induce GVHD after injection into 6-Gy-irradiated B6D2F1: noninjected mice (X, n = 11), mice receiving 107 fresh donor T cells (day 0, ▵, n = 17) were used as controls. Results are presented as Kaplan-Meier survival graph after T-cell injection. (E) Effect of the length of T-cell culture: 107 cells were injected after day 2 (▪,n = 8), day 4 (•, n = 4), or day 6 (▴, n = 13) of culture. ▵ indicates day 0 (noncultured cells). (F) Effect of injecting increased amounts of 6-day cultured T cells: 107 (▴, n = 13), 2 × 107 (•, n = 4), 2.5 to 3 × 107 (○, n = 8), 4 × 107 (▪, n = 7), and 8 × 107 (▾, n = 4). ▵ indicates day 0 (noncultured cells).
In vivo development and functions of murine T cells after various period of culture. (A, B) T-cell development in CD3-KO mice after injection of 107 fresh (day 0) or cultured mT cells (days 2-6). Each point represents percentages (○) and numbers (•) of CD4 (A) and CD8 (B) T cells in the blood and spleen from individual mice (n = 4 per group) 3.5 and 5 months after adoptive transfer, respectively. Of the 4 mice injected with day 6 cultured cells, 3 died 4 months after transfer. *Indicates blood and **spleen: significant values as compared to those of day 0 (P < .05 Fisher PLSD test). (C) Allogeneic MLR of mT cells from reconstituted CD3-KO mice: the allogeneic proliferative response of splenocytes from CD3-KO mice against BALB/c-irradiated splenocytes was measured 5 months after injection of cultured T cells. Cell proliferation was determined by 3H-Tdr incorporation after 4 days of coculture. □ indicates an individual mouse. *Significant values as compared to those of day 0 (P < .05 Fisher PLSD test). (D) 107 fresh day 0 (▵, n = 8), day 2 (▪, n = 5), day 4 (•, n = 7), or day 6 (▴, n = 6) cultured mT cells were injected into B6 nude mice that were grafted with allogeneic skin tail fragments from BALB/c mice 2 weeks later. Skin graft survival was monitored over time. Results are presented as Kaplan-Meier skin survival graph. (E-F) Ability of cultured donor mT cells to induce GVHD after injection into 6-Gy-irradiated B6D2F1: noninjected mice (X, n = 11), mice receiving 107 fresh donor T cells (day 0, ▵, n = 17) were used as controls. Results are presented as Kaplan-Meier survival graph after T-cell injection. (E) Effect of the length of T-cell culture: 107 cells were injected after day 2 (▪,n = 8), day 4 (•, n = 4), or day 6 (▴, n = 13) of culture. ▵ indicates day 0 (noncultured cells). (F) Effect of injecting increased amounts of 6-day cultured T cells: 107 (▴, n = 13), 2 × 107 (•, n = 4), 2.5 to 3 × 107 (○, n = 8), 4 × 107 (▪, n = 7), and 8 × 107 (▾, n = 4). ▵ indicates day 0 (noncultured cells).
In another set of experiments the functionality of cultured T cells was tested for their ability to reject allogeneic skin graft implanted 2 weeks after donor T-cell infusion in syngeneic B6 athymic nude mice (Figure 2D). All allogeneic skin grafts were acutely rejected with the same kinetics by mice that received either noncultured or day-2 cultured T cells. In contrast, more than 40% (P = .01, log-rank test compared to mice receiving noncultured cells) and 100% of mice that received day-4 or day-6 cultured T cells did not reject allogeneic skin graft, respectively. Interestingly, in both CD3-KO mice and nude mice that survived 5 months after injection of day-6 cultured cells, only CD4 cells were detected, and alterations of the T-cell repertoire were noticed (data not shown).
The functionality of cultured T cells also was tested in a model of GVHD induction. The injection of 107 noncultured day-2 or day-4 cultured B6 T cells into semi-allogeneic sublethally irradiated B6D2F1 mice led to an acute GVHD, with a mean survival time (MST) of 15.7 ± 0.6, 14.7 ± 1.1, and 16.1 ± 0.0 days, respectively (Figure 2E). By contrast, no acute GVHD could be observed in 13 mice that received day-6 cultured T cells, while 2 developed a late chronic cutaneous nonlethal GVHD and were killed (weight loss ≥ 13%). We investigated whether injecting larger amounts of day-6 cultured T cells could reverse this reduced alloreactivity. While no GVHD was observed even after injection of 2 × 107 T cells, GVHD was restored in 7 of 8 mice after injection of 2.5 to 3 × 107 day 6 cultured T cells (Figure 2F). Moreover, injection of 4 × 107 or 8 × 107 day-6 cultured T cells fully restored the allogeneic response in 100% of the recipients, with an MST of 20.2 ± 0.6 and 18.0 ± 0.0 days, respectively. Thus, the prolonged culturing of T cells appears to quantitatively decrease their alloreactivity, while a 3- to 4-fold increase in the number of grafted cells restored the in vivo allogeneic immune response. Several nonexclusive hypotheses can explain this result: (1) effector cells have lost their function after culture, (2) their engraftment capacities in vivo are altered, or (3) there is an imbalance between effector T cells and Treg's, which dominate.
Initial depletion of CD25+ T cells preserves T-cell functionality of murine and human T cells
The majority of natural Treg constitutively express the alpha subunit of the IL-2 receptor (CD25). Thus, we investigated whether CD25+ T-cell depletion prior to the activation/expansion of T cells would preserve the functionality of murine and human T cells by preventing a potential imbalance between effector and Treg's. Concerning mT cells, the injection of 107 day-6 cultured CD25-depleted mT cells into irradiated semi-allogeneic B6D2F1 mice, led to an efficient alloreactive response. However, death from GVHD was slightly delayed (MST of 23.4 ± 1.6 days) as compared to noncultured CD25-depleted T cells (MST 18.1 ± 0.9 days) (Figure 3A). Moreover, the GVHD was accelerated when higher numbers of CD25-depleted T cells were injected, with an MST of 20.4 ± 1.8 and 16.7 ± 0.6 days for 2 × 107 (not shown) and 3 × 107 day-6 cultured CD25-depleted mT cells, respectively.
Concerning hT cells, we compared the alloreactive response of total T cells and day-0 CD25-depleted T cells, cultured with CD3 + IL-2 for 6 days. Interestingly, the proliferative response of CD25-depleted hT cells against irradiated allogeneic EBV B cells was comparable to those obtained with noncultured T cells at day 6 of culture (Figure 3B).
CD25 depletion before cell culture preserves the allogeneic responses of cultured murine and human T cells. (A) Induction of GVHD in mice: mT cells were initially depleted of CD25+ cells before culture and 6 days later 107 (▪, n = 5) and 3 × 107 (□, n = 4) day 6 cultured cells were injected into B6D2F1 irradiated mice. Controls were mice receiving 107 CD25-depleted noncultured cells (▵, n = 8), 107 nondepleted day 6 cultured cells (▴, n = 13), and noninjected mice (X, n = 11). (B) Proliferative response of hT cells to EBV B cells: total hT cells (▪) and initially CD25-depleted hT cells (□) were plated either directly (day 0) or after culture with CD3 + IL-2 (day 6) in the presence of allogeneic irradiated EBV B cells (T cells/EBV B cells ratio: 5:1). The proliferative response was measured by 3H-Tdr incorporation. Results presented as mean cpm ± SEM of triplicate cultures and are from 1 representative experiment of 3 using cells from 3 different donors.
CD25 depletion before cell culture preserves the allogeneic responses of cultured murine and human T cells. (A) Induction of GVHD in mice: mT cells were initially depleted of CD25+ cells before culture and 6 days later 107 (▪, n = 5) and 3 × 107 (□, n = 4) day 6 cultured cells were injected into B6D2F1 irradiated mice. Controls were mice receiving 107 CD25-depleted noncultured cells (▵, n = 8), 107 nondepleted day 6 cultured cells (▴, n = 13), and noninjected mice (X, n = 11). (B) Proliferative response of hT cells to EBV B cells: total hT cells (▪) and initially CD25-depleted hT cells (□) were plated either directly (day 0) or after culture with CD3 + IL-2 (day 6) in the presence of allogeneic irradiated EBV B cells (T cells/EBV B cells ratio: 5:1). The proliferative response was measured by 3H-Tdr incorporation. Results presented as mean cpm ± SEM of triplicate cultures and are from 1 representative experiment of 3 using cells from 3 different donors.
Thus, the initial depletion of CD25+ cells preserves, both in vivo and in vitro, the functionality of murine or human T cells expanded in the presence of IL-2. This suggests that the conditions used for the culture of total T cells could imbalance the ratio between effector cells and Treg's.
Culture of hT cells favors the preferential expansion of Treg's
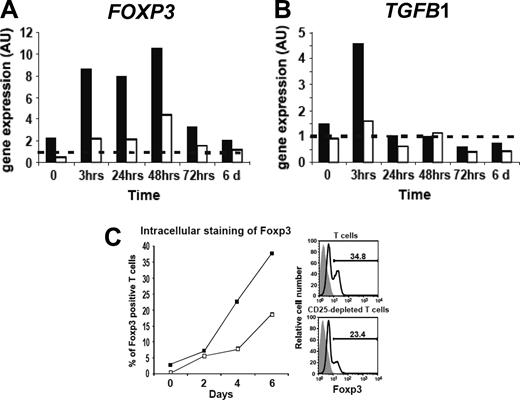
As it is difficult to evaluate the number of Treg's present in T-cell cultures due to the lack of specific cell-surface markers, we first addressed this question by carrying out a quantitative measurement of FOXP3 and TGFB1 mRNA on hT cells and CD25-depleted hT cells at different time points of their culture (Figure 4A,B). FOXP3 and TGFB1 mRNA levels in total cultured T cells, compared to noncultured cells, increased by 4- and 3-fold as early as 3 hours of culture, respectively. Afterward, levels of Foxp3 mRNA remained stably expressed in high levels up to 48 hours after the beginning of the hT cell culture. Moreover, intracellular staining of FOXP3 at different time points of the culture (Figure 4C) showed a significant increase of the percentage of FOXP3+ cells in cultured T cells as compared to CD25-depleted T cells (P < .05, paired Student t test). However, a delayed and lower increase of both the FOXP3 mRNA levels and percentage of FOXP3+ cells also was observed in CD25-depleted hT cells. Altogether, the augmentation of FOXP3+ hT-cell frequency suggests that the number of Treg's rapidly increased within hT-cell cultures under CD3 + IL-2 stimulation.
Next, we asked whether the high frequency of FOXP3+ hT cells after culture could be related to cell division. Therefore, we investigated whether dividing cells may have increased suppressor function. Thus CD3-purified hT cells were labeled with CFSE and cultured under different conditions. At day 4, the percentage of dividing cells cultured under CD3 + IL-2, CD3/CD28 + IL-2, and CD3/CD28 stimulation represented 8.4%, 10.5%, and 7.4% of hT cells, respectively. Therefore, dividing (CFSElow) and nondividing (CFSEhigh) cells were sorted (Figure 5A) and assayed for their functionality. Interestingly, the allogeneic response of dividing cells to EBV B cells stimulation was significantly lower (P < .01, unpaired Student t test) than those of nondividing T cells, regardless of the culture conditions, suggesting the presence of anergic and/or suppressive T cells in the CFSElow cell fraction (Figure 5B). To demonstrate that dividing cells were enriched in Treg's, CFSElow cells were tested for their suppressive activity. The results from 2 independent experiments showed that CFSElow cells strongly suppressed the proliferation of CFSEhigh cells in response to allogeneic EBV B-cell stimulation (Figure 5C). Moreover we demonstrated that this inhibition was dependent upon the number of CFSElow cells present in the culture (Figure 5D). Finally, the fact that CFSElow cells acted through a cell-cell, contact-dependent mechanism was further demonstrated by inhibiting their suppressive activity in MLR assays carried out using transwell plates (Figure 5E). Altogether, our findings demonstrate that the culture conditions used favor a preferential differentiation/expansion of Treg's that subsequently leads to suppression of effector T-cell functions.
Increase of FOXP3 and TGFB1 expression after hT-cell culture can be prevented by initial depletion of CD25+ hT cells. Relative expression of FOXP3 (A) and TGFB1 (B) genes in hT cells (▪) and CD25-depleted hT cells (□) were analyzed by real-time quantitative RT-PCR before culture and at different time points of the culture in the presence of CD3 + IL2. Results from a representative experiment performed in duplicate are expressed in arbitrary units (AU). FOXP3 and TGFB1 gene expressions in calibrator cells (dashed line) were arbitrarily set at 1. (C) Foxp3 protein expression in CD3+ T cells and CD3+ CD25-depleted T cells cultured in the presence of CD3 + IL2. Intracellular staining of Foxp3 was analyzed by flow cytometry in CD3+ gated cells from total cultured T cells (▪) and from CD25-depleted cultured T cells (□) at different time points. One representative curve of 2 separate experiments is shown. Right panels illustrate a typical Foxp3 analysis of T cells and CD25-depleted T cells cultured for 6 days and a negative isotypic control (gray histogram).
Increase of FOXP3 and TGFB1 expression after hT-cell culture can be prevented by initial depletion of CD25+ hT cells. Relative expression of FOXP3 (A) and TGFB1 (B) genes in hT cells (▪) and CD25-depleted hT cells (□) were analyzed by real-time quantitative RT-PCR before culture and at different time points of the culture in the presence of CD3 + IL2. Results from a representative experiment performed in duplicate are expressed in arbitrary units (AU). FOXP3 and TGFB1 gene expressions in calibrator cells (dashed line) were arbitrarily set at 1. (C) Foxp3 protein expression in CD3+ T cells and CD3+ CD25-depleted T cells cultured in the presence of CD3 + IL2. Intracellular staining of Foxp3 was analyzed by flow cytometry in CD3+ gated cells from total cultured T cells (▪) and from CD25-depleted cultured T cells (□) at different time points. One representative curve of 2 separate experiments is shown. Right panels illustrate a typical Foxp3 analysis of T cells and CD25-depleted T cells cultured for 6 days and a negative isotypic control (gray histogram).
Discussion
By studying the immune functions of cultured mT cells and hT cells, we demonstrate here that in both species, T-cell culture leads to alteration of effector functions because of a preferential expansion of Treg's. Thus, our results show for the first time that the ex vivo manipulation of T cells under classic culture conditions leads to the disturbance of the delicate balance between effector and suppressor T cells. Consequently, the initial depletion of CD25+ T cells appears to be a simple method to improve their functionality.
Although no parallel studies between mice and humans have been carried out, our results are in line with other reports showing that ex vivo manipulation of T cells favor (1) functional immune defects,4,5 (2) T-cell repertoire alterations,2,25 and (3) immunophenotypical changes such as acquisition of activation (CD25, CD69) and effector (CD45RO) cell-surface markers (data not shown).5 Of note, the retroviral transduction by itself has no deleterious effects, as already reported by us as well as others.21,26
We emphasize here that when injected in vivo, cultured mT cells strongly diminished their capacity to engraft and to drive immune functions in immunodeficient animals, for example, to reject allogeneic skin graft and to provoke a GVHD. Similarly, hT cells displayed alterations of effector functions such as decreased proliferative responses to recall and allogeneic antigens. Furthermore, these culture-induced functional defects appeared dependent upon the method of stimulation used and increased with the culture length. Thus, stimulation of hT cells in the presence of exogeneous IL-2, as with anti-CD3 + IL-2 or anti-CD3/CD28 + IL-2, gave rise to both higher cell proliferation and expansion and better gene transfer efficiency than with CD3/CD28 alone. Conversely, conditions that favor cell expansion and transduction efficiency were deleterious for further T-cell Ag-specific responsiveness. In fact, the alteration of in vitro T-cell responsiveness was further amplified between day 4 and day 15 of culture. From these findings, one can envision that the culture conditions favor exhaustion of effector T cells, and/or an imbalance between effector cells and Treg's. It is known that TCR-CD3 engagement in the absence of co-stimulation using immobilized anti-CD3 mAbs leads to apoptosis and anergy of T cells both in mice and humans.7,27 However, CD28 triggering, which in turn drives IL-2 production,28 allows productive hT-cell proliferation.29 This could explain that by using both CD3/CD28 stimulation without IL-2 addition, we were able to observe a slow proliferation of T cell, albeit not sustained enough, and a relative better maintenance of the immune functions. By contrast, addition of IL-2 considerably increased the cell proliferation but dramatically altered effector functions. Interestingly, increasing by approximately 3-fold the number of mT cells injected in mice corrected the immune defects. This suggests that ex vivo cultures quantitatively impaired effector functions. This hypothesis was reinforced by the fact that depletion of CD25+ cells prior to the cultures of murine or human T cells allowed maintaining efficient effector functions. This result is in line with a recent publication that shows in a murine model that the removal of Treg prior to immunization against vaccine preparations enhanced CD8 T-cell reactivity.30
Functional activity of dividing and nondividing hT cells after 4 days of culture. CD3-purified hT cells were initially stained with CFSE and then cultured in the presence of CD3 + IL-2 (▪); CD3/CD28 + IL-2 (▦); and CD3/CD28 (□). At day 4 of culture, dividing and nondividing cells were sorted in order to assay their functionality. (A) A typical CFSE analysis of day 4 cultured T cells with CD3 + IL-2, indicating how dividing (CFSElow) and nondividing (CFSEhigh) cells were sorted. (B) Proliferation of nondividing and dividing T-cell fractions in response to allogeneic EBV B-cell stimulation. Noncultured T cells (▨) are also shown. The cell proliferation was measured by 3H-Tdr incorporation at day 4 of culture. Results are presented as mean cpm ± SEM of triplicate wells. *Significant values as compared to those of nondividing cells (P < .01, unpaired Student t test). (C) Suppressive activity of CFSElow sorted T cells on the proliferation of autologous CFSEhigh T cells in response to allogeneic EBV B-cell stimulation. The cell proliferation was measured by 3H-Tdr incorporation at day 4. Results from triplicates were normalized for each culture condition to the positive control (ie, proliferation of CFSEhigh cells alone in the presence of allogeneic EBV B-cell stimulation is considered as 0% of the suppression activity). One representative of 2 independent experiments performed with cells from 2 different donors is shown. (D) Dose-dependent suppressive activity of CFSElow cells sorted from T cells cultured in the presence of CD3 + IL2 for 4 days. CFSElow sorted cells were mixed at various cell ratios with autologous nondividing (CFSEhigh) cells stimulated by 50 Gy-irradiated allogeneic EBV B cells. Cell proliferation was measured by 3H-Tdr incorporation 4 days later. Results are presented as mean cpm ± SEM of triplicate wells. (E) Role of the cell-cell contact on the suppressive activity of CFSElow sorted T cells on the proliferation of autologous CFSEhigh T cells in response to allogeneic EBV B-cell stimulation. MLR suppressive T-cell assays were performed as in panel C, cells being separated (+) or not separated (-) using transwell plates. One representative of 2 independent experiments performed in triplicate is shown.
Functional activity of dividing and nondividing hT cells after 4 days of culture. CD3-purified hT cells were initially stained with CFSE and then cultured in the presence of CD3 + IL-2 (▪); CD3/CD28 + IL-2 (▦); and CD3/CD28 (□). At day 4 of culture, dividing and nondividing cells were sorted in order to assay their functionality. (A) A typical CFSE analysis of day 4 cultured T cells with CD3 + IL-2, indicating how dividing (CFSElow) and nondividing (CFSEhigh) cells were sorted. (B) Proliferation of nondividing and dividing T-cell fractions in response to allogeneic EBV B-cell stimulation. Noncultured T cells (▨) are also shown. The cell proliferation was measured by 3H-Tdr incorporation at day 4 of culture. Results are presented as mean cpm ± SEM of triplicate wells. *Significant values as compared to those of nondividing cells (P < .01, unpaired Student t test). (C) Suppressive activity of CFSElow sorted T cells on the proliferation of autologous CFSEhigh T cells in response to allogeneic EBV B-cell stimulation. The cell proliferation was measured by 3H-Tdr incorporation at day 4. Results from triplicates were normalized for each culture condition to the positive control (ie, proliferation of CFSEhigh cells alone in the presence of allogeneic EBV B-cell stimulation is considered as 0% of the suppression activity). One representative of 2 independent experiments performed with cells from 2 different donors is shown. (D) Dose-dependent suppressive activity of CFSElow cells sorted from T cells cultured in the presence of CD3 + IL2 for 4 days. CFSElow sorted cells were mixed at various cell ratios with autologous nondividing (CFSEhigh) cells stimulated by 50 Gy-irradiated allogeneic EBV B cells. Cell proliferation was measured by 3H-Tdr incorporation 4 days later. Results are presented as mean cpm ± SEM of triplicate wells. (E) Role of the cell-cell contact on the suppressive activity of CFSElow sorted T cells on the proliferation of autologous CFSEhigh T cells in response to allogeneic EBV B-cell stimulation. MLR suppressive T-cell assays were performed as in panel C, cells being separated (+) or not separated (-) using transwell plates. One representative of 2 independent experiments performed in triplicate is shown.
The identification of both CD25-positive and CD25-negative subsets of FOXP3+ cells with Treg activity and the demonstration by Fontenot et al31 that FOXP3 is the definitive marker of Treg were taken into account in our experiments. Thus, we observed that Treg's were rapidly activated and expanded in our cultures. Indeed, the increase of FOXP3 mRNA occurred as soon as 3 hours after cell-culture initiation, and Foxp3 protein was detected in one third of cultured cells at day 6. Nevertheless, the fact that approximately 20% of the CD25-depleted T-cell fraction also express FOXP3 at day 6 raises the question of the specificity of FOXP3 expression by human T cells after activation32 and/or of the de novo generation of Treg's from CD25-depleted T cells as already published by others.33 The small increase of FOXP3, albeit delayed, in CD25-depleted cultures, which may reflect de novo generation of Treg's33,34 or expansion of rare CD25+ contaminants, was, however, not sufficient enough to impair effector functions.
In our hands, the suppressive activity of total cultured T cells was further amplified in the fraction of cells that have divided within the first days. Indeed, these T cells proliferated less to allogeneic stimulation and contained a high suppressor activity capable of preventing the expansion of effector cells in a contact-dependent manner. The first 2 days of culture are of interest, even though it is prior to cell division, as this appears to be a determining period for T-cell behavior and, thus, the rapid increase of FOXP3 expression can explain the decline of the T-cell functionality during this time period. The preferential expansion/generation of Treg also is associated with an increased production of TGFβ that would prevent conventional T-cell activation and endogenous IL-2 production. This expansion of Treg can be particularly favored under culture conditions where exogenous IL-2 has been added. Thornton et al8 recently reported on the critical role of IL-2 in the in vitro activation of CD4+CD25+ T-cell suppressor function. In this study, under CD3/IL-2 stimulation, both CD4+CD25- and CD4+CD25+ T-cell populations are equally induced to divide, and CD4+CD25+ cells inhibit both the transcription of IL-2 mRNA in CD4+CD25- T cells and their proliferation. Our results are in accordance with in vivo experimental data showing that a higher proportion of Treg's divide and progress through cell cycle than other T cells when injected in T-cell-depleted mice.11
Finally, it would be worthwhile to test other culture conditions in which cytokines such as IL-15, IL-18, and IL-21 known to activate the cycling, the survival, and as well as some effector functions of T cells,35-38 are added or combined with CD3/CD28 ± IL-2 stimulation either after or not the removal of CD25+ T cells. Recently, the use of magnetic beads coated with anti-CD3 and anti-CD28 mAbs has been proven to be very efficient for stimulating and expanding T cells39 and Treg's.40 However, this technique seems to be more efficient for expanding CD4+ T cells than CD8+ T cells.39
Our results are of interest for clinical settings such as allogeneic hematopoietic stem cell (HSC) transplantation or donor leukocyte infusions, where T cells of donor origin beneficially contribute to the immune reconstitution and/or the control of leukemia relapse, but deleteriously induce GVHD.41 Since our data indicate that CD25-depleted cultured T cells would favor engraftment and effector functions, an increased risk of GVHD cannot be neglected. Of note, several experimental and clinical data have shown that specific elimination of alloreactive T cells using a thymidine kinase (TK) “suicide” gene therapy can efficiently control GVHD.42,43 Therefore, one can propose to the patients who have to receive transplants of allogeneic HSC and T cells a combined strategy in which cultured T cells would be Treg (CD25) depleted and genetically engineered with a TK gene. We notice that the proportion of cells expressing intracellular FOXP3 in cultures that have been initially depleted of CD25+ cells rises about 5%, a percentage that is not far from physiologic values. Thus, the risk with time to develop autoimmune diseases related to Treg depletion44 would be limited. Otherwise, another approach could be to add back Treg's that have been independently expanded in vitro. Indeed, recent experimental data have shown that nonspecific and/or alloantigen-specific Treg's can control GVHD while preserving the graft versus leukemia effect.45,46
Overall, our findings underline that the unexpected expansion of Treg is responsible for the culture-induced T-cell defects and suggest new strategies for improving the functions of T cells designed for cell and/or gene therapy protocols.
Prepublished online as Blood First Edition Paper, September 13, 2005; DOI 10.1182/blood-2005-07-2658.
Supported in part by grants from Assistance Publique—Hôpitaux de Paris (AP-HP), Le Programme Hospitalier de Recherche Clinique, and Agence Française contre les Myopathies. Supported in part by a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) fellowship from Brazil and from Association Française contre les Myopathies (M. M.-L.).
V.T.-V. and F.M.L. contributed equally to this work. S.L.G. and M.G. contributed equally as third authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr J. Maral, from Chiron Corporation, for providing us with rhIL-2; and to Immunex for the generous gift of trimeric CD40-ligand. We thank Cecile Robin and François Berrehar for their expert technical assistance. We thank Stacie Abdalla for her helpful comments.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal