Abstract
Several reports indicate that there might be differences in clinical features between Asian and Western myelodysplastic syndrome (MDS) cases. We analyzed refractory anemia (RA) in French-American-British (FAB) classification cases diagnosed in Japan and Germany to perform a more exact comparison between Asian and Western MDS types. In the first step, we analyzed agreement of morphologic diagnosis between Japanese and German hematologists. Blood and bone marrow slides of 129 patients diagnosed with FAB-RA, FAB-RA with ringed sideroblasts (RARS), or aplastic anemia were selected randomly and evaluated separately by each group. The agreements of diagnoses according to FAB and World Health Organization (WHO) classifications were 98.4% and 83.8%, respectively. Second, we compared clinical features between 131 Japanese and 597 German patients with FAB-RA. Japanese patients were significantly younger than German patients. Japanese patients had more severe cytopenias. However, prognosis of Japanese patients was significantly more favorable than that of German patients. Japanese patients had a significantly lower cumulative risk of acute leukemia evolution than did German patients. Frequency of WHO-RA in Japanese patients with FAB-RA was significantly higher than that in German patients. In conclusion, our results indicate that the clinical features of Japanese patients with FAB-RA differ from those of German patients.
Introduction
Myelodysplastic syndromes (MDSs) are acquired clonal stem cell disorders characterized by ineffective hematopoiesis with myelodysplasia1 and are associated with a high risk of progression to acute leukemias.2 MDSs are very heterogeneous in terms of their morphology, clinical features, and survival.3 Refractory anemia (RA) according to the French-American-British (FAB) classification is generally classified as a low-risk group in MDS,4 comprising 30% to 40% of all MDS cases. It was reported that the International Prognostic Scoring System (IPSS) was useful for assessing the prognosis in the whole group of patients with MDS according to the FAB classification.5 According to the World Health Organization (WHO) classification,6 most patients with FAB-RA are classified as refractory cytopenia with multilineage dysplasia (RCMD) or, less frequently, as WHO-RA. It was reported that patients with WHO-RA had more favorable prognoses than did patients with RCMD.7-9
There are several reports indicating possible differences in clinical features between Western MDS types and Eastern MDS types. It has been reported that the median age of Japanese patients with MDS is 60 years.10 The median age of patients with MDS in Korea and Thailand and the mean age of those in Central Africa were reported to be 57,11 56,12 and 57 years,13 respectively. However, large MDS studies from Western countries showed a median or mean age of 68 to 73 years.5,14-16 We have reported that the clinical features of RA with excess of blasts (RAEB) or RAEB in transformation according to FAB classification seemed to be similar between Japanese and Western patients.17 However, previous reports indicate that Japanese patients with MDS have a lower frequency of RA with ringed sideroblasts (RARS) according to FAB classification and a higher frequency of FAB-RA than the Western IPSS study17,18 and that there are different prognostic factors between Japanese and Western patients with MDS.10,17 From cytogenetic analysis, it was indicated that the frequency of Japanese MDS with isolated del(5q) was lower than that in the IPSS study.18 We additionally reported that patients with FAB-RA demonstrated favorable outcomes compared with those of the IPSS study.17 We consider that there are different clinical features between Asian and Western patients with low-risk MDS. In the present study, after conducting an interobserver morphologic variation study for diagnosis of MDS subgroups between the Japanese and German hematologists, we compared in detail the clinical features of Japanese and German patients in FAB-RA.
Patients and methods
Patients
A total of 728 consecutive patients (Japan, 131 cases; Germany, 597 cases) with a diagnosis of primary RA according to FAB classification (FAB-RA) were included in this retrospective analysis. Japanese patients were diagnosed at the Saitama Medical School Hospital, Nagasaki University Hospital or affiliated hospitals in Japan between April 1976 and January 1997. German patients were diagnosed at the Department of Hematology, Oncology, and Clinical Immunology of the Heinrich-Heine University in Germany between January 1973 and December 2002. Patients who had previously been treated with antineoplastic drugs or ionizing radiation were excluded from the study. Informed consent was provided according to the Declaration of Helsinki. This study was performed according to the guideline of the institutional review board of the Saitama Medical School.
Interobserver variation study
Hematologic examinations were performed using standard methods (peripheral blood [PB] and bone marrow [BM] Wright-Giemsa or May-Giemsa stained films). PB and BM differential counts were performed on 100 and 500 cells, respectively. Evaluations of bone marrow cellularity were performed using the specimens of BM trephine biopsy and/or clot section.
In the first step, we reviewed all the training slides of FAB-RA (25 Japanese and 20 German cases) by Japanese and German hematologists separately. After this training review, the first joint review meeting for morphologic consensus was performed by 4 Japanese and 4 Germany hematologists for 4 days in February 2002 at Heinrich-Heine-University. At the first joint review, we mainly discussed evaluation of dysplasia and diagnosis using the training slides.
In the second step, the slides of 129 patients (110 FAB-RA, 7 FAB-RARS, 12 aplastic anemia [AA] diagnosed by Japanese or German groups) were selected randomly and were evaluated for morphologic diagnosis according to FAB and WHO classifications by the Japanese and German groups separately in each country. Patients with FAB-RA were reclassified to WHO subgroups according to the criteria of a previous German report.7 After this separate review, the second joint review meeting for morphologic consensus was performed by 4 Japanese and 4 German hematologists for 4 days in October 2004 at Heinrich-Heine-University. The observers were blinded to the clinical and laboratory data, including cytogenetics, until finishing this separate review. Diagnoses of FAB classification or AA were performed using only morphologic findings. Concerning diagnoses of WHO classification, morphologic and cytogenetic findings were used. In the second joint review meeting, the concordance rate of morphologic diagnosis according to the FAB and WHO classifications between Japanese and German hematologists was analyzed, and we discussed cases whose diagnoses did not agree between Japanese and German hematologists in this separate review.
Cytogenetic analysis
Cytogenetic analyses were performed with a trypsin-Giemsa banding technique on BM cells from aspirates. Ordinarily 20 to 30 metaphases were examined. Cytogenetic aberrations were grouped according to the IPSS publication.5
Clinical studies
Comparisons of the clinical features and the prognostic factors between 131 Japanese and 597 German patients with FAB-RA were analyzed. Patients were followed for overall survival (OS) and leukemic progression through June 2004 in Japanese cases and July 2003 in German cases. OS was measured from the date of diagnosis until death from any cause, the date of stem cell transplantation, or until the last patient contact. Leukemic progression was measured from the date of diagnosis until the date of diagnosis of acute leukemia. We classified causes of death into 3 subtypes: MDS related (MDS death), MDS unrelated (non-MDS death), and unclear cause (unclear death). MDS death was defined as acute leukemia, infection, bleeding, or heart failure resulting from anemia or iron overload. Non-MDS death was defined as causes of certain independence from MDS. Unclear death was defined as causes of death without any obvious MDS-related sign and without causes of certain independence from MDS. We also measured survival period censored non-MDS death or unclear death (modified survival). Measurements of modified survival were censored at the date of death in patients with non-MDS death or unclear death.
Statistical methods
The chi-square test and the nonparametric Mann-Whitney test were used to compare the proportions of patients and continuous data, respectively. The Kaplan-Meier method was used to generate the estimate of cumulative probabilities of OS, modified survival, and cumulative risk of acute leukemia evolution. The difference in the cumulative probabilities within subcategories of patients was compared using a 2-sided log-rank test. Age- and sex-adjusted effects of clinical parameters on outcomes were performed with use of 2 different models of multivariate Cox proportional hazards regression. Model A included age category, sex, dichotomized peripheral blood counts, and chromosome category of IPSS. Model B included age category, sex, and IPSS score. An examination for interaction between parameters was performed with the inclusion of interaction terms into each model. The effects of clinical parameters were evaluated as hazard ratios and their 95% confidence intervals. The interobserver concordance was evaluated using the simple κ coefficient. A 2-sided P value of less than .05 was considered to be statistically significant. All statistical analyses were performed with the use of SAS software (version 8.2; SAS Institute, Cary, NC), and all graphic presentations were performed with the use of StatView (version 5.0; SAS Institute).
Results
Morphologic consensus
Of the 129 cases reviewed, the agreement of morphologic diagnosis according to FAB classification between Japanese and German hematologists was 98.4%. A significant concordance was achieved while using FAB classification (κ, 0.94; P < .001). There were 2 cases whose diagnoses did not agree between Japanese and German hematologists by separate review. One case was diagnosed as AA by the Japanese group, but the diagnosis by the German group was FAB-RA. The final diagnosis of this case as AA was reached by consensus among the Japanese and German groups by joint review. Another case was diagnosed as RAEB by the Japanese group, but the diagnosis by the German group was FAB-RA. The Japanese hematologists judged that percentage of BM blasts of this patient was slightly higher than 5%. Each group performed morphologic examination again. As a result, the blasts percentage was judged to be less than 5%. The final diagnosis of this case as FAB-RA was reached by consensus among the Japanese and German groups by joint review. Of the 110 FAB-RA and 7 FAB-RARS cases reviewed for WHO classification, the agreement of morphologic diagnosis according to WHO classification between Japanese and German hematologists was 83.8%. A significant concordance was achieved while using WHO classification (κ, 0.73; P < .001).
Comparison of clinical features and prognostic factors between Japanese and German patients with FAB-RA
Clinical and laboratory features at the time of diagnosis. The age of Japanese patients with FAB-RA was significantly younger than that of German patients with FAB-RA (P < .001). The sex ratios were not significantly different between the 2 countries. Japanese patients with FAB-RA had significantly lower absolute neutrophil counts (ANCs), lower hemoglobin (Hb) concentrations, lower platelet (PLT) counts, and higher frequency of 2 or 3 lineage cytopenias according to the IPSS definition than did German patients with FAB-RA (Table 1). Cytogenetic analysis was performed in 102 Japanese and 199 German patients. In the Japanese FAB-RA group, the frequency of cytogenetic abnormalities was 30 patients (29%). In contrast, cytogenetic abnormalities were found in 105 (53%) of the German patients with FAB-RA. Japanese patients with FAB-RA had a significantly lower frequency of cytogenetic abnormalities than did German patients with FAB-RA. The subgroups of cytogenetic abnormalities according to IPSS are summarized in Table 2. The distribution of the cytogenetic subgroups according to IPSS showed no significant difference between Japanese and German patients with FAB-RA. Japanese patients with FAB-RA had a significantly lower frequency of FAB-RA associated with an isolated del(5q) cytogenetic abnormality (5q–syndrome) than did German patients with FAB-RA. Japanese patients with FAB-RA were highly categorized into the intermediate-1 (INT-1) risk subgroup, whereas German patients were equally categorized into the low-risk and INT-1 risk subgroups. The frequency of patients with intermediate-2 (INT-2) risk was low in both countries (Table 3).
Laboratory features at the time of diagnosis and clinical features in patients with RA classified according to the FAB criteria
. | Japan, n = 131 . | Germany, n = 597 . | P . |
|---|---|---|---|
| Sex, male/female | 70/61 | 309/288 | .73 |
| Age, y | 57 (12-88) | 71 (7-93) | < .001 |
| Neutrophil count, × 109/L | 1.58 (0.05-10.24) | 1.98 (0.06-23.00) | < .001 |
| Hemoglobin concentration, g/L | 84 (25-143) | 94 (30-169) | .002 |
| Platelet count, × 109/L | 41 (4-390) | 127 (2-1540) | < .001 |
| 2- or 3-lineage cytopenias, %* | 68 | 39 | < .001 |
| Abnormal karyotype, % | 29 | 53 | < .001 |
| Median survival, mo | 175 | 40 | < .001 |
. | Japan, n = 131 . | Germany, n = 597 . | P . |
|---|---|---|---|
| Sex, male/female | 70/61 | 309/288 | .73 |
| Age, y | 57 (12-88) | 71 (7-93) | < .001 |
| Neutrophil count, × 109/L | 1.58 (0.05-10.24) | 1.98 (0.06-23.00) | < .001 |
| Hemoglobin concentration, g/L | 84 (25-143) | 94 (30-169) | .002 |
| Platelet count, × 109/L | 41 (4-390) | 127 (2-1540) | < .001 |
| 2- or 3-lineage cytopenias, %* | 68 | 39 | < .001 |
| Abnormal karyotype, % | 29 | 53 | < .001 |
| Median survival, mo | 175 | 40 | < .001 |
Values for presentation characteristics are given as median and range (in parentheses) where applicable.
Cytopenia according to IPSS: hemoglobin concentration less than 100 g/L, absolute neutrophil count less than 1.5 × 109/L, platelet count less than 100 × 109/L.
Cytogenetic findings at the time of diagnosis in patients with RA classified according to the FAB criteria
. | Japan, n = 102 . | Germany, n = 199 . |
|---|---|---|
| Good, no. (%) | 79 (77.5) | 143 (71.8) |
| Normal | 72 | 94 |
| -Y | 1 | 4 |
| del(5q) | 3 | 39 |
| del(20q) | 3 | 6 |
| Intermediate, no. (%) | 15 (14.7) | 31 (15.6) |
| Poor, no. (%) | 8 (7.9) | 25 (12.6) |
| Complex (3 or more abnormalities) | 5 | 16 |
| Chromosome 7 anomalies | 3 | 9 |
. | Japan, n = 102 . | Germany, n = 199 . |
|---|---|---|
| Good, no. (%) | 79 (77.5) | 143 (71.8) |
| Normal | 72 | 94 |
| -Y | 1 | 4 |
| del(5q) | 3 | 39 |
| del(20q) | 3 | 6 |
| Intermediate, no. (%) | 15 (14.7) | 31 (15.6) |
| Poor, no. (%) | 8 (7.9) | 25 (12.6) |
| Complex (3 or more abnormalities) | 5 | 16 |
| Chromosome 7 anomalies | 3 | 9 |
Intermediate indicates other abnormalities not listed in good and poor classifications.
Univariate analysis of overall survival and cumulative risk of acute leukemia in patients with RA classified according to the FAB criteria
. | . | Percentile of OS (mo) . | . | . | Percentile of cumulative risk of AML (mo) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable, by country of origin . | No. of patients . | 75% . | 50% . | P . | 10% . | 25% . | 50% . | P . | |||
| Japanese patients | |||||||||||
| Age, y | |||||||||||
| 60 or younger | 72 | 114 | 217 | <.001 | NR | NR | NR | .12 | |||
| Older than 60 | 59 | 18 | 59 | 51 | NR | NR | |||||
| Sex | |||||||||||
| Male | 70 | 42 | 176 | .85 | 74 | NR | NR | .53 | |||
| Female | 61 | 53 | 129 | 104 | NR | NR | |||||
| Neutrophil count | |||||||||||
| Fewer than 1.5 × 109/L | 63 | 52 | 157 | .84 | 51 | NR | NR | .16 | |||
| At least 1.5 × 109/L | 68 | 53 | 176 | NR | NR | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 100 g/L | 81 | 52 | 114 | .24 | 92 | NR | NR | .95 | |||
| At least 100 g/L | 50 | 53 | 202 | 38 | NR | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 70 g/L | 45 | 23 | 100 | .01 | 104 | NR | NR | .81 | |||
| At least 70 g/L | 86 | 62 | 202 | 92 | NR | NR | |||||
| Platelet count | |||||||||||
| Fewer than 100 × 109/L | 109 | 52 | 175 | .37 | 92 | NR | NR | .35 | |||
| At least 100 × 109/L | 22 | 54 | 109 | 14 | NR | NR | |||||
| Cytopenia (IPSS) | |||||||||||
| 0/1 | 42 | 53 | 202 | .84 | NR | NR | NR | .83 | |||
| 2/3 | 89 | 52 | 157 | 92 | NR | NR | |||||
| Chromosome (IPSS) | |||||||||||
| Good | 79 | 76 | 175 | .17 | 104 | NR | NR | <.001 | |||
| Intermediate | 15 | 19 | NR | NR | NR | NR | |||||
| Poor | 8 | 27 | 102 | 4 | 37 | NR | |||||
| IPSS* | |||||||||||
| Low | 21 | 76 | 202 | .29 | NR | NR | NR | <.001 | |||
| INT-1 | 73 | 52 | 175 | 104 | NR | NR | |||||
| INT-2 | 8 | 27 | 102 | 4 | 22 | NR | |||||
| German patients | |||||||||||
| Age, y | |||||||||||
| 60 or younger | 133 | 26 | 66 | <.001 | 13 | 91 | NR | .85 | |||
| Older than 60 | 461 | 14 | 35 | 21 | 136 | 173 | |||||
| Sex | |||||||||||
| Male | 309 | 16 | 41 | .92 | 21 | 78 | 173 | .41 | |||
| Female | 288 | 16 | 43 | 19 | NR | NR | |||||
| Neutrophil count | |||||||||||
| Fewer than 1.5 × 109/L | 162 | 14 | 43 | .54 | 17 | 52 | 173 | .014 | |||
| At least 1.5 × 109/L | 301 | 16 | 37 | 25 | NR | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 100 g/L | 337 | 9 | 30 | <.001 | 14 | 136 | NR | .18 | |||
| At least 100 g/L | 217 | 23 | 57 | 42 | 173 | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 90 g/L | 235 | 8 | 29 | <.001 | 17 | 136 | NR | .15 | |||
| At least 90 g/L | 319 | 20 | 51 | 40 | 173 | NR | |||||
| Platelet count | |||||||||||
| Fewer than 100 × 109/L | 207 | 9 | 23 | <.001 | 11 | 50 | 136 | <.001 | |||
| At least 100 × 109/L | 339 | 23 | 53 | 35 | NR | NR | |||||
| Cytopenias (IPSS) | |||||||||||
| 0/1 | 288 | 23 | 55 | <.001 | 63 | NR | NR | <.001 | |||
| 2/3 | 188 | 7 | 22 | 10 | 28 | 136 | |||||
| Chromosome (IPSS) | |||||||||||
| Good | 143 | 27 | 66 | <.001 | 25 | NR | NR | <.001 | |||
| intermediate | 31 | 26 | 44 | 10 | 91 | 91 | |||||
| Poor | 25 | 7 | 16 | 4 | 14 | 52 | |||||
| IPSS* | |||||||||||
| Low | 82 | 43 | 82 | <.001 | NR | NR | NR | <.001 | |||
| INT-1 | 78 | 12 | 31 | 10 | 27 | 91 | |||||
| INT-2 | 11 | 4 | 7 | 2 | 5 | 52 | |||||
. | . | Percentile of OS (mo) . | . | . | Percentile of cumulative risk of AML (mo) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable, by country of origin . | No. of patients . | 75% . | 50% . | P . | 10% . | 25% . | 50% . | P . | |||
| Japanese patients | |||||||||||
| Age, y | |||||||||||
| 60 or younger | 72 | 114 | 217 | <.001 | NR | NR | NR | .12 | |||
| Older than 60 | 59 | 18 | 59 | 51 | NR | NR | |||||
| Sex | |||||||||||
| Male | 70 | 42 | 176 | .85 | 74 | NR | NR | .53 | |||
| Female | 61 | 53 | 129 | 104 | NR | NR | |||||
| Neutrophil count | |||||||||||
| Fewer than 1.5 × 109/L | 63 | 52 | 157 | .84 | 51 | NR | NR | .16 | |||
| At least 1.5 × 109/L | 68 | 53 | 176 | NR | NR | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 100 g/L | 81 | 52 | 114 | .24 | 92 | NR | NR | .95 | |||
| At least 100 g/L | 50 | 53 | 202 | 38 | NR | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 70 g/L | 45 | 23 | 100 | .01 | 104 | NR | NR | .81 | |||
| At least 70 g/L | 86 | 62 | 202 | 92 | NR | NR | |||||
| Platelet count | |||||||||||
| Fewer than 100 × 109/L | 109 | 52 | 175 | .37 | 92 | NR | NR | .35 | |||
| At least 100 × 109/L | 22 | 54 | 109 | 14 | NR | NR | |||||
| Cytopenia (IPSS) | |||||||||||
| 0/1 | 42 | 53 | 202 | .84 | NR | NR | NR | .83 | |||
| 2/3 | 89 | 52 | 157 | 92 | NR | NR | |||||
| Chromosome (IPSS) | |||||||||||
| Good | 79 | 76 | 175 | .17 | 104 | NR | NR | <.001 | |||
| Intermediate | 15 | 19 | NR | NR | NR | NR | |||||
| Poor | 8 | 27 | 102 | 4 | 37 | NR | |||||
| IPSS* | |||||||||||
| Low | 21 | 76 | 202 | .29 | NR | NR | NR | <.001 | |||
| INT-1 | 73 | 52 | 175 | 104 | NR | NR | |||||
| INT-2 | 8 | 27 | 102 | 4 | 22 | NR | |||||
| German patients | |||||||||||
| Age, y | |||||||||||
| 60 or younger | 133 | 26 | 66 | <.001 | 13 | 91 | NR | .85 | |||
| Older than 60 | 461 | 14 | 35 | 21 | 136 | 173 | |||||
| Sex | |||||||||||
| Male | 309 | 16 | 41 | .92 | 21 | 78 | 173 | .41 | |||
| Female | 288 | 16 | 43 | 19 | NR | NR | |||||
| Neutrophil count | |||||||||||
| Fewer than 1.5 × 109/L | 162 | 14 | 43 | .54 | 17 | 52 | 173 | .014 | |||
| At least 1.5 × 109/L | 301 | 16 | 37 | 25 | NR | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 100 g/L | 337 | 9 | 30 | <.001 | 14 | 136 | NR | .18 | |||
| At least 100 g/L | 217 | 23 | 57 | 42 | 173 | NR | |||||
| Hemoglobin concentration | |||||||||||
| Less than 90 g/L | 235 | 8 | 29 | <.001 | 17 | 136 | NR | .15 | |||
| At least 90 g/L | 319 | 20 | 51 | 40 | 173 | NR | |||||
| Platelet count | |||||||||||
| Fewer than 100 × 109/L | 207 | 9 | 23 | <.001 | 11 | 50 | 136 | <.001 | |||
| At least 100 × 109/L | 339 | 23 | 53 | 35 | NR | NR | |||||
| Cytopenias (IPSS) | |||||||||||
| 0/1 | 288 | 23 | 55 | <.001 | 63 | NR | NR | <.001 | |||
| 2/3 | 188 | 7 | 22 | 10 | 28 | 136 | |||||
| Chromosome (IPSS) | |||||||||||
| Good | 143 | 27 | 66 | <.001 | 25 | NR | NR | <.001 | |||
| intermediate | 31 | 26 | 44 | 10 | 91 | 91 | |||||
| Poor | 25 | 7 | 16 | 4 | 14 | 52 | |||||
| IPSS* | |||||||||||
| Low | 82 | 43 | 82 | <.001 | NR | NR | NR | <.001 | |||
| INT-1 | 78 | 12 | 31 | 10 | 27 | 91 | |||||
| INT-2 | 11 | 4 | 7 | 2 | 5 | 52 | |||||
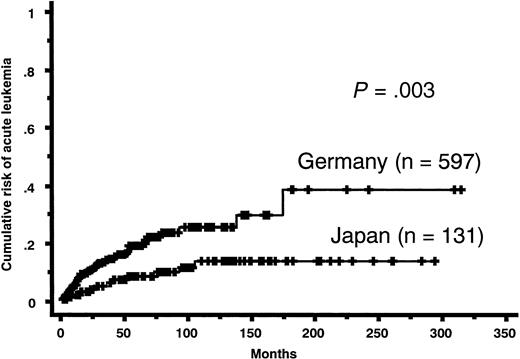
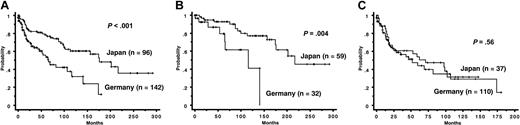
Prognosis. Follow-up periods ranged from 1 to 292 months (median, 69 months) in Japanese patients with FAB-RA. Follow-up periods in German patients with FAB-RA ranged from 0 to 313 months (median, 13 months). During the follow-up period, 50 Japanese patients and 252 German patients died, and 10 Japanese patients and 56 German patients transformed to acute leukemia. Japanese patients showed a significantly lower cumulative risk of acute leukemia evolution than did German patients (Figure 1). Concerning causes of death, German patients were classified as 153 cases of MDS death (50 acute leukemia, 25 bleeding, 64 infection, 14 heart failure), 24 cases of non-MDS death, and 75 cases of unclear death. Japanese patients were classified as 40 cases of MDS death (11 acute leukemia, 9 bleeding, 19 infection, 1 heart failure), 7 cases of non-MDS death, and 3 cases of unclear death. In both OS and modified survival, all Japanese patients with FAB-RA had a more favorable prognosis than did all German patients with FAB-RA (OS median survival: Japan, 175 months; Germany, 40 months; P < .001; modified survival median survival: Japan, 202 months; Germany, 73 months; P < .001) (Figure 2A). In OS, for those aged 60 years or younger, Japanese patients with FAB-RA had a more favorable OS than did German patients with FAB-RA (median survival: Japan, 217 months; Germany, 66 months; P < .001) and for those aged older than 60 years, Japanese patients with FAB-RA had a more favorable OS than did German patients with FAB-RA (median survival: Japan, 59 months; Germany, 35 months; P = .025). In modified survival, for those aged 60 years or younger, Japanese patients with FAB-RA had a more favorable modified survival than did German patients with FAB-RA (median survival: Japan, > 292 months; Germany, 108 months; P < .001). However, for those aged older than 60 years, Japanese patients with FAB-RA did not show a more favorable modified survival than did German patients with FAB-RA (median survival: Japan, 102 months; Germany, 69 months; P = .46) (Figure 2B-C).
Prognostic factors. In Japanese patients with FAB-RA, the clinical variables of age older than 60 years and Hb concentration less than 70 g/L were significantly correlated with OS. Sex, Hb concentration less than 100 g/L, PLT count fewer than 100 × 109/L, ANC fewer than 1.5 × 109/L, cytopenias (2 or 3 lineages), and IPSS cytogenetic subgroups were not significantly correlated with OS (Table 3). In German patients with FAB-RA, age older than 60 years, Hb concentration less than 100 g/L, PLT count fewer than 100 × 109/L, cytopenias (2 or 3 lineages), and IPSS cytogenetic subgroups were significantly correlated with OS. Sex and ANC fewer than 1.5 × 109/L were not significantly correlated with OS (Table 3). The IPSS cytogenetic subgroups and IPSS subgroup were significantly correlated with cumulative risk of acute leukemia evolution in Japanese patients with FAB-RA (Table 3). The other clinical variables in Table 3 were not significantly correlated with cumulative risk of acute leukemia evolution. ANC fewer than 1.5 × 109/L, PLT count fewer than 100 × 109/L, cytopenias (2 or 3 lineages), IPSS cytogenetic subgroups, and IPSS subgroup were significantly correlated with cumulative risk of acute leukemia evolution in German patients with FAB-RA. Age, sex, and Hb concentrations were not significantly correlated with cumulative risk of acute leukemia evolution (Table 3).
In the age- and sex-adjusted multivariate analyses for OS, there was no clinical parameter that associated with OS in Japanese patients in all models, whereas cytopenias (especially, thrombocytopenia and anemia) and poor IPSS cytogenetic subgroup, and INT-1 and INT-2 IPSS risk subgroups retained as significantly adverse clinical parameters for OS in German patients. For acute leukemia evolution, poor IPSS cytogenetic subgroup and INT-2 IPSS risk subgroup were retained as significant parameters in the cumulative risk of acute leukemia evolution in Japanese patients after age and sex adjustment, whereas in German patients ANC fewer than 1.5 × 109/L was no longer associated with acute leukemia evolution, but other parameters in the univariate analyses were retained as poor prognostic factors (Table 4).
Multivariate analysis of parameters that affected overall survivors and acute leukemia evolution in patients with RA classified according to the FAB criteria
. | Overall survival . | . | Leukemic transformation . | . | ||
|---|---|---|---|---|---|---|
| Characteristic, by model . | Japanese HR (95% CI) . | German HR (95% CI) . | Japanese HR (95% CI) . | German HR (95% CI) . | ||
| Model A | ||||||
| Age older than 60 y | 5.1 (2.6-9.9)* | 2.2 (1.5-3.0)* | 1.6 (0.4-6.4) | 1.7 (0.9-3.3) | ||
| Sex, male | 1.2 (0.7-2.2) | 1.0 (0.8-1.4) | 1.6 (0.4-7.1) | 1.2 (0.7-2.1) | ||
| ANC fewer than 1.5 × 109/L | 1.2 (0.7-2.2) | 1.0 (0.7-1.3) | 2.0 (0.5-8.2) | 1.7 (0.9-3.1) | ||
| Platelet count fewer than 100 × 109/L | 1.3 (0.6-2.7) | 1.9 (1.4-2.5)* | 0.4 (0.1-1.9) | 2.2 (1.2-4.1)* | ||
| Hemoglobin concentration less than 100 g/L | 1.5 (0.8-2.8) | 1.8 (1.4-2.4)* | 1.0 (0.2-3.9) | 1.9 (1.1-3.5)* | ||
| Chromosome (IPSS), intermediate | 1.5 (0.6-3.6) | 1.1 (0.6-1.9) | 1.5 (0.2-14) | 2.3 (0.9-5.6) | ||
| Chromosome (IPSS), poor | 1.4 (0.5-4.2) | 2.8 (1.6-4.9)* | 11.9 (2.4-59)* | 6.6 (2.8-16)* | ||
| Model B | ||||||
| Age older than 60 y | 4.6 (2.5-8.7)* | 2.1 (1.5-2.9)* | 1.7 (0.5-6.3) | 1.6 (0.8-3.0) | ||
| Sex, male | 1.1 (0.6-2.0) | 1.2 (0.9-1.6) | 1.7 (0.4-6.7) | 1.2 (0.7-2.1) | ||
| IPSS, INT-1 | 1.0 (0.5-1.8) | 1.4 (1.0-2.0)* | 1.0 (0.2-4.7) | 3.1 (1.6-5.7)* | ||
| IPSS, INT-2 | 1.6 (0.5-4.7) | 4.0 (2.0-8.0)* | 8.6 (1.7-43)* | 9.5 (3.2-27)* | ||
. | Overall survival . | . | Leukemic transformation . | . | ||
|---|---|---|---|---|---|---|
| Characteristic, by model . | Japanese HR (95% CI) . | German HR (95% CI) . | Japanese HR (95% CI) . | German HR (95% CI) . | ||
| Model A | ||||||
| Age older than 60 y | 5.1 (2.6-9.9)* | 2.2 (1.5-3.0)* | 1.6 (0.4-6.4) | 1.7 (0.9-3.3) | ||
| Sex, male | 1.2 (0.7-2.2) | 1.0 (0.8-1.4) | 1.6 (0.4-7.1) | 1.2 (0.7-2.1) | ||
| ANC fewer than 1.5 × 109/L | 1.2 (0.7-2.2) | 1.0 (0.7-1.3) | 2.0 (0.5-8.2) | 1.7 (0.9-3.1) | ||
| Platelet count fewer than 100 × 109/L | 1.3 (0.6-2.7) | 1.9 (1.4-2.5)* | 0.4 (0.1-1.9) | 2.2 (1.2-4.1)* | ||
| Hemoglobin concentration less than 100 g/L | 1.5 (0.8-2.8) | 1.8 (1.4-2.4)* | 1.0 (0.2-3.9) | 1.9 (1.1-3.5)* | ||
| Chromosome (IPSS), intermediate | 1.5 (0.6-3.6) | 1.1 (0.6-1.9) | 1.5 (0.2-14) | 2.3 (0.9-5.6) | ||
| Chromosome (IPSS), poor | 1.4 (0.5-4.2) | 2.8 (1.6-4.9)* | 11.9 (2.4-59)* | 6.6 (2.8-16)* | ||
| Model B | ||||||
| Age older than 60 y | 4.6 (2.5-8.7)* | 2.1 (1.5-2.9)* | 1.7 (0.5-6.3) | 1.6 (0.8-3.0) | ||
| Sex, male | 1.1 (0.6-2.0) | 1.2 (0.9-1.6) | 1.7 (0.4-6.7) | 1.2 (0.7-2.1) | ||
| IPSS, INT-1 | 1.0 (0.5-1.8) | 1.4 (1.0-2.0)* | 1.0 (0.2-4.7) | 3.1 (1.6-5.7)* | ||
| IPSS, INT-2 | 1.6 (0.5-4.7) | 4.0 (2.0-8.0)* | 8.6 (1.7-43)* | 9.5 (3.2-27)* | ||
Model A included age category, sex, dichotomized peripheral blood counts, and chromosome category of IPSS. Model B included age category, sex, and IPSS score.
HR indicates hazard ratio; 95% CI, 95% confidence interval; ANC, absolute neutrophil count; IPSS, International Prognosis Score System; INT-1, Intermediate-1; INT-2, intermediate-2.
Statistically significant hazard ratio.
Cumulative risk of acute leukemia evolution of patients with FAB-RA. Japanese patients had a lower cumulative risk of acute leukemia evolution than did German patients (P = .003).
Cumulative risk of acute leukemia evolution of patients with FAB-RA. Japanese patients had a lower cumulative risk of acute leukemia evolution than did German patients (P = .003).
WHO classification. The original diagnoses according to the WHO classification by each group in the present series show that the frequency of WHO-RA in Japanese patients with FAB-RA (73%) was significantly higher than in the German patients with FAB-RA (24%) (P < .001). In Japanese patients, patients with WHO-RA were significantly younger and had significantly lower PLT counts than did patients with RCMD. The OS of Japanese patients with WHO-RA was significantly more favorable than that of Japanese patients with RCMD (Table 5). The OS of all Japanese patients with WHO-RA was significantly more favorable than that of all German patients with WHO-RA (Figure 3A). For those aged 60 years or younger, the OS of Japanese patients with WHO-RA was significantly more favorable than that of German patients with WHO-RA. However, for those older than 60 years, Japanese patients with WHO-RA did not show a more favorable OS than did German patients with WHO-RA (Figure 3B-C). Frequencies of poor karyotype according to IPSS in Japanese patients with WHO-RA and RCMD were 4% and 20%, respectively. Japanese patients with WHO-RA had a lower cumulative risk of acute leukemia evolution than did Japanese patients with RCMD (10% cumulative risk: WHO-RA, not reached; RCMD, 38 months; 25% cumulative risk: RCMD, 104 months; P = .018).
Laboratory features at the time of diagnosis and clinical features in Japanese patients with WHO-RA and RCMD classified according to the WHO criteria
. | WHO-RA, n = 96 . | RCMD, n = 32 . | P . |
|---|---|---|---|
| Sex, male/female | 53/43 | 15/17 | .41 |
| Age, y | 55 (12-86) | 66 (16-88) | .038 |
| Neutrophil count, × 109/L | 1.62 (0.26-4.69) | 1.28 (0.05-10.24) | .76 |
| Hemoglobin concentration, g/L | 87 (30-143) | 71 (25-140) | .094 |
| Platelet count, × 109/L | 38 (4-246) | 127 (13-390) | .026 |
| 2- or 3-lineage cytopenias, %* | 67 | 75 | .38 |
| Abnormal karyotype, % | 24 | 36 | .26 |
| Median survival, mo | 176 | 52 | .023 |
. | WHO-RA, n = 96 . | RCMD, n = 32 . | P . |
|---|---|---|---|
| Sex, male/female | 53/43 | 15/17 | .41 |
| Age, y | 55 (12-86) | 66 (16-88) | .038 |
| Neutrophil count, × 109/L | 1.62 (0.26-4.69) | 1.28 (0.05-10.24) | .76 |
| Hemoglobin concentration, g/L | 87 (30-143) | 71 (25-140) | .094 |
| Platelet count, × 109/L | 38 (4-246) | 127 (13-390) | .026 |
| 2- or 3-lineage cytopenias, %* | 67 | 75 | .38 |
| Abnormal karyotype, % | 24 | 36 | .26 |
| Median survival, mo | 176 | 52 | .023 |
Values for presentation characteristics are given as median and range (in parentheses) where applicable.
Cytopenia according to IPSS: hemoglobin concentration less than 100 g/L, absolute neutrophil count less than 1.5 × 109/L, platelet count less than 100 × 109/L.
Cumulative survival of patients with FAB-RA. (Top) Overall survival (OS). (Bottom) Modified survival. (A) In all patients with FAB-RA, Japanese patients had a more favorable prognosis than did German patients in OS (P < .001). Japanese patients had a more favorable prognosis than did German patients in modified survival (P < .001). (B) In patients aged 60 years or younger, Japanese patients had a more favorable prognosis than did German patients in OS (P < .001). Japanese patients had a more favorable prognosis than did German patients in modified survival (P < .001). (C) In patients aged older than 60 years, Japanese patients had a more favorable prognosis than did German patients in OS (P = .025). Japanese patients did not show a more favorable prognosis than did German patients in modified survival (P = .46).
Cumulative survival of patients with FAB-RA. (Top) Overall survival (OS). (Bottom) Modified survival. (A) In all patients with FAB-RA, Japanese patients had a more favorable prognosis than did German patients in OS (P < .001). Japanese patients had a more favorable prognosis than did German patients in modified survival (P < .001). (B) In patients aged 60 years or younger, Japanese patients had a more favorable prognosis than did German patients in OS (P < .001). Japanese patients had a more favorable prognosis than did German patients in modified survival (P < .001). (C) In patients aged older than 60 years, Japanese patients had a more favorable prognosis than did German patients in OS (P = .025). Japanese patients did not show a more favorable prognosis than did German patients in modified survival (P = .46).
Discussion
Different clinical features between Asian and Western patients with MDS have been reported by several studies.10,17 However, these data are based on local series of patients. Speculation about certain differences is problematic because there might be differences in the interpretation of dysplasia in blood and bone marrow by different observers. The present study aimed to characterize the racial features of Western and Asian MDS cases. We thought that an assessment of interpretation of morphologic findings and definition of diagnostic criteria was warranted to check that the diagnoses by the Japanese group were in line with those of the German group, before comparing the clinical features between Japanese and German patients with FAB-RA. In the present study, the agreement of morphologic diagnosis between Japanese and German hematologists was 98.4%. It was confirmed that the diagnoses according to FAB classification or AA were not different between the Japanese and German groups. After morphologic consensus was obtained at the first joint review meeting, we performed this separate review. The concordance rate according to the FAB classification of morphologic diagnosis between Japanese and German hematologists was thus excellent. However, the subjects of this separate review were only FAB-RA, FAB-RARS, and AA cases that had already been diagnosed by the Japanese or German groups. We think that it is most difficult to distinguish FAB-RA and disorders with secondary dysplasia (collagen diseases, viral infectious diseases, and liver cirrhosis, etc). If we included these diseases in the present separate review, the concordance rate of morphologic diagnosis would likely have been lower.
Our results indicate that the clinical features of Japanese FAB-RA cases differ from those of German cases. Comparing Japanese and German FAB-RA cases we found that the median age of Japanese patients with FAB-RA was lower than that of German patients with FAB-RA. The population pyramids (negative growth type) and life expectancies (Japan, 80.7 years; Germany, 77.4 years) at 2000 in Japan and Germany are almost the same.19 Therefore, we think that this difference of median age is real. Furthermore, Japanese patients with FAB-RA had more pronounced cytopenia, especially more severe thrombocytopenia, and a higher frequency of pancytopenia or bicytopenia, as compared with German patients with RA. Also the cytogenetic characteristics differed between Japanese and German RA cases. Although there was no difference in the distribution of cytogenetic subgroups according to IPSS, the frequency of chromosomal abnormalities was lower in Japanese patients with RA; notably that of isolated del (5q) was lower in Japan. Toyama et al18 and Matsushima et al20 reported that Japanese patients with MDS had a lower frequency of isolated del (5q) than Western reports (2.0% and 1.5%, respectively). Morel et al21 and Greenberg et al5 reported that the frequencies of isolated del (5q) in all MDS cases were 4.7% and 5.9%, respectively. The majority of patients with 5q–syndrome are diagnosed as FAB-RA at diagnosis. If the percentage that patients with FAB-RA compared with all MDS is assumed to be 35%, that of 5q–syndrome in the present German patients with FAB-RA becomes 6.9% of all MDS. Although this frequency of 5q–syndrome present in German patients with FAB-RA was slightly higher than the reports of Morel et al21 and Greenberg et al,5 we believe that the result of the present study supported Japanese previous reports.
In OS, regardless of age, Japanese patients with FAB-RA had a more favorable prognosis than did their German counterparts. In modified survival, for those aged 60 years or younger, Japanese patients with FAB-RA had a more favorable modified survival than did German patients with FAB-RA. Therefore, we believe that the favorable prognosis of younger patients with FAB-RA (≤ 60 years) is certain. In modified survival, for those older than 60 years, Japanese patients with FAB-RA did not show a more favorable modified survival than did German patients with FAB-RA. Therefore, the prognostic difference between Japan and Germany may result from the characteristics of young Japanese patients with FAB-RA (≤ 60 years).
Characteristics of Japanese patients with WHO-RA were younger and had lower PLT counts. These characteristics were similar to those of MDS responders for immunosuppressive therapy (IST) in a report by Molldrem et al.22 The response rate for IST from a Japanese report23 was higher than from Western reports.22,24 However, only 8 Japanese cases received IST and only 3 responded in our present study. We think that a large-scale study is necessary to establish the relationship between Japanese WHO-RA and response for IST.
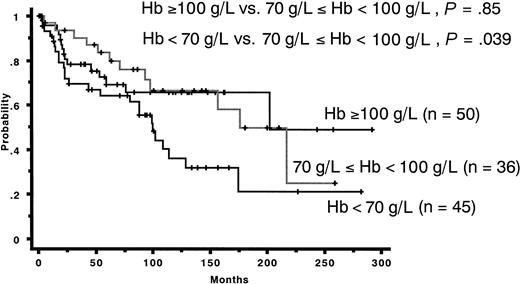
In the present study, IPSS was useful for assessing OS in German FAB-RA cases but not in Japanese FAB-RA cases. This was mainly due to the lack of a significant correlation between the number and degree of cytopenias and OS in Japanese patients with FAB-RA. In the IPSS publication, the researchers reported that cytopenias (2 or 3 lineages) were related with poor survival. In this study, however, Japanese patients showed more favorable prognoses despite possessing more pronounced cytopenia. Management of thrombocytopenia seems to be similar between Japan and Germany. Concerning the prognostic effect of Hb concentration, the threshold was different between Japanese and German patients with FAB-RA. Most of the Japanese patients with Hb concentrations greater than 70 g/L had no symptoms related to anemia and did not require red cell transfusion. In fact, most Japanese patients with Hb concentration lower than 70 g/L had received red cell transfusion. In contrast, most German patients with Hb concentration lower than 90 g/L had received red cell transfusion. We presumed that the cause of the different prognostic Hb concentration thresholds by Japanese and German patients may be related to these red cell transfusion procedures. We also presume that the difference in Hb concentration used as a threshold for red cell transfusion may be related to the different general characteristics among races rather than the different characteristics of FAB-RA between Asian and Western patients with FAB-RA. The Italian guideline recommends that all patients with Hb concentration lower than 80 g/L should receive red cell transfusion.25 Japanese patients with FAB-RA with Hb concentration greater than 70 g/L do not usually require regular red cell transfusion. We compared Japanese patients with RA with Hb concentrations greater than 100 g/L and those with Hb concentrations of 70 to 100 g/L. In fact, the latter group (70-100 g/L) did not differ in clinical course from patients with Hb concentrations greater than 100 g/L (P = .86). Moreover, Japanese patients with Hb concentrations of 70 to 100 g/L had a significantly more favorable prognosis than those with Hb concentrations lower than 70 g/L (P = .039) (Figure 4). This result indicates that the Hb threshold below which transfusion should be recommended may be different between Asian and Western patients with FAB-RA.
Cumulative overall survival of patients with WHO-RA. (A) Among all patients with WHO-RA, Japanese patients had a more favorable prognosis than did German patients (P < .001). (B) In patients aged 60 years or younger, Japanese patients had a more favorable prognosis than did German patients (P = .004). (C) In patients aged older than 60 years, Japanese patients did not show a more favorable prognosis than did German patients (P = .56).
Cumulative overall survival of patients with WHO-RA. (A) Among all patients with WHO-RA, Japanese patients had a more favorable prognosis than did German patients (P < .001). (B) In patients aged 60 years or younger, Japanese patients had a more favorable prognosis than did German patients (P = .004). (C) In patients aged older than 60 years, Japanese patients did not show a more favorable prognosis than did German patients (P = .56).
We think that our results concerning the prognostic OS effect of chromosomal findings may be insufficient and may include some problematic issues. In particular, the observation periods of Japanese patients with poor karyotype according to IPSS may be problematic. Four of 8 Japanese patients with poor karyotype are surviving. However, the observation periods of the 2 surviving patients were insufficient (1 and 6 months, respectively). Concerning acute leukemia evolution, the effect of chromosomal findings was not different between Japanese and German patients. We think that the prognostic effect on OS of chromosomal findings may not be different between Japanese and German patients, if sufficient observation periods for Japanese patients with poor karyotype are available.
We made great efforts to achieve morphologic consensus in the present study. The original diagnoses according to FAB and WHO classifications were not different between the Japanese and German groups. In the present series, the original diagnoses according to the WHO classification by each group show the frequency of WHO-RA in Japanese patients to be higher than that in German patients. In Japanese patients, the prognosis of patients with WHO-RA was more favorable than that of patients with RCMD, and patients with WHO-RA had a lower cumulative risk of acute leukemia evolution than did patients with RCMD. In a previous report of a German group,7 the same results had been reported. This finding indicates that one reason for the better prognosis of Japanese patients may be the different distribution of subgroups by WHO classification between Asian and Western patients with FAB-RA, namely a higher frequency of patients with WHO-RA in Japan. In Japanese patients, patients with WHO-RA were younger and had lower PLT counts than did patients with RCMD, significantly. Furthermore, the prognosis of Japanese patients with WHO-RA was significantly more favorable than that of Japanese patients with RCMD. For those aged 60 years or younger, the prognosis of Japanese patients with WHO-RA was significantly more favorable than that of German patients with WHO-RA. However, for those older than 60 years, Japanese patients with WHO-RA did not show a more favorable prognosis than did German patients with WHO-RA. These findings in young Japanese patients with WHO-RA (≤ 60 years) might indicate the differences in clinical features between Japanese and German patients with FAB-RA.
This is the first report to compare clinical features between Asian and Western patients with FAB-RA after confirming a morphologic consensus. Our results indicate that the clinical features of Japanese FAB-RA cases differ from those of German cases. These differences are not due to the different interpretation of morphologic features by different observers. Several guidelines25,26 have been published in Western countries. To adapt these Western guidelines to Asian patients, some modifications may be required, taking into account ethnic characteristics.
Cumulative overall survival of Japanese patients with FAB-RA. The group with hemoglobin concentration of 70 to 100 g/L showed no significant prognostic difference from the group with hemoglobin greater than 100 g/L in patients with FAB-RA (P = .85). The group with hemoglobin concentrations of 70 to 100 g/L had a more favorable prognosis than did the group with hemoglobin concentrations lower than 70 g/L in patients with FAB-RA (P = .039).
Cumulative overall survival of Japanese patients with FAB-RA. The group with hemoglobin concentration of 70 to 100 g/L showed no significant prognostic difference from the group with hemoglobin greater than 100 g/L in patients with FAB-RA (P = .85). The group with hemoglobin concentrations of 70 to 100 g/L had a more favorable prognosis than did the group with hemoglobin concentrations lower than 70 g/L in patients with FAB-RA (P = .039).
Prepublished online as Blood First Edition Paper, June 21, 2005; DOI 10.1182/blood-2005-01-0040.
Supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 16639013) (I.J.) and Kompetenznetzwerk “Akute und Chronische Leukämien” des Bundesforschungsministeriums.
A.M. designed the research, performed morphologic analyses, collected the data, analyzed the data, and wrote the manuscript; U.G. and I.J. designed the research, performed morphologic analyses, collected the data, and analyzed the data; M.T. designed the research, performed morphologic analyses, and analyzed the data; M.I. collected the data, performed morphologic analyses, and analyzed the data; M.B. designed the research and analyzed the data; M.M., A.K., S.K., and M.A. performed morphologic analyses and collected the data; and Y.M., H.T., and M.S. collected the data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal