Abstract
Previously, we described the age-dependent accumulation of mitochondrial DNA (mtDNA) mutations, leading to a high degree of mtDNA heterogeneity among normal marrow and blood CD34+ clones and in granulocytes. We established a method for sequence analysis of single cells. We show marked, distinct mtDNA heterogeneity from corresponding aggregate sequences in isolated cells of 5 healthy adult donors—37.9% ± 3.6% heterogeneity in circulating CD34+ cells, 36.4% ± 14.1% in T cells, 36.0% ± 10.7% in B cells, and 47.7% ± 7.4% in granulocytes. Most heterogeneity was caused by poly-C tract variability; however, base substitutions were also prevalent, as follows: 14.7% ± 5.7% in CD34+ cells, 15.2% ± 9.0% in T cells, 15.4% ± 6.7% in B cells, and 32.3% ± 2.4% in granulocytes. Many poly-C tract length differences and specific point mutations seen in these same donors but assayed 2 years earlier were still present in the new CD34+ samples. Additionally, specific poly-C tract differences and point mutations were frequently shared among cells of the lymphoid and myeloid lineages. Secular stability and lineage sharing of mtDNA sequence variability suggest that mutations arise in the lymphohematopoietic stem cell compartment and that these changes may be used as a natural genetic marker to estimate the number of active stem cells.
Introduction
Mitochondria play many important roles in cellular homeostasis, including the production of adenosine triphosphate (ATP) by the process of oxidative phosphorylation (the respiratory chain); metabolism of amino acids, fatty acids, cholesterol, steroids, heme, and nucleotides; and the release of death-promoting factors including cytochrome c leading to apoptosis. The generation of cellular energy in the form of ATP is probably the most crucial function of mitochondria.1,2 Hundreds of mitochondria are present within each cell's cytoplasm, and 2 to 10 copies of mitochondrial DNA (mtDNA) are packaged in each mitochondrion. Human mtDNA is a double-stranded circular molecule containing 16 569 base pairs (bp) encoding for 37 genes: the 12S and 16S ribosomal RNA genes, the 22 transfer RNA genes required for mitochondrial protein synthesis, and 13 polypeptide genes that generate subunits of the respiratory chain.3 However, most mitochondrial respiratory chain polypeptides are coded by nuclear DNA (nDNA). Mitochondrial DNA is distinguished from nDNA by several characteristics, including the properties of heteroplasmy, maternal inheritance, relaxed replication, and mitotic segregation.2 In addition, mtDNA has a modified genetic code4 and a paucity of introns, and it lacks histone protection.5 Oxidative phosphorylation for ATP generation is believed to be the main source of the reactive oxygen species (ROSs), such as superoxide anions, hydrogen peroxide, and hydroxyl radicals, produced in mitochondria. Chronic ROS exposure induces oxidative damage to mitochondrial and cellular macromolecules, including DNA, proteins, and lipids.6 The 10- to 20-fold higher mutation rate in mtDNA compared with nDNA is believed to be caused by its proximity to sites of ROS generation, lack of histones, and limited DNA repair capacity,6 though recent data have shown that excision repair mechanisms operate for mammalian mtDNA.7,8
Hundreds of mtDNA mutations (deletions, insertions, and base substitutions) have been identified in many degenerative diseases; other mutations that impact mitochondrial function have been associated with nuclear genes. Thus, mitochondrial dysfunction can stem from a mitochondrial maternal inheritance pattern, a nuclear Mendelian inheritance pattern, or a combination of them.9 A large deletion of mtDNA is a hallmark of Pearson syndrome, a congenital disorder, one manifestation of which is sideroblastic anemia.10 Somatically acquired mtDNA mutations also have been linked to aging, degenerative diseases, cancer, and autoimmunity.9 MtDNA mutations were reported in apparently acquired sideroblastic anemia and in myelodysplastic syndromes in general,11 though our laboratory was unable to confirm these results.12 Somatic mtDNA mutations are attributed mainly to mitochondrial oxidative damage, which increases with age.6 In the aging process, individual postmitotic cells initially acquire a single mutant mtDNA among an overwhelming majority of wild-type species (heteroplasmy), and probably different mutant mtDNA clonally expand to homoplasmy in parallel in individual cells through relaxed replication, leading to mitochondrial dysfunction.13,14 In mitotic tissues, such as bone marrow, intracellular random genetic drift is probably caused by relaxed replication and mitotic segregation.13 Computer modeling has suggested that random processes are sufficient to explain the incidence of mtDNA mutations in dividing cells.15 Moreover, single-cell analysis of mtDNA in proliferating epithelial tissue showed high proportions of age-associated homoplasmic mutant cells.16,17
We have described an age-dependent accumulation of mtDNA mutations leading to a high degree of mtDNA sequence heterogeneity in normal marrow and blood CD34+ cells, but not in umbilical cord blood.18 The accumulation of mtDNA mutations over time and the clonal expansion of cells with somatic mtDNA mutations appear to be relatively common in marrow progenitor cells and possibly even in marrow stem cells.19 In our previous studies, CD34+ individual cells were cloned during a brief in vitro culture period, after which their mtDNAs were sequenced.18,19 Single-cell analysis—the examination of individual cells without culture— ensures that even limited growth in vitro does not introduce mutations; furthermore, some mature blood cells are either cumbersome to cultivate (T and B cells) or will not undergo mitosis (granulocytes). We now have established a method for mtDNA sequence analysis in single cells, obtained by flow cytometric sorting and analyzed using gene amplification with primers specific for the control region of mtDNA. In the present study, single blood CD34+ cells, granulocytes, T cells, and B cells were obtained from the same healthy donors who had volunteered for our previous studies, thus permitting us to examine changes in the polymorphic spectra of the mtDNA control region over time. We also used the single-cell method to assess the mtDNA sequence in differentiated cells derived from lymphohematopoietic stem cells.
Materials and methods
Tissue samples
Peripheral blood (PB) from 5 healthy adult donors was collected after informed consent and according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute. Sequence analyses of the mtDNA control region from CD34+ clones and single granulocytes have been published and are included here for comparative purposes only.18,19
Single-cell sorting for CD34+ cells, lymphocytes, and granulocytes
Mononuclear cells and granulocytes from PB were separated by density gradient centrifugation. After washing with phosphate-buffered saline (PBS), the number of cells was adjusted to 1 × 107 cells/mL with PBS containing 0.5% bovine serum albumin (BSA; Invitrogen, Carlsbad, CA). Fifteen microliters of anti-CD34 phycoerythrin (PE)–conjugated monoclonal antibody, anti-CD3 allophycocyanin (APC)–conjugated antibody, or anti-CD20 fluorescein isothiocyanate (FITC) antibody (BD Biosciences, San Jose, CA) were added to 100 μL mononuclear cell suspension, and 15 μL anti-CD33 PE-conjugated antibody (BD Biosciences) was added to a 100-μL granulocyte suspension. After incubation for 30 minutes at 4°C, cells were washed and resuspended in 0.5 mL PBS with 0.5% BSA.
Cell sorting was performed on a MoFlo Cytometer (Dako-Cytomation, Fort Collins, CO), using 100 mW of the 488-nm line of an argon laser (I-90; Coherent, Palo Alto, CA) for excitation. Forward scatter was the triggering parameter. Single-cell deposition was accomplished using the CyClone automated cloner (Dako-Cytomation) in the 0.5 single-drop mode with gating based on forward scatter and fluorescence. Single CD34+ cells, T cells, B cells, and granulocytes were placed into each well of an optical 96-well reaction plate (MicroAmp; Applied Biosystems, Foster City, CA) containing 50 μL of 1 × lysis buffer (10 mM Tris-HCl [pH 8.0], 50 mM KCl, 100 μg/mL Proteinase K, and 1% Triton X-100).
DNA extraction from single CD34+ cells, lymphocytes, and granulocytes
Single cells in the lysis buffer were incubated at 56°C for 30 minutes to liberate total DNA, and then proteinase K was inactivated by incubation at 96°C for 5 minutes in the 96-well reaction plate. Lysates were briefly centrifuged and stored at -20°C.
PCR amplification of the mtDNA control region
Cell lysates of single cells were subjected to amplification of mtDNA using the LA polymerase chain reaction (PCR) kit (TaKaRa LA Taq; TaKaRa Bio, Madison, WI). Two-step nested PCR amplification was performed with outer and inner pairs of primers to generate sufficient template from the single CD34+ cells, lymphocytes, and granulocytes for sequencing of the mtDNA control region. Primer pairs for PCR amplification of the mtDNA control region were previously described.19 The primary PCR mixture contained 400 μM each dNTP,2 U LA Taq (TaKaRa LA Taq), 0.8 μM outer primers, and 5 μL cell lysates. Primary PCR amplification was performed in an optical 96-well reaction plate (MicroAmp) using the GeneAmp PCR system 9700 (Applied Biosystems) as follows: one cycle of 96°C for 1 minute, then 35 cycles of 96°C for 30 seconds, 52°C for 50 seconds, and 72°C for 1 minute, with a 5-second increase per cycle, and ending with one cycle of 72°C for 5 minutes. Secondary PCR was performed in 50 μL reaction mixture containing 400 μM each dNTP, 2 U LATaq,0.8 μM inner nested primers, and 3 μL primary PCR product under the same amplification conditions described. Secondary PCR samples were purified using the QIA quick PCR purification kit (Qiagen, Valencia, CA).
Sequence analysis
BigDye Terminator version 3.1 ready reaction kit and ABI Prism 3100 Genetic Analyzer (Applied Biosystems) were used for direct sequencing of the amplified mtDNA PCR products. Sequencing primers have been described.19 mtDNA sequences experimentally obtained were compared with the Revised Cambridge Reference Sequence (RCRS) (http://www.mitomap.org/mitomap/mitoseq.html)20 using the Blast2 program (http://www.ncbi.nlm.nih.gov/blast/bl2seq/bl2.html) and the database search tool, MitoAnalyzer (http://www.cstl.nist.gov/biotech/strbase/mitoanalyzer.html, 2001),21 to determine polymorphisms and mutations.
Confirmation of mtDNA nucleotide changes
mtDNA mutations in all donors were confirmed by reanalysis of the first PCR product. For cells showing a sequence difference from the donor's corresponding aggregate mtDNA sequence, 2 or more independent PCR reamplifications were performed, followed by sequencing. Amplification and sequencing from the original single-cell lysates were not feasible for granulocytes because of poor recovery of DNA and the appearance of apparently new base substitutions (see “Discussion”).
TA cloning
To confirm heteroplasmy and mixed nucleotide signals in the mtDNA control region resulting from the existence of a deletion, PCR products were separated by agarose gel electrophoresis and extracted from the gels using the QIAquick gel extraction kit (Qiagen). The purified PCR products were inserted into the pCR2.1-TOPO vector and transformed into competent Escherichia coli (TOP10 cells) using the TOPO TA cloning kit (Invitrogen). Recombinant plasmids were isolated from 10 to 20 white bacterial colonies and sequenced.
Statistical analysis
One-way analysis of variance (ANOVA) was performed to examine whether the mtDNA heterogeneity (total frequency, unique differences, base substitutions, and length alterations of the polymeric C tracts) observed in single CD34+ cells, lymphocytes, and granulocytes from the 5 healthy donors constituted significant statistical differences (P < .05).
Results
Aggregate genotype of the mtDNA control region
We analyzed new blood samples from 5 of the 6 healthy donors examined previously; donors 1 to 5 here correspond to donors 1 and 3 to 6 in the published study.18,19 The aggregate cell mitochondrial genotype of each donor was compared with the mtDNA heterogeneity found in single CD34+ cells, T cells, B cells, and granulocytes. There was marked variation in the number of nucleotide changes in the control region of aggregate cells' mtDNA among the 5 healthy volunteers, with a range from 6 (donor 1) to 24 (donor 3), as previously reported (Table 1).18,19 Fifty-nine mtDNA sequence variants were noted in the control region among the 5 donors; 58 of these variants were listed in the polymorphism database (http://www.mitomap.org), and the nucleotide variant (517A>G) was again found in donor 5 (donor 6 in the previous study18,19 ). Donor 2 had length variations of the homopolymeric C tracts at nucleotide positions 303 to 315 in the aggregate genotype (Table 1).
Nucleotide sequences change of mtDNA control region from aggregate cells
Donor no. (age, y/sex) and polymorphism . | Affected genes . |
|---|---|
| Donor no. 1 (48/F) | |
| 73A > G | HV2, 7S |
| 150C > T | HV2, 7S, OH |
| 263A > G | HV2, OH |
| 8CT6C* | HV2, OH, CSB2 |
| 16192C > T* | HV1, 7S |
| 16270C > T | HV1, 7S |
| Donor no. 2 (44/M) | |
| 73A > G | HV2, 7S |
| 146T > C | HV2, 7S, OH |
| 152T > C | HV2, 7S, OH |
| 195T > C | HV2, OH |
| 263A > G | HV2, OH |
| 8CT6C,* 9CT6C* | HV2, OH, CSB2 |
| 514-515delCA | NA |
| 16223C > T | HV1, 7S |
| 16278C > T | HV1, 7S |
| 16294C > T | HV1, 7S |
| 16390G > A | 7S |
| Donor no. 3 (36/M) | |
| 93A > G | HV2, 7S |
| 95A > C | HV2, 7S |
| 185G > A | HV2, 7S, OH |
| 189A > G | HV2, 7S, OH |
| 236T > C | HV2, OH |
| 8CT6C* | HV2, OH, CSB2 |
| 247G > A | HV2, OH, TFB1 |
| 263A > G | HV2, OH |
| 514-515delCA | NA |
| 16093T > C | HV1 |
| 16129G > A | HV1, 7S |
| 16148C > T | HV1, 7S |
| 16168C > T | HV1, 7S, TAS |
| 16172T > C | HV1, 7S, TAS |
| 16187C > T* | HV1, 7S |
| 16188C > G* | HV1, 7S |
| 16189T > C* | HV1, 7S |
| 16223C > T | HV1, 7S |
| 16230A > G | HV1, 7S |
| 16278C > T | HV1, 7S |
| 16293A > G | HV1, 7S |
| 16311T > C | HV1, 7S |
| 16320C > T | HV1, 7S |
| Donor no. 4 (55/M) | |
| 73A > G | HV2, 7S |
| 263A > G | HV2, OH |
| 7CT6C* | HV2, OH, CSB2 |
| 514-515delCA | NA |
| 16126T > C | HV1, 7S |
| 16294C > T | HV1, 7S |
| 16296C > T | HV1, 7S |
| 16519T > C | 7S |
| Donor no. 5 (35/F) | |
| 73A > G | HV2, 7S |
| 150C > T | HV2, 7S, OH |
| 263A > G | HV2, OH |
| 8CT6C* | HV2, OH, CSB2 |
| 517A > G† | NA |
| 16270C > T | HV1, 7S |
| 16292C > T | HV1, 7S |
| 16362T > C | HV1, 7S |
Donor no. (age, y/sex) and polymorphism . | Affected genes . |
|---|---|
| Donor no. 1 (48/F) | |
| 73A > G | HV2, 7S |
| 150C > T | HV2, 7S, OH |
| 263A > G | HV2, OH |
| 8CT6C* | HV2, OH, CSB2 |
| 16192C > T* | HV1, 7S |
| 16270C > T | HV1, 7S |
| Donor no. 2 (44/M) | |
| 73A > G | HV2, 7S |
| 146T > C | HV2, 7S, OH |
| 152T > C | HV2, 7S, OH |
| 195T > C | HV2, OH |
| 263A > G | HV2, OH |
| 8CT6C,* 9CT6C* | HV2, OH, CSB2 |
| 514-515delCA | NA |
| 16223C > T | HV1, 7S |
| 16278C > T | HV1, 7S |
| 16294C > T | HV1, 7S |
| 16390G > A | 7S |
| Donor no. 3 (36/M) | |
| 93A > G | HV2, 7S |
| 95A > C | HV2, 7S |
| 185G > A | HV2, 7S, OH |
| 189A > G | HV2, 7S, OH |
| 236T > C | HV2, OH |
| 8CT6C* | HV2, OH, CSB2 |
| 247G > A | HV2, OH, TFB1 |
| 263A > G | HV2, OH |
| 514-515delCA | NA |
| 16093T > C | HV1 |
| 16129G > A | HV1, 7S |
| 16148C > T | HV1, 7S |
| 16168C > T | HV1, 7S, TAS |
| 16172T > C | HV1, 7S, TAS |
| 16187C > T* | HV1, 7S |
| 16188C > G* | HV1, 7S |
| 16189T > C* | HV1, 7S |
| 16223C > T | HV1, 7S |
| 16230A > G | HV1, 7S |
| 16278C > T | HV1, 7S |
| 16293A > G | HV1, 7S |
| 16311T > C | HV1, 7S |
| 16320C > T | HV1, 7S |
| Donor no. 4 (55/M) | |
| 73A > G | HV2, 7S |
| 263A > G | HV2, OH |
| 7CT6C* | HV2, OH, CSB2 |
| 514-515delCA | NA |
| 16126T > C | HV1, 7S |
| 16294C > T | HV1, 7S |
| 16296C > T | HV1, 7S |
| 16519T > C | 7S |
| Donor no. 5 (35/F) | |
| 73A > G | HV2, 7S |
| 150C > T | HV2, 7S, OH |
| 263A > G | HV2, OH |
| 8CT6C* | HV2, OH, CSB2 |
| 517A > G† | NA |
| 16270C > T | HV1, 7S |
| 16292C > T | HV1, 7S |
| 16362T > C | HV1, 7S |
HV1 indicates hypervariable segment 1; HV2, hypervariable segment 2; 7S, 7S DNA; OH, H-strand origin; CSB2, conserved sequence block II; TAS, termination-association sequence; TFB1, mitochondrial transcription factor 1 binding site; NA, not applicable.
Homopolymeric C tracts at nucleotides 303 to 315 (for example, 7CT6C defined CCCCCCCTCCCCCC) and 16184 to 16193 in HV2 and HV1, respectively
New mtDNA polymorphisms (not listed in MitoMap database)
Single-cell PCR
Individual CD34+ cells, T cells, B cells, and granulocytes from the 5 healthy donors were isolated by flow cytometric sorting, and the mtDNA control region was amplified (see “Materials and methods”). We performed nested mtDNA PCR amplification of 96 single cells of CD34+ cells, T cells, B cells, and granulocytes for each donor. PCR amplification efficiency of the individual CD34+ cells, T cells, B cells, and granulocytes was 95.2% (457 of 480 cells), 95.4% (458 of 480 cells), 92.7% (445 of 480 cells), and 79.4% (381 of 480 cells), respectively (Table 2). Although there were variations in PCR efficiency among the donors, the PCR efficiency of granulocytes was lower than that for other cell types (Table 2). By using cell lysis buffer rather than heating for DNA extraction, the PCR amplification efficiency of granulocytes in this study (79.4%) was greatly improved compared with the 7.5% found previously.19
Nested mtDNA PCR efficiency of single CD34+ cells, lymphocytes and granulocytes from healthy donors
. | . | PCR efficiency . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | CD34+ cells . | . | T cells . | . | B cells . | . | Granulocytes . | . | |||||||
| Donor no. . | Age, y/sex . | No. total . | No. positive (%) . | No. total . | No. positive (%) . | No. total . | No. positive (%) . | No. total . | No. positive (%) . | |||||||
| 1 | 48/F | 96 | 85 (88.5) | 96 | 88 (91.7) | 96 | 87 (90.6) | 96 | 66 (68.8) | |||||||
| 2 | 44/M | 96 | 92 (95.8) | 96 | 94 (97.9) | 96 | 78 (81.2) | 96 | 82 (85.4) | |||||||
| 3 | 36/M | 96 | 94 (97.9) | 96 | 86 (89.6) | 96 | 94 (97.9) | 96 | 72 (75.0) | |||||||
| 4 | 55/M | 96 | 93 (96.9) | 96 | 94 (97.9) | 96 | 93 (96.9) | 96 | 77 (80.2) | |||||||
| 5 | 35/F | 96 | 93 (96.9) | 96 | 96 (100.0) | 96 | 93 (96.9) | 96 | 84 (87.5) | |||||||
| Total | NA/NA | 480 | 457 (95.2) | 480 | 458 (95.4) | 480 | 445 (92.7) | 480 | 381 (79.4) | |||||||
. | . | PCR efficiency . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | CD34+ cells . | . | T cells . | . | B cells . | . | Granulocytes . | . | |||||||
| Donor no. . | Age, y/sex . | No. total . | No. positive (%) . | No. total . | No. positive (%) . | No. total . | No. positive (%) . | No. total . | No. positive (%) . | |||||||
| 1 | 48/F | 96 | 85 (88.5) | 96 | 88 (91.7) | 96 | 87 (90.6) | 96 | 66 (68.8) | |||||||
| 2 | 44/M | 96 | 92 (95.8) | 96 | 94 (97.9) | 96 | 78 (81.2) | 96 | 82 (85.4) | |||||||
| 3 | 36/M | 96 | 94 (97.9) | 96 | 86 (89.6) | 96 | 94 (97.9) | 96 | 72 (75.0) | |||||||
| 4 | 55/M | 96 | 93 (96.9) | 96 | 94 (97.9) | 96 | 93 (96.9) | 96 | 77 (80.2) | |||||||
| 5 | 35/F | 96 | 93 (96.9) | 96 | 96 (100.0) | 96 | 93 (96.9) | 96 | 84 (87.5) | |||||||
| Total | NA/NA | 480 | 457 (95.2) | 480 | 458 (95.4) | 480 | 445 (92.7) | 480 | 381 (79.4) | |||||||
NA indicates not applicable.
Sequence heterogeneity within the mtDNA control region
A total of 457 CD34+ cells, 458 T cells, 445 B cells, and 381 granulocytes from each of the 5 donors were subjected to sequencing of the 1121-bp mtDNA control region, known to include mutational hot spots, in order to assess the heterogeneity of individual cell mtDNA sequences.22,23 A very small number of sequences (1 from CD34+ cells, 2 from T cells, 3 from B cells, and 5 from granulocytes) were discarded because of likely contamination (inferred from the absence of the donor's identifying polymorphisms present in the respective aggregate sequence). Our results, based on the analysis of 456 CD34+ cells, 456 T cells, 442 B cells, and 376 granulocytes from the 5 donors, showed that 37.9% ± 3.6% (mean ± SD) of CD34+ cells, 36.4% ± 14.1% of T cells, 36.0% ± 10.7% of B cells, and 47.7% ± 7.4% of granulocytes had mtDNA heterogeneities distinct from the donor's corresponding aggregate mtDNA sequences (Tables 3, 4). Although the amount of mtDNA heterogeneity varied among the cell types, only the greater amount of heterogeneity detected in the granulocytes was statistically significant when compared with CD34+ cells (P < .05).
Mutational spectra of mtDNA control region in single CD34+ cells, T cells, B cells, and granulocytes
. | . | Heterogeneity . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Donor no. (age, y/sex) and mtDNA sequence differences from aggregate sequence . | Cell no. . | Frequency . | . | Unique . | . | |||
| . | . | No. . | % . | No. . | % . | |||
| 1 (48/F) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 53 | — | — | — | — | |||
| Nonaggregate sequence | — | 32 | 37.6 | 13 | 15.3 | |||
| +8CT6C,* 9CT6C* | 20 | — | — | — | — | |||
| +71G > G/del,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16192C > T/C | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16207A > G/A | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16355C > C/T | 1 | — | — | — | — | |||
| +8CT6C* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16129G > G/A | 1 | — | — | — | — | |||
| +551A > G§ | 1 | — | — | — | — | |||
| +556A > G§ | 1 | — | — | — | — | |||
| +16129G > A/G | 1 | — | — | — | — | |||
| +16192C > T/C | 1 | — | — | — | — | |||
| +16482A > A/G, 16525A > G§ | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 53 | — | — | — | — | |||
| Nonaggregate sequence | — | 34 | 39.1 | 20 | 23 | |||
| +8CT6C,* 9CT6C* | 14 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 2 | — | — | — | — | |||
| +57T > T/C,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +71G > del,§ 8CT6C,* 9CT6C,* 16132A > A/G§ | 1 | — | — | — | — | |||
| +143G > G/A, 16278C > C/T | 1 | — | — | — | — | |||
| +162C > C/T,§ 16381T > T/C | 1 | — | — | — | — | |||
| +246T > T/C | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16390G > A | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16149A > A/G§ | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16192C† | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 16184C > T | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 1 | — | — | — | — | |||
| +526G > G/A§ | 1 | — | — | — | — | |||
| +16210A > A/G§ | 1 | — | — | — | — | |||
| +16222C > T | 1 | — | — | — | — | |||
| +16222C > C/T | 1 | — | — | — | — | |||
| +16267C > C/T§ | 1 | — | — | — | — | |||
| +16277A > A/G§ | 1 | — | — | — | — | |||
| +16390G > G/A | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 69 | — | — | — | — | |||
| Nonaggregate sequence | — | 17 | 19.8 | 11 | 12.8 | |||
| +8CT6C,* 9CT6C* | 6 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 2 | — | — | — | — | |||
| +7CT6C* | 1 | — | — | — | — | |||
| +189A > G | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +7CT6C,* 16262C > C/T | 1 | — | — | — | — | |||
| +16112C > C/A§ | 1 | — | — | — | — | |||
| +16147C > T | 1 | — | — | — | — | |||
| +16148C > C/T | 1 | — | — | — | — | |||
| +16189T > C, 16190C > C/T | 1 | — | — | — | — | |||
| +16390G > A | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 27 | — | — | — | — | |||
| Nonaggregate sequence | — | 37 | 57.8 | 27 | 42.2 | |||
| +8CT6C,* 9CT6C* | 8 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 4 | — | — | — | — | |||
| +37A > G/A§ | 1 | — | — | — | — | |||
| +61C > A/C§ | 1 | — | — | — | — | |||
| +72T > T/C | 1 | — | — | — | — | |||
| +96C > T/C,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +141C > C/T§ | 1 | — | — | — | — | |||
| +168T > T/C§ | 1 | — | — | — | — | |||
| +168T > T/C,§ 217T > T/C | 1 | — | — | — | — | |||
| +181A > A/G§ | 1 | — | — | — | — | |||
| +192T > T/C, 8CT6C,* 9CT6C,* 16203A > A/G | 1 | — | — | — | — | |||
| +204T > T/C, 16111C > G/C§ | 1 | — | — | — | — | |||
| +221G > A/G§ | 1 | — | — | — | — | |||
| +265T > T/C, 16213G > A, 16363C > C/T§ | 1 | — | — | — | — | |||
| +7CT6C,* 16445T > T/C§ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16227A > A/G | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16371A > A/G§ | 1 | — | — | — | — | |||
| +316G > G/A | 1 | — | — | — | — | |||
| +514-515delCA | 1 | — | — | — | — | |||
| +16036G > G/del§ | 1 | — | — | — | — | |||
| +16064T > T/C§ | 1 | — | — | — | — | |||
| +16066A > A/G | 1 | — | — | — | — | |||
| +16086T > C | 1 | — | — | — | — | |||
| +16106G > G/C§ | 1 | — | — | — | — | |||
| +16120A > A/G§ | 1 | — | — | — | — | |||
| +16344C > T | 1 | — | — | — | — | |||
| +16468T > T/C§ | 1 | — | — | — | — | |||
| 2 (44/M) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 60 | — | — | — | — | |||
| Nonaggregate sequence | — | 32 | 34.8 | 15 | 16.3 | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 12 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 5 | — | — | — | — | |||
| +8CT6C* | 3 | — | — | — | — | |||
| +64C > C/T | 1 | — | — | — | — | |||
| +228G > A/G | 1 | — | — | — | — | |||
| +236T > T/C | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 1 | — | — | — | — | |||
| +385A > G | 1 | — | — | — | — | |||
| +468C > C/A | 1 | — | — | — | — | |||
| +16140T > T/C, 16411C > C/T§ | 1 | — | — | — | — | |||
| +16269A > A/G | 1 | — | — | — | — | |||
| +16273G > G/A, 16544T > C§ | 1 | — | — | — | — | |||
| +16311T > T/C | 1 | — | — | — | — | |||
| +16321C > C/T | 1 | — | — | — | — | |||
| +16431C > C/T§ | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 77 | — | — | — | — | |||
| Nonaggregate sequence | — | 17 | 18.1 | 6 | 6.4 | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 10 | — | — | — | — | |||
| +8CT6C* | 3 | — | — | — | — | |||
| +204T > C/T | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 1 | — | — | — | — | |||
| +487A > A/G§ | 1 | — | — | — | — | |||
| +16399A > A/G | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 48 | — | — | — | — | |||
| Nonaggregate sequence | — | 30 | 38.5 | 14 | 17.9 | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 13 | — | — | — | — | |||
| +8CT6C* | 5 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16093T > C | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +368A > G§ | 1 | — | — | — | — | |||
| +465C > C/T§ | 1 | — | — | — | — | |||
| +16131T > T/C | 1 | — | — | — | — | |||
| +16170A > A/G | 1 | — | — | — | — | |||
| +16183A > G | 1 | — | — | — | — | |||
| +16183A > A/del | 1 | — | — | — | — | |||
| +16191C > C/T | 1 | — | — | — | — | |||
| +16353C > C/T | 1 | — | — | — | — | |||
| +16399A > G | 1 | — | — | — | — | |||
| +16423A > A/G§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 44 | — | — | — | — | |||
| Nonaggregate sequence | — | 38 | 46.3 | 26 | 31.7 | |||
| +9CT6C,* 10CT6C* | 7 | — | — | — | — | |||
| +8CT6C* | 4 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 3 | — | — | — | — | |||
| +122C > C/T§ | 2 | — | — | — | — | |||
| +49A > A/G,§ 8CT6C,* 16443T > T/C§ | 1 | — | — | — | — | |||
| +108A > G/A, 16335A > T/A§ | 1 | — | — | — | — | |||
| +119T > T/C, 16193C > C/T | 1 | — | — | — | — | |||
| +204T>C, 16513T > T/C§ | 1 | — | — | — | — | |||
| +263A > G/A, 8CT6C,* 9CT6C,* 10CT6C,* 402A > G/A§ | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16454C > C/T§ | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 16338A > A/G§ | 1 | — | — | — | — | |||
| +421T > T/C,§ 493A > A/G | 1 | — | — | — | — | |||
| +470A > A/G§ | 1 | — | — | — | — | |||
| +523A > G/A,§ 16494C > T/C§ | 1 | — | — | — | — | |||
| +16026C > T§ | 1 | — | — | — | — | |||
| +16081A > A/G | 1 | — | — | — | — | |||
| +16095C > T§ | 1 | — | — | — | — | |||
| +16143T > T/C,§ 16450G > G/A§ | 1 | — | — | — | — | |||
| +16184C > C/A | 1 | — | — | — | — | |||
| +16196G > G/A | 1 | — | — | — | — | |||
| +16285A > A/G§ | 1 | — | — | — | — | |||
| +16293A > A/G | 1 | — | — | — | — | |||
| +16343A > A/G | 1 | — | — | — | — | |||
| +16356T > T/C | 1 | — | — | — | — | |||
| +16454C > C/T§ | 1 | — | — | — | — | |||
| +16507C > C/A§ | 1 | — | — | — | — | |||
| 3 (36/M) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 59 | — | — | — | — | |||
| Nonaggregate sequence | — | 34 | 36.6 | 11 | 11.8 | |||
| +8CT6C,* 9CT6C* | 19 | — | — | — | — | |||
| +16093T > C/T | 4 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 2 | — | — | — | — | |||
| +30T > T/G§ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16093T > C/T | 1 | — | — | — | — | |||
| +16063T > T/C | 1 | — | — | — | — | |||
| +16093T > T/C | 1 | — | — | — | — | |||
| +16259C > C/T | 1 | — | — | — | — | |||
| +16305A > A/G | 1 | — | — | — | — | |||
| +16309A > A/G | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 41 | — | — | — | — | |||
| Nonaggregate sequence | — | 45 | 52.3 | 18 | 20.9 | |||
| +8CT6C,* 9CT6C* | 23 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 3 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 3 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 2 | — | — | — | — | |||
| +96C > C/T,§ 155T > T/C§ | 1 | — | — | — | — | |||
| +118G > G/A,§ 8CT6C,* 9CT6C,* 10CT6C,* 16224C > T/C | 1 | — | — | — | — | |||
| +143G > G/A, 8CT6C,* 9CT6C,* 16093T† | 1 | — | — | — | — | |||
| +150C > C/T, 8CT6C,* 9CT6C,* 16093T† | 1 | — | — | — | — | |||
| +309C > C/T, 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16442C > C/T§ | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C,* 12CT6C* | 1 | — | — | — | — | |||
| +399T > T/C,§ 16200A > A/G§, 16307A > A/G§ | 1 | — | — | — | — | |||
| +529G > A/G§ | 1 | — | — | — | — | |||
| +16093T > T/C, 16293A > G/A | 1 | — | — | — | — | |||
| +16292C > C/T | 1 | — | — | — | — | |||
| +16401C > C/T§ | 1 | — | — | — | — | |||
| +16410C > C/T§ | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 48 | — | — | — | — | |||
| Nonaggregate sequence | — | 46 | 48.9 | 17 | 18.1 | |||
| +8CT6C,* 9CT6C* | 29 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 2 | — | — | — | — | |||
| +18C > C/T§ | 1 | — | — | — | — | |||
| +103G > A | 1 | — | — | — | — | |||
| +103G > G/A, 8CT6C,* 9CT6C,* 16390G > A/G | 1 | — | — | — | — | |||
| +200A > A/G | 1 | — | — | — | — | |||
| +283A > A/G§ | 1 | — | — | — | — | |||
| +7CT6C,* 16261C > T | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16222C > C/T | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16456G > G/A | 1 | — | — | — | — | |||
| +16092T > T/C | 1 | — | — | — | — | |||
| +16093T > T/C | 1 | — | — | — | — | |||
| +16390G > A | 1 | — | — | — | — | |||
| +16400C > C/T | 1 | — | — | — | — | |||
| +16423A > A/G§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 42 | — | — | — | — | |||
| Nonaggregate sequence | — | 30 | 41.7 | 25 | 34.7 | |||
| +8CT6C,* 9CT6C* | 6 | — | — | — | — | |||
| +116A > A/G§ | 1 | — | — | — | — | |||
| +127T > T/C, 16357T > T/A§ | 1 | — | — | — | — | |||
| +162C > C/T§ | 1 | — | — | — | — | |||
| +181A > A/G§ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16561A > A/G§ | 1 | — | — | — | — | |||
| +318T > T/C | 1 | — | — | — | — | |||
| +437C > T/C,§ 16197C > C/T§ | 1 | — | — | — | — | |||
| +502C > T/C | 1 | — | — | — | — | |||
| +533A > G§ | 1 | — | — | — | — | |||
| +570C > C/T§ | 1 | — | — | — | — | |||
| +16064T > T/C,§ 16445T > T/C§ | 1 | — | — | — | — | |||
| +16075T > T/G§ | 1 | — | — | — | — | |||
| +16109A > A/C§ | 1 | — | — | — | — | |||
| +16129G > A/G | 1 | — | — | — | — | |||
| +16132A > A/G§ | 1 | — | — | — | — | |||
| +16149A > A/G§ | 1 | — | — | — | — | |||
| +16339C > T/C§ | 1 | — | — | — | — | |||
| +16350A > A/G | 1 | — | — | — | — | |||
| +16357T > T/C | 1 | — | — | — | — | |||
| +16365C > C/T | 1 | — | — | — | — | |||
| +16396T > T/C§ | 1 | — | — | — | — | |||
| +16411C > C/T§ | 1 | — | — | — | — | |||
| +16457G > G/A§ | 1 | — | — | — | — | |||
| 4 (55/M) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 52 | — | — | — | — | |||
| Nonaggregate sequence | 41 | 44.1 | 11 | 11.8 | ||||
| +264C > T | 12 | — | — | — | — | |||
| +514insCA† | 10 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 5 | — | — | — | — | |||
| +264C > T/C | 2 | — | — | — | — | |||
| +514insCA, 514-515delCA‡ | 2 | — | — | — | — | |||
| +43C > C/T,§ 264C > T | 1 | — | — | — | — | |||
| +146T > C/T | 1 | — | — | — | — | |||
| +146T > C/T, 514insCA† | 1 | — | — | — | — | |||
| +146T > T/C, 514insCA† | 1 | — | — | — | — | |||
| +150C > T/C, 514insCA, 514-515delCA‡ | 1 | — | — | — | — | |||
| +264C > C/T | 1 | — | — | — | — | |||
| +264C > T, 16327C > C/T | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 514insCA† | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡, 16287C > C/T | 1 | — | — | — | — | |||
| +16156G > G/A§ | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 50 | — | — | — | — | |||
| Nonaggregate sequence | — | 43 | 46.2 | 25 | 26.9 | |||
| +514insCA† | 13 | — | — | — | — | |||
| +264C > T | 5 | — | — | — | — | |||
| +3T > T/C,§ 264C > C/T, 16518G > G/A§ | 1 | — | — | — | — | |||
| +146T > C, 514insCA† | 1 | — | — | — | — | |||
| +178A > A/G§ | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +214A > G | 1 | — | — | — | — | |||
| +217T > T/C, 6CT6C,* 7CT6C* | 1 | — | — | — | — | |||
| +264C > C/T | 1 | — | — | — | — | |||
| +264C > T 401T > T/C,§ 425A > A/G§ | 1 | — | — | — | — | |||
| +6CT6C,* 7CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡ | 1 | — | — | — | — | |||
| +514-515delCA, 514insCA,‡ 16200A > A/G§ | 1 | — | — | — | — | |||
| +514insCA,† 16292C > T | 1 | — | — | — | — | |||
| +514insCA,† 16206A > A/G,§ 16452T > T/C§ | 1 | — | — | — | — | |||
| +514insCA,† 16569G > G/A§ | 1 | — | — | — | — | |||
| +526G > G/A§ | 1 | — | — | — | — | |||
| +16155A > A/G | 1 | — | — | — | — | |||
| +16174C > C/T | 1 | — | — | — | — | |||
| +16206A > A/G,§ 16328C > C/T | 1 | — | — | — | — | |||
| +16292C > C/T | 1 | — | — | — | — | |||
| +16296C† | 1 | — | — | — | — | |||
| +16299A > A/G | 1 | — | — | — | — | |||
| +16385A > A/G§ | 1 | — | — | — | — | |||
| +16428G > G/A§ | 1 | — | — | — | — | |||
| +16537C > C/T§ | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 56 | — | — | — | — | |||
| Nonaggregate sequence | 37 | 39.8 | 14 | 15.1 | ||||
| +264C > T | 14 | — | — | — | — | |||
| +514insCA† | 9 | — | — | — | — | |||
| +6C > C/T,§ 514insCA† | 1 | — | — | — | — | |||
| +146T > C, 514insCA† | 1 | — | — | — | — | |||
| +152T > C, 170C > C/T§ | 1 | — | — | — | — | |||
| +169A > A/G§ | 1 | — | — | — | — | |||
| +214A > G | 1 | — | — | — | — | |||
| +237A > A/G, 264C > T | 1 | — | — | — | — | |||
| +264C > T/C | 1 | — | — | — | — | |||
| +264C > T, 7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +264C > T, 16338A > A/G§ | 1 | — | — | — | — | |||
| +7CT6C* 8CT6C* | 1 | — | — | — | — | |||
| +6CT6C,* 7CT6C,* 16338A > A/G§ | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡ | 1 | — | — | — | — | |||
| +514insCA,† 7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +514insCA,† 16382C > C/T§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 36 | — | — | — | — | |||
| Nonaggregate sequence | — | 40 | 52.6 | 25 | 32.9 | |||
| +514insCA† | 11 | — | — | — | — | |||
| +264C > T | 5 | — | — | — | — | |||
| +146T > C | 1 | — | — | — | — | |||
| +146T > C, 514insCA† | 1 | — | — | — | — | |||
| +146T > T/C, 514insCA† | 1 | — | — | — | — | |||
| +146T > C, 514insCA†, 16292C > C/T | 1 | — | — | — | — | |||
| +174C > T,§ 514insCA† | 1 | — | — | — | — | |||
| +188A > A/G, 264C > C/T, 420C > C/T | 1 | — | — | — | — | |||
| +210A > T/A§ | 1 | — | — | — | — | |||
| +215A > G, 514insCA,† 16434G > G/A§ | 1 | — | — | — | — | |||
| +264C > T, 407T > T/C§ | 1 | — | — | — | — | |||
| +264C > T, 16415A > A/G§ | 1 | — | — | — | — | |||
| +294T > T/C, 514-515delCA, 514insCA‡ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 514insCA† | 1 | — | — | — | — | |||
| +8CT6C* | 1 | — | — | — | — | |||
| +368A > A/G,§ 523A > A/G§ | 1 | — | — | — | — | |||
| +382C > T§ | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡, 16362T > T/C | 1 | — | — | — | — | |||
| +536C > T/C§ | 1 | — | — | — | — | |||
| +16096G > T,§ 16379C > C/T§ | 1 | — | — | — | — | |||
| +16166A > A/del§ | 1 | — | — | — | — | |||
| +16189T > T/del§ | 1 | — | — | — | — | |||
| +16196G > G/A, 16224T > T/C | 1 | — | — | — | — | |||
| +16199T > T/C,§ 16334T > T/C§ | 1 | — | — | — | — | |||
| +16314A > A/G | 1 | — | — | — | — | |||
| 5 (35/F) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 59 | — | — | — | — | |||
| Nonaggregate sequence | — | 34 | 36.6 | 11 | 11.8 | |||
| +8CT6C,* 9CT6C* | 19 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 4 | — | — | — | — | |||
| +200A > A/G | 2 | — | — | — | — | |||
| +146T > C/T | 1 | — | — | — | — | |||
| +186C > C/T | 1 | — | — | — | — | |||
| +200A > G | 1 | — | — | — | — | |||
| +200A > A/G, 7CT6C,* 8CT6C,* 16362T > C/T | 1 | — | — | — | — | |||
| +200A > G, 16326A > A/G | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +220T > T/C§ | 1 | — | — | — | — | |||
| +263A > G/A | 1 | — | — | — | — | |||
| +385A > G | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 71 | — | — | — | — | |||
| Nonaggregate sequence | — | 25 | 26 | 15 | 15.6 | |||
| +8CT6C,* 9CT6C* | 10 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 9CT6C* | 2 | — | — | — | — | |||
| +14T > T/C§ | 1 | — | — | — | — | |||
| +134T > T/C,§ 7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +200A > G | 1 | — | — | — | — | |||
| +228G > A/G | 1 | — | — | — | — | |||
| +7CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16140T > T/C | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16092T > T/C | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16212A > A/G | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16360C > C/T | 1 | — | — | — | — | |||
| +316G > G/A, 16111C > C/A§ | 1 | — | — | — | — | |||
| +16225C > C/T§ | 1 | — | — | — | — | |||
| +16230A > A/G | 1 | — | — | — | — | |||
| +16463A > A/G | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 61 | — | — | — | — | |||
| Nonaggregate sequence | — | 30 | 33 | 17 | 18.7 | |||
| +8CT6C,* 9CT6C* | 11 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 3 | — | — | — | — | |||
| +146T > T/C | 2 | — | — | — | — | |||
| +20T > T/C§ | 1 | — | — | — | — | |||
| +105C > C/T,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +200A > A/G | 1 | — | — | — | — | |||
| +204T > C, 8CT6C,* 9CT6C,* 16278C > C/T | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16072C > C/G§ | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +16151C > C/T§ | 1 | — | — | — | — | |||
| +16166A > A/del§ | 1 | — | — | — | — | |||
| +16213G > G/A | 1 | — | — | — | — | |||
| +16254A > A/del§ | 1 | — | — | — | — | |||
| +16275A > A/T§ | 1 | — | — | — | — | |||
| +16292C > T/C§ | 1 | — | — | — | — | |||
| +16310G > G/A | 1 | — | — | — | — | |||
| +16340A > A/G§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 49 | — | — | — | — | |||
| Nonaggregate sequence | — | 33 | 40.2 | 29 | 35.4 | |||
| +8CT6C,* 9CT6C* | 2 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 2 | — | — | — | — | |||
| +68G > G/A | 1 | — | — | — | — | |||
| +71G > G/del§ | 1 | — | — | — | — | |||
| +80C/T,§ 164C/T§ | 1 | — | — | — | — | |||
| +82A > A/G,§ 200A > G | 1 | — | — | — | — | |||
| +93A > A/G, 16185C > T | 1 | — | — | — | — | |||
| +122C > T§ | 1 | — | — | — | — | |||
| +139T > C/T§ | 1 | — | — | — | — | |||
| +172T > T/O§ | 1 | — | — | — | — | |||
| +199T > C, 16217T > T/C, 16439C > T§ | 1 | — | — | — | — | |||
| +200A > G | 1 | — | — | — | — | |||
| +200A > G/A | 1 | — | — | — | — | |||
| +200A > A/G | 1 | — | — | — | — | |||
| +200A > G, 223T > T/C | 1 | — | — | — | — | |||
| +229G > C§ | 1 | — | — | — | — | |||
| +266T > T/C | 1 | — | — | — | — | |||
| +304C > C/T§ | 1 | — | — | — | — | |||
| +310T > C/T | 1 | — | — | — | — | |||
| +378C > C/T,§ 16189T > T/C | 1 | — | — | — | — | |||
| +385A > A/del§ | 1 | — | — | — | — | |||
| +457C > C/T§, 517A > G§ | 1 | — | — | — | — | |||
| +16112C > C/T,§ 16420A > G/A§ | 1 | — | — | — | — | |||
| +16113A > G§ | 1 | — | — | — | — | |||
| +16123T > T/C§ | 1 | — | — | — | — | |||
| +16285A > A/G§ | 1 | — | — | — | — | |||
| +16305A > A/G | 1 | — | — | — | — | |||
| +16325T > T/C | 1 | — | — | — | — | |||
| +16350A > A/G | 1 | — | — | — | — | |||
| +16426C > T§ | 1 | — | — | — | — | |||
| +16527C > C/T§ | 1 | — | — | — | — | |||
. | . | Heterogeneity . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Donor no. (age, y/sex) and mtDNA sequence differences from aggregate sequence . | Cell no. . | Frequency . | . | Unique . | . | |||
| . | . | No. . | % . | No. . | % . | |||
| 1 (48/F) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 53 | — | — | — | — | |||
| Nonaggregate sequence | — | 32 | 37.6 | 13 | 15.3 | |||
| +8CT6C,* 9CT6C* | 20 | — | — | — | — | |||
| +71G > G/del,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16192C > T/C | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16207A > G/A | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16355C > C/T | 1 | — | — | — | — | |||
| +8CT6C* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16129G > G/A | 1 | — | — | — | — | |||
| +551A > G§ | 1 | — | — | — | — | |||
| +556A > G§ | 1 | — | — | — | — | |||
| +16129G > A/G | 1 | — | — | — | — | |||
| +16192C > T/C | 1 | — | — | — | — | |||
| +16482A > A/G, 16525A > G§ | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 53 | — | — | — | — | |||
| Nonaggregate sequence | — | 34 | 39.1 | 20 | 23 | |||
| +8CT6C,* 9CT6C* | 14 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 2 | — | — | — | — | |||
| +57T > T/C,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +71G > del,§ 8CT6C,* 9CT6C,* 16132A > A/G§ | 1 | — | — | — | — | |||
| +143G > G/A, 16278C > C/T | 1 | — | — | — | — | |||
| +162C > C/T,§ 16381T > T/C | 1 | — | — | — | — | |||
| +246T > T/C | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16390G > A | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16149A > A/G§ | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16192C† | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 16184C > T | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 1 | — | — | — | — | |||
| +526G > G/A§ | 1 | — | — | — | — | |||
| +16210A > A/G§ | 1 | — | — | — | — | |||
| +16222C > T | 1 | — | — | — | — | |||
| +16222C > C/T | 1 | — | — | — | — | |||
| +16267C > C/T§ | 1 | — | — | — | — | |||
| +16277A > A/G§ | 1 | — | — | — | — | |||
| +16390G > G/A | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 69 | — | — | — | — | |||
| Nonaggregate sequence | — | 17 | 19.8 | 11 | 12.8 | |||
| +8CT6C,* 9CT6C* | 6 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 2 | — | — | — | — | |||
| +7CT6C* | 1 | — | — | — | — | |||
| +189A > G | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +7CT6C,* 16262C > C/T | 1 | — | — | — | — | |||
| +16112C > C/A§ | 1 | — | — | — | — | |||
| +16147C > T | 1 | — | — | — | — | |||
| +16148C > C/T | 1 | — | — | — | — | |||
| +16189T > C, 16190C > C/T | 1 | — | — | — | — | |||
| +16390G > A | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 27 | — | — | — | — | |||
| Nonaggregate sequence | — | 37 | 57.8 | 27 | 42.2 | |||
| +8CT6C,* 9CT6C* | 8 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 4 | — | — | — | — | |||
| +37A > G/A§ | 1 | — | — | — | — | |||
| +61C > A/C§ | 1 | — | — | — | — | |||
| +72T > T/C | 1 | — | — | — | — | |||
| +96C > T/C,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +141C > C/T§ | 1 | — | — | — | — | |||
| +168T > T/C§ | 1 | — | — | — | — | |||
| +168T > T/C,§ 217T > T/C | 1 | — | — | — | — | |||
| +181A > A/G§ | 1 | — | — | — | — | |||
| +192T > T/C, 8CT6C,* 9CT6C,* 16203A > A/G | 1 | — | — | — | — | |||
| +204T > T/C, 16111C > G/C§ | 1 | — | — | — | — | |||
| +221G > A/G§ | 1 | — | — | — | — | |||
| +265T > T/C, 16213G > A, 16363C > C/T§ | 1 | — | — | — | — | |||
| +7CT6C,* 16445T > T/C§ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16227A > A/G | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16371A > A/G§ | 1 | — | — | — | — | |||
| +316G > G/A | 1 | — | — | — | — | |||
| +514-515delCA | 1 | — | — | — | — | |||
| +16036G > G/del§ | 1 | — | — | — | — | |||
| +16064T > T/C§ | 1 | — | — | — | — | |||
| +16066A > A/G | 1 | — | — | — | — | |||
| +16086T > C | 1 | — | — | — | — | |||
| +16106G > G/C§ | 1 | — | — | — | — | |||
| +16120A > A/G§ | 1 | — | — | — | — | |||
| +16344C > T | 1 | — | — | — | — | |||
| +16468T > T/C§ | 1 | — | — | — | — | |||
| 2 (44/M) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 60 | — | — | — | — | |||
| Nonaggregate sequence | — | 32 | 34.8 | 15 | 16.3 | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 12 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 5 | — | — | — | — | |||
| +8CT6C* | 3 | — | — | — | — | |||
| +64C > C/T | 1 | — | — | — | — | |||
| +228G > A/G | 1 | — | — | — | — | |||
| +236T > T/C | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 1 | — | — | — | — | |||
| +385A > G | 1 | — | — | — | — | |||
| +468C > C/A | 1 | — | — | — | — | |||
| +16140T > T/C, 16411C > C/T§ | 1 | — | — | — | — | |||
| +16269A > A/G | 1 | — | — | — | — | |||
| +16273G > G/A, 16544T > C§ | 1 | — | — | — | — | |||
| +16311T > T/C | 1 | — | — | — | — | |||
| +16321C > C/T | 1 | — | — | — | — | |||
| +16431C > C/T§ | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 77 | — | — | — | — | |||
| Nonaggregate sequence | — | 17 | 18.1 | 6 | 6.4 | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 10 | — | — | — | — | |||
| +8CT6C* | 3 | — | — | — | — | |||
| +204T > C/T | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 1 | — | — | — | — | |||
| +487A > A/G§ | 1 | — | — | — | — | |||
| +16399A > A/G | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 48 | — | — | — | — | |||
| Nonaggregate sequence | — | 30 | 38.5 | 14 | 17.9 | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 13 | — | — | — | — | |||
| +8CT6C* | 5 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16093T > C | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +368A > G§ | 1 | — | — | — | — | |||
| +465C > C/T§ | 1 | — | — | — | — | |||
| +16131T > T/C | 1 | — | — | — | — | |||
| +16170A > A/G | 1 | — | — | — | — | |||
| +16183A > G | 1 | — | — | — | — | |||
| +16183A > A/del | 1 | — | — | — | — | |||
| +16191C > C/T | 1 | — | — | — | — | |||
| +16353C > C/T | 1 | — | — | — | — | |||
| +16399A > G | 1 | — | — | — | — | |||
| +16423A > A/G§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 44 | — | — | — | — | |||
| Nonaggregate sequence | — | 38 | 46.3 | 26 | 31.7 | |||
| +9CT6C,* 10CT6C* | 7 | — | — | — | — | |||
| +8CT6C* | 4 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 3 | — | — | — | — | |||
| +122C > C/T§ | 2 | — | — | — | — | |||
| +49A > A/G,§ 8CT6C,* 16443T > T/C§ | 1 | — | — | — | — | |||
| +108A > G/A, 16335A > T/A§ | 1 | — | — | — | — | |||
| +119T > T/C, 16193C > C/T | 1 | — | — | — | — | |||
| +204T>C, 16513T > T/C§ | 1 | — | — | — | — | |||
| +263A > G/A, 8CT6C,* 9CT6C,* 10CT6C,* 402A > G/A§ | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16454C > C/T§ | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 16338A > A/G§ | 1 | — | — | — | — | |||
| +421T > T/C,§ 493A > A/G | 1 | — | — | — | — | |||
| +470A > A/G§ | 1 | — | — | — | — | |||
| +523A > G/A,§ 16494C > T/C§ | 1 | — | — | — | — | |||
| +16026C > T§ | 1 | — | — | — | — | |||
| +16081A > A/G | 1 | — | — | — | — | |||
| +16095C > T§ | 1 | — | — | — | — | |||
| +16143T > T/C,§ 16450G > G/A§ | 1 | — | — | — | — | |||
| +16184C > C/A | 1 | — | — | — | — | |||
| +16196G > G/A | 1 | — | — | — | — | |||
| +16285A > A/G§ | 1 | — | — | — | — | |||
| +16293A > A/G | 1 | — | — | — | — | |||
| +16343A > A/G | 1 | — | — | — | — | |||
| +16356T > T/C | 1 | — | — | — | — | |||
| +16454C > C/T§ | 1 | — | — | — | — | |||
| +16507C > C/A§ | 1 | — | — | — | — | |||
| 3 (36/M) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 59 | — | — | — | — | |||
| Nonaggregate sequence | — | 34 | 36.6 | 11 | 11.8 | |||
| +8CT6C,* 9CT6C* | 19 | — | — | — | — | |||
| +16093T > C/T | 4 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 2 | — | — | — | — | |||
| +30T > T/G§ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16093T > C/T | 1 | — | — | — | — | |||
| +16063T > T/C | 1 | — | — | — | — | |||
| +16093T > T/C | 1 | — | — | — | — | |||
| +16259C > C/T | 1 | — | — | — | — | |||
| +16305A > A/G | 1 | — | — | — | — | |||
| +16309A > A/G | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 41 | — | — | — | — | |||
| Nonaggregate sequence | — | 45 | 52.3 | 18 | 20.9 | |||
| +8CT6C,* 9CT6C* | 23 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 3 | — | — | — | — | |||
| +9CT6C,* 10CT6C* | 3 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C* | 2 | — | — | — | — | |||
| +96C > C/T,§ 155T > T/C§ | 1 | — | — | — | — | |||
| +118G > G/A,§ 8CT6C,* 9CT6C,* 10CT6C,* 16224C > T/C | 1 | — | — | — | — | |||
| +143G > G/A, 8CT6C,* 9CT6C,* 16093T† | 1 | — | — | — | — | |||
| +150C > C/T, 8CT6C,* 9CT6C,* 16093T† | 1 | — | — | — | — | |||
| +309C > C/T, 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16442C > C/T§ | 1 | — | — | — | — | |||
| +9CT6C,* 10CT6C,* 11CT6C,* 12CT6C* | 1 | — | — | — | — | |||
| +399T > T/C,§ 16200A > A/G§, 16307A > A/G§ | 1 | — | — | — | — | |||
| +529G > A/G§ | 1 | — | — | — | — | |||
| +16093T > T/C, 16293A > G/A | 1 | — | — | — | — | |||
| +16292C > C/T | 1 | — | — | — | — | |||
| +16401C > C/T§ | 1 | — | — | — | — | |||
| +16410C > C/T§ | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 48 | — | — | — | — | |||
| Nonaggregate sequence | — | 46 | 48.9 | 17 | 18.1 | |||
| +8CT6C,* 9CT6C* | 29 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 2 | — | — | — | — | |||
| +18C > C/T§ | 1 | — | — | — | — | |||
| +103G > A | 1 | — | — | — | — | |||
| +103G > G/A, 8CT6C,* 9CT6C,* 16390G > A/G | 1 | — | — | — | — | |||
| +200A > A/G | 1 | — | — | — | — | |||
| +283A > A/G§ | 1 | — | — | — | — | |||
| +7CT6C,* 16261C > T | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16222C > C/T | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C,* 16456G > G/A | 1 | — | — | — | — | |||
| +16092T > T/C | 1 | — | — | — | — | |||
| +16093T > T/C | 1 | — | — | — | — | |||
| +16390G > A | 1 | — | — | — | — | |||
| +16400C > C/T | 1 | — | — | — | — | |||
| +16423A > A/G§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 42 | — | — | — | — | |||
| Nonaggregate sequence | — | 30 | 41.7 | 25 | 34.7 | |||
| +8CT6C,* 9CT6C* | 6 | — | — | — | — | |||
| +116A > A/G§ | 1 | — | — | — | — | |||
| +127T > T/C, 16357T > T/A§ | 1 | — | — | — | — | |||
| +162C > C/T§ | 1 | — | — | — | — | |||
| +181A > A/G§ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16561A > A/G§ | 1 | — | — | — | — | |||
| +318T > T/C | 1 | — | — | — | — | |||
| +437C > T/C,§ 16197C > C/T§ | 1 | — | — | — | — | |||
| +502C > T/C | 1 | — | — | — | — | |||
| +533A > G§ | 1 | — | — | — | — | |||
| +570C > C/T§ | 1 | — | — | — | — | |||
| +16064T > T/C,§ 16445T > T/C§ | 1 | — | — | — | — | |||
| +16075T > T/G§ | 1 | — | — | — | — | |||
| +16109A > A/C§ | 1 | — | — | — | — | |||
| +16129G > A/G | 1 | — | — | — | — | |||
| +16132A > A/G§ | 1 | — | — | — | — | |||
| +16149A > A/G§ | 1 | — | — | — | — | |||
| +16339C > T/C§ | 1 | — | — | — | — | |||
| +16350A > A/G | 1 | — | — | — | — | |||
| +16357T > T/C | 1 | — | — | — | — | |||
| +16365C > C/T | 1 | — | — | — | — | |||
| +16396T > T/C§ | 1 | — | — | — | — | |||
| +16411C > C/T§ | 1 | — | — | — | — | |||
| +16457G > G/A§ | 1 | — | — | — | — | |||
| 4 (55/M) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 52 | — | — | — | — | |||
| Nonaggregate sequence | 41 | 44.1 | 11 | 11.8 | ||||
| +264C > T | 12 | — | — | — | — | |||
| +514insCA† | 10 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 5 | — | — | — | — | |||
| +264C > T/C | 2 | — | — | — | — | |||
| +514insCA, 514-515delCA‡ | 2 | — | — | — | — | |||
| +43C > C/T,§ 264C > T | 1 | — | — | — | — | |||
| +146T > C/T | 1 | — | — | — | — | |||
| +146T > C/T, 514insCA† | 1 | — | — | — | — | |||
| +146T > T/C, 514insCA† | 1 | — | — | — | — | |||
| +150C > T/C, 514insCA, 514-515delCA‡ | 1 | — | — | — | — | |||
| +264C > C/T | 1 | — | — | — | — | |||
| +264C > T, 16327C > C/T | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 514insCA† | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡, 16287C > C/T | 1 | — | — | — | — | |||
| +16156G > G/A§ | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 50 | — | — | — | — | |||
| Nonaggregate sequence | — | 43 | 46.2 | 25 | 26.9 | |||
| +514insCA† | 13 | — | — | — | — | |||
| +264C > T | 5 | — | — | — | — | |||
| +3T > T/C,§ 264C > C/T, 16518G > G/A§ | 1 | — | — | — | — | |||
| +146T > C, 514insCA† | 1 | — | — | — | — | |||
| +178A > A/G§ | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +214A > G | 1 | — | — | — | — | |||
| +217T > T/C, 6CT6C,* 7CT6C* | 1 | — | — | — | — | |||
| +264C > C/T | 1 | — | — | — | — | |||
| +264C > T 401T > T/C,§ 425A > A/G§ | 1 | — | — | — | — | |||
| +6CT6C,* 7CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡ | 1 | — | — | — | — | |||
| +514-515delCA, 514insCA,‡ 16200A > A/G§ | 1 | — | — | — | — | |||
| +514insCA,† 16292C > T | 1 | — | — | — | — | |||
| +514insCA,† 16206A > A/G,§ 16452T > T/C§ | 1 | — | — | — | — | |||
| +514insCA,† 16569G > G/A§ | 1 | — | — | — | — | |||
| +526G > G/A§ | 1 | — | — | — | — | |||
| +16155A > A/G | 1 | — | — | — | — | |||
| +16174C > C/T | 1 | — | — | — | — | |||
| +16206A > A/G,§ 16328C > C/T | 1 | — | — | — | — | |||
| +16292C > C/T | 1 | — | — | — | — | |||
| +16296C† | 1 | — | — | — | — | |||
| +16299A > A/G | 1 | — | — | — | — | |||
| +16385A > A/G§ | 1 | — | — | — | — | |||
| +16428G > G/A§ | 1 | — | — | — | — | |||
| +16537C > C/T§ | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 56 | — | — | — | — | |||
| Nonaggregate sequence | 37 | 39.8 | 14 | 15.1 | ||||
| +264C > T | 14 | — | — | — | — | |||
| +514insCA† | 9 | — | — | — | — | |||
| +6C > C/T,§ 514insCA† | 1 | — | — | — | — | |||
| +146T > C, 514insCA† | 1 | — | — | — | — | |||
| +152T > C, 170C > C/T§ | 1 | — | — | — | — | |||
| +169A > A/G§ | 1 | — | — | — | — | |||
| +214A > G | 1 | — | — | — | — | |||
| +237A > A/G, 264C > T | 1 | — | — | — | — | |||
| +264C > T/C | 1 | — | — | — | — | |||
| +264C > T, 7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +264C > T, 16338A > A/G§ | 1 | — | — | — | — | |||
| +7CT6C* 8CT6C* | 1 | — | — | — | — | |||
| +6CT6C,* 7CT6C,* 16338A > A/G§ | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡ | 1 | — | — | — | — | |||
| +514insCA,† 7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +514insCA,† 16382C > C/T§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 36 | — | — | — | — | |||
| Nonaggregate sequence | — | 40 | 52.6 | 25 | 32.9 | |||
| +514insCA† | 11 | — | — | — | — | |||
| +264C > T | 5 | — | — | — | — | |||
| +146T > C | 1 | — | — | — | — | |||
| +146T > C, 514insCA† | 1 | — | — | — | — | |||
| +146T > T/C, 514insCA† | 1 | — | — | — | — | |||
| +146T > C, 514insCA†, 16292C > C/T | 1 | — | — | — | — | |||
| +174C > T,§ 514insCA† | 1 | — | — | — | — | |||
| +188A > A/G, 264C > C/T, 420C > C/T | 1 | — | — | — | — | |||
| +210A > T/A§ | 1 | — | — | — | — | |||
| +215A > G, 514insCA,† 16434G > G/A§ | 1 | — | — | — | — | |||
| +264C > T, 407T > T/C§ | 1 | — | — | — | — | |||
| +264C > T, 16415A > A/G§ | 1 | — | — | — | — | |||
| +294T > T/C, 514-515delCA, 514insCA‡ | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 514insCA† | 1 | — | — | — | — | |||
| +8CT6C* | 1 | — | — | — | — | |||
| +368A > A/G,§ 523A > A/G§ | 1 | — | — | — | — | |||
| +382C > T§ | 1 | — | — | — | — | |||
| +514insCA, 514-515delCA‡, 16362T > T/C | 1 | — | — | — | — | |||
| +536C > T/C§ | 1 | — | — | — | — | |||
| +16096G > T,§ 16379C > C/T§ | 1 | — | — | — | — | |||
| +16166A > A/del§ | 1 | — | — | — | — | |||
| +16189T > T/del§ | 1 | — | — | — | — | |||
| +16196G > G/A, 16224T > T/C | 1 | — | — | — | — | |||
| +16199T > T/C,§ 16334T > T/C§ | 1 | — | — | — | — | |||
| +16314A > A/G | 1 | — | — | — | — | |||
| 5 (35/F) | ||||||||
| CD34+ cells | ||||||||
| Aggregate sequence | 59 | — | — | — | — | |||
| Nonaggregate sequence | — | 34 | 36.6 | 11 | 11.8 | |||
| +8CT6C,* 9CT6C* | 19 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 4 | — | — | — | — | |||
| +200A > A/G | 2 | — | — | — | — | |||
| +146T > C/T | 1 | — | — | — | — | |||
| +186C > C/T | 1 | — | — | — | — | |||
| +200A > G | 1 | — | — | — | — | |||
| +200A > A/G, 7CT6C,* 8CT6C,* 16362T > C/T | 1 | — | — | — | — | |||
| +200A > G, 16326A > A/G | 1 | — | — | — | — | |||
| +204T > C | 1 | — | — | — | — | |||
| +220T > T/C§ | 1 | — | — | — | — | |||
| +263A > G/A | 1 | — | — | — | — | |||
| +385A > G | 1 | — | — | — | — | |||
| T cells | ||||||||
| Aggregate sequence | 71 | — | — | — | — | |||
| Nonaggregate sequence | — | 25 | 26 | 15 | 15.6 | |||
| +8CT6C,* 9CT6C* | 10 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 9CT6C* | 2 | — | — | — | — | |||
| +14T > T/C§ | 1 | — | — | — | — | |||
| +134T > T/C,§ 7CT6C,* 8CT6C* | 1 | — | — | — | — | |||
| +200A > G | 1 | — | — | — | — | |||
| +228G > A/G | 1 | — | — | — | — | |||
| +7CT6C* | 1 | — | — | — | — | |||
| +7CT6C,* 8CT6C,* 16140T > T/C | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16092T > T/C | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16212A > A/G | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16360C > C/T | 1 | — | — | — | — | |||
| +316G > G/A, 16111C > C/A§ | 1 | — | — | — | — | |||
| +16225C > C/T§ | 1 | — | — | — | — | |||
| +16230A > A/G | 1 | — | — | — | — | |||
| +16463A > A/G | 1 | — | — | — | — | |||
| B cells | ||||||||
| Aggregate sequence | 61 | — | — | — | — | |||
| Nonaggregate sequence | — | 30 | 33 | 17 | 18.7 | |||
| +8CT6C,* 9CT6C* | 11 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 3 | — | — | — | — | |||
| +146T > T/C | 2 | — | — | — | — | |||
| +20T > T/C§ | 1 | — | — | — | — | |||
| +105C > C/T,§ 8CT6C,* 9CT6C* | 1 | — | — | — | — | |||
| +200A > A/G | 1 | — | — | — | — | |||
| +204T > C, 8CT6C,* 9CT6C,* 16278C > C/T | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 16072C > C/G§ | 1 | — | — | — | — | |||
| +8CT6C,* 9CT6C,* 10CT6C* | 1 | — | — | — | — | |||
| +16151C > C/T§ | 1 | — | — | — | — | |||
| +16166A > A/del§ | 1 | — | — | — | — | |||
| +16213G > G/A | 1 | — | — | — | — | |||
| +16254A > A/del§ | 1 | — | — | — | — | |||
| +16275A > A/T§ | 1 | — | — | — | — | |||
| +16292C > T/C§ | 1 | — | — | — | — | |||
| +16310G > G/A | 1 | — | — | — | — | |||
| +16340A > A/G§ | 1 | — | — | — | — | |||
| Granulocytes | ||||||||
| Aggregate sequence | 49 | — | — | — | — | |||
| Nonaggregate sequence | — | 33 | 40.2 | 29 | 35.4 | |||
| +8CT6C,* 9CT6C* | 2 | — | — | — | — | |||
| +7CT6C,* 8CT6C* | 2 | — | — | — | — | |||
| +68G > G/A | 1 | — | — | — | — | |||
| +71G > G/del§ | 1 | — | — | — | — | |||
| +80C/T,§ 164C/T§ | 1 | — | — | — | — | |||
| +82A > A/G,§ 200A > G | 1 | — | — | — | — | |||
| +93A > A/G, 16185C > T | 1 | — | — | — | — | |||
| +122C > T§ | 1 | — | — | — | — | |||
| +139T > C/T§ | 1 | — | — | — | — | |||
| +172T > T/O§ | 1 | — | — | — | — | |||
| +199T > C, 16217T > T/C, 16439C > T§ | 1 | — | — | — | — | |||
| +200A > G | 1 | — | — | — | — | |||
| +200A > G/A | 1 | — | — | — | — | |||
| +200A > A/G | 1 | — | — | — | — | |||
| +200A > G, 223T > T/C | 1 | — | — | — | — | |||
| +229G > C§ | 1 | — | — | — | — | |||
| +266T > T/C | 1 | — | — | — | — | |||
| +304C > C/T§ | 1 | — | — | — | — | |||
| +310T > C/T | 1 | — | — | — | — | |||
| +378C > C/T,§ 16189T > T/C | 1 | — | — | — | — | |||
| +385A > A/del§ | 1 | — | — | — | — | |||
| +457C > C/T§, 517A > G§ | 1 | — | — | — | — | |||
| +16112C > C/T,§ 16420A > G/A§ | 1 | — | — | — | — | |||
| +16113A > G§ | 1 | — | — | — | — | |||
| +16123T > T/C§ | 1 | — | — | — | — | |||
| +16285A > A/G§ | 1 | — | — | — | — | |||
| +16305A > A/G | 1 | — | — | — | — | |||
| +16325T > T/C | 1 | — | — | — | — | |||
| +16350A > A/G | 1 | — | — | — | — | |||
| +16426C > T§ | 1 | — | — | — | — | |||
| +16527C > C/T§ | 1 | — | — | — | — | |||
Frequency indicates total mtDNA heterogeneity different from aggregate sequences; unique, uniquely different heterogeneity; +, mtDNA nucleotide changes compared with aggregate cell sequence; and —, not applicable.
Homopolymeric C tract at nucleotide positions at 303 to 315 (eg, 7CT6C defined CCCCCCCTCCCCCC) in HV2
Same as the Revised Cambridge Reference Sequence but different from the aggregate sequence
Mixed pattern of 514-515delCA and 514insCA
mtDNA mutations (not listed in accepted polymorphism database)
Summary of mtDNA heterogeneity in single CD34+ cells, T cells, B cells, and granulocytes
. | . | Heterogeneity . | . | . | Poly-C tract . | ||
|---|---|---|---|---|---|---|---|
| Cell type . | Assay, no.* . | Total rate,% (no.) . | Unique, % (no.) . | Substitution, % (no.) . | np 303-315, % (no.) . | ||
| CD34+ cells | 456 | 37.9 ± 3.6 (173)† | 13.4 ± 2.2 (61)† | 14.7 ± 5.7 (67)† | 22.3 ± 9.3 (101) | ||
| T cells | 456 | 36.4 ± 14.1 (164) | 18.6 ± 7.9 (84)† | 15.2 ± 9.0 (69)† | 21.5 ± 15.4 (96) | ||
| B cells | 442 | 36.0 ± 10.7 (160) | 16.5 ± 2.5 (73)† | 15.4 ± 6.7 (69)† | 19.7 ± 13.4 (87) | ||
| Granulocytes | 376 | 47.7 ± 7.4 (178) | 35.4 ± 4.1 (132) | 32.3 ± 2.4 (121) | 13.7 ± 10.2 (50) | ||
. | . | Heterogeneity . | . | . | Poly-C tract . | ||
|---|---|---|---|---|---|---|---|
| Cell type . | Assay, no.* . | Total rate,% (no.) . | Unique, % (no.) . | Substitution, % (no.) . | np 303-315, % (no.) . | ||
| CD34+ cells | 456 | 37.9 ± 3.6 (173)† | 13.4 ± 2.2 (61)† | 14.7 ± 5.7 (67)† | 22.3 ± 9.3 (101) | ||
| T cells | 456 | 36.4 ± 14.1 (164) | 18.6 ± 7.9 (84)† | 15.2 ± 9.0 (69)† | 21.5 ± 15.4 (96) | ||
| B cells | 442 | 36.0 ± 10.7 (160) | 16.5 ± 2.5 (73)† | 15.4 ± 6.7 (69)† | 19.7 ± 13.4 (87) | ||
| Granulocytes | 376 | 47.7 ± 7.4 (178) | 35.4 ± 4.1 (132) | 32.3 ± 2.4 (121) | 13.7 ± 10.2 (50) | ||
Np indicates nucleotide position.
All assays were from donor no. 5
Statistically significant difference (P < .05) from granulocytes
We estimated the incidence of nucleotide changes in 96 single cells by using the number of uniquely different patterns of mtDNA, on the conservative assumption that cells bearing the same mutant mtDNA were derived from a common original clone. The heterogeneous mtDNAs of CD34+ cells, T cells, B cells, and granulocytes were classified into several unique patterns of heterogeneity. The incidence rates of unique heterogeneous patterns of mtDNA among CD34+ cells, T cells, B cells, and granulocytes were 13.4% ± 2.2%, 18.6% ± 7.9%, 16.5% ± 2.5%, and 35.4% ± 4.1%, respectively (Tables 3, 4). The frequent patterns of mtDNA heterogeneity (major subpopulations of mutant mtDNA) in each donor were detected as 2 or 3 nucleotide changes in addition to the polymorphisms in the respective aggregate mtDNA. All 4 cell types had the same frequent differences in most cases (Figure 1; Table 3). Furthermore, although for this study donor blood was sampled 2 years after marrow from the same donors had been assayed for our previous studies, the same minor mtDNA species that we reported earlier were still present in all donors (Figure 2; Table 3).18,19
Fifty-five percent to 60% of the genetic alterations in CD34+ cells, T cells, and B cells were length changes in the homopolymeric C tract (poly-C tract) at nucleotide positions 303 to 315 in hypervariable region 2 (101 of 173 in CD34+ cells; 96 of 164 in T cells; 87 of 160 in B cells). In contrast, only approximately 30% of mtDNA heterogeneity in granulocytes was attributed to length alterations in the poly-C tract (50 of 178 in granulocytes) (Table 4). In contrast, the mean proportions of cells in which the control region of mtDNA had base substitutions were 14.7%, 15.2%, 15.4%, and 32.3% in single CD34+ cells, T cells, B cells, and granulocytes, respectively (Table 4). The incidence of mtDNA base substitutions in granulocytes was significantly higher than that in CD34+ cells, T cells, and B cells (P < .05).
Discussion
Low-frequency individual mtDNA mutations will always be under-represented in aggregate samples and will be detected only by analysis of individual cells in which the mutant mtDNA copies are clonally expanded (or with specially designed detection techniques for specific mutations).24 Previously, to examine differences among individual cells and for comparison with the aggregate, we performed mtDNA sequence analysis of CD34+ clones after a brief period of in vitro cell culture of individual cells.18,19 However, to prevent artifacts that might arise from the cell cloning process and to analyze mature blood cells that are difficult to propagate in tissue culture or that do not undergo mitosis, we developed a method for sequence analysis of single cells. Single cells were obtained by flow cytometry, and their lysates were amplified with primers specific for the control region of mtDNA. In the analysis of granulocytes, the efficiency of the nested PCR amplification was considerably improved (79.4%) over results obtained in our earlier cloned single-cell PCR method (7.5%); one reason for the improvement may be the lysis buffer in the present study used in extracting DNA from single cells, compared to the heating procedure used previously (Table 2).
In other experiments to develop a heteroplasmic Standard Reference Material (see SRM 2394 at www.nist.gov/srm), 285-bp amplicons were generated by gene amplification of mtDNA with and without a single base substitution and then mixed in varying proportions.25 According to the sequencing data of the mixtures, the minor species of mtDNA could be detected when it was present at approximately 20%; therefore, mixed nucleotide signals on sequencing electropherograms, when reproduced by mtDNA reamplification from the original single-cell lysate, were assumed to represent at least 20% heteroplasmy. When nucleotide changes occurred, they appeared first as a single copy among most of the aggregate mtDNA sequence. To observe low-frequency mtDNA mutations in detail, a sensitive detection method for the minor population of mtDNA mutants is necessary. Single-cell analysis may be preferable to our previously described method, which was based on a short period of cloning of the individual cells in tissue culture, because the total rate of mtDNA heterogeneity in circulating CD34+ cells in the present study (37.9%) was higher than it was for CD34+ cell clones (25%).19 We hypothesize that this apparent increase in sensitivity was caused by our ability to sample minor mtDNA species among the heteroplasmic population of mtDNA in a single cell. In other words, minor species of mutated mtDNA, even if present in most cells in a population but at levels lower than 20%, would not be reliably detected by conventional sequencing; however, such a population in theory would be identified in approximately one fifth of the cells by sampling if each cell's mtDNA were examined. Consistent with this premise was the difficulty we experienced in reliably amplifying mtDNA from granulocytes using the single-cell lysate as a source of DNA. For one donor, we repeatedly amplified the DNA from the original CD34+ cell lysates and then compared the mtDNA sequence among triplicates. Ten percent to 15% of the second and third amplicons differed from the original sequence, indicating that some DNA differences that originally appeared to be distinct from the donor's corresponding aggregate sequences were not always detected in all 3 sets of replicate amplicons. However, because we did not detect any new nucleotide substitutions in the replicates (only the aggregate sequence was observed), the most parsimonious interpretation of these results is that heteroplasmy (multiple mtDNA species) was present in the original cell lysate. For granulocytes, we were unable to consistently recover sufficient DNA from the starting sample (approximately 80% success compared with 100% for CD34+ cells) to detect the same nucleotide substitutions repeatedly. When DNA was reamplified from the original sample, more than 40% of triplicates differed from the original sequence, and approximately 10% showed apparently new nucleotide substitutions (data not shown). We doubt that these differences are artifactual for several reasons. LA taq DNA polymerase has proofreading activity, and its fidelity (error rate) is approximately 1 to 2 × 10-6 (errors /nucleotides).26 The expected rate of artifactual nucleotide changes during primary PCR amplification is theoretically very low. Indeed, we failed to detect many additional mutations or polymorphisms arising from the secondary PCR, despite the notorious susceptibility of nested sequencing to reamplification artifacts. One possible explanation for these results is the presence of multiple minor species of mtDNA mutants, barely detectable at the level of single-cell PCR and sequencing, in granulocytes compared with CD34+ cells.
In our initial experiments, we observed considerable differences in marrow CD34+ cell mtDNA nucleotide sequences among a group of healthy donors, suggesting that aggregate mtDNA sequences in hematopoietic tissue differed considerably from the standard Cambridge reference sequence and among healthy persons.12 When we next examined clones obtained by single-cell sorting of CD34+ cells followed by a brief period of tissue culture, unexpected mtDNA heterogeneity was observed, first for marrow18 and later for peripheral blood,19 from hematologically healthy adults. To examine mtDNA heterogeneity in the lymphohematopoietic system in more detail, we have now compared individual CD34+ cells, T cells, B cells, and granulocytes from the same 5 healthy adult donors. The sequence of each cell's mtDNA was compared with the aggregate mtDNA for the respective cell type and for the individual donor. Differences were expressed as a measure of mtDNA heterogeneity among cells. Overall, heterogeneity was 37.9% ± 3.6% for circulating CD34+ cells, 36.4% ± 14.1% for T cells, 36.0% ± 10.7% for B cells, and 47.7% ± 7.4% for granulocytes.
Fifty-five percent to 60% of the mtDNA alterations in CD34+ cells, T cells, and B cells represented length changes in the poly-C tract at nucleotide positions 303 to 315 in hypervariable region 2 (101 of 173 in CD34+ cells, 96 of 164 in T cells, 87 of 160 in B cells). In contrast, only approximately 30% of mtDNA heterogeneity in granulocytes was caused by poly-C tract length alterations (50 of 178 in granulocytes) (Table 4). The mean proportions of cells in which the control region of mtDNA showed base substitutions was 14.7%, 15.2%, 15.4%, and 32.3% in single CD34+ cells, T cells, B cells, and granulocytes, respectively (Table 4). The prevalence of mtDNA base substitutions in granulocytes was significantly higher than that in other cells (P < .05). T and B cells are also mature cells, but the incidence of base substitution in these cells was similar to that in CD34+ cells. Mutations in mtDNA are believed to result from secondary physiologic exposure to reactive oxygen species in the mitochondrion.6
Comparison of the major subpopulations of mtDNA mutants among CD34+ cells, T cells, B cells, and granulocytes. Major subpopulations of mtDNA differences in each cell type were compared in each donor (donors 1-5). Each vertical axis represents the proportion of the cells bearing the major subpopulations of mtDNA mutants. “Other” indicates the total proportion of minor populations of mtDNA mutants.
Comparison of the major subpopulations of mtDNA mutants among CD34+ cells, T cells, B cells, and granulocytes. Major subpopulations of mtDNA differences in each cell type were compared in each donor (donors 1-5). Each vertical axis represents the proportion of the cells bearing the major subpopulations of mtDNA mutants. “Other” indicates the total proportion of minor populations of mtDNA mutants.
Results of the granulocyte analysis—a higher proportion of cells containing mutations and apparently greater heteroplasmic diversity of multiple minor species in single cells—suggest that mutations may arise during late myeloid differentiation, probably as a result of their greater exposure to the reactive oxygen species in the mitochondria.27 Striking direct evidence for this hypothesis has come from recent experiments using transgenic mice that overexpressed the antioxidant enzyme catalase localized to mitochondria: mtDNA deletions were decreased, peroxide production was attenuated, age-related pathology was reduced, and animal survival was improved.28 On the assumption that mtDNA heterogeneity reflects environmental mutagen exposure, our single-cell methodology allows us to test this hypothesis in tissue culture of primary cells and cell lines exposed to mutagens and toxic oxygen molecules. It could also serve as a measure of the adverse effects of those therapies known to induce mutations such as chemotherapy and radiation treatments.29
The data that we describe in this paper and that have been published previously18,19 are consistent with the age-related origin of ultimately homoplasmic mtDNA genetic alterations within the lymphohematopoietic stem cell compartment. As we have reported, umbilical cord blood–derived CD34+ cells show remarkable homogeneity in mtDNA sequence, in great contrast to results with adult blood CD34+ cells.18 In humans, the accumulation of mtDNA point mutations in the same control region of the mtDNA sequence with aging has been reported by Michikawa et al30 in human fibroblasts and in leukocytes from young and old persons. For fibroblasts, point mutations in general and the T414G transversion were more frequent and in longitudinally collected samples were only detected in samples from elderly persons.30 In vitro, T414G appeared to be favored for replication over the wild-type sequence in cell lines under specific culture conditions,31 offering an explanation for the development over time of homoplasmy for this nucleotide alteration in vivo. In white blood cells, T414G changes were not detected, but a different mutation, C150T, was more prevalent as a homoplasmic change among centenarians.28 In these experiments, bulk cultures of fibroblasts or samples of white blood cells were analyzed, followed by denaturant gel gradient electrophoresis, gene amplification, and either bacterial cloning followed by sequencing or direct sequencing to detect mutations.
Transition of the major subpopulations of mtDNA mutants in CD34+ cells from each healthy donor in 2 years. Our present data about the proportion of major subpopulations of mutant mtDNA in single CD34+ cells from 5 healthy donors were compared with our previous publication data. We used the sequence data of mtDNA control region in CD34+ clones derived from the same donors' bone marrow 2 years before this study (2002)18 and derived from peripheral blood 1 year before this study (2003).19
Transition of the major subpopulations of mtDNA mutants in CD34+ cells from each healthy donor in 2 years. Our present data about the proportion of major subpopulations of mutant mtDNA in single CD34+ cells from 5 healthy donors were compared with our previous publication data. We used the sequence data of mtDNA control region in CD34+ clones derived from the same donors' bone marrow 2 years before this study (2002)18 and derived from peripheral blood 1 year before this study (2003).19
In our current study of single cells, most of the frequent patterns of mtDNA heterogeneity (the major subpopulations of mutant mtDNA with base substitutions or poly-C tract length alterations) in CD34+ cells appeared in diverse lineages, including T cells, B cells, and granulocytes (Figure 1; Table 3). The transversion also was detected in separated lymphomonocytes and in granulocytes from the same donors.32 Our approach allows a variety of inferences of potential biological importance related specifically to hematopoiesis and to aging in general. Except for granulocytes, we did not observe a higher frequency of mtDNA heterogeneity or new mutations in differentiated cells. Most likely, mutations arose in primitive stem cells and the then labeled stem cell's progeny after clonal expansion (alternatively, it is possible that the major subpopulation of mutant mtDNA might represent maternally inherited mtDNA, requiring years after birth to segregate among progeny cells).17 These results and conclusions are consistent with the continuous replication of mtDNA independent of the cell cycle and, therefore, the correlation of accumulation of mtDNA genetic differences with time rather than the proliferative status of a cell compartment. Moreover, though our healthy donors' blood was sampled 2 years after their marrow had been first tested, the major subpopulations of mutant mtDNA identified by base substitutions or poly-C tract length alterations in CD34+ cells were relatively stable in all 5 donors (2 mutations [+8CT6C, 9CT6C, 10CT6C] had appeared in donor 2 [Figure 2; Table 3]).18,19 (Stability was also suggested in the comparison of longitudinal bulk white blood cells, obtained many decades apart, in the experiments of Zhang et al32 ). Although our longitudinal samples occurred over a shorter interval of approximately 2 years, the relative proportion of the major subpopulations in CD34+ cells appeared to be actively maintained, suggesting that the same minor stem cells marked by mtDNA mutation/polymorphism were operating over this period of time. Clonality and mutation studies of CD34+ cells and their progeny in paroxysmal nocturnal hemoglobinuria33 and in myeloproliferative diseases34 such as polycythemia vera35 indicate that a single stem cell can support hematopoiesis for many years. Our data would suggest the capacity and stability of individual normal stem cell clones to similarly contribute to hematopoiesis.
mtDNA in blood cells might be usable as a natural genetic “marker” of hematopoietic progenitors and stem cells to estimate the number of active stem cells in the steady state, as has been performed using glucose-6-phosphate dehydrogenase isotypes for the cat,36 and also to predict the appearance and the extinction of individual hematopoietic stem cell clones over time. We are studying mtDNA heterogeneity in recipient hematopoietic cells after allogeneic stem cell transplantation and will statistically compare the results to the healthy donor's unperturbed hematopoietic system. In recent years, CD34+ cells have also been used as a stem cell source for regenerative medicine, and the examination of mtDNA heterogeneity may be useful for evaluating the quality of CD34+ cells and as an aging marker. Furthermore, fixed mtDNA changes in leukemic cells might provide a simple and sensitive method for monitoring minimal residual disease.37
Prepublished online as Blood First Edition Paper, July 14, 2005; DOI 10.1182/blood-2005-01-0150.
Supported by the Intramural Research Program of the NIH, NHLBI.
This paper is a contribution of the U.S. National Institutes of Health (NIH) and the National Institute of Standards and Technology (NIST) and is not subject to copyright.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Certain commercial equipment, instruments, materials, or companies are identified in this paper to specify the experimental procedure. Such identification does not imply recommendation or endorsement by the NIH or the NIST, nor does it imply that the materials or equipment identified are the best available for this purpose. We thank Dr Yong-Gang Yao for helpful comments and criticism during the revision of this manuscript.

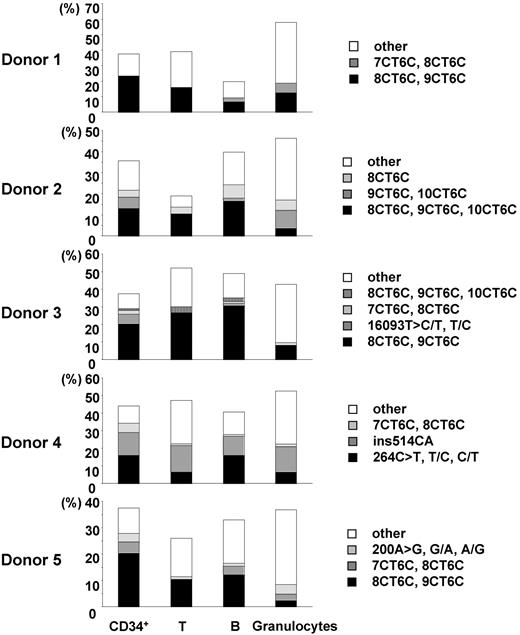
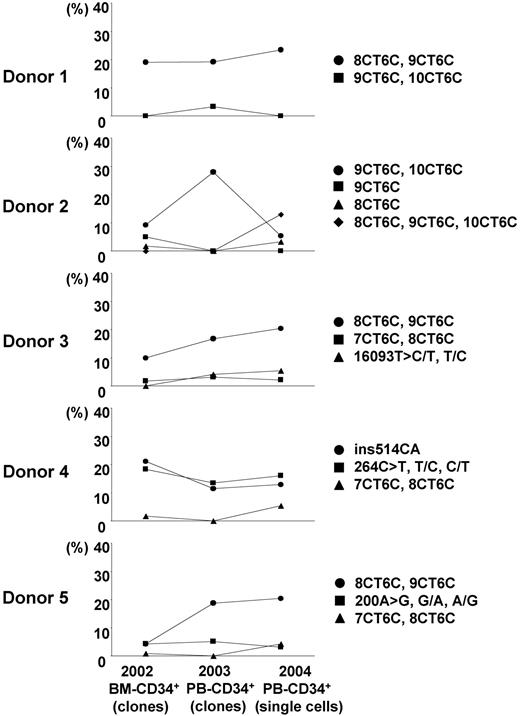
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal