Abstract
The homeostatic adult bone marrow (BM) is a complex tissue wherein physical and biochemical interactions serve to maintain a balance between the hematopoietic and nonhematopoietic compartments. To focus on soluble factor interactions occurring between mesenchymal and hematopoietic cells, a serum-free adhesion-independent culture system was developed that allows manipulation of the growth of both mesenchymal and hematopoietic human BM-derived progenitors and the balance between these compartments. Factorial experiments demonstrated a role for stem cell factor (SCF) and interleukin 3 (IL-3) in the concomitant growth of hematopoietic (CD45+) and nonhematopoietic (CD45–) cells, as well as their derivatives. Kinetic tracking of IL-3α receptor (CD123) and SCF receptor (CD117) expression on a sorted CD45– cell population revealed the emergence of CD45–CD123+ cells capable of osteogenesis. Of the total fibroblast colony-forming units (CFU-Fs) and osteoblast colony-forming units (CFU-O), approximately 24% of CFU-Fs and about 22% of CFU-Os were recovered from this population. Cell-sorting experiments demonstrated that the CD45+ cell population secreted soluble factors that positively affect the survival and proliferation of CFU-Fs and CFU-Os generated from the CD45– cells. Together, our results provide insight into the intercellular cytokine network between hematopoietic and mesenchymal cells and provide a strategy to mutually culture both mesenchymal and hematopoietic cells in a defined scalable bioprocess.
Introduction
Hematopoiesis in the adult bone marrow (BM) is governed by a complex interplay between soluble regulatory molecules, cell-tocell contact, and interactions with extracellular matrix components.1-6 Although self-regulation within the hematopoietic niche is undoubtedly critical, it has also been clearly demonstrated that hematopoiesis is directly regulated by mesenchymal stem/progenitor cells (MPCs) and their descendents.7,8 Despite the role that MPCs play in hematopoiesis, much less is understood about MPC regulation in vivo by hematopoietic cells (HCs), largely due to difficulties in prospectively identifying putative MPCs in their native microenvironment. MPCs can be detected in vitro using retrospective assays, such as the fibroblast colony-forming unit (CFU-F) assay.9-11 Interestingly, despite observations suggesting a role for HCs in MPC development,12,13 HCs are commonly regarded as “contaminants” in MPC culture and are typically discarded during MPC propagation.
“Dexter-type” cultures were designed to recapitulate both the HC and non-HC compartments of the BM microenvironment.14,15 However, limited control of the dynamic balance between HCs and non-HCs, as well as the inability of Dexter-type cultures to support long-term hematopoietic and nonhematopoietic progenitor cell growth,14,16 limits the suitability of this culture system for studying the regulation of mesengenesis by HCs. Accordingly, an in vitro model capable of maintaining the developmental potential of both BM-derived mesenchymal cells (MCs), including MPCs, and HCs, including hematopoietic progenitor cells (HPCs), would be ideal for studying the soluble factor interplay between these cell systems. Understanding this interaction may provide insight into the biologic mechanisms governing MPC regulation.
We previously reported an approach to grow adult BM-derived HPCs17 and MPCs as single cells in adhesion-independent, stirred suspension cultures (SSCs).18 This approach supports the growth of both populations and allows the quantitative examination of the role of isolated (in the absence of adhesion-mediated signaling) soluble factor interactions in the interplay between the HC and MC compartments. Our studies revealed that the addition of specific growth factors to the serum-containing culture medium influenced the survival and growth of both MPCs and HPCs in suspension. Herein, we extend our work to demonstrate that SSC-grown cells maintain the ability to differentiate along multiple mesenchymal lineages, including fibroblastic adipogenic, osteogenic, and myogenic cells, and that such multilineage progenitors can proliferate in SSCs. Furthermore, using high-content screening approaches, we remove the confounding influences of serum and identify soluble factor combinations that influence the growth of MPCs in serum-free (SF) SSCs. This SF-SSC system was then used to study the mechanism by which factors, directly or indirectly secreted by HCs, influence MPC growth. Our analyses revealed that supplementation of interleukin 3 (IL-3) is an effective mediator of MPC survival and, when supplemented with stem cell factor (SCF), supports the expansion of MPCs in SSCs. SCF did not influence the growth and expansion of MPCs, but the effects observed when combined with IL-3 suggest that SCF may act on HCs, stimulating the secretion of as yet undetermined soluble factors capable of modulating MPC responses, including survival and growth responses. Kinetic tracking of IL-3α receptor (CD123) and SCF receptor (CD117) expression on the CD45– cell population revealed the culture type-specific emergence of CD45–CD123+ cells capable of forming a functional bone phenotype. Cell-sorting studies demonstrated a supportive role for CD45+ cells on MPC growth during suspension culture. These findings provide insight into the regulatory role that HCs (via their endogenous release of factors) have on the survival and growth of MPCs in vitro.
Materials and methods
Progenitor cell assays
To test the developmental repertoire of BM-derived cells grown under adhesion-independent SSC conditions, the following functional assays were performed. In all assay systems, SSCs were initiated according to the methods described by Baksh et al.18 CFU-F and osteoblast colony-forming unit (CFU-O) assays were performed according to the methods previously described.18
Adipogenesis. Suspension cells were removed from SSCs supplemented with SCF and IL-3 at 7, 14, and 21 days and plated at a density of 1 × 104 cells/cm2 in adipogenic-inducing medium containing Dulbecco modified Eagle medium (DMEM; high glucose, 4.5 g/L), 1 μM dexamethasone, 0.5 mM indomethacin, 10 g/mL insulin, 100 mM 3-isobutyl-1methylxantine (all from Sigma, St Louis, MO) and 10% fetal bovine serum (FBS). Cells were cultured for 3 weeks and then stained with Oil Red O (Sigma). The expression of the adipogenic-specific gene, peroxisome proliferator-activated receptor γ-2 (PPARγ-2) was analyzed on day 21 by reverse transcription-polymerase chain reaction (RT-PCR).
Myoblast-CFU assay. Cell suspensions harvested from adhesion-independent SSCs supplemented with either SCF plus IL-3, SCF plus IL-3 plus 20 ng/mL platelet-derived growth factor (PDGF-BB) or without cytokines were prepared at a concentration of 1 × 104 cells/cm2 and plated in 35-mm tissue culture dishes. The cells were maintained for 2 weeks in MCDB custom-made molecular and developmental biology 120 medium (provided by Dr J. Tremblay, Centre de Recherche en Génétique Humaine, Montreal, QC, Canada) containing 15% FBS, after which the serum level was decreased to 2% for a further 7 days. At termination, cultures were incubated with purified mouse antidesmin (1:40 in phosphate-buffered saline [PBS], BD PharMingen, San Diego, CA) for 1 hour, washed 3 times with PBS, and incubated with the secondary antibody (Alexa Fluor 488 goat antimouse antibody [1:200, Molecular Probes, Eugene, OR]) for 1 hour, washed again with PBS, and resuspended in 0.1 M sodium cacodylate buffer (pH 7.4). Positive desmin colonies were imaged and enumerated at × 4(4 ×/0.1 NA objective lens) using an inverted phase microscope.
Serum-free–small-scale suspension culture configuration
To screen multiple growth factors and growth factor combinations, a small-scale suspension culture configuration was implemented. BM samples were obtained from healthy donors (n = 3) and fractionated on a Ficoll-Paque gradient (Sigma) as previously described.18 Approval was obtained from the University of Toronto Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. BM-derived mononuclear cells (BM MNCs) were cultured at 37°C with 5% humidified CO2 in StemSpan medium (StemCell Technologies, Vancouver, BC, Canada), in polystyrene 6-well plates (Falcon, Becton Dickinson Labware, Franklin Lake, NJ) with and without the addition of cytokines. The 6-well plates were placed on a shaker platform (Hoeffer, San Francisco, CA) rotating at low speed. The cultures were fed according to the methods previously described.18 Cell suspensions were assayed on days 0, 7, 14, and 21 for CFU-F and CFU-O potential.
Factorial experiments and analysis
A 2-level factorial design (FD) matrix was constructed as described by Box et al19 to evaluate the effects of basic fibroblast growth factor (FGF), PDGF-BB, SCF, and IL-3 and their respective 24 possible combinations on total cell, CFU-F, and CFU-O expansion. In this study, optimal (high: 20 ng/mL) or zero dose levels (0 ng/mL) for PDGF and FGF were chosen based on previous studies.13,20 A dose level of 100 ng/mL and 20 ng/mL was chosen for SCF and IL-3, respectively. Each of the 16 cytokine combinations was tested on 3 independent sources of cells in triplicate. All statistical analyses of the FD experiments was performed using SPSS 11.0 (SPSS, Chicago, IL). A univariate analysis of variance (ANOVA) was performed and the significance level of each combination was determined by performing F tests. The parameter values of the general linear model19 (which contains parameter coefficients denoting the affects (β) of each cytokine and cytokine combination on the expansion of MPCs as assayed by CFU-F and CFU-O development) were estimated by regression analysis; significance (P < .05) was determined by performing F tests. To satisfy the statistical assumption of normality all responses analyzed were log-transformed. Box plots constructed with the log-transformed data confirmed data symmetry.
SF suspension cultures initiated with unsorted, CD45+, or CD45–cells. To study the regulation of MPC growth in suspension and eliminate confounding influences attributed to the presence of HCs, the CD45– cell fraction of BM MNCs was isolated. Specifically, BM MNCs (n = 3) were incubated with saturating concentrations (1:100) of conjugated mouse IgG1κ anti–human CD45-fluorescein isothiocyanate (FITC; BD Biosciences, Mississauga, ON, Canada) and 7-amino-actinomycin D (7-AAD; viability dye; BD Biosciences). Both viable CD45– and CD45+ cells were sorted on a Beckman-Coulter (Hialeah, FL) EPICS Cell Sorter at a rate of 2000 to 3000 cells/sec at 10.5 psi to 99.5% purity. SSCs (n = 3, for each group; Baksh et al18 ) were initiated with unsorted, CD45–, and CD45+ cells and cultured in StemSpan medium supplemented with 20 ng/mL IL-3 and 100 ng/mL SCF. Control suspension cultures (no cytokine addition) were initiated in parallel. At 7, 14, and 21 days, live cells were determined by trypan blue exclusion and counted using a hemocytometer. An aliquot of cells from each condition were initiated in CFU-F and CFU-O assays (n = 3, in triplicate).
Flow cytometric sorting of suspension-derived CD45–CD117+and CD45–CD123+cells. On days 7, 14, and 21, cells from SSCs (no cytokine and SCF plus IL-3 treatment groups) that were initiated with CD45– cells on day 0 were incubated with saturating concentrations of mouse IgG1κ anti–human CD45-FITC and CD123-phycoerythrin (PE; IL-3α receptor) or CD45-FITC and CD117-PE (SCF receptor; BD Biosciences) to recover populations of CD45–CD123+ and CD45–CD117+ cells on a Beckman-Coulter EPICS Cell Sorter at a rate of 2000 to 3000 cells/sec at 10.5 psi to 99.5% purity. The CFU-F and CFU-O developmental potential of these sorted cell populations were assayed as described.
Results
Suspension-cultured cells retain multilineage developmental capacities
The developmental potential of the suspension-grown cells was assessed in functional assays. First, the osteogenic potential of suspension-grown cells was assessed in a CFU-O assay.12,21 Imaging of tetracycline-labeled cultures21 was used to quantify bone nodule colonies (Figure 1A) and to determine the CFU-O expansion over the time period studied. Bone nodules were also confirmed by alkaline phosphatase, procollagen Ia, osteopontin, and osteocalcin gene expression.18 When cells derived from SSCs, incubated with SCF plus IL-3, were maintained in adipogenic culture conditions for 3 weeks, cells containing fat globules (Figure 1B) were observed that expressed mRNA transcripts for PPARγ-2 (Figure 1C). We also observed other phenotypes when our suspension-derived cells were grown under CFU-F assay conditions. For example, when suspension cells derived from the SCF plus IL-3 plus 20 ng/mL PDGF-BB group were plated in CFU-F assays, in addition to discrete colonies of fibroblastic-like cells (Figure 1D), smaller cells with thin cellular protrusions (Figure 1E) and expressing NeuN22 (not shown) were also observed.
Myogenic (myoblast-CFU [CFU-M]) developmental potential was also assayed. Plated cells were stained with Giemsa to visualize the colonies generated (Figure 1F), and staining the cells with desmin resulted in 73% ± 10% of the colonies labeling positive (Figure 1G). Kinetic analysis of the number of desmin-positive colonies revealed a statistically significant difference (P < .05) in CFU-M expansion in the different cytokine treatment groups on day 21 (Figure 1H). RT-PCR analysis confirmed that the desmin-positive colonies also expressed mRNA transcripts for myosin D1 (Figure 1I).
A serum-free suspension culture system for the propagation of mesenchymal and hematopoietic progenitors
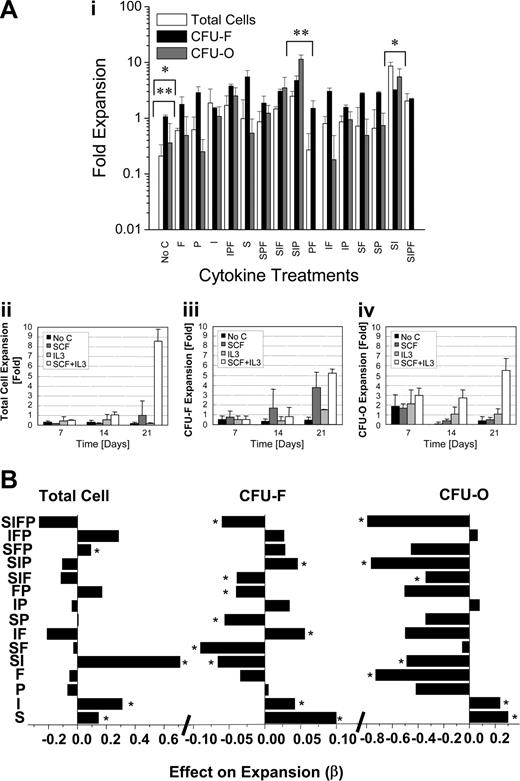
The adhesion-independent SSC system used here allows the independent manipulation of HCs and MPCs using phenotypic markers. To study the interplay between MPCs and HPCs effectively, as well as to attribute cell responses to specific cytokines and not to unknown serum components and cell adhesion effects, we used SF conditions. A 24 FD was used to quantitatively explore the effects of exogenously added factors and to detect synergies among factors. FGF, PDGF, SCF, and IL-3 and their respective combinations were screened for their ability to form CFU-Fs and CFU-Os on output cells from SSCs. At 7, 14, and 21 days, the total number of cells was calculated and CFU-F and CFU-O assays were initiated with cells derived from each condition. The greatest expansion effects on total cell, CFU-F, and CFU-O expansion were achieved in the SCF plus IL-3 and SCF plus IL-3 plus PDGF treatment conditions, whereas minimal growth was achieved in the single-factor groups (Figure 2A, in which day-21 results are shown). Interestingly, we have reported that the combination of SCF and IL-3 also supports the growth of HPCs (in serum-containing medium),17 suggesting that this approach may allow the growth of both types of marrow-derived cell populations.
Multidifferentiation potential of suspension-derived cells. (A) In addition to bone nodule formation detected by UV-fluorescence excitation of tetracycline-labeled cultures (week 1 SCF plus IL-3 suspension-derived cells cultured in CFU-O conditions shown here), (B) cells containing fat globules formed under adipogenic culture conditions (× 64). (C) RT-PCR confirmed that adipogenic cultures expressed PPAR-γ. (D) Typical cells that grew in a CFU-F assay that were initiated with cells removed from SSCs of the SCF plus IL-3 treatment group after 1 week (× 32.1). CFU-F cultures were stained with α-naphthyl acetate esterase followed by a counterstain with hematoxylin solution at 2 weeks. (E) The typical cells from CFU-F assays that were initiated with suspension-derived cells cultured in the presence of SCF plus IL-3 plus 20 ng/mL PDGF-BB (×32.1). (F) At 7, 14, and 21 days of suspension culture, test populations were removed from each suspension culture and plated in CFU-M assays; at termination (day 21), the cultures were stained with Giemsa to visualize the colonies generated. (G) Extent of desmin staining within individual cells comprising the fibroblastic colonies observed in panel F (× 20). (H) Graphic display of the fold expansion in CFU-Ms. A statistically significant difference (P < .05) in CFU-M expansion achieved in the different cytokine treatment groups on day 21 was observed. Each bar represents the mean ± SD (n = 3). (I) RT-PCR confirmed that CFU-M cultures expressed MyoD1 after 21 days. GAPDH indicates glyceraldehyde phosphate dehydrogenase.
Multidifferentiation potential of suspension-derived cells. (A) In addition to bone nodule formation detected by UV-fluorescence excitation of tetracycline-labeled cultures (week 1 SCF plus IL-3 suspension-derived cells cultured in CFU-O conditions shown here), (B) cells containing fat globules formed under adipogenic culture conditions (× 64). (C) RT-PCR confirmed that adipogenic cultures expressed PPAR-γ. (D) Typical cells that grew in a CFU-F assay that were initiated with cells removed from SSCs of the SCF plus IL-3 treatment group after 1 week (× 32.1). CFU-F cultures were stained with α-naphthyl acetate esterase followed by a counterstain with hematoxylin solution at 2 weeks. (E) The typical cells from CFU-F assays that were initiated with suspension-derived cells cultured in the presence of SCF plus IL-3 plus 20 ng/mL PDGF-BB (×32.1). (F) At 7, 14, and 21 days of suspension culture, test populations were removed from each suspension culture and plated in CFU-M assays; at termination (day 21), the cultures were stained with Giemsa to visualize the colonies generated. (G) Extent of desmin staining within individual cells comprising the fibroblastic colonies observed in panel F (× 20). (H) Graphic display of the fold expansion in CFU-Ms. A statistically significant difference (P < .05) in CFU-M expansion achieved in the different cytokine treatment groups on day 21 was observed. Each bar represents the mean ± SD (n = 3). (I) RT-PCR confirmed that CFU-M cultures expressed MyoD1 after 21 days. GAPDH indicates glyceraldehyde phosphate dehydrogenase.
Using the standard methods of analyzing FD experiments, ANOVA and linear regression analysis,19 the significance of the output responses (ie, total cell, CFU-F, and CFU-O expansion) from each cytokine combination was determined at each time point. Statistical analysis obtained from the 2-level (none and high-dose) FD experiment reported coefficients (β values) and their respective P values for single-factor (main) effects as well as for second- and higher-order interactions. The β value was used to determine the type of the effect (ie, negative or positive) that a treatment condition had on the desired outcomes. This approach allowed us to score significant differences (at day 21) in the magnitude of the effect (β) of each treatment condition on the measured outcomes (Figure 2B).
Single-factor positive effects on total cell, CFU-F, and CFU-O development were identified only when SCF or IL-3 was added (Figure 2B). Despite this, SCF or IL-3 alone was not sufficient in supporting a significant expansion of total cell, CFU-O, or CFU-F numbers at any of the time points studied (Figure 2Aii-iv). However, it would appear that SCF alone was capable of supporting CFU-F expansion over the study period, but the extent of this expansion was not statistically significant (P > .05) between days 14 and 21. Importantly, CFU-F expansion was not associated with an increase in the total number of cells in the presence of SCF alone (Figure 2Aii-iii). However, when combined, SCF and IL-3 supported a significant expansion in total cells, CFU-Fs, and CFU-Os (Figure 2Aii-iv). Our statistical analysis reported that the combination of SCF plus IL-3 yielded a negative effect on both CFU-F and CFU-O expansion (Figure 2B), despite the fact that the actual values calculated for the fold expansion of CFU-Fs and CFU-Os were more than 5-fold (Figure 2Ai). The negative parametric values associated with CFU-F and CFU-O expansion (Figure 2B) suggest that single treatment with SCF or IL-3, at the doses examined, elicited overlapping responses and, as such, when combined did not achieve a result greater than the additive outputs (relative to no factor supplementation).
Subpopulation interactions in heterogeneous SSC conditions
To explore the mechanism by which SCF and IL-3 affect MPC growth, experiments to examine the cross-talk between the different cell populations in this heterogeneous culture were undertaken. CD45+ and CD45– cells were cultured in SSCs in the presence of 100 ng/mL SCF and 20 ng/mL IL-3 in SF conditions (SSCs initiated with unsorted cells and in the absence of added cytokines served as controls). SSC cells from each group remained as single suspended cells throughout the study period as determined by analyzing the cell suspension after removing an aliquot from each SSC and examining the cells directly under a microscope. Parallel SSCs were initiated with adherent-derived MPCs. These cultures were used as positive controls for cell aggregate formation and generated aggregates measuring greater than 70 to 200 μm in SSCs, whereas our SSCs initiated with BM MNCs failed to form such cell aggregates.
Fold expansion in total cells, CFU-Fs, and CFU-Os as a function of cytokine combination and the effect of cytokine combination on total cell, CFU-F, and CFU-O expansion. (A) The fold expansions in total cell, CFU-Fs, and CFU-Os on day 21 were calculated relative to the day 0 values of each 24 cytokine combination. Statistical analysis was performed using the Student t test between the no cytokine and SCF plus IL-3 treatment conditions and between the SCF plus IL-3 and SCF plus IL-3 plus PDGF treatment conditions. Statistical differences (P < .05) were observed in total cell, CFU-F, and CFU-O expansion between the no cytokine and SCF plus IL-3 groups (denoted by the asterisk) and between the no cytokine and SCF plus IL-3 plus PDGF treatment groups (denoted by double asterisks). No C indicates no cytokine; F, FGF; P, PDGF; I, IL-3, and S, SCF. Each bar represents the mean ± SD (n = 3, performed in triplicate). Panels ii-iv show the fold expansion in total cells, CFU-Fs, and CFU-Os as a function of time in the no cytokine, SCF, IL-3, and SCF plus IL-3 conditions. (B) Graphic display of the effects (β) of SCF, IL-3, PDGF, and FGF and their 24 combinations on total cell, CFU-F, and CFU-O expansion. Bars to the left of the center axis in each graph represent negative β values, whereas positive β values are represented on the right. Statistical significance is denoted by the asterisk (P < .05).
Fold expansion in total cells, CFU-Fs, and CFU-Os as a function of cytokine combination and the effect of cytokine combination on total cell, CFU-F, and CFU-O expansion. (A) The fold expansions in total cell, CFU-Fs, and CFU-Os on day 21 were calculated relative to the day 0 values of each 24 cytokine combination. Statistical analysis was performed using the Student t test between the no cytokine and SCF plus IL-3 treatment conditions and between the SCF plus IL-3 and SCF plus IL-3 plus PDGF treatment conditions. Statistical differences (P < .05) were observed in total cell, CFU-F, and CFU-O expansion between the no cytokine and SCF plus IL-3 groups (denoted by the asterisk) and between the no cytokine and SCF plus IL-3 plus PDGF treatment groups (denoted by double asterisks). No C indicates no cytokine; F, FGF; P, PDGF; I, IL-3, and S, SCF. Each bar represents the mean ± SD (n = 3, performed in triplicate). Panels ii-iv show the fold expansion in total cells, CFU-Fs, and CFU-Os as a function of time in the no cytokine, SCF, IL-3, and SCF plus IL-3 conditions. (B) Graphic display of the effects (β) of SCF, IL-3, PDGF, and FGF and their 24 combinations on total cell, CFU-F, and CFU-O expansion. Bars to the left of the center axis in each graph represent negative β values, whereas positive β values are represented on the right. Statistical significance is denoted by the asterisk (P < .05).
The purity of CD45– cells, obtained using cell sorting, was greater than 99% (Figure 3A). The expression of CD45 within the SSCs initiated with CD45+ and CD45– cells was tracked to ensure that the starting population remained CD45+ and CD45–, respectively. Flow cytometric analysis confirmed that SSCs initiated with CD45– cells maintained a more than 97% CD45– phenotype and cultures initiated with CD45+ cells remained more than 99% CD45+.
At 7, 14, and 21 days, cells were removed and analyzed from each culture condition (Figure 3B). SSCs initiated with unsorted and CD45–-sorted cells gave rise to discrete colonies of CFU-Fs and CFU-Os (Figure 3Bi-iv). No detectable CFU-F or CFU-O activity was observed from the CD45+-sorted subpopulation. Representative bone nodules derived from the unsorted cells (Figure 3Bii) and CD45– cells (Figure 3Biv) were examined by scanning electron microscopy (SEM) to visualize the elaborated mineralized collagenous extracellular matrix. Cells derived at day 7 generated average CFU-F colony sizes of 7.2 ± 1.9 mm and 6.6 ± 1.2 mm from the unsorted and CD45–-sorted cell populations, respectively. No significant difference in the CFU-F colony sizes from these cell fractions (unsorted versus CD45–-sorted cells) was observed at any time point studied, suggesting that SSC did not compromise the capacity of suspension-grown MPCs to proliferate upon adhesion.
Quantitatively tracking CFU-F and CFU-O development revealed that the unsorted cell group (containing > 60% CD45+ cells) resulted in a higher fold-increase in total, CFU-F, and CFU-O cells when compared to the numbers obtained from the CD45–-sorted cell population (Figure 3C). Significant differences were calculated between the unsorted and CD45–-sorted cell groups with respect to the fold expansion in total cells, CFU-Fs, and CFU-Os at various time points (Figure 3C), with minimal CFU-F and CFU-O growth observed in the absence of CD45+ cells. On average, a 58% ± 11% increase in CFU-F and CFU-O development was observed when CD45– cells were cultured in the presence of CD45+ cells. Additionally, CD45+ cells cultured in the absence of CD45– cells demonstrated a significantly lower total cell expansion (Figure 3Ci) and no capacity to form CFU-Fs (Figure 3Cii) or CFU-Os (Figure 3Ciii).
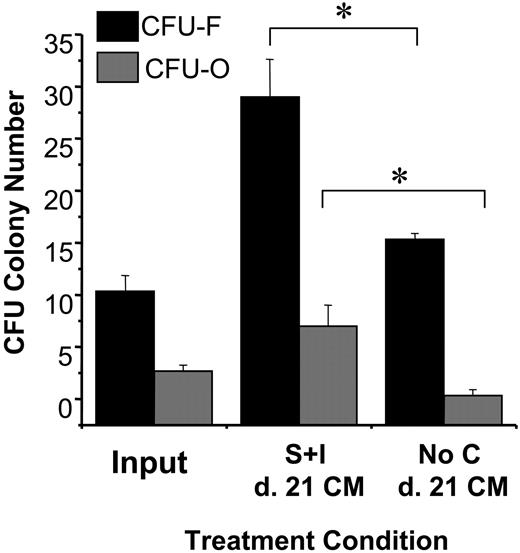
As an initial screen to determine if there were factors secreted by HCs (CD45+) that caused the growth of cells capable of CFU-F and CFU-O development, conditioned medium (CM) from SSCs supplemented with, or without, SCF and IL-3 were harvested and used as the basal medium in CFU-F and CFU-O assays. BM MNCs cultured in CM harvested from day 21 SCF plus IL-3–supplemented SF SSCs that were initiated with sorted BM MNC-derived CD45+ cells yielded significantly greater numbers of CFU-F and CFU-O cells, relative to CM harvested from parallel day 21 “no cytokine” suspension cultures (Figure 4). These experiments allow us to conclude that factors secreted by the CD45+ cells, and elicited by the addition of SCF and IL-3 supplementation, positively affect CFU-F and CFU-O growth.
SCF and IL-3 act by different mechanisms to support suspension culture MPC growth
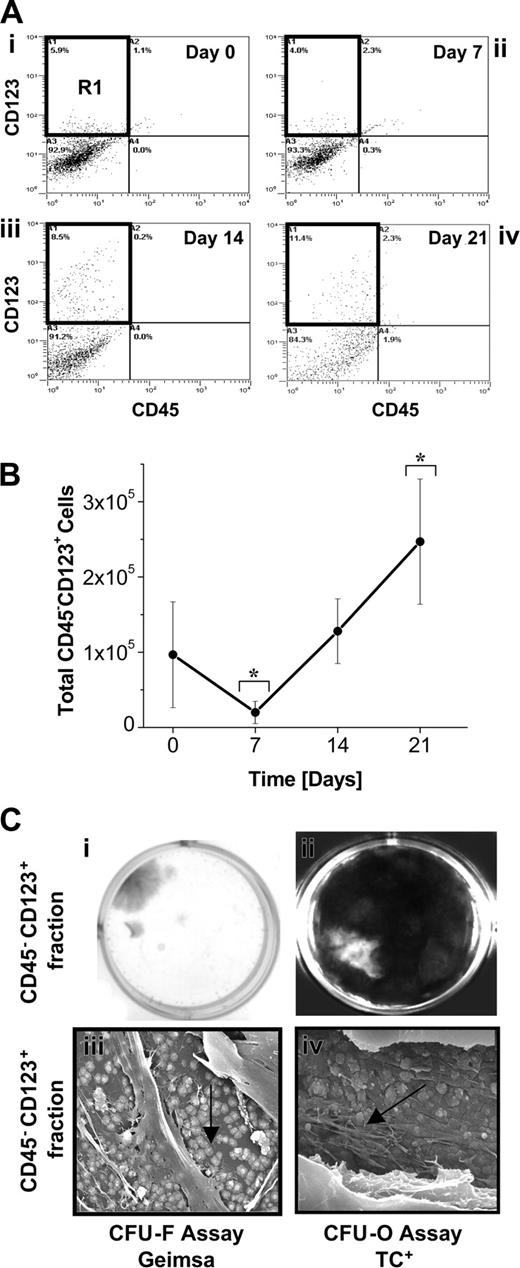
The results indicated that both IL-3 and SCF supplementation and the presence of the CD45+ cell population were necessary components for maximal propagation of CD45–-derived CFU-Fs and CFU-Os in SSCs. To investigate whether IL-3 or SCF transduces signals directly on CD45– cells, the expression of CD123 (IL-3α receptor) and CD117 (SCF receptor) was tracked using flow cytometry. Propagation with SCF plus IL-3 resulted in a significant increase in the percentage of cells expressing CD123 between days 7 and 21 (Figure 5A). The relative expression of CD45–CD123+ cells increased from 3.5% ± 1.3% on day 7 to 8.9% ± 2.3% by day 21. Furthermore, the fluorescent intensity of CD45–CD123+ cells also increased with time (Figure 5Aiv), suggesting that a higher density of CD123 receptor complexes may be present on single CD45– cells with time in suspension culture.
The influence of CD45+ cells on the yield of CFU-F and CFU-O development from CD45– cells. (A) Representative flow cytometric histogram plots demonstrate the distribution of both CD45– and CD45+ cell populations from input BM-derived cells. (i) Day 0 BM-derived cells were incubated with saturating concentrations of CD45-PE and (ii) the CD45– cells were recovered. R1 represents the isotype control gate corresponding to the CD45– cell fraction, and R2 represents the region used to sort CD45+ cells. The purity of the recovered CD45– fraction was more than 99%, as determined by flow cytometry. (B) CFU-F and CFU-O development from unsorted and CD45– suspension-derived cells. Cells harvested from day 7 suspension cultures initiated with unsorted and CD45– cells were plated in CFU-F and CFU-O assays. CFU-F assays were stained with Giemsa to visualize discrete colonies of fibroblastic cells (i,iii), and CFU-O assays (ii,iv) were incubated with tetracycline to visualize newly formed bone nodules (seen as white areas) under UV-fluorescence microscopy. Scanning electron micrographs revealed a layer of globular accretions (v) and mineralized extracellular matrix (vi). Field width (FW) for panels v and vi = 70 μm and 14μm, respectively. Note: CFU-F and CFU-O assays were initiated with 1 × 103 cells/cm2. (C) The calculated fold expansion in total cells, CFU-Fs, and CFU-Os attained in SSCs initiated with unsorted, CD45–, and CD45+ cells. Calculated (i) total cell, (ii) CFU-F, and (iii) CFU-O expansions as a function of suspension culture time. The cells harvested from the CD45+ suspension cultures did not give rise to CFU-Fs and CFU-Os. Statistically significant differences (P < .05) are denoted with an asterisk. Each bar represents the mean ± SD (n = 3, performed in triplicate).
The influence of CD45+ cells on the yield of CFU-F and CFU-O development from CD45– cells. (A) Representative flow cytometric histogram plots demonstrate the distribution of both CD45– and CD45+ cell populations from input BM-derived cells. (i) Day 0 BM-derived cells were incubated with saturating concentrations of CD45-PE and (ii) the CD45– cells were recovered. R1 represents the isotype control gate corresponding to the CD45– cell fraction, and R2 represents the region used to sort CD45+ cells. The purity of the recovered CD45– fraction was more than 99%, as determined by flow cytometry. (B) CFU-F and CFU-O development from unsorted and CD45– suspension-derived cells. Cells harvested from day 7 suspension cultures initiated with unsorted and CD45– cells were plated in CFU-F and CFU-O assays. CFU-F assays were stained with Giemsa to visualize discrete colonies of fibroblastic cells (i,iii), and CFU-O assays (ii,iv) were incubated with tetracycline to visualize newly formed bone nodules (seen as white areas) under UV-fluorescence microscopy. Scanning electron micrographs revealed a layer of globular accretions (v) and mineralized extracellular matrix (vi). Field width (FW) for panels v and vi = 70 μm and 14μm, respectively. Note: CFU-F and CFU-O assays were initiated with 1 × 103 cells/cm2. (C) The calculated fold expansion in total cells, CFU-Fs, and CFU-Os attained in SSCs initiated with unsorted, CD45–, and CD45+ cells. Calculated (i) total cell, (ii) CFU-F, and (iii) CFU-O expansions as a function of suspension culture time. The cells harvested from the CD45+ suspension cultures did not give rise to CFU-Fs and CFU-Os. Statistically significant differences (P < .05) are denoted with an asterisk. Each bar represents the mean ± SD (n = 3, performed in triplicate).
The total number of CD45–CD123+ cells that were generated throughout SSCs was calculated using the percent expression obtained by flow cytometry (Figure 5A). A decrease in the number of CD45–CD123+ cells was initially observed; however, after 7 days, the number of CD45–CD123+ cells significantly increased (P < .05) in number resulting in about a 12-fold increase in total CD45–CD123+ cells by day 21 (relative to day 7 values; Figure 5B).
Effects of conditioned medium derived from HCs grown in suspension on CFU-F and CFU-O development. BM-MNCs were plated directly in CFU-F and CFU-O assay conditions that used conditioned medium harvested from day 21 suspension cultures initiated with CD45+ cells that were supplemented with and without SCF plus IL-3. Statistical significance (P < .05) is denoted by an asterisk. Each bar represents the mean ± SD (n = 2 BM samples, performed in triplicate).
Effects of conditioned medium derived from HCs grown in suspension on CFU-F and CFU-O development. BM-MNCs were plated directly in CFU-F and CFU-O assay conditions that used conditioned medium harvested from day 21 suspension cultures initiated with CD45+ cells that were supplemented with and without SCF plus IL-3. Statistical significance (P < .05) is denoted by an asterisk. Each bar represents the mean ± SD (n = 2 BM samples, performed in triplicate).
To determine whether IL-3 was acting directly on MPCs, the CFU-F and CFU-O developmental potential of the CD45–CD123+ cells was tested. Representative tetracycline-labeled CFU-O cells were processed for SEM and demonstrated hallmarks of de novo bone formation, as we have previously described23 (Figure 5Ciiiiv). No CFU-Fs or CFU-Os were detected from input (day 0) BM-derived CD45–CD123+ cells at the cell-seeding dilution used (ie, 1 × 103 cells/cm2). However, following 7 days in suspension culture, about 1 in 104 CD45–CD123+ cells had the capacity to form CFU-Fs and CFU-Os (Figure 5C). CD45– cells were also evaluated for their expression of CD117; less than 1% of the total CD45– population was CD117+ (data not shown). Furthermore, CD45–CD117+ cells, isolated on day 7 of suspension culture, failed to give rise to CFU-Fs and CFU-Os. Importantly, a CD45–CD123+CD117+ or CD45–CD123–CD117+ cell population was not detected in these studies, suggesting that the observed contribution of SCF to the MPC growth is indirect.
A comparison in the yield of CFU-Fs and CFU-Os from unsorted, CD45–, and CD45–CD123+ cell populations
Analysis of the frequency of CFU-Fs and CFU-Os over the 21-day culture period in the unsorted, CD45–-, and CD45–CD123+-sorted cell fractions revealed an increase in the frequency of both CFU-Fs and CFU-Os by day 21 (relative to day 7 values; Table 1). Importantly, the frequency of CFU-Fs and CFU-Os measured in SSCs initiated with CD45– cells (alone) was statistically less than that obtained in the unsorted cell population, emphasizing, again, the importance of CD45+ cells in the growth and maintenance of these clonogenic MPCs.
CFU-F and CFU-O colony numbers/104 cells from suspension-derived cells of various cell fractions
. | CFU-F . | . | . | . | CFU-O . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time, d . | Unsorted cells . | CD123+CD45- cells* . | CD45- cells sorted† . | CD123+CD45- cells‡ . | Unsorted cells . | CD123+CD45- cells* . | CD45- cells sorted† . | CD123+CD45- cells‡ . | ||||||
| 0 | 10 ± 2 | 0 ± 0 | 8 ± 0 | 0 ± 0 | 5 ± 2 | 0 ± 0 | 4 ± 0 | 0 ± 0 | ||||||
| 7 | 14 ± 2 | 2.3 ± 0.6 | 1 ± 0 | 0.3 ± 0.6 | 8.6 ± 1.5 | 0.67 ± 0 | 0.7 ± 0.6 | 0.3 ± 0.6 | ||||||
| 14 | 11 ± 4 | 3 ± 1 | 1.3 ± 0.6 | 1.0 ± 0 | 15 ± 3 | 1.33 ± 0.6 | 2.3 ± 0.6 | 0.3 ± 0.6 | ||||||
| 21 | 19 ± 5 | 4.3 ± 0.6 | 7 ± 1 | 1.7 ± 0.6 | 20 ± 3.5 | 3.6 ± 1 | 3 ± 0.6 | 0.67 ± 0.6 | ||||||
. | CFU-F . | . | . | . | CFU-O . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time, d . | Unsorted cells . | CD123+CD45- cells* . | CD45- cells sorted† . | CD123+CD45- cells‡ . | Unsorted cells . | CD123+CD45- cells* . | CD45- cells sorted† . | CD123+CD45- cells‡ . | ||||||
| 0 | 10 ± 2 | 0 ± 0 | 8 ± 0 | 0 ± 0 | 5 ± 2 | 0 ± 0 | 4 ± 0 | 0 ± 0 | ||||||
| 7 | 14 ± 2 | 2.3 ± 0.6 | 1 ± 0 | 0.3 ± 0.6 | 8.6 ± 1.5 | 0.67 ± 0 | 0.7 ± 0.6 | 0.3 ± 0.6 | ||||||
| 14 | 11 ± 4 | 3 ± 1 | 1.3 ± 0.6 | 1.0 ± 0 | 15 ± 3 | 1.33 ± 0.6 | 2.3 ± 0.6 | 0.3 ± 0.6 | ||||||
| 21 | 19 ± 5 | 4.3 ± 0.6 | 7 ± 1 | 1.7 ± 0.6 | 20 ± 3.5 | 3.6 ± 1 | 3 ± 0.6 | 0.67 ± 0.6 | ||||||
CD123+CD45- cells isolated from unsorted cells that were grown in stirred suspension.
CD45- cells were sorted from input BM samples and were directly grown in stirred suspension.
CD123+CD45- cells isolated from CD45--sorted cells that were grown in stirred suspension.
Developmental potential of CD45–CD123+ cells. (A) Representative flow cytometric dot plots demonstrating the relative increase in the percentage of CD45–CD123+ cells throughout the 21-day culture period. CD45– cells cultured in suspension were dual-labeled for CD45 and CD123 to track CD45–CD123+-expressing cells. The CD45–CD123+ cells, represented by R1, were sorted on day 0 (i), 7 (ii), 14 (iii), and 21 (iv) and the developmental potential of these isolated populations were determined in CFU-F and CFU-O assays. Representative quad plots of day 0, 7, 14, and 21 suspension-derived cells (of a suspension culture initiated with CD45– cells) stained with CD45 and CD123 are shown here. Positive expression was determined as more than 99% of isotype control and about 15 000 gated events were collected. (B) Total number of CD45–CD123+ cells generated in SF suspension culture conditions supplemented with 100 ng/mL SCF and 20 ng/mL IL-3. Dual fluorescence labeling for CD45 and CD123 was used to track the percentage of CD45–CD123+cells in suspension cultures initiated with CD45– cells. These values were used to calculate the total number of CD45–CD123+cells generated throughout suspension culture. Statistical significance (P < .05) is denoted by an asterisk. Each data point represents the mean ± SD (n = 3). (C) CFU-F and CFU-O development from CD45–CD123+ suspension-derived cells. CD45–CD123+-sorted cells (grown in CD45– SSCs) were plated in CFU-F and CFU-O assays following 7 days of suspension culture. (i) CFU-F assays were stained with Giemsa to visualize discrete colonies of fibroblastic cells; (ii) CFU-O assays were incubated with tetracycline (TC), to visualize newly formed bone nodules (seen as white areas) under UV-fluorescence microscopy. Representative scanning electron micrographs of revealing (iii) cement line matrix deposition (arrow) and (iv) collagen mineralization (arrow) derived from CD45–CD123+ sorted cells grown in CFU-O assay conditions (FW for iii and iv = 53 μm and 21 μm, respectively). Note: CFU-F and CFU-O assays were initiated with 1 × 103 cells/cm2.
Developmental potential of CD45–CD123+ cells. (A) Representative flow cytometric dot plots demonstrating the relative increase in the percentage of CD45–CD123+ cells throughout the 21-day culture period. CD45– cells cultured in suspension were dual-labeled for CD45 and CD123 to track CD45–CD123+-expressing cells. The CD45–CD123+ cells, represented by R1, were sorted on day 0 (i), 7 (ii), 14 (iii), and 21 (iv) and the developmental potential of these isolated populations were determined in CFU-F and CFU-O assays. Representative quad plots of day 0, 7, 14, and 21 suspension-derived cells (of a suspension culture initiated with CD45– cells) stained with CD45 and CD123 are shown here. Positive expression was determined as more than 99% of isotype control and about 15 000 gated events were collected. (B) Total number of CD45–CD123+ cells generated in SF suspension culture conditions supplemented with 100 ng/mL SCF and 20 ng/mL IL-3. Dual fluorescence labeling for CD45 and CD123 was used to track the percentage of CD45–CD123+cells in suspension cultures initiated with CD45– cells. These values were used to calculate the total number of CD45–CD123+cells generated throughout suspension culture. Statistical significance (P < .05) is denoted by an asterisk. Each data point represents the mean ± SD (n = 3). (C) CFU-F and CFU-O development from CD45–CD123+ suspension-derived cells. CD45–CD123+-sorted cells (grown in CD45– SSCs) were plated in CFU-F and CFU-O assays following 7 days of suspension culture. (i) CFU-F assays were stained with Giemsa to visualize discrete colonies of fibroblastic cells; (ii) CFU-O assays were incubated with tetracycline (TC), to visualize newly formed bone nodules (seen as white areas) under UV-fluorescence microscopy. Representative scanning electron micrographs of revealing (iii) cement line matrix deposition (arrow) and (iv) collagen mineralization (arrow) derived from CD45–CD123+ sorted cells grown in CFU-O assay conditions (FW for iii and iv = 53 μm and 21 μm, respectively). Note: CFU-F and CFU-O assays were initiated with 1 × 103 cells/cm2.
Flow cytometric analysis revealed that the CD45–CD123+ subpopulation represented less than about 10% of the total CD45– cell population (Figure 5). We also observed a similar percent expression (∼10%) of CD45–CD123+ cells in the total unsorted BM cell population after 21 days of SSC (not shown). We determined using clonogenic assays that the CD45–CD123+ subpopulation, after sorting from the CD45– cell population, contained about 24% and about 22% of the total CFU-Fs and CFU-Os, respectively, when compared to the number generated from the CD45–-sorted cell population at day 21 (Table 1). Additionally, we observed that the CD45–CD123+ subpopulation sorted from the total cell population (unsorted cells) generated about 23% and about 18% of the CFU-Fs and CFU-Os, respectively, relative to the unsorted cell population, by day 21 (Table 1) and not 100% recovery, which we might have expected. Taken together, our analysis (Table 1) revealed that CD123+CD45– cells might also require the presence of CD45+ cells to attain their maximal colony number output. Despite this, the observed increase in the frequency of CFU-Fs and CFU-Os, as a function of time, suggests that a CD45– CD123+ cell subpopulation may contain a cell type directly responsive to IL-3, which has the capacity to give rise to both CFU-Fs and CFU-Os and that this population also requires factors secreted by HCs to support their growth in suspension.
Discussion
Despite increasing experimental and clinical interest in MSCs, our understanding of the underlying mechanisms regulating MSC fate remains elusive. The established approach to the isolation and culture of MSCs involves their propagation on tissue culture plastic with the concomitant removal of HCs during adherent culturemediated expansion. However, one important unanswered question in this approach is whether HCs play a role in signaling to MPCs, regulating their growth and development, similar to the converse that is seen in hematopoiesis by MC types.7 To address this question, we investigated the regulatory role that HCs may play in MPC development by designing a suspension culture system that would enable the coculture of both HCs and MCs.
First, we confirmed that our SSC maintained MPCs capable of developing into multiple cell types by demonstrating their ability to differentiate into the fibroblast, osteogenic, adipogenic, and myogenic lineages. These findings provided motivation to examine specific factors that might influence the survival and growth of multipotential MPCs in suspension such as cytokine supplementation and accessory cell involvement, specifically HCs. Therefore, to limit the effects of adhesion and focus on the regulatory role of specific cytokines in supporting the growth and expansion of MPCs in suspension, as well as to determine the influence of HCs (via endogenous secretion of soluble factors) on the growth of MPCs, experiments were performed in a SF SSC.
FD was used rather than “one-factor-at-a-time” experiments to evaluate the effects of multiple growth factors on total cell and mesenchymal colony-forming potential. Our factorial also allowed interactions between factors to be detected. Analysis revealed that SCF and IL-3 had positive effects on the survival of total cells, CFU-Fs, and CFU-Os in SF SSCs initiated with (unsorted) BM-derived cells. IL-3 alone demonstrated the capacity to maintain input numbers throughout the 21-day culture period; however, this growth condition was insufficient in stimulating cell and progenitor expansion. Expansion was obtained with the combination of SCF plus IL-3 (> 5-fold). Interestingly, the combined effect of SCF plus IL-3 was less than additive (Figure 2B), suggesting that these factors may elicit their effects via overlapping mechanisms. This observation, along with the cell-sorting and cell surface receptor analysis studies, provide insight into the complex intercellular network regulating MPC and HPC responses in vitro.
In HSC culture systems, it has been well established that the survival and proliferation of HSCs is controlled by a number of interacting cytokines including SCF and IL-3,24 which act directly on progenitor cells expressing receptors for these ligands.25 IL-3 is considered an early acting growth factor necessary for the proliferation of multipotential colony-forming cells that include granulocytes, erythroid cells, monocytes, and the like,26 whereas SCF has been shown to promote the survival of hematopoietic stem/progenitor cells.27 In combination, these 2 cytokines are thought to act synergistically to inhibit apoptosis and promote proliferation.25,28 Based on the presumed colocalization of putative MSCs and their descendents and HSCs in the BM compartment, it is plausible that these cytokines play similar roles in vivo.
To investigate the mechanism by which IL-3 transduced stimulatory signals to MPCs, SSCs were initiated under cytokinesupplemented SF conditions with subsets of the whole BM population. Tests were performed to determine if IL-3 affects MPCs in an indirect fashion through HCs (CD45+) and their subsequent release of soluble factors or by direct action on CD45– cells. Cells responsive to IL-3 express CD123 (IL-3α receptor) and are typically of the hematopoietic lineage (ie, CD45+).26,29,30 To explore the potential direct action of IL-3 on MPCs, CD45– cells were cultured in SF SSC supplemented with SCF and IL-3. Flow cytometric analysis confirmed that more than 97% of the cells maintained a CD45– phenotype throughout suspension culture and propagation with SCF and IL-3 resulted in a significant increase in the percentage and total number of CD45– cells expressing IL-3α receptor (CD123) over 21 days (Figure 4A-B). Importantly, we demonstrated that the CD45– cell fraction included a small percentage of CD123+ cells.
Interestingly, there were no CFU-Fs or CFU-Os detected in the CD45– CD123+ cell fraction at input, although about 1 in 10 000 CD4–CD123+ cells was capable of giving rise to CFU-Fs and CFU-Os as early as day 7 of suspension culture (Table 1). These data suggest that the CD123+ cells giving rise to CFU-Fs and CFU-Os may be at a very low frequency in BM, undetectable in our assay conditions or, perhaps MPCs “acquire” this phenotype after an extended time in SSC. In response to IL-3 (with SCF) this rare cell type may not only selectively grow and thus be readily detected by immunophenotyping techniques such as flow cytometry, but also be within the detection limit of conventional functional assays over time in culture. Surprisingly, despite their low frequency, about 24% and about 22% of the total attainable CFU-Fs and CFU-Os, respectively, were recoverable in the CD45–CD123+ cell population (at day 21). This represents a significant fraction of the total number of available CFU-Fs and CFU-Os. These findings suggest that MPCs may be phenotypically heterogeneous, an observation corroborated in other studies demonstrating that adherent-derived MSC populations display heterogeneity with respect to their self-renewal31,32 and multilineage differentiation potential.11,33 However, whether the apparent heterogeneity ascribed to suspension-derived MPCs is attributed to lineage hierarchy that exists in the putative in vivo MSC compartment (ie, CD45–CD123– to CD45–CD123+ or vice versa), or whether a functional CD45–CD123+ population is present, undetected, in the BM compartment, remains to be elucidated. Nevertheless, the results presented provide evidence that IL-3 has the potential to act directly on a unique subset of MPCs with CFU-F and CFU-O developmental potential and provides a mechanism by which subpopulations of MPCs have the capacity to grow in noncontact suspension culture conditions. Determination of the multilineage potential of the CD45–CD123+ cell population (studies underway) would help to clearly delineate the stem versus progenitor cell potential of this population.
These studies also revealed a role for HCs in the stimulation of CFU-F and CFU-O growth in SSCs (Figure 3C). Analysis of the combined fold expansion of total cells attained in SSCs initiated with CD45+ or CD45– cells alone was calculated to be less than that obtained from suspension cultures initiated with unsorted cells. Specifically, a 58% ± 11% increase in the number of CFU-F–and CFU-O–detectable colonies was calculated over the 21-day study period when CD45– cells were grown in the presence of CD45+ cells. These results support the existence of positive interactions between CD45+ and CD45– cells, likely mediated by their respective endogenous secretion of soluble factors, similar to that which is observed between early HCs and their progenitors.34
The differential responsiveness of MPCs to endogenously produced and exogenously supplemented factors. IL-3 exerts its action (+) on both HCs and non-HCs throughout SF suspension culture, resulting in the expansion of cells in both these compartments. IL-3 has also been shown to promote the growth of CD45–CD123+ cells, which are capable of giving rise to CFU-Fs and CFU-Os. However, on average, only about 24% and about 22% of the total attainable CFU-Fs and CFU-Os, respectively, are recovered from a CD45–CD123+ cell population by day 21 of the study period, suggesting that the mesenchymal stem cell compartment is phenotypically heterogeneous. Furthermore, the growth of HCs is also directly influenced by IL-3 (and SCF). It has also been demonstrated from these studies that HCs potentiate the growth of MPCs via the release of endogenous factors (factor(s) X) that target MPCs, resulting in their growth.
The differential responsiveness of MPCs to endogenously produced and exogenously supplemented factors. IL-3 exerts its action (+) on both HCs and non-HCs throughout SF suspension culture, resulting in the expansion of cells in both these compartments. IL-3 has also been shown to promote the growth of CD45–CD123+ cells, which are capable of giving rise to CFU-Fs and CFU-Os. However, on average, only about 24% and about 22% of the total attainable CFU-Fs and CFU-Os, respectively, are recovered from a CD45–CD123+ cell population by day 21 of the study period, suggesting that the mesenchymal stem cell compartment is phenotypically heterogeneous. Furthermore, the growth of HCs is also directly influenced by IL-3 (and SCF). It has also been demonstrated from these studies that HCs potentiate the growth of MPCs via the release of endogenous factors (factor(s) X) that target MPCs, resulting in their growth.
The soluble factor composition of the SSC medium was not analyzed in this study. However, preliminary experiments revealed a significant increase in the yield of CFU-Fs and CFU-Os when BM-derived cells were cultured with conditioned media harvested from SF suspension cultures (Figure 4). Taken together, we hypothesize that suspension-grown HCs (> 60% of the unsorted suspension cell population) secrete numerous regulatory molecules that form the basis of intercellular cross-talk networks, which then regulate the survival and growth of MPCs in an autocrine or paracrine manner.34,35 Assessing the protein composition of the media from which HCs are grown may provide further insight into the important role that CD45+ cells play on MPC growth and allow further optimization of stimulatory signals necessary for expanding an MPC population in this controlled SSC system.
In summary, the development of an SF suspension culture configuration provided the opportunity to examine soluble factor interactions between the mesenchymal and hematopoietic compartments. Collectively, these results demonstrate the ability of IL-3 to enhance MPC expansion in SSCs and suggest that it may act directly with CD123+ MPCs (Figure 6). The observed competitive emergence of CFU-F–and CFU-O–capable CD45– CD123+ cells in direct response to IL-3 supplementation revealed heterogeneity in the MSC compartment. The functional relevance of this phenotypic heterogeneity remains to be determined.
Prepublished online as Blood First Edition Paper, July 19, 2005; DOI 10.1182/blood-2005-01-0433.
Supported by operating grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada (P.W.Z.) and the Ontario Research and Development Challenge Fund (J.E.D.). D.B. was a recipient of a NSERC postgraduate scholarship and held an Ontario Graduate Scholarship. D.B. designed and performed research, analyzed data, and wrote the paper. J.E.D. assisted in experimental design and interpretation, as well as manuscript writing and editing. P.W.Z. assisted in experimental design and interpretation, as well as manuscript writing and editing.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank StemCell Technologies (Vancouver, BC, Canada) for generously providing the reagents and medium used in this work and Dr A. Keating (Toronto Hospital Network, Toronto, ON, Canada) for providing the BM samples. We would also like to acknowledge Dr Jacque Tremblay (Centre de Recherche en Génétique Humaine, QC, Canada) and Dr Jane Aubin (Department of Medical Biophysics, University of Toronto) for providing culture media for and advice concerning the myogenic cultures. Minke Hoeksma provided technical assistance in the factorial design portion of this work.
P.W.Z. is the Canada Research Chair in Stem Cell Bioengineering.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal