Abstract
Juvenile myelomonocytic leukemia (JMML) is a clonal myeloproliferative/myelodysplastic disorder of early childhood with a poor prognosis. JMML cells are characterized by hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) caused by a continuously activated GM-CSF receptor–retrovirus-associated sequence (RAS) signal transduction pathway through various molecular mechanisms, resulting in spontaneous GM colony formation in vitro. Bisphosphonate zoledronic acid (ZOL), a RAS-blocking compound, suppressed colony formation from bone marrow (BM) cells of 8 patients with JMML and 5 healthy control subjects without and with GM-CSF (10 ng/mL), respectively, in a dose-dependent manner in clonal culture. At 10 μM ZOL, however, spontaneous GM colony formation from JMML BM cells decreased to 3%, but the formation of G colonies containing granulocytes, but no macrophages, was enhanced, whereas 40% of GM colonies were retained and G colony formation was not affected in culture of normal BM cells with GM-CSF. In suspension culture, cytochemical and flow cytometric analyses showed that 10 μM ZOL also inhibited spontaneous proliferation and differentiation along monocyte/macrophage lineage of JMML BM cells but not the development of normal BM cells by GM-CSF. The inhibitory effect of ZOL on JMML cells was confirmed at a single-clone level and observed even at 3 μM. The current result offers a novel approach to therapy in JMML.
Introduction
Juvenile myelomonocytic leukemia (JMML) is a rare clonal myeloproliferative/myelodysplastic disorder of infancy and early childhood.1-3 Despite various approaches to therapy, the mortality rate in patients with JMML is still high. Intensive chemotherapeutic regimens have largely proved futile in inducing durable remissions.4 Low-dose chemotherapy with 6-mercaptopurine, for example, has been temporarily effective in some patients, but generally it has not been shown to result in long-term disease control.5,6 Allogeneic hematopoietic stem cell transplantation (SCT) is presently the only therapy capable of producing durable remissions, but the 4- or 5-year probability of event-free survival in patients with JMML treated by SCT is approximately 50%.5,7 Therefore, the development of a new treatment for patients with JMML is awaited.
JMML cells are characterized by the ability to spontaneously proliferate in the absence of hematopoietic growth factors in vitro,8 giving rise to granulocyte-macrophage (GM) colonies caused by hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) released from monocytes/macrophages.9 Several investigators have shown that deregulated GM-CSF signal transduction through the retrovirus-associated sequence (RAS) pathway plays a key role in the characteristic property of JMML cells. Activating mutations of the RAS gene were found in 15% to 30% of patients with JMML.10-14 It was also reported that 30% of patients with JMML had mutations of the gene NF1 encoding neurofibromin, a guanosine triphosphatase acting protein that inactivates RAS, leading to a continuously activated GM-CSF receptor (GM-CSFR)–RAS pathway.15 Furthermore, somatic mutations of PTPN11 encoding a cytoplasmic Src homology-2 domain containing protein that controls RAS functions were found in 34% of patients with JMML without Noonan syndrome.16 Given these reports, linking the pathogenesis of JMML to the continuously activated GM-CSFR–RAS signal transduction pathway, it is reasonable to explore molecular mechanism-based therapy for JMML.
A GM-CSF antagonist, E21R, was earlier shown to inhibit JMML-cell growth in vitro and JMML-cell engraftment in immunodeficient mice.17,18 Another feasible way to inhibit JMML-cell development is to block the GM-CSFR–RAS signaling intracellular pathway. The first obligatory step in the signal transduction, which is essential for RAS activity, is the membrane localization of RAS accomplished through a prenylation reaction mediated by farnesyltransferase (FTase), which involves the covalent linking of a 15-carbon isoprenyl (farnesyl) group to RAS.19-21 FTase inhibitors (FTIs) have been evaluated in several in vitro and in vivo preclinical systems and demonstrated some antitumor effects.22,23 However, even when FTase is inhibited, RAS can be transferred to the membrane by an alternative pathway using geranylgeranyl transferase-1.24 Bisphosphonate (BP) has been used to treat bone diseases and acted as an anticancer drug by inhibiting the activation of RAS through the suppression of both farnesylation and geranylgeranylation.25,26 More recently, zoledronic acid (ZOL), a third-generation BP, has been shown to be more effective in blocking RAS activity.27 We then investigated the effect of ZOL on JMML-cell growth in vitro to clarify the function of continuously activated RAS in JMML cells and to evaluate the feasibility of using ZOL as an antileukemia drug for JMML.
Materials and methods
Zoledronic acid
Zoledronic acid (2-(imidazol-1-yl)-hydroxy-ethylidene-1, 1-bisphosphonic acid, disodium salt, 4.75 hydrate) was supplied by Novartis Pharma AG (Basel, Switzerland), dissolved in a stock solution of water at a concentration of 100 mM and stored at –20°C in plastic (not glass) containers.
Acquisition of donor samples
With personal and parental consent, bone marrow (BM) samples were obtained from children with JMML diagnosed based on the criteria of the International JMML Working Group.28 Normal controls were healthy adult volunteers who donated BM samples after providing informed consent. The study protocol was approved by the MDS Committee of the Japanese Society of Pediatric Hematology. Plastic syringes (10 mL) coated with preservative-free heparin were used for the acquisition of BM samples. Nonphagocytic mononuclear cells (NPMNCs) were separated by Ficoll-Hypaque density centrifugation after the depletion of phagocytes with Silica (Immuno Biological Laboratories, Fujioka, Japan). The NPMNCs were washed twice and suspended in α-medium (Flow Laboratories, Rockville, MD). Nonerythroid cells were obtained by lysis with NH4CL.
Clonal culture
The isolated BM NPMNCs were incubated in methylcellulose culture in triplicate using a technique described previously29 with some modifications. Briefly, 1 mL culture mixture containing 2 × 104 cells, α-medium, 0.9% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% fetal bovine serum (FBS; Hyclone, Logan, UT), 1% deionized fraction V bovine serum albumin (BSA; Sigma, Saint Louis, MO), 5 × 10–5 M mercaptoethanol, and various concentrations of ZOL was plated in each 35-mm standard nontissue culture dish (Nunc, Roskilde, Denmark) and incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. In some experiments, GM-CSF (Stem Cell Technologies, Vancouver, Canada) was added to the culture mixture at a concentration (10 ng/mL) that induced an optimal response in methylcellulose culture of human BM hematopoietic cells.30 The size and type of colonies (> 40 cells) formed in the culture were assessed at day 14 of culture. The size of small colonies was determined by direct cell counting in situ under an inverted microscope. When colonies contained more than 200 cells, they were removed individually with an Eppendorf micropipette and prepared as single-cell suspensions. Colony size was estimated using a counting chamber with the cell suspension. Colony types were determined according to criteria reported previously31 : granulocyte (G), monocyte/macrophage (M), and GM colonies that consist of granulocytes, including neutrophils, eosinophils, and basophils, monocytes/macrophages, and both granulocytes and monocytes/macrophages, respectively. To assess the accuracy of the in situ identification for the colony types, individual colonies were lifted, spread on glass slides using a cytocentrifuge (Cytospin 2; Shandon Southern Instruments, Sewickley, PA), and treated with May-Grünwald-Giemsa staining and cytochemical staining with α-naphthyl butyrate esterase (α-NBE) according to conventional methods. Differential counts of the cells were done with more than 100 cells on the cytospin smears in all experiments.
Suspension culture
Twenty thousand nonerythroid BM cells were incubated in a suspension culture using a modified version of a technique described previously.32,33 Briefly, 1 mL culture mixture containing 5 × 104 cells, α-medium, 30% FBS, 1% deionized fraction V BSA, and various concentrations of ZOL was incubated in 12-well tissue plates (Nunc) at 37°C in a humidified atmosphere flushed with 5% CO2 in air. The number of cultured cells and the cellular composition were determined at days 0, 5, and 10. The cell number was assessed by direct cell counting of liquid culture aliquots in a hemocytometer. The cellular composition was determined on cytospin smears of the cultured cells stained and by flow cytometric analysis (see “Flow cytometry”). In the special experiment, colonies developed in clonal culture at day 5 were individually collected in a microtube containing 100 μL α-medium and then divided into 2 aliquots. Each half was incubated in a suspension culture with or without 10 μM ZOL for 10 days after the addition of 50 μL respective culture medium.
Flow cytometry
Flow cytometric analysis was carried out using myelomonocytic cell-differentiation–related antigens; fluorescein isothiocyanate (FITC)–conjugated CD14, CD16, and human leukocyte antigen (HLA)–DR; phycoerythrin (PE)–conjugated CD11b and CD13; and peridininchlorophyll (PerCP)–conjugated CD45. All monoclonal antibodies used were purchased from Becton Dickinson Immunocytometry Systems (San Jose, CA). The staining procedure was described previously.34 All incubations with antibodies were carried out for 30 minutes on ice. Flow cytometric data were acquired on a fluorescence-activated cell scan (Becton Dickinson) using the Lysis-II software program. In a standard 3-color immunofluorescence protocol, forward and side scatters (FSC and SSC, respectively) were collected along with 3-color antibody combinations. Electronic gating on the basis of FSC and SSC excluded cellular debris and nonviable cells. Cell population percentages of monocytes/macrophages, granulocytes, lymphocytes, and blastic cells in nonerythroid cells were assessed by SSC and CD45 staining as described.35 The flow cytometric profile was obtained by either directly gating discrete populations identified by SSC and PerCP-conjugated CD45 staining, or by back-gating using FITC- and PE-conjugated antibody combinations to identify cells of interest.
Statistical analysis
Student t test was used to determine the significance of differences among groups of unpaired samples in all experiments. P values were derived from 2-sided tests, and P less than .05 was considered statistically significant.
Results
Effect of ZOL on colony growth from JMML and normal BM cells in clonal culture
Eight children with JMML were enrolled in this study (6 boys and 2 girls). Their median age was 3 years and 1 month (range, 1 month to 5 years and 9 months).
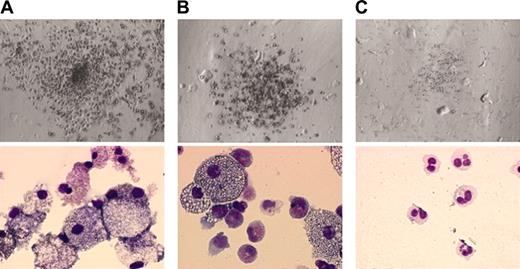
In clonal culture, BM cells of all 8 patients produced a large number of spontaneous colonies in the absence of hematopoietic factors, and the number of colonies was comparable to that formed in the presence of 10 ng/mL GM-CSF in 6 of 8 patients (Table 1). In cases 3 and 6, however, the number of colonies increased with the addition of 10 ng/mL GM-CSF to the culture. Most colonies formed in the absence or presence of 10 ng/mL GM-CSF were GM colonies that consisted of both granulocytes and macrophages (Table 1; Figure 1A). By contrast, BM cells of healthy adults produced no colonies in the absence of hematopoietic factors. On the addition of GM-CSF, normal BM cells produced colonies. Eighty-seven percent of the colonies were GM colonies whose appearance revealed a diminished number of macrophages (Figure 1B) as compared with spontaneous GM colonies generated from JMML BM cells, and the rest were G colonies (12% ± 11%, n = 6) with only a few M colonies (< 1%).
Colony formation from BM cells of patients with JMML and healthy adults
. | No. of colonies formed, 2 × 104 BM NPMNCs . | . | GM colonies in total colonies, % . | . | ||
|---|---|---|---|---|---|---|
| Case no. . | GM-CSF absent . | GM-CSF present* . | GM-CSF absent . | GM-CSF present* . | ||
| Patients with JMML | ||||||
| 1 | 135 ± 23 | 160 ± 14 | 97 | 99 | ||
| 2 | 153 ± 33 | 190 ± 22 | 100 | 100 | ||
| 3 | 44 ± 12 | 121 ± 13 | 98 | 100 | ||
| 4 | 193 ± 34 | 168 ± 33 | 97 | 99 | ||
| 5 | 131 ± 32 | 177 ± 32 | 95 | 99 | ||
| 6 | 46 ± 11 | 94 ± 21 | 100 | 100 | ||
| 7 | 152 ± 21 | 206 ± 16 | 96 | 99 | ||
| 8 | 217 ± 31 | 198 ± 22 | 99 | 98 | ||
| Mean ± SD | 137 ± 41 | 160 ± 36 | 98 ± 2 | 99 ± 0.2 | ||
| Healthy adults | ||||||
| 1 | 0 ± 0 | 155 ± 22 | NA | 93 | ||
| 2 | 0 ± 0 | 28 ± 13 | NA | 65 | ||
| 3 | 0 ± 0 | 234 ± 35 | NA | 84 | ||
| 4 | 0 ± 0 | 104 ± 21 | NA | 91 | ||
| 5 | 0 ± 0 | 200 ± 22 | NA | 95 | ||
| 6 | 0 ± 0 | 203 ± 19 | NA | 97 | ||
| Mean ± SD | 0 ± 0 | 154 ± 23 | NA | 87 ± 11 | ||
. | No. of colonies formed, 2 × 104 BM NPMNCs . | . | GM colonies in total colonies, % . | . | ||
|---|---|---|---|---|---|---|
| Case no. . | GM-CSF absent . | GM-CSF present* . | GM-CSF absent . | GM-CSF present* . | ||
| Patients with JMML | ||||||
| 1 | 135 ± 23 | 160 ± 14 | 97 | 99 | ||
| 2 | 153 ± 33 | 190 ± 22 | 100 | 100 | ||
| 3 | 44 ± 12 | 121 ± 13 | 98 | 100 | ||
| 4 | 193 ± 34 | 168 ± 33 | 97 | 99 | ||
| 5 | 131 ± 32 | 177 ± 32 | 95 | 99 | ||
| 6 | 46 ± 11 | 94 ± 21 | 100 | 100 | ||
| 7 | 152 ± 21 | 206 ± 16 | 96 | 99 | ||
| 8 | 217 ± 31 | 198 ± 22 | 99 | 98 | ||
| Mean ± SD | 137 ± 41 | 160 ± 36 | 98 ± 2 | 99 ± 0.2 | ||
| Healthy adults | ||||||
| 1 | 0 ± 0 | 155 ± 22 | NA | 93 | ||
| 2 | 0 ± 0 | 28 ± 13 | NA | 65 | ||
| 3 | 0 ± 0 | 234 ± 35 | NA | 84 | ||
| 4 | 0 ± 0 | 104 ± 21 | NA | 91 | ||
| 5 | 0 ± 0 | 200 ± 22 | NA | 95 | ||
| 6 | 0 ± 0 | 203 ± 19 | NA | 97 | ||
| Mean ± SD | 0 ± 0 | 154 ± 23 | NA | 87 ± 11 | ||
NA indicates not applicable.
GM-CSF was used at a concentration of 10 ng/mL, which induced an optimal response among normal BM cells.
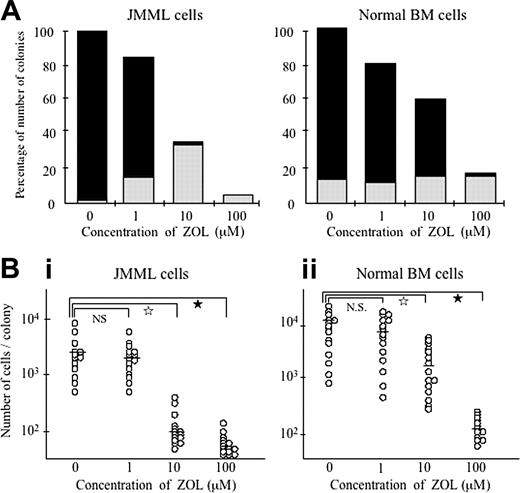
When added to the culture, ZOL suppressed the spontaneous colony formation from BM cells of all 8 patients with JMML in a dose-dependent manner (Figure 2A). The number of spontaneous colonies formed from JMML BM cells decreased to a 3rd and a 15th at concentrations of 10 and 100 μM ZOL, respectively. In particular, the formation of GM colonies was much decreased at 10 μM ZOL (3% ± 3%), and no GM colonies were produced at 100 μM ZOL. Furthermore, the size of spontaneous colonies was smaller at greater than 10 μM ZOL. As shown in Figure 2B, the numbers of constituent cells in individual spontaneous colonies (n = 15 at each concentration of ZOL) randomly chosen from the cultures of BM cells of 3 patients with JMML (cases 4, 6, and 7) with 10 and 100 μM ZOL were much smaller than those with no or 1 μM ZOL. Interestingly, the addition of ZOL enhanced the formation of small G colonies consisting of only granulocytes, with no macrophages (Figures 1C and 2A). The effect of ZOL on the formation of colonies from BM cells of patients with JMML (cases 4 and 7) in the clonal culture containing 10 ng/mL GM-CSF was also examined. We again observed that 10 μM ZOL inhibited GM colony formation and enhanced G colony formation in a manner similar to that observed for the spontaneous colony formation (data not shown).
Colonies formed under various conditions. Top and bottom panels show the appearance of colonies under an inverted microscope (ITM; Olympus, Tokyo, Japan) equipped with a camera module (DP50; Olympus) (objective lens, SPlan 4PL; numerical aperture, 0.13; magnification, × 4) and their constituent cells stained with May-Grünwald-Giemsa solution (microscope, BX51, Olympus; camera module, XC-003, Sony, Tokyo, Japan; objective lens, UPlan F1, Olympus; numerical aperture, 1.3; magnification, × 100), respectively. (A) A spontaneous GM colony in the culture of JMML BM cells without hematopoietic factors. (B) A GM colony in the culture of normal BM cells with 10 ng/mL GM-CSF. (C) A spontaneous G colony in the culture of JMML BM cells with 10 μM ZOL.
Colonies formed under various conditions. Top and bottom panels show the appearance of colonies under an inverted microscope (ITM; Olympus, Tokyo, Japan) equipped with a camera module (DP50; Olympus) (objective lens, SPlan 4PL; numerical aperture, 0.13; magnification, × 4) and their constituent cells stained with May-Grünwald-Giemsa solution (microscope, BX51, Olympus; camera module, XC-003, Sony, Tokyo, Japan; objective lens, UPlan F1, Olympus; numerical aperture, 1.3; magnification, × 100), respectively. (A) A spontaneous GM colony in the culture of JMML BM cells without hematopoietic factors. (B) A GM colony in the culture of normal BM cells with 10 ng/mL GM-CSF. (C) A spontaneous G colony in the culture of JMML BM cells with 10 μM ZOL.
However, when 10 and 100 μM ZOL were added to the culture of normal BM cells with 10 ng/mL GM-CSF, the number of colonies decreased to approximately 60% and 20%, respectively (Figure 2A), but at 10 μM ZOL, GM colonies still occupied the larger proportion of the total colonies retained (69% ± 14%, significantly different from the percentage [8% ± 7%] in spontaneous GM colony formation by JMML BM cells [P < .01]). ZOL did not have a significant effect on the formation of G colonies from normal BM cells in the presence of 10 ng/mL GM-CSF. The size of the GM colonies also decreased in a dose-dependent manner, but it was much larger than that of the spontaneous colonies from JMML samples at 10 μM ZOL (P < .01) (Figure 2B).
Effect of ZOL on development of JMML and normal BM cells in suspension culture
The result of the clonal culture suggested that ZOL inhibited the proliferation and differentiation of both JMML and normal BM cells, but the inhibitory effect was stronger in the former. We then carried out suspension culture to examine the effect of ZOL on the development of BM cells of patients with JMML and healthy donors in more detail.
Effect of ZOL on colony formation from JMML and normal BM cells. (A) The percentages of the number of colonies in the culture of JMML and normal BM cells without and with 10 ng/mL GM-CSF, respectively, in the presence of various concentrations of ZOL to that in the absence of ZOL, and the proportion of G and GM colonies in total colonies under the respective conditions. The values indicate the means calculated from the data for 8 patients with JMML and 6 healthy donors in Table 1. ▪ indicates GM colonies; ▦, G colonies. (B) The numbers of cells contained in individual colonies cultured from JMML and normal BM cells without and with 10 ng/mL GM-CSF, respectively. Fifteen colonies were randomly chosen in the culture of BM cells of 3 patients with JMML and 3 healthy donors under the respective conditions. Bars indicate the means of cell numbers of the 15 colonies. In i, NS indicates not significant; ☆, P < .01; ★, P < .001. In ii, ☆ indicates P < .05; ★, P < .001.
Effect of ZOL on colony formation from JMML and normal BM cells. (A) The percentages of the number of colonies in the culture of JMML and normal BM cells without and with 10 ng/mL GM-CSF, respectively, in the presence of various concentrations of ZOL to that in the absence of ZOL, and the proportion of G and GM colonies in total colonies under the respective conditions. The values indicate the means calculated from the data for 8 patients with JMML and 6 healthy donors in Table 1. ▪ indicates GM colonies; ▦, G colonies. (B) The numbers of cells contained in individual colonies cultured from JMML and normal BM cells without and with 10 ng/mL GM-CSF, respectively. Fifteen colonies were randomly chosen in the culture of BM cells of 3 patients with JMML and 3 healthy donors under the respective conditions. Bars indicate the means of cell numbers of the 15 colonies. In i, NS indicates not significant; ☆, P < .01; ★, P < .001. In ii, ☆ indicates P < .05; ★, P < .001.
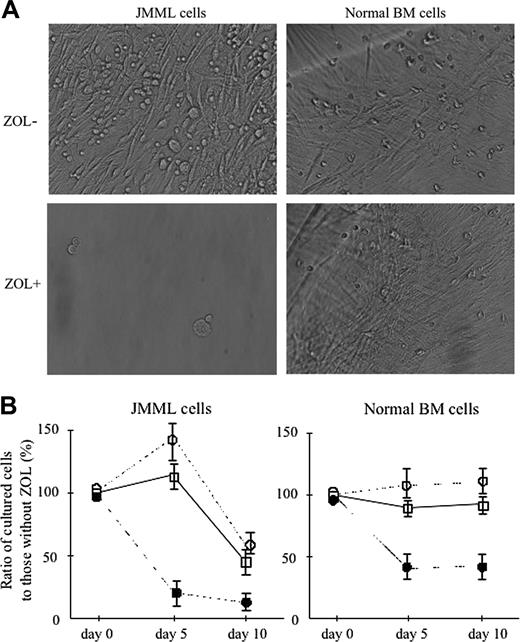
In suspension culture without hematopoietic factors, nonerythroid BM cells of 3 patients with JMML (cases 4, 6, and 7) proliferated, whereas no increase of the cells cultured from BM cells of 3 healthy donors (cases 1, 3, and 5) was observed. With the addition of 10 ng/mL GM-CSF to the culture, normal BM cells proliferated. When incubated for 15 days, both JMML and normal BM cells showed the development of a number of adherent cells in the suspension culture without and with 10 ng/mL GM-CSF, respectively, as shown in Figure 3A. Therefore, we could not accurately calculate the number of cells contained in the cultures at day 15. When ZOL was added, no development of adherent cells was observed at greater than 10 μM in the culture of JMML BM cells at day 15. In the culture of normal BM cells, however, adherent cells developed at 10 μM ZOL in the presence of 10 ng/mL GM-CSF although they did not develop at 100 μM.
Figure 3B shows the effect of ZOL on the proliferation of BM cells of 3 patients with JMML (cases 4, 6, and 7) and 3 healthy donors (cases 1, 3, and 5) in the suspension culture. Each value represents the mean ± SD of the percentages of the cells cultured at the respective concentrations of ZOL to those without ZOL at days 5 and 10 of culture. At day 5, 100 μM, but neither 1 nor 10 μM, ZOL inhibited the proliferation of JMML BM cells, but at day 10, 1 to 100 μM ZOL inhibited it. However, 1 and 10 μM ZOL revealed no inhibitory effect on the proliferation of normal BM cells even at day 10 of culture, whereas 100 μM ZOL inhibited it at days 5 and 10.
Figure 4A shows the effect of ZOL on the proportion of α-NBE–positive macrophages and –negative cells in the suspension culture of JMML and normal BM cells. JMML BM cells contained 34% ± 6% of monocytes/macrophages whose α-NBE–positive granules were fine as compared with those in normal BM monocytes/macrophages, and α-NBE–negative cells included granulocytes, lymphocytes, immature blastic cells, and nucleated erythroid cells. In the culture of JMML BM cells without ZOL and with 1 μM ZOL, most of the cells generated were α-NBE–positive macrophages at day 10, and α-NBE–negative populations contained a few granulocytes and immature blastic cells (Figure 4A-B). At 10 and 100 μM ZOL, however, the proportion of α-NBE–positive macrophages significantly decreased and that of α-NBE–negative cells increased. The majority of the α-NBE–negative cells were granulocytes, but there was a substantial number of blastic cells.
However, a large proportion of normal BM cells were α-NBE–negative granulocytes. The α-NBE–negative cells also included nucleated erythroid cells, lymphocytes, and a few blastic cells. In the culture of normal BM cells with 10 ng/mL GM-CSF, α-NBE–negative granulocytes accounted for three quarters of the cells generated, and the other quarter were α-NBE–positive macrophages (Figure 4A-B). The addition of ZOL did not affect the proportion of α-NBE–positive macrophages and α-NBE–negative granulocytes at concentrations of 1 and 10 μM, but, in the culture with 100 μM ZOL, most of the cultured cells were α-NBE–negative granulocytes (Figure 4A-B).
Effect of ZOL on the proliferation of JMML and normal BM cells in the suspension culture. (A) Phase microscopy of adherent cells developed from BM cells of a patient with JMML (case 4) and a healthy donor (case 3) at day 15 of suspension culture without and with 10 ng/mL ZOL (microscope, ITM2; camera module, DP50; objective lens, DPlan Ap10 UV, Olympus; numerical aperture, 0.4; magnification, × 40). A number of adherent cells were observed in the culture of JMML cells without ZOL, but their development was suppressed by the addition of ZOL. The growth of adherent cells in the culture of normal BM cells was not affected by the addition of ZOL. (B) The inhibition rate by 1, 10, and 100 μM ZOL in the proliferation of JMML and normal BM cells in the suspension culture without and with 10 ng/mL GM-CSF, respectively, at days 5 and 10. Each value indicates the mean ± SD calculated from the data in 3 patients with JMML and 3 healthy donors. □ indicates ZOL 1 μM; ○, ZOL 10 μM; •, ZOL 100 μM.
Effect of ZOL on the proliferation of JMML and normal BM cells in the suspension culture. (A) Phase microscopy of adherent cells developed from BM cells of a patient with JMML (case 4) and a healthy donor (case 3) at day 15 of suspension culture without and with 10 ng/mL ZOL (microscope, ITM2; camera module, DP50; objective lens, DPlan Ap10 UV, Olympus; numerical aperture, 0.4; magnification, × 40). A number of adherent cells were observed in the culture of JMML cells without ZOL, but their development was suppressed by the addition of ZOL. The growth of adherent cells in the culture of normal BM cells was not affected by the addition of ZOL. (B) The inhibition rate by 1, 10, and 100 μM ZOL in the proliferation of JMML and normal BM cells in the suspension culture without and with 10 ng/mL GM-CSF, respectively, at days 5 and 10. Each value indicates the mean ± SD calculated from the data in 3 patients with JMML and 3 healthy donors. □ indicates ZOL 1 μM; ○, ZOL 10 μM; •, ZOL 100 μM.
Effect of ZOL on the differentiation of JMML and normal BM cells in the suspension culture. (A) Effect of ZOL on the proportion of α-NBE–positive and –negative cells in the suspension culture of JMML and normal BM cells. Each value indicates the mean calculated from the data for 3 patients with JMML and 3 healthy donors. NS indicates not significant. ▪ indicates α-NBE-positive cells; ▦, α-NBE-negative cells. (B) Appearance of α-NBE–positive and –negative cells in the suspension culture of BM cells of a patient with JMML (case 4) and a healthy donor (case 5) without and with 10 ng/mL GM-CSF (microscope, BH2, Olympus; camera module, DP70, Olympus; objective lens, Span, Olympus; numerical aperture, 0.7; magnification, × 40). α-NBE–positive macrophages possess brown granules in their cytoplasm.
Effect of ZOL on the differentiation of JMML and normal BM cells in the suspension culture. (A) Effect of ZOL on the proportion of α-NBE–positive and –negative cells in the suspension culture of JMML and normal BM cells. Each value indicates the mean calculated from the data for 3 patients with JMML and 3 healthy donors. NS indicates not significant. ▪ indicates α-NBE-positive cells; ▦, α-NBE-negative cells. (B) Appearance of α-NBE–positive and –negative cells in the suspension culture of BM cells of a patient with JMML (case 4) and a healthy donor (case 5) without and with 10 ng/mL GM-CSF (microscope, BH2, Olympus; camera module, DP70, Olympus; objective lens, Span, Olympus; numerical aperture, 0.7; magnification, × 40). α-NBE–positive macrophages possess brown granules in their cytoplasm.
The results in the suspension culture indicate that 100 μM ZOL inhibits the development of both JMML and normal BM cells, but 10 μM ZOL predominantly inhibits the spontaneous proliferation and differentiation along monocyte/macrophage lineage of JMML BM cells.
Flow cytometric analysis of the cells in the suspension culture of JMML and normal BM cells
The effect of ZOL on the differentiation of JMML and normal BM cells in the suspension culture was analyzed by flow cytometry. Figure 5 shows a representative flow cytometric profile of nonerythroid BM cells of a patient with JMML (case 4) before and after 10 days of suspension culture with and without 10 μM ZOL, and Table 2 summarizes the ratio of granulocytes, monocytes/macrophages, lymphocyte, and blastic cells in the population excluding CD45-negative nucleated erythroid cells before and after 10 days of the suspension culture of BM cells from 3 patients with JMML (cases 4, 6, and 7) and 3 healthy donors (cases 1, 3, and 5), which was determined by using SSC and CD45 staining.
Proportion of each cell population in nonerythroid cells cultured from JMML and normal BM cells
. | ZOL concentration, μM . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Day 0 . | Day 10 . | . | . | . | ||||
. | 0 . | 0 . | 1 . | 10 . | 100 . | ||||
| JMML cells, % | |||||||||
| Granulocytes | 46 ± 7 | 2 ± 1 | 3 ± 2 | 30 ± 7 | 43 ± 5 | ||||
| Monocytes/macrophages | 30 ± 9 | 92 ± 2 | 88 ± 6 | 62 ± 9 | 51 ± 9 | ||||
| Lymphocytes | 11 ± 2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||
| Blasts | 13 ± 4 | 6 ± 2 | 9 ± 4 | 8 ± 1 | 6 ± 2 | ||||
| Normal BM cells, % | |||||||||
| Granulocytes | 77 ± 9 | 68 ± 9 | 69 ± 7 | 62 ± 6 | 75 ± 3 | ||||
| Monocytes/macrophages | 6 ± 4 | 28 ± 3 | 26 ± 4 | 38 ± 6 | 25 ± 3 | ||||
| Lymphocytes | 12 ± 4 | 4 ± 5 | 5 ± 4 | 0 ± 0 | 0 ± 0 | ||||
| Blasts | 5 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||
. | ZOL concentration, μM . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Day 0 . | Day 10 . | . | . | . | ||||
. | 0 . | 0 . | 1 . | 10 . | 100 . | ||||
| JMML cells, % | |||||||||
| Granulocytes | 46 ± 7 | 2 ± 1 | 3 ± 2 | 30 ± 7 | 43 ± 5 | ||||
| Monocytes/macrophages | 30 ± 9 | 92 ± 2 | 88 ± 6 | 62 ± 9 | 51 ± 9 | ||||
| Lymphocytes | 11 ± 2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||
| Blasts | 13 ± 4 | 6 ± 2 | 9 ± 4 | 8 ± 1 | 6 ± 2 | ||||
| Normal BM cells, % | |||||||||
| Granulocytes | 77 ± 9 | 68 ± 9 | 69 ± 7 | 62 ± 6 | 75 ± 3 | ||||
| Monocytes/macrophages | 6 ± 4 | 28 ± 3 | 26 ± 4 | 38 ± 6 | 25 ± 3 | ||||
| Lymphocytes | 12 ± 4 | 4 ± 5 | 5 ± 4 | 0 ± 0 | 0 ± 0 | ||||
| Blasts | 5 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | ||||
Each value (mean ± SD) was calculated from data for 3 patients with JMML (cases 4, 6, and 7) and healthy donors (cases 1, 3, and 5).
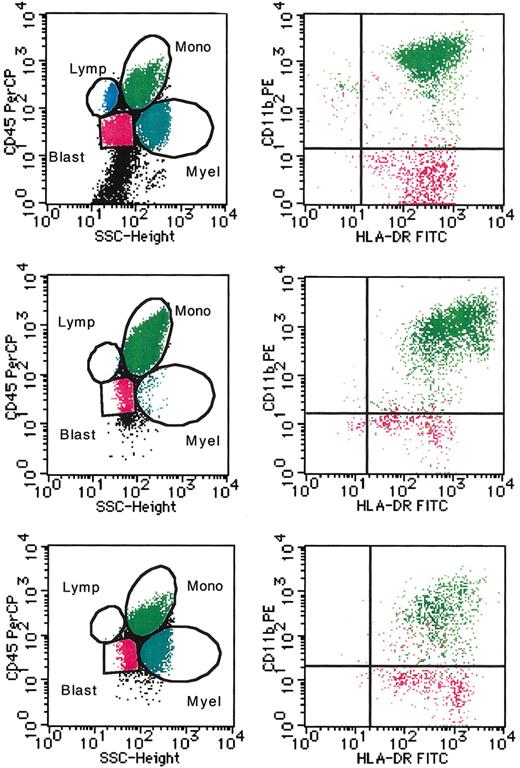
Approximately one third of the nonerythroid cells obtained from BM cells of patients with JMML were monocytes/macrophages, characterized by high SSC and bright CD45 expression, which also expressed CD11b, CD13, CD14, CD16, and HLA-DR but no CD34, and half were high SSC and lower-density CD45-expressing granulocytes that were CD13+, CD16+, and CD33+, but CD14– and CD34– (Figure 5; Table 2; data not shown). There was also a substantial number of lower SSC and bright CD45-expressing lymphocytes and lower SSC and lower CD45-expressing blastic cells that expressed HLA-DR and CD34 but neither CD11b, CD13, CD14, CD16, nor CD33. When cultured for 10 days, JMML BM cells generated a large number of mature macrophages revealing more bright CD45 expression. Granulocytes accounted for only 2% ± 1%, but 6% ± 2% of the population were blastic cells. On the addition of greater than 10 μM ZOL, however, the production of monocytes/macrophages was significantly suppressed, and the monocyte/macrophage population contained a proportion of lower CD11b-expressing immature monocytes/macrophages. A third of the cultured cells were granulocytes, and a substantial number of blastic cells were still present (8% ± 1%). Nonerythroid cells obtained from normal BM cells consisted of 77% ± 9% granulocytes, 6% ± 4% CD11bbright/HLA-DR+ mature monocytes/macrophages, 12% ± 4% lymphocytes, and a few blastic cells expressing CD34 (Table 2; data not shown). In the culture of normal BM cells with GM-CSF, macrophages also increased, but the percentage was 28% ± 3%. A large number of granulocytes existed, but no or few blastic cells did. The addition of ZOL did not have a significant effect on the composition of the population cultured from normal BM cells.
Representative flow cytometric profile of the cells in the suspension culture of JMML BM cells. The nonerythroid BM cells of a patient with JMML (case 4) before (top row) and after 10 days of culture without and with 10 μM ZOL (middle and bottom rows, respectively) were examined by flow cytometry. Populations of granulocytes (Myel, dark blue dots), monocytes/macrophages (Mono, green dots), lymphocytes (Lymp, bright blue dots), and blastic cells (Blast, red dots) were identified by a combination of SSC and CD45 staining (left). CD11b and HLA-DR expression in monocytes/macrophages and blastic cells was further analyzed (right).
Representative flow cytometric profile of the cells in the suspension culture of JMML BM cells. The nonerythroid BM cells of a patient with JMML (case 4) before (top row) and after 10 days of culture without and with 10 μM ZOL (middle and bottom rows, respectively) were examined by flow cytometry. Populations of granulocytes (Myel, dark blue dots), monocytes/macrophages (Mono, green dots), lymphocytes (Lymp, bright blue dots), and blastic cells (Blast, red dots) were identified by a combination of SSC and CD45 staining (left). CD11b and HLA-DR expression in monocytes/macrophages and blastic cells was further analyzed (right).
Thus, 10 μM ZOL affected the predominant differentiation along monocyte/macrophage lineage of JMML BM cells but not the development of normal BM cells. Furthermore, although 10 μM ZOL inhibited the massive expansion of mature monocytes/macrophages, an immature population of JMML cells survived.
Effect of ZOL on clonal cells derived from JMML BM cells
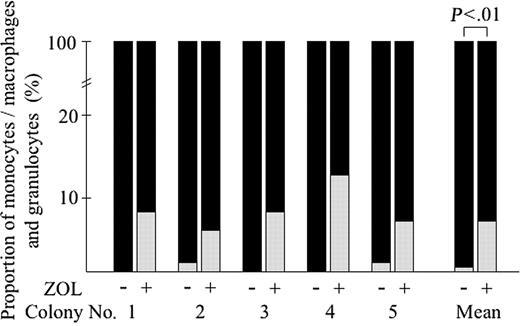
To confirm the inhibitory effect of ZOL on JMML BM cells, we carried out suspension culture of clonal cells derived from single spontaneous colonies formed in culture of BM cells of a patient with JMML (case 4). Five spontaneous colonies containing 100 to 200 cells generated from the JMML BM sample at day 5 of clonal culture were randomly chosen, individually removed, and divided equally. Each half was incubated in a suspension culture with or without 10 μM ZOL for 10 days. In all 5 colonies examined, the number of cells generated with ZOL was approximately a tenth of that without ZOL. At 10 μM, ZOL also affected the differentiation of clonal cells derived from the same clones (Figure 6). Most of the cells contained in the half cultured without ZOL were macrophages (99% ± 0%), whereas the half with 10 μM ZOL contained a substantial number of granulocytes in all 5 experiments (6% ± 3%). This result clearly indicates that 10 μM ZOL inhibits the proliferation and differentiation along monocyte/macrophage lineage of JMML cells at a clone level.
Effect of ZOL on the clonal cells derived from single spontaneous colonies in the culture of JMML BM cells. Five spontaneous colonies generated from BM cells of 3 patients with JMML at day 5 of clonal culture were individually lifted and divided equally, then each half was incubated in the suspension culture with or without 10 μM ZOL for 10 days. The proportion of monocytes/macrophages and granulocytes was determined on cytospin smears of the cultured cells cytochemically stained with α-NBE. ▪ indicates monocytes/macrophages; ▦, granulocytes.
Effect of ZOL on the clonal cells derived from single spontaneous colonies in the culture of JMML BM cells. Five spontaneous colonies generated from BM cells of 3 patients with JMML at day 5 of clonal culture were individually lifted and divided equally, then each half was incubated in the suspension culture with or without 10 μM ZOL for 10 days. The proportion of monocytes/macrophages and granulocytes was determined on cytospin smears of the cultured cells cytochemically stained with α-NBE. ▪ indicates monocytes/macrophages; ▦, granulocytes.
Effect of ZOL on JMML and normal BM cells at lower concentrations
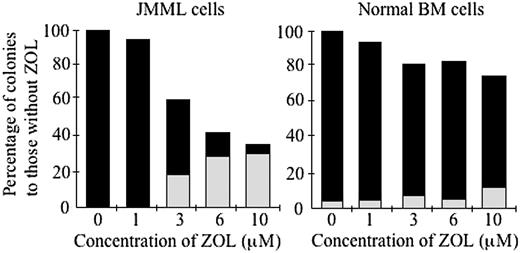
Finally, we examined the effect of ZOL at concentrations of 1 to 10 μM on the growth of JMML and normal BM cells because it was shown that the serum concentration of ZOL was less than 10 μMin a clinical study of the treatment of osteoporosis.26 As shown in Figure 7, when 1, 3, 6, and 10 μM ZOL were added to the clonal culture of BM cells of a patient with JMML (case 7), the number and size of spontaneous colonies were depressed at concentrations of more than 3 μM ZOL. The number of spontaneous GM colonies significantly decreased and that of G colonies increased even at 3 μM ZOL (P < .05). By contrast, 3 μM ZOL had little effect on the colony formation from normal BM cells (case 6) by 10 ng/mL GM-CSF.
Discussion
BM cells of all 8 patients with JMML enrolled in this study produced spontaneous colonies in the culture without hematopoietic factors in accordance with previous reports.8,36 Because normal BM cells generated no colonies under the same condition, the spontaneous colonies were considered to be derived from the abnormal JMML clones. The several studies showed that the spontaneous colony formation by JMML cells is caused by hypersensitivity to GM-CSF through various molecular mechanisms continuously activating the GM-CSFR–RAS signal transduction pathway.10,11,15 Our findings, that the number of spontaneous colonies was comparable to that of colonies formed in the presence of GM-CSF in most patients with JMML and that there was no remarkable difference in the inhibitory effects of ZOL, a RAS-blocking compound, between the colony formation from JMML cells with and without GM-CSF, are consistent with these studies. In the present study, however, the number of spontaneous colonies varied among patients with JMML. For example, the number was relatively small in cases 3 and 6, in both of which the number of colonies increased to more than 2-fold with the addition of GM-CSF to the culture.
Effect of ZOL on the colony formation from JMML and normal BM cells at less than 10 μM. BM cells of a patient with JMML (case 7) and a healthy adult (case 6) were incubated in the clonal culture with concentrations of 0, 1, 3, 6, and 10 μM ZOL for 14 days in the absence and presence of GM-CSF (10 ng/mL), respectively. ▪ indicates GM colonies; ▦, G colonies.
Effect of ZOL on the colony formation from JMML and normal BM cells at less than 10 μM. BM cells of a patient with JMML (case 7) and a healthy adult (case 6) were incubated in the clonal culture with concentrations of 0, 1, 3, 6, and 10 μM ZOL for 14 days in the absence and presence of GM-CSF (10 ng/mL), respectively. ▪ indicates GM colonies; ▦, G colonies.
Most of the spontaneous colonies were GM colonies consisting of both macrophages and granulocytes. We also observed that 10 μM ZOL induced the emergence of a significant number of erythroid colonies from JMML cells in the presence of only erythropoietin (Y.O., A.M., H.K., Feng Ma, T.T., and K.T., manuscript in preparation). These observations indicate the multipotentiality of JMML clones.8 However, the proportion of macrophages in the spontaneous GM colonies derived from JMML cells was higher than that in GM colonies induced from normal BM cells by GM-CSF. Furthermore, the proportion of macrophages in the suspension culture of JMML BM cells was also higher than that in the culture of normal BM cells with GM-CSF. Thus, JMML cells, although multipotential, exhibited a predominant expansion of monocytes/macrophages in in vitro culture, compatible with the symptoms in patients with JMML, such as monocytosis and monocytic-cell infiltration to organs.
ZOL, as well as FTIs shown previously,23 inhibited the spontaneous GM colony formation from JMML BM cells in a dose-dependent manner. It is of interest that G colonies consisting of only granulocytes, no macrophages, emerged with the addition of ZOL to the clonal culture. Because these G colonies were spontaneous colonies produced in the absence of hematopoietic factors, the spontaneous G colonies induced by the addition of ZOL seemed to originate from JMML clones, not normal hematopoietic progenitors. Cytochemical and flow cytometric analyses of the cells in the suspension culture of JMML cells also showed the decrease of mature monocytes/macrophages, but granulocytes and immature cells survived even in the presence of ZOL. Taken together, these results indicate that ZOL predominantly inhibited the differentiation of JMML cells into monocytes/macrophages but not granulocytes. This finding was confirmed in a single JMML clone, suggesting that continuously activated RAS preferentially stimulates the differentiation of JMML cells along monocyte/macrophage lineage. This notion is supported by the report that murine myeloid precursor cells with v-Ha-Ras were induced to differentiate along monocyte/macrophage lineage.37
There are at least 2 possibilities regarding the immature JMML cells that the continuously activated RAS acts on. The first one is that JMML cells already committed to the monocyte/macrophage lineage may be hastened along the pathway by the activated RAS. Second, continuously activated RAS may act on the multipotential JMML cells to commit them to differentiate into monocytes/macrophages but not other lineages, including granulocytes, because it was shown that mutational activation of RAS in murine multipotential precursor cells inhibits their differentiation into, not macrophages, but neutrophils.38 In any case, ZOL, a RAS-blocker, arrested the JMML cells in the early stages by suppressing their differentiation into mature monocytes/macrophages.
The growth of monocytes/macrophages from normal BM cells was suppressed by 100 μM ZOL in both clonal and suspension cultures, but the effect of ZOL on granulocyte development was minor even at a higher concentration. This suggests that activated RAS also acts on immature hematopoietic cells to preferentially differentiate along monocyte/macrophage lineage in normal hematopoiesis. RAS, continuously activated in JMML cells, may accelerate their differentiation into monocytes/macrophages, although monocytes/macrophages derived from JMML cells were dysfunctional because they reacted weakly to α-NBE staining as compared with those from normal BM cells. Therefore, the massive expansion of macrophages in the culture of JMML cells may be the result of their accelerated differentiation into monocytes/macrophages. However, there remains a possibility that aberrant signaling through the RAS stimulates not only the differentiation of immature JMML cells but also the proliferation of mature monocytes/macrophages derived from immature JMML cells directly.
Aside from providing insight into molecular mechanisms behind the pathogenesis of JMML, our observation offers a novel approach to therapy in JMML because it has been proven that ZOL can be safely used in the treatment of bone disease or some cancers.26,39 In the present study, indeed, the effect of ZOL on normal BM cells was weak as compared with that on JMML cells. Even though ZOL does not kill the immature population of JMML cells as shown by the flow cytometric analysis of the cultured JMML cells, ZOL, which prevents the massive expansion of monocytes/macrophages derived from JMML cells, could be useful for the therapy of JMML, because death in patients with JMML usually occurs as the result of monocytic-cell infiltration to organs, leading to organ dysfunction, infection, and bleeding.1
We mainly used the concentrations of more than 10 μM ZOL to define the difference of the effect of ZOL between JMML and normal BM cells in the present study. In the clinical situation, however, it is critical whether the serum concentration of ZOL in vivo is able to inhibit the JMML-cell growth. In the experiment using lower concentrations, ZOL had an inhibitory effect on JMML cells, although little effect on normal BM cells, even at a concentration of 3 μM, which was within the range of serum concentrations reported in a previous clinical study for the therapy of osteoporosis.26 Although the inhibitory effect of ZOL on JMML-cell growth does not reach a maximum at such a concentration in vivo, ZOL could enhance the effectiveness of other antileukemia drugs targeting immature JMML cells in combination with such drugs.25,40 To translate our finding to the clinical setting, further studies to clarify the molecular mechanism of the effect of ZOL and the in vivo effect of ZOL are needed.
Prepublished online as Blood First Edition Paper, July 26, 2005; DOI 10.1182/blood-2005-03-0972.
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Ministry of Health, Labor, and Welfare of Japan (K.T.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal