Comment on Nolan et al, page 3264
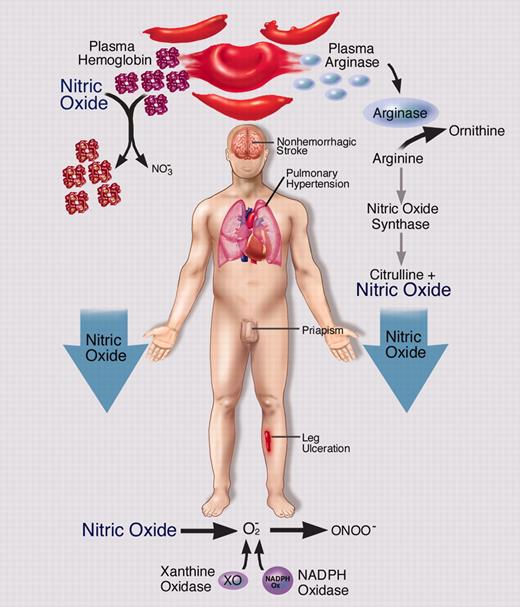
Hemolytic anemia, while silent from a vaso-occlusive pain crisis standpoint, leads to sustained nitric oxide depletion, oxidant stress, vasoconstriction and proliferative vasculopathy in a number of organ systems, ultimately contributing to the development of priapism, cutaneous leg ulceration, pulmonary hypertension, sudden death, and possibly stroke.
Our survival depends on the efficient delivery of oxygen to tissues. This is achieved by concentrating both ferrous hemeiron and oxygen to approximately 20 mM in red cells. Both substances are extremely redox active and toxic, generating superoxide anion via heme-mediated auto-oxidation and Fenton chemistry, and, when released into plasma during hemolysis, react with and consume endothelial-derived nitric oxide (NO).1,2 Thus the concentration of oxygen and heme-iron in blood has evolved in concert with remarkable antioxidant, anti-inflammatory, and antiproliferative systems that limit stress responses to these redox active species.
The first line of defense is the erythrocyte itself. The physical separation of hemoglobin, methemoglobin, and heme-bound oxygen from endothelium creates diffusional barriers around the red blood cell that limit NO and superoxide diffusion between the red cell and endothelium and prevent hemoglobin extravasation into the interstitial compartment.3 A major second line of defense is the hemoglobin scavenging system: hemoglobin binds with extremely high affinity to haptoglobin, a high-molecular-weight multimeric protein that limits hemoglobin extravasation and NO scavenging. CD163, the hemoglobin scavenger protein, recognizes a neoepitope on the hemoglobin-haptoglobin complex and then binds the complex for internalization into the reticuloendothelial system.4 This binding also signals the downstream expression and activation of interleukin-10 (IL-10), hemeoxygenase 1, biliverdin reductase, and P21,4-8 all systems possessing catalytic antioxidant, anti-inflammatory, and antiproliferative properties.FIG1
It is therefore not surprising that diseases or therapies that overwhelm these systems, such as the steady-state intravascular hemolysis characteristic of the hereditary and acquired hemolytic anemias, as well as therapy with stroma-free hemoglobin-based blood substitutes, lead to NO scavenging, superoxide formation, endothelial dysfunction, vasculopathy, and increased risk of death.9 In addition to NO scavenging by plasma cell–free hemoglobin, hemolysis releases red cell arginase into plasma, which degrades arginine, the substrate for endothelial NO synthesis.10 Indeed, an increasing number of reports of pulmonary hypertension in patients with hereditary and acquired hemolytic anemias suggest that there may exist a syndrome of hemolytic anemia–associated pulmonary hypertension. Patients with disparate hemolytic anemias also suffer from cutaneous leg ulceration and, to a lesser extent, priapism. Of interest, priapism was strongly associated with pulmonary hypertension in a recent cohort study of patients with sickle cell disease11 and has been described in both the endothelial nitric oxide synthase (eNOS)/neuronal nitric oxide synthase (nNOS) double knock-out mouse and sickle cell transgenic mouse.12
In the current issue of Blood, Nolan and colleagues expand this paradigm with the finding that priapism was strongly linked to high hemolytic rate in patients enrolled in the Cooperative Study for Sickle Cell Disease. These investigators performed a case-control study and found that patients with priapism were more likely to be homozygous for hemoglobin S (HbSS) disease (less likely to have HbSC [compound heterozygosity for HbS and HbC] disease and HbSS-α thalassemia) and to have elevated markers of hemolysis and inflammation. Multivariate analysis identified lactate dehydrogenase, reticulocyte count, and platelet count as independently associated with priapism. The authors conclude that priapism, pulmonary hypertension, and possibly ischemic stroke are all associated with low steady-state hemoglobin levels, protection by α thalassemia, and HbSC disease, and may be considered a subphenotype of sickle cell disease mechanistically linked to hemolytic anemia, reduced NO bioavailability, and vasculopathy.
In conclusion, biochemical, physiologic, and epidemiologic data suggest that chronic intravascular hemolytic anemia, while silent from a vaso-occlusive pain crisis standpoint, leads to sustained NO depletion, oxidant stress, vasoconstriction, and proliferative vasculopathy in a number of organ systems, ultimately contributing to the development of priapism, cutaneous leg ulceration, pulmonary hypertension, sudden death, and possibly stroke (Figure). The existence of such a subphenotype suggests new directions for therapy (targeting hemolytic anemia with higher levels and pancellular penetrance of hemoglobin F, inhibiting the Gardos channel to limit hemolysis, increasing NO bioavailability, and reducing superoxide formation) and combination therapy, with transfusions and hydroxyurea aimed at inhibiting erythropoiesis. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal