Abstract
Donor-derived T cells have been proposed to play a role in pathogenesis of chronic graft-versus-host disease (cGVHD). The impact of ex vivo T-cell depletion (TCD) on cGVHD was analyzed in a randomized multicenter trial involving unrelated donor marrow transplants. A total of 404 patients diagnosed with hematologic malignancies received a total body irradiation-based myeloablative conditioning regimen. GVHD prophylaxis included TCD plus cyclosporine (CSA) or unmodified grafts with CSA plus methotrexate (M/C). Median recipient age was 31.2 years (range, 0.5-55.6 years); median follow-up time since randomization was 4.2 years. The mean number of T cells infused was 1 log lower on the TCD arm. The incidence of cGVHD at 2 years was similar between the TCD and M/C arms, 29% versus 34% (P = .27), respectively. Survival at 3 years from diagnosis of cGVHD was also similar, (TCD 51% versus M/C 58%; P = .29). The proportion of patients with cGVHD who discontinued immunosuppression at 5 years was not different (TCD 72% versus M/C 63%; P = .27), and incidence of serious infections and leukemia relapse were similar on both treatment arms. In spite of a significant reduction of acute GVHD, TCD did not reduce the incidence of cGVHD or improve survival in patients who developed cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is a multiorgan system immune disorder that is a major complication after allogeneic hematopoietic stem cell transplantation (HSCT).1 Chronic GVHD is also a leading cause of ongoing posttransplantation morbidity and mortality.2,3 Each year about 7000 patients undergo HSCT in North America for the treatment of malignant or nonmalignant diseases.4 In patients surviving at least 100 days, approximately 50% develop cGVHD. Due to greater donor recipient genetic disparity, the risk of acute and chronic GVHD is increased after unrelated donor (URD) transplantations when compared with HSCTs from HLA-matched sibling donors.5-8 Pharmacologic methods of immunosuppression that successfully prevent acute GVHD (aGVHD) are not equally effective in preventing cGVHD, underscoring the need for better understanding and management of cGVHD.9-11 It has been postulated that donor-derived alloreactive T cells play a role in the pathogenesis of both aGVHD and cGVHD.12 In cohort studies or retrospective registry analyses, ex vivo T-cell depletion (TCD) of the donor bone marrow or in vivo administration of antilymphocyte antibodies consistently reduces aGVHD, but not always cGVHD.6,13-16 Since donor T cells also play a key role in mediating graft-versus-tumor (GVT) effects, aggressive GVHD prevention strategies in patients with malignant disease may compromise beneficial antineoplastic GVT effects.17,18
In 1995, the National Heart, Lung, and Blood Institute initiated a prospective, randomized multicenter trial to evaluate the impact of ex vivo TCD of marrow as compared with unmodified grafts on disease-free survival in recipients of URD bone marrow transplants.19 The focus of this report is to examine the effect of TCD, marrow cell doses, and other prognostic factors on the development of cGVHD and to describe clinical manifestations and outcomes in patients who develop cGVHD. Since no prospective studies have addressed risk factors associated with cGVHD in general, or specifically in URD marrow transplantation, factors predicting survival after cGVHD are also presented.
Patients, materials, and methods
Patients and donors
The Unrelated Donor Marrow Transplantation Trial included 15 participating transplantation centers. Between March 1995 and October 2000, 410 patients with hematologic malignancies were randomized; 203 patients were randomized to receive T-cell-depleted marrow and cyclosporine (TCD arm) and 207 to receive methotrexate and cyclosporine after transplantation of T-cell-replete marrow (M/C arm). The study protocol was approved by the institutional review boards (IRBs) at each transplantation center, and all patients signed IRB-approved consent forms prior to initiation of treatment. Of the 410 patients randomized, 5 died before undergoing transplantation (TCD, n = 2; M/C, n = 3) and one patient underwent transplantation 2 years later. Median recipient age was 31.2 years (range, 0.5-55.6 years). Diagnoses included chronic myelogenous leukemias (CML; n = 182), acute myelocytic leukemia (AML; n = 103), acute lymphocytic leukemia (ALL; n = 88), myelodysplastic syndrome (MDS; n = 23), non-Hodgkin lymphoma (NHL; n = 3), and other leukemia (n = 11). The mean infused CD3+ cell doses were 2.8 ± 12.9 (standard deviation [SD]) × 106/kg and 30.1 ± 22.0 × 106/kg in the TCD and M/C arms, respectively. The mean infused CD34+ cell doses were 2.0 ± 1.8 × 106/kg and 3.8 ± 3.4 × 106/kg in the TCD and M/C arms, respectively. The protocol required donors to be selected based on matching of HLA-A and -B determined by serologic level typing and HLA-DRB1 determined by high-resolution molecular typing. Overall, 298 (73%) patients received an HLA 6 of 6 match. In patients with an HLA 5 of 6 match, 10% were mismatched at HLA-A (n = 40), 9% at HLA-B (n = 36), and 9% at HLA-DRB1 (n = 36). The median donor age was 36 years (range, 19-59 years); 61% of donors were male.19
Transplantation procedures
Two methods of TCD were employed: counterflow centrifugal elutriation (Beckman, Palo Alto, CA), a physical method of separating T cells from hematopoietic stem and progenitor cells, and T10B9 (MEDI-500; Medimmune, Gaithersburg, MD), an antibody method of targeting the αβ subunit of the T-cell receptor, which lyses bound cells in the presence of rabbit complement.20,21
Because conditioning regimen varied by type of GVHD prophylaxis, the study evaluated the treatment package. Recipients of TCD received additional therapy in order to promote engraftment. Patients who received marrow T-cell depleted by T10B9 plus complement (n = 134) received conditioning consisting of 1410 cGy fractionated total body irradiation (TBI) over 3 days, 9 gm/m2 cytarabine over 3 days, and 100 mg/kg cyclophosphamide over 2 days. Patients who received TCD by elutriation (n = 67) received a conditioning regimen consisting of 1320 cGy to 1375 cGy TBI over 4 days, 120 mg/kg cyclophosphamide over 2 days, and 60 mg/kg per day equine antithymocyte globulin over 2 days. Patients randomized to M/C received 1320 cGy to 1375 cGy fractionated TBI and 120 mg/kg cyclophosphamide over 2 days. For GVHD prophylaxis, all patients received cyclosporine after transplantation. Patients on the M/C arm also received intravenous methotrexate: 15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11.10
Data collection
National Marrow Donor Program (NMDP) data forms were prospectively collected at baseline, 100 days, 6 months, 1 year, and annually thereafter along with supplemental data forms developed by the Medical Coordinating Center (The EMMES Corporation, Rockville, MD).
The data on each patient were reviewed (blinded to treatment arm) by expert panels to assign an aGVHD score,22 infection scores for types and severities of infection (Jo-Anne van Burik, S.L.C., Allison G. Freifeld, manuscript in preparation), and a cause of death defined by prespecified criteria. The occurrence of cGVHD was determined from the first report of cGVHD diagnosis on the NMDP forms. Subsequent queries were sent to the transplantation centers to obtain the dates of completion of systemic therapy for cGVHD.
Statistical analysis
The primary end point was the incidence of any stage (extensive or limited) cGVHD. To describe the actual risk of cGVHD at the time of transplantation, the complement of the Kaplan-Meier (1-KM) and the cumulative incidence estimate (CINC) for cGVHD were determined.23 The 1-KM and the CINC are both marginal estimates of the probability of failure due to the event of interest but differ in the way they handle the competing risk of death, and have different interpretations. The 1-KM estimate is uniformly higher than the CINC because in the computation of 1-KM, patients who die early are censored and their probability of failing from the defined end point is redistributed across later time points, whereas in the computation of the CINC estimate, these individuals are no longer at risk for the end point. The 1-KM predicts the cumulative probability of the end point in the absence of any competing risk. The CINC estimates the cumulative probability of the end point when the competing risk is present.23,24 Kaplan-Meier estimates were used to estimate survival,25 and differences between groups were compared using the log-rank statistic.26 The Cox proportional hazards model with time-dependent covariates was used to create prognostic models that considered multiple variables.27 Variables considered were: treatment arm; TCD method; transplantation center; total CD3+, CD34+, and nucleated cell doses; recipient and donor demographics; primary disease; risk status; degree of HLA match; recipient and donor cytomegalovirus (CMV) serologic status; median days to neutrophil engraftment; previous maximum aGVHD grade; and organs involved. For the analyses of patients diagnosed with cGVHD, additional variables included Karnofsky-Lansky performance score, serum bilirubin level and platelet count at the time of diagnosis, and the organs involved. Incidence of relapse was estimated with death in remission as a competing risk. Time to termination of all systemic immunosuppression was estimated with death while receiving immunosuppression considered as a competing risk.
Results
Overall
With a median 4.2 years (range, 1.5-7.0 years) follow-up from date of randomization, the primary study end point, 3-year disease-free survival (DFS) was not statistically different between the TCD (27%; 95% confidence interval [CI], 21%-33%) and M/C (34%; 95% CI, 27%-40%) arms (P = .16). Overall survival for all randomized patients at 3 years after HSCT was also not significantly different between treatment arms (TCD: 34%; 95% CI, 27%-40%; M/C: 36%; 95% CI, 29%-43%). The proportion of patients experiencing infection, time to first infection, and types of infections were similar. Severity of infections (particularly CMV infections) was greater in TCD recipients (van Burik JH, Carter SL, Freifeld AG, et al, manuscript submitted 2005). Using the Bearman toxicity scale, the incidence and severity of mucositis, hepatic, pulmonary, renal, and central nervous system (CNS) toxicities were greater among recipients of M/C.19
Acute GVHD
The cumulative incidence estimates of acute GVHD grades II-IV at day 100 were significantly lower in the TCD arm than in the M/C arm, 39% (95% CI, 33%-46%) versus 63% (95% CI, 56%-69%), respectively (P < .01). Incidence of acute GVHD grades III-IV was also lower in the TCD arm, 18% (95% CI, 13%-24%) versus 37% (95% CI, 30%-44%), respectively (P < .01).
Incidence of chronic GVHD
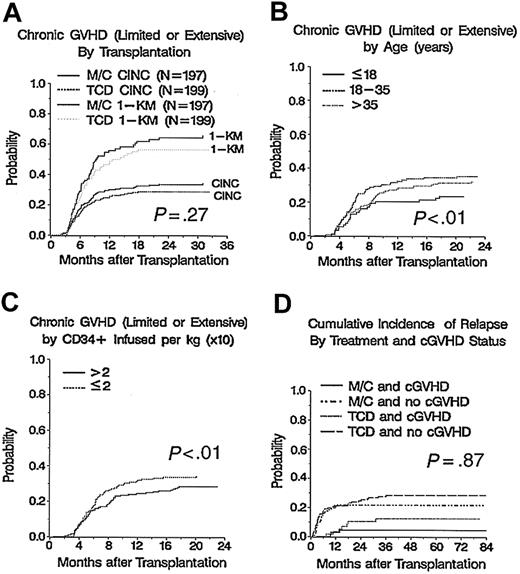
Overall, 124 patients developed cGVHD (TCD = 57, M/C = 67). The median time of occurrence of cGVHD was 180 days (range, 64-943 days) after transplantation, with no difference in the median time of cGVHD onset between treatment arms (TCD, 181 days versus M/C, 179 days; P = .71). For all patients, the CINC of cGVHD at 2 years was 31% (95% CI, 27%-36%) and the 1-KM estimate was 61% (95% CI, 54%-68%). There was no statistically significant difference at 2 years in the cGVHD CINC estimates between treatment arms: 29% (95% CI, 22%-35%) in recipients with TCD and 34% (95% CI, 27%-40%) in recipients of M/C (P = .27). Similarly, there was no difference in the 1-KM estimates of cGVHD between TCD and M/C: 56% (95% CI, 46%-67%) versus 64% (95% CI, 55%-74%), respectively (P = .27; Figure 1A).
Cumulative incidence of chronic GVHD and relapse by covariates. (A) Cumulative incidence of chronic GVHD by treatment arm, P = .27. (B) Incidence of chronic GVHD by recipient age, P < .01. (C) Incidence of chronic GVHD by CD34+ dose, P < .01. (D) Cumulative incidence of relapse by treatment arm and chronic GVHD status, P = .87.
Cumulative incidence of chronic GVHD and relapse by covariates. (A) Cumulative incidence of chronic GVHD by treatment arm, P = .27. (B) Incidence of chronic GVHD by recipient age, P < .01. (C) Incidence of chronic GVHD by CD34+ dose, P < .01. (D) Cumulative incidence of relapse by treatment arm and chronic GVHD status, P = .87.
Analysis of factors associated with risk of developing cGVHD is shown in Table 1.Although, in univariate analysis, primary disease other than CML was significant, in a multivariate Cox proportional hazards model, significant and independently favorable risk factors for decreased risk of cGVHD are younger recipient age (P < .01; Figure 1B), higher infused CD34+ dose (P ≤ .01; Figure 1C), and prior aGVHD of grade of 0 or I (P ≤ .01).
Prognostic factors for developing cGVHD
. | All patients, N = 404 . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Development of cGVHD . | CINC of cGVHD at 2 years . | 95% CI . | Hazard ratio* . | P . | Favorable factors . | ||||
| Treatment arm | |||||||||
| M/C | 0.34 | 0.27-0.40 | 1.22 | .27 | NA | ||||
| TCD | 0.29 | 0.22-0.35 | 1.00 | NA | NA | ||||
| Acute GVHD grade† | No prior aGVHD (0-I) | ||||||||
| II-IV | NA | NA | 1.84 | < .01 | NA | ||||
| 0-I | NA | NA | 1.00 | NA | NA | ||||
| Recipient age | Younger recipients | ||||||||
| Less than 19 years | 0.23 | 0.14-0.32 | 1.00 | NA | NA | ||||
| 18-35 years | 0.35 | 0.27-0.43 | 2.51 | < .01 | NA | ||||
| Greater than 35 years | 0.32 | 0.25-0.40 | 2.44 | < .01 | NA | ||||
| Primary disease | Diseases other than CML | ||||||||
| CML | 0.40 | 0.33-0.48 | 1.75 | < .01 | NA | ||||
| Other | 0.23 | 0.18-0.29 | 1.00 | NA | NA | ||||
| CD34+, infused/kg (× 106) | Higher CD34+ infused | ||||||||
| Less than or equal to 2.0 | 0.34 | 0.27-0.41 | 1.73 | < .01 | NA | ||||
| Greater than 2.0 | 0.28 | 0.22-0.35 | 1.00 | NA | NA | ||||
. | All patients, N = 404 . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Development of cGVHD . | CINC of cGVHD at 2 years . | 95% CI . | Hazard ratio* . | P . | Favorable factors . | ||||
| Treatment arm | |||||||||
| M/C | 0.34 | 0.27-0.40 | 1.22 | .27 | NA | ||||
| TCD | 0.29 | 0.22-0.35 | 1.00 | NA | NA | ||||
| Acute GVHD grade† | No prior aGVHD (0-I) | ||||||||
| II-IV | NA | NA | 1.84 | < .01 | NA | ||||
| 0-I | NA | NA | 1.00 | NA | NA | ||||
| Recipient age | Younger recipients | ||||||||
| Less than 19 years | 0.23 | 0.14-0.32 | 1.00 | NA | NA | ||||
| 18-35 years | 0.35 | 0.27-0.43 | 2.51 | < .01 | NA | ||||
| Greater than 35 years | 0.32 | 0.25-0.40 | 2.44 | < .01 | NA | ||||
| Primary disease | Diseases other than CML | ||||||||
| CML | 0.40 | 0.33-0.48 | 1.75 | < .01 | NA | ||||
| Other | 0.23 | 0.18-0.29 | 1.00 | NA | NA | ||||
| CD34+, infused/kg (× 106) | Higher CD34+ infused | ||||||||
| Less than or equal to 2.0 | 0.34 | 0.27-0.41 | 1.73 | < .01 | NA | ||||
| Greater than 2.0 | 0.28 | 0.22-0.35 | 1.00 | NA | NA | ||||
Variables that were considered and found not significant were date of transplantation, center, Karnofsky-Lansky performance status, sex of recipient and donor, donor age, HLA match, risk status, recipient and donor CMV status, recipient and donor race, method of T-cell depletion, T cells infused/kg, and total nucleated cell dose infused/kg.
NA indicates not applicable.
Cox proportional hazards univariate analysis
Point estimates for aGVHD are not presented since it is a time-varying covariate
Relapse
For all patients at 3 years, the relapse rate was 24% (95% CI, 18%-29%) for TCD patients and 16% (95% CI, 11%-20%) for M/C patients (P = .08). Patients who developed cGVHD had a significantly lower probability of relapse within both the TCD (28% versus 12%, P < .01) and M/C (22% versus 4%, P < .01) treatment arms (Figure 1D).
Characteristics of patients with chronic GVHD
Of 124 patients who developed cGVHD (TCD = 57, M/C = 67), 60% had diagnoses supported by histologic evidence. At the time of cGVHD diagnosis, 58% of the patients had more than one organ involved; 80% had a serum bilirubin less than 2.0 mg/dL. In 42% of cGVHD patients, platelet counts were less than 100 000/μL. Recipients with cGVHD in the TCD arm had less frequent prior acute GVHD (TCD 54% versus M/C 87%; P < .01) and a trend toward poorer performance status (< 80% Karnofsky score; TCD 50% versus M/C 32%; P = .05).
As shown in Figure 2, among those patients with cGVHD, more TCD patients had cutaneous involvement (TCD 68% versus M/C 50%; P = .05) and weight loss (TCD 21% versus M/C 6%; P = .01), but less often oral involvement (TCD 25% versus M/C 45%; P = .02). Rates of gastrointestinal or hepatic involvement, xerophthalmia, or obstructive lung disease were similar in both treatment arms.
Treatment of chronic GVHD
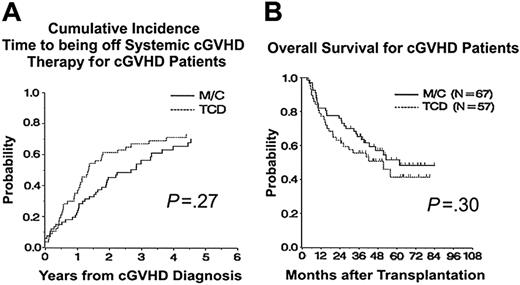
Most patients with cGVHD received prolonged systemic treatment with cyclosporine (95%), corticosteroids (87%), mycophenolate (26%), tacrolimus (21%), or azathioprine (13%). At 3 years from transplantation in patients with cGVHD, the CINC of being off all systemic immunosuppressive therapy was 63% (95% CI, 51%-75%) for TCD and 45% (95% CI, 34%-57%) for M/C (P < .01), but by 5 years the discontinuation rates were similar (TCD 72% versus M/C 63%; P = .27; Figure 3A).
Effect of chronic GVHD on incidence of serious infections
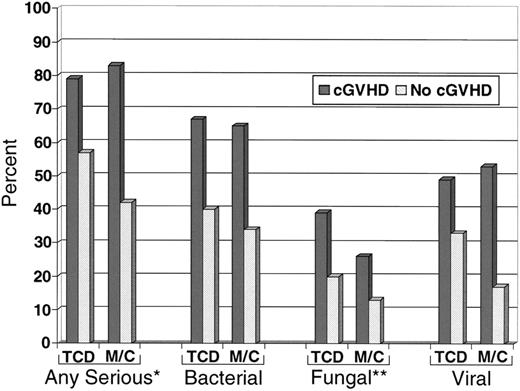
Among patients surviving at 100 days after transplantation, 81% of patients with cGVHD had a serious (severe, life-threatening, or fatal) infection as compared with 50% of patients who did not develop cGVHD (P < .01), irrespective of treatment arm. In patients diagnosed with cGVHD, treatment arm did not alter the frequency of serious infections (P = .47) or of bacterial (P = .77) or viral (P = .57) infections. However, among patients with cGVHD, those in the TCD arm had more fungal infections than those in the M/C arm (P = .05; Figure 4).
Survival and cause of death in patients with cGVHD
As shown in Figure 3B, the 3-year estimates of overall survival from transplantation for patients who developed cGVHD showed no significant difference between treatments: TCD 56% (95% CI, 43%-69%) and M/C 65% (95% CI, 54%-77%), P = .30.
Prognostic factors for survival from cGVHD onset are shown in Table 2.Although platelet count, primary disease risk status, and recipient CMV serology status, but not treatment arm, were suggestively important in univariate analysis, multivariate regression demonstrated that none of these factors had an independently significant impact on survival after the development of cGVHD. Multivariate analysis (Table 3; stratified on treatment arm) demonstrated that higher (≥ 80%) Karnofsky-Lansky performance status (P = .01), prior aGVHD grade 0-I (P = .03), and HLA6 of 6 match (P = .03) each favorably influenced overall survival in patients with cGVHD. The prognostic factors were the same in both arms.
Prognostic factors for survival in patients with cGVHD: univariate analysis
Patients with chronic GVHD, N = 124 . | Kaplan-Meier survival probability at 3 years . | 95% CI . | Hazard ratio . | P . | Favorable factors . |
|---|---|---|---|---|---|
| Treatment arm | |||||
| M/C | 0.58 | 0.45-0.70 | 0.78 | .29 | NA |
| TCD | 0.51 | 0.38-0.64 | 1.00 | NA | NA |
| Performance status at cGVHD diagnosis | Performance status of 80-100 | ||||
| Less than 80 | 0.34 | 0.20-0.48 | 2.66 | < .01 | NA |
| Greater than or equal to 80 | 0.68 | 0.57-0.80 | 1.00 | NA | NA |
| Platelet count at cGVHD diagnosis | Platelet count ≥ 100 000/μL | ||||
| Less than 100 000 | 0.41 | 0.28-0.55 | 2.41 | < .01 | NA |
| Greater than or equal to 100 000 | 0.71 | 0.59-0.83 | 1.00 | NA | NA |
| Prior acute GVHD | Acute GVHD grade 0 or 1 | ||||
| Grades II-IV | 0.45 | 0.32-0.58 | 1.84 | .02 | NA |
| 0 or 1 | 0.65 | 0.52-0.77 | 1.00 | NA | NA |
| HLA match | 6 of 6 HLA match | ||||
| 6 of 6 | 0.58 | 0.48-0.69 | 0.59 | .06 | NA |
| 5 of 6 | 0.42 | 0.23-0.60 | 1.00 | NA | NA |
| Risk status | Good risk status | ||||
| Poor | 0.38 | 0.17-0.58 | 2.04 | .02 | NA |
| Good | 0.58 | 0.48-0.68 | 1.00 | NA | NA |
| Recipient CMV serostatus | Seronegative | ||||
| Negative | 0.63 | 0.52-0.75 | 0.60 | .05 | NA |
| Positive | 0.42 | 0.28-0.57 | 1.00 | NA | NA |
Patients with chronic GVHD, N = 124 . | Kaplan-Meier survival probability at 3 years . | 95% CI . | Hazard ratio . | P . | Favorable factors . |
|---|---|---|---|---|---|
| Treatment arm | |||||
| M/C | 0.58 | 0.45-0.70 | 0.78 | .29 | NA |
| TCD | 0.51 | 0.38-0.64 | 1.00 | NA | NA |
| Performance status at cGVHD diagnosis | Performance status of 80-100 | ||||
| Less than 80 | 0.34 | 0.20-0.48 | 2.66 | < .01 | NA |
| Greater than or equal to 80 | 0.68 | 0.57-0.80 | 1.00 | NA | NA |
| Platelet count at cGVHD diagnosis | Platelet count ≥ 100 000/μL | ||||
| Less than 100 000 | 0.41 | 0.28-0.55 | 2.41 | < .01 | NA |
| Greater than or equal to 100 000 | 0.71 | 0.59-0.83 | 1.00 | NA | NA |
| Prior acute GVHD | Acute GVHD grade 0 or 1 | ||||
| Grades II-IV | 0.45 | 0.32-0.58 | 1.84 | .02 | NA |
| 0 or 1 | 0.65 | 0.52-0.77 | 1.00 | NA | NA |
| HLA match | 6 of 6 HLA match | ||||
| 6 of 6 | 0.58 | 0.48-0.69 | 0.59 | .06 | NA |
| 5 of 6 | 0.42 | 0.23-0.60 | 1.00 | NA | NA |
| Risk status | Good risk status | ||||
| Poor | 0.38 | 0.17-0.58 | 2.04 | .02 | NA |
| Good | 0.58 | 0.48-0.68 | 1.00 | NA | NA |
| Recipient CMV serostatus | Seronegative | ||||
| Negative | 0.63 | 0.52-0.75 | 0.60 | .05 | NA |
| Positive | 0.42 | 0.28-0.57 | 1.00 | NA | NA |
NA indicates not applicable.
Final multivariate analysis: survival from cGVHD diagnosis
Survival . | Hazard ratio . | 95% CI . | P . | Favorable factors . |
|---|---|---|---|---|
| Performance status at diagnosis | ||||
| Less than 80 | 2.67 | 1.54-4.60 | < .01 | Performance status of 80-100 |
| Greater than or equal to 80 | 1.00 | NA | NA | NA |
| Acute GVHD grade | Acute GVHD grade 0 or 1 | |||
| II, III, or IV | 1.99 | 1.09-3.63 | .03 | NA |
| 0 or I | 1.00 | NA | NA | NA |
| HLA match | 6 of 6 HLA match | |||
| 5 of 6 | 1.92 | 1.05-3.57 | .03 | NA |
| 6 of 6 | 1.00 | NA | NA | NA |
Survival . | Hazard ratio . | 95% CI . | P . | Favorable factors . |
|---|---|---|---|---|
| Performance status at diagnosis | ||||
| Less than 80 | 2.67 | 1.54-4.60 | < .01 | Performance status of 80-100 |
| Greater than or equal to 80 | 1.00 | NA | NA | NA |
| Acute GVHD grade | Acute GVHD grade 0 or 1 | |||
| II, III, or IV | 1.99 | 1.09-3.63 | .03 | NA |
| 0 or I | 1.00 | NA | NA | NA |
| HLA match | 6 of 6 HLA match | |||
| 5 of 6 | 1.92 | 1.05-3.57 | .03 | NA |
| 6 of 6 | 1.00 | NA | NA | NA |
Stratified on treatment because of nonproportional hazards.
NA indicates not applicable.
Overall, 59 of the 124 patients with cGVHD died. Chronic GVHD was the most frequent primary cause of death resulting in 51 (86%) deaths (TCD, 25 and M/C, 26; Table 4). Infections were the major secondary cause of death. Only 6 patients died from relapse.
Causes of death for patients with cGVHD
Primary and secondary causes of death . | TCD arm, n (%) . | M/C arm, n (%) . |
|---|---|---|
| Chronic GVHD | 14 (48) | 13 (43) |
| Chronic GVHD with infection* | 11 (38) | 13 (43) |
| Malignancy relapse | 4 (14) | 2 (7) |
| Other† | 0 (0) | 2 (3) |
| Total | 29 (100) | 30 (100) |
Primary and secondary causes of death . | TCD arm, n (%) . | M/C arm, n (%) . |
|---|---|---|
| Chronic GVHD | 14 (48) | 13 (43) |
| Chronic GVHD with infection* | 11 (38) | 13 (43) |
| Malignancy relapse | 4 (14) | 2 (7) |
| Other† | 0 (0) | 2 (3) |
| Total | 29 (100) | 30 (100) |
Chronic GVHD with a fatal infection
Breast cancer (n = 1), myocardial infarction (n = 1)
Discussion
Chronic GVHD remains a major obstacle for the long-term success of allogeneic HSCT.1 Multiple studies have demonstrated the negative impact of cGVHD on survival and on quality of life and functional status in patients who survive and are cured of their hematologic malignancy. The primary objective of the present study was to determine the effects of marrow TCD on the incidence, clinical manifestations, and consequences of cGVHD in a prospectively followed cohort of URD transplant recipients. Extensive and limited stages of cGVHD were combined, since these staging definitions have been poorly reproducible between transplantation centers.8
Time to being off systemic immunosuppressive therapy and overall survival from transplantation for patients with chronic GVHD. (A) Time to being off systemic immunosuppressive therapy for chronic GVHD, P = .27 at 5 years. (B) Overall survival from time of transplantation for patients with chronic GVHD, P = .30.
Time to being off systemic immunosuppressive therapy and overall survival from transplantation for patients with chronic GVHD. (A) Time to being off systemic immunosuppressive therapy for chronic GVHD, P = .27 at 5 years. (B) Overall survival from time of transplantation for patients with chronic GVHD, P = .30.
Serious infections in patients surviving 100 days after transplantation. All serious infections were more frequent in patients with cGVHD, *P < .01. Fungal infections were more frequent in patients with cGVHD after TCD, **P = .05.
Serious infections in patients surviving 100 days after transplantation. All serious infections were more frequent in patients with cGVHD, *P < .01. Fungal infections were more frequent in patients with cGVHD after TCD, **P = .05.
This analysis found that the incidence and time to development of cGVHD was similar in transplant patients receiving either TCD or M/C for GVHD prophylaxis after URD marrow transplantation. This differs from some earlier retrospective analyses in which URD TCD was usually associated with lesser risks of both acute and chronic GVHD.6,28 Of importance, in a randomized trial methotrexate was shown to not impact cGVHD incidence.29 The mean T-cell depletion in this study was 1log, which may be insufficient to protect against the development of cGVHD. However, ineffective prophylaxis of cGVHD using TCD despite lower risks of aGVHD may reflect differing pathogeneses of these 2 GVHD syndromes.30,31 The results of this prospective randomized trial are consistent with an earlier retrospective registry analysis in 870 mismatched related and URD HSCTs, which demonstrated consistently effective aGVHD prevention by a variety of ex vivo TCD methods, but no protection against cGVHD.16 In that study, the disparate effect of TCD in preventing acute but not chronic GVHD was particularly evident using TCD with narrow specificity anti-T-cell antibodies. These narrow spectrum techniques yielded an increased risk of cGVHD. This suggests that infusion of non-T-accessory cell populations may play a role in promoting cGVHD.16 In the current trial, two different TCD methodologies were used and conditioning regimens varied by the type of GVHD prophylaxis in order to promote engraftment; however, the study was designed to evaluate the whole treatment package and not its specific components.
Significant factors associated with cGVHD include older patient age and prior aGVHD. These have been identified in earlier reports.2 The association of a higher CD34+ marrow cell dose with lower incidence of cGVHD is a new observation in URD transplantation and needs to be confirmed in future studies. One study of 50 patients after HLA-identical sibling bone marrow transplantation found a negative correlation between a higher number of marrow CD34+ cells (> 3.12 × 106/kg) and probability of cGVHD.32 Two large cohort studies reported no correlation between the CD34+ cell dose and cGVHD in recipients of HLA-identical sibling bone marrow.33,34 In contrast, very high CD34+ cell dose (> 8 × 106/kg) is a recognized risk factor for higher incidence and severity of cGVHD after peripheral blood allogeneic stem cell (PBSC) transplantation.35-37 This may reflect different importance of CD34+ cells or accompanying cell populations in the pathogenesis of cGVHD after marrow versus PBSC transplantation. The current study also confirms the protective effect of cGVHD in prevention of relapse. An average 1log TCD of the bone marrow does not abrogate this cGVHD-associated antineoplastic effect.
About 60% of patients with cGVHD had more than one organ involved, most commonly skin and/or oral mucosa. Chronic GVHD after TCD transplantation was associated with more frequent skin involvement and weight loss, but less oral involvement. Other regimen-related factors may confound interpretation of these differences. For example, a higher incidence of oral mucositis observed in the M/C cohort might predispose patients to a higher incidence of oral cGVHD.19
Time to discontinuation of systemic immunosuppression is a marker for success of therapy for cGVHD.38,39 At 5 years there was no difference in the proportion of patients completing immunosuppression, reflecting similar rates of cGVHD resolution in the 2 treatment arms.
Serious infections were more frequent in patients with cGVHD and were a major contributing cause of morbidity and mortality. More frequent fungal infections occurred in TCD patients with cGVHD, but the net adverse effect of cGVHD and its therapy were largely independent of the initial randomized treatment. The exact mechanism of immune compromise due to cGVHD or therapeutic treatment requires further research and new techniques to limit immune compromise.
Overall survival after diagnosis of cGVHD was similar in the TCD and M/C groups. Lower performance status, HLA mismatch, and preceding aGVHD were each independently associated with poorer survival in patients with cGVHD. Karnofsky score and aGVHD grade have been recognized as adverse prognostic factors in prior retrospective cohort studies.2 A recent analysis emphasized the increased risks of HLA-mismatch on nonrelapse mortality in patients with cGVHD after URD transplantation.39
In summary, in this prospective randomized trial, despite reduction of aGVHD, an average 1log ex vivo TCD failed to reduce the incidence of cGVHD. The TCD methodologies used in this study were less intense than many TCD methodologies currently used, and these results may not necessarily be extrapolated to other TCD techniques. In patients developing cGVHD, overall survival was not impacted by treatment arm. Chronic GVHD was associated with more frequent serious infections, but also with effective protection against relapse in both the TCD and M/C cohorts. Nonrelapse mortality remains excessively high after cGVHD diagnosis, and developing better cGVHD prevention and treatment strategies represents a major task. Improved understanding of cGVHD biology and more refined graft manipulations are needed to increase the long-term success of URD marrow transplantation.
Appendix
Participating institutions and coinvestigators were University of Minnesota (Elutriation Center, n = 103; John E. Wagner, Jo-Anne van Burik, Stella M. Davies, Shawn Fuller), Memorial Sloan-Kettering Cancer Center (T10B9 Center, n = 70; Richard O'Reilly, Nancy Collins), Medical College of Virginia (T10B9 Center, n = 53), Wake Forest University Baptist Medical Center (T10B9 Center, n = 36; David Hurd), University of Nebraska (Elutriation Center, n = 34; Thomas Gross, Michael Bishop), University of Utah (T10B9 Center, n = 33; Finn Petersen, Patrick Beatty), Stanford University (T10B9 Center, n = 25; Robert Negrin), University of Iowa (T10B9 Center, n = 19), University of South Carolina (T10B9 Center, n = 13; Adrian Gee), Ohio State University (T10B9 Center, n = 6; Edward Copelan), Duke University (T10B9 Center, n = 6; Joanne Kurtzberg), University of Kentucky (T10B9 Center, n = 5; John S. Thompson, Gordon Phillips), Medical College of Wisconsin (T10B9 Center, n = 4; Carolyn Keever-Taylor, William Drobyski, Neal Flomenberg), Western Pennsylvania Hospital (T10B9 Center, n = 2; Richard Shadduck), and University of Pittsburgh (T10B9 Center, n = 1; Albert Donnenberg); Craig Howe, Steering Committee Chairperson; Paul J. Martin, Fred Hutchinson Cancer Research Center; The EMMES Corporation (Donald Stablein, Elizabeth Wagner); and NHLBI (LeeAnn Jensen, Nancy Geller, Paul McCurdy).
Prepublished online as Blood First Edition Paper, July 26, 2005; DOI 10.1182/blood-2005-04-1614.
Supported by a contract from the National Heart, Lung, and Blood Institute (N01-HB-47095 [S.Z.P., D.W.], N01-HB-47097 [J.H.-D., R.G., J.C., S.Y.], N01-HB-47094 [S.L.C., A.M.M.], and N01-HB-47098 [N.A.K., E.P.]).
A complete list of the members of the National Heart, Lung, and Blood Institute Unrelated Donor Marrow Transplantation Trial appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are indebted to the work of many clinical investigators who have advanced the field and the many physicians and nurses who have diligently cared for these complex patients. In addition, we gratefully acknowledge the work of the many search coordinators and the dedicated staff of the National Marrow Donor Program.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal