Abstract
Multicentric Castleman disease (MCD) is an atypical lymphoproliferative disorder characterized by systemic lymphadenopathy and constitutional inflammatory symptoms. Dysregulated overproduction of interleukin-6 is responsible for the clinical abnormalities. This multicenter prospective study was undertaken to evaluate the safety and efficacy of a humanized anti–human interleukin-6 (IL-6) receptor monoclonal antibody (MRA) in patients with MCD. We report here results of the first 60 weeks of the study enrolling 28 patients. The initial dosing period consisted of 8 infusions of 8 mg/kg MRA administered biweekly. Adjustments in the dose and treatment interval were allowed for each patient in an extension phase after 16 weeks. Within 16 weeks, treatment with MRA consistently alleviated lymphadenopathy and all the inflammatory parameters. Hemoglobin, albumin, and total cholesterol levels, high-density lipoprotein cholesterol values, and body mass index all increased significantly. In addition, fatigue diminished. Chronic inflammatory symptoms were successfully managed over 60 weeks. In 8 (28.6%) patients, the MRA dose was decreased or the treatment interval was extended without exacerbation. Eleven (73.3%) of 15 patients who had received oral corticosteroids before study entry were able to do well on a reduced corticosteroid dose. Most adverse events were mild to moderate in severity. MRA was tolerated well and significantly alleviated chronic inflammatory symptoms and wasting in patients with MCD.
Introduction
Castleman disease is a rare atypical lymphoproliferative disorder1 classified according to the histopathologic findings of the affected lymph nodes as hyaline–vascular, plasma-cell type, or a mixed-type variant of the two.2,3 Patients with the plasma-cell or the mixed-type variant frequently have systemic manifestations, such as low-grade fever, fatigue, loss of appetite, and weight loss. Abnormal laboratory findings include anemia, hypoalbuminemia, hypocholesterolemia, hypergammaglobulinemia, and increased acute-phase proteins. As the disease progresses, patients often undergo severe wasting.2-4
Although multicentric Castleman disease (MCD) is nonneoplasmic, without treatment the prognosis is poor. Infections, renal failure, and malignancies, including malignant lymphoma and Kaposi sarcoma, are common causes of death in patients with MCD.5 Kaposi sarcoma–associated herpesvirus (also called human herpesvirus type 8 [KSHV/HHV-8]) is reported to be an etiologic agent of Castleman disease, especially in patients infected with HIV.6-8
Although clinical abnormalities may resolve after excision of the affected lymph nodes in patients with localized Castleman disease,3,4,9,10 MCD is often refractory to treatment with corticosteroids or chemotherapy.5 Treatment with interferon-α or with the anti-CD20 monoclonal antibody rituximab (Rituxan; Hoffman-La Roche, Basel, Switzerland) has resulted in durable clinical remission of the disease; however, these findings are based on a small number of patients.11,12
Interleukin-6 (IL-6) is a pleiotropic cytokine with a wide range of biologic activities, including support of hematopoiesis and regulation of immune responses.13 Dysregulated overproduction of IL-6 from germinal center B cells is implicated in the pathogenesis of plasma-cell–type Castleman disease.9 Previous studies demonstrated that anti–IL-6 and anti–IL-6 receptor antibodies dramatically alleviated the symptoms and biochemical abnormalities (though the disease usually relapsed on cessation of therapy).10,14 Thus, the blockade of IL-6 signaling is considered an attractive approach to treat MCD. The purpose of this open-label, prospective study was to evaluate the long-term efficacy and safety of the humanized anti–human IL-6 receptor monoclonal antibody (MRA, formerly called rhPM-1) in patients with MCD. The study is to continue until 2007. Results are reported from the first 60 weeks of treatment.
Patients, materials, and methods
Patients
The study began in June 2001, and patients were enrolled from July 19, 2001, until October 11, 2001. Patients were eligible if they were at least 20 years of age and had clinically and pathologically confirmed diagnoses of MCD of the plasma-cell or mixed-type variant. An independent pathologist examined the histologic features of the lymph nodes collected from the patients and confirmed the histologic diagnosis. None of the patients had autoimmune diseases such as rheumatoid arthritis or Sjögren syndrome. Patients had to have C-reactive protein (CRP) values of at least 20 mg/L and white blood cell counts of at least 3.5 × 109/L at screening (2 weeks before study dosing). Patients using antitumor agents, immunosuppressants, and corticosteroids were eligible if the dose had been stable for 4 weeks before study dosing. Patients were excluded if any radiation therapy or surgical procedure had been performed within 4 weeks of entry. Patients who had any history of serious allergy or concurrent ischemic heart diseases or who were pregnant or lactating were excluded from the study.
This study complied with all provisions of the Declaration of Helsinki and its current amendments and was conducted in accordance with Good Clinical Practice guidelines. All patients gave written informed consent before participating in this study.
Study drug
MRA is a humanized anti–human IL-6 receptor monoclonal antibody of immunoglobulin G1κ (IgG1κ) subtype (Chugai Pharmaceutical Roche Group, Tokyo, Japan). The antibody was humanized by complementarity-determining region (CDR) grafting, whereby the CDR from a mouse anti–human IL-6 receptor monoclonal antibody is grafted to a human-IgG framework and transfected into Chinese hamster ovary cells for production.15 The resultant humanized antibody is specific for the human IL-6 receptor but contains minimal mouse protein, reducing the likelihood of the host developing anti-MRA antibodies. MRA inhibits IL-6 function by competing for the membrane-bound and the soluble forms of the human IL-6 receptor.16
Study protocol
This study was designed as an open-label trial. The protocol was approved by the Ministry of Health, Labor and Welfare of Japan and by the local institutional review board at each of the 13 participating centers.
Based on previous dose-finding studies,17 patients were treated with 8 mg/kg MRA every 2 weeks for 16 weeks. Patients then entered an open-label extension of the study during which, at the discretion of the investigator, the dose and schedule could be individualized according to inflammatory symptoms and laboratory parameters. The maximum MRA dose was 8 mg/kg, and the minimum treatment interval was 1 week.
Total dose of the reconstituted products was diluted to 250 mL saline, which was infused intravenously over 1 hour. Patients were observed for 1 hour after infusion. To avoid infusion-related reactions, no particular pretreatment was applied. Patients who developed anti-MRA antibodies were to be withdrawn from the study.
At screening, lymph node biopsy samples were sent to an independent pathologist for diagnosis confirmation. Clinical and laboratory assessments were conducted at screening and on dosing days. Antibodies against HHV-8 and HIV were measured by enzyme-linked immunosorbent assay (ELISA; SRL, Tokyo, Japan) at screening. Antibodies (IgG and IgE types) against MRA and serum concentrations of free MRA were measured using ELISA (SRL) on every dosing day.
Measurement of response
The primary efficacy end point was improvement in disease activity assessed by biochemical markers such as CRP, hemoglobin (Hb), and serum albumin (Alb), and general fatigue was measured using a visual analog scale (VAS). Changes in size of swollen lymph nodes, number of red blood cells (RBCs), hematocrit, body weight, serum amyloid A protein, and immunoglobulins (IgG, IgA, IgM) were also measured as subsidiary efficacy parameters.
Short-axis lengths of swollen lymph nodes were measured by computed tomography. At baseline for each patient, each affected lymph node whose short-axis length was greater than or equal to 10.0 mm was chosen. These lymph node sizes were monitored at 4 months and 1 year after the start of treatment. Two radiologists independently assessed the results.
Safety was assessed on the basis of recorded adverse events, laboratory measures, and electrocardiography findings. An adverse event was defined as any adverse change from the patient's baseline condition, regardless of whether it was considered related to treatment. Frequencies of infusion-related reactions, which were defined as any adverse experience occurring during or after infusion on the treatment day, were also evaluated. The severity of an adverse event was graded as mild (the event does not interfere with the patient's usual activity), moderate (the event interferes with the patient's usual activity), or severe (the event prevents the patient from undertaking usual activity and necessitates therapeutic cessation of the study drug).
Statistical analysis
The overall effect of treatment was evaluated based on changes from baseline in the efficacy parameters with paired t tests. The significance level was set at P less than or equal to .05 (2-sided). No adjustment was made for multiple comparisons.
Safety was evaluated according to the frequency of adverse events and by laboratory abnormalities. The frequency of adverse events was calculated by system organ class, and the preferred term was coded by the Medical Dictionary for Regulatory Activities (MedDRA). Any laboratory abnormality was graded by National Cancer Institute (NCI) common terminology criteria for adverse events version 3.0.
Results
Patient disposition, baseline characteristics, and previous or concomitant medication
Baseline demographics and clinical characteristics of the patients are listed in Table 1. In total, 28 patients (17 men, 11 women) were enrolled in the study. All patients were diagnosed with the plasma-cell type and had systemic manifestations of the disease. The average serum IL-6 concentration was 34.8 pg/mL (SD = 34.5; n = 28) at baseline (The normal upper limit of serum IL-6 is 4 pg/mL). The average serum soluble IL-6 receptor concentration was 28.5 ng/mL (SD = 11.6; n = 28) at baseline. Patients had symptoms and abnormal laboratory findings compatible with Castleman disease at screening and at predose measurements. Median patient age was 38 years, and median disease duration was 4 years. Two patients were seropositive for KSHV/HHV-8; none was seropositive for HIV. Fifteen patients received corticosteroids concomitantly, and no patient received immunosuppressants during the study period. The average corticosteroid dose was 15.5 mg/body per day (SD = 3.5 mg/body; n = 15) of prednisolone equivalent. Four patients (Table 1; patients 3, 15, 23, 24) received chemotherapy with such agents as cyclophosphamide and melphalan plus prednisolone before this study. In 3 of the 4 patients (Table 1; patients 3, 15, 23), chemotherapy was discontinued more than 4 months before the MRA treatment started. In the other patient (Table 1; patient 24), chemotherapy was stopped 6 weeks before enrollment in this study.
Baseline demographic and clinical characteristics
. | . | . | . | . | Laboratory data at baseline . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age at entry into trial, y . | Duration of disease, y . | Histologic diagnosis . | CRP, mg/L . | IgG, g/L . | Hb, g/L . | Alb, g/L . | T-cho, mg/dL . | HHV-8 . | IL-6, pg/mL* . | Manifestation/complication . | ||||||
| 1 | F | 33 | 9 | PC | 100 | 41.0 | 77 | 27 | 52 | + | 31.9 | Splenomegaly, skin disorder | ||||||
| 2 | F | 36 | 4 | PC | 122 | 48.8 | 68 | 28 | 89 | - | 56.2 | Splenomegaly, hepatomegaly, LIP, skin disorder | ||||||
| 3 | M | 64 | 1 | PC | 24 | 58.9 | 113 | 29 | 148 | - | 7.5 | Splenomegaly, LIP | ||||||
| 4 | M | 53 | 11 | PC | 92 | 48.5 | 102 | 24 | 89 | - | 25.2 | LIP, skin disorder | ||||||
| 5 | M | 37 | 1 | PC | 70 | 39.9 | 126 | 34 | 131 | - | 16.5 | LIP | ||||||
| 6 | M | 66 | 2 | PC | 24 | 11.5 | 113 | 34 | 231 | - | 15.9 | Skin disorder, CMMoL | ||||||
| 7 | M | 23 | 3 | PC | 135 | 48.3 | 101 | 23 | 116 | - | 36.8 | Skin disorder | ||||||
| 8 | M | 55 | 11 | PC | 110 | 42.6 | 108 | 31 | 91 | - | 30.6 | Splenomegaly, hepatomegaly | ||||||
| 9 | F | 27 | 2 | PC | 136 | 73.7 | 93 | 30 | 94 | - | 44.8 | Splenomegaly, hepatomegaly, LIP, skin disorder | ||||||
| 10 | F | 37 | 5 | PC | 89 | 33.6 | 115 | 31 | 169 | - | 10.7 | Splenomegaly, LIP | ||||||
| 11 | F | 51 | 9 | PC | 50 | 102.0 | 73 | 17 | 80 | - | 21.4 | None | ||||||
| 12 | M | 27 | 3 | PC | 107 | 74.0 | 112 | 26 | 97 | - | 19.8 | LIP | ||||||
| 13 | F | 50 | 14 | PC | 215 | 66.5 | 59 | 21 | 101 | - | 90.6 | Splenomegaly, hepatomegaly, LIP | ||||||
| 14 | F | 35 | 14 | PC | 27 | 24.6 | 112 | 36 | 144 | - | 7.0 | Splenomegaly, skin disorder | ||||||
| 15 | M | 60 | 4 | PC | 24 | 65.2 | 72 | 32 | 85 | - | 5.9 | LIP | ||||||
| 16 | F | 53 | 3 | PC | 104 | 48.8 | 71 | 22 | 111 | - | 35.8 | LIP | ||||||
| 17 | M | 49 | 4 | PC | 120 | 58.5 | 99 | 25 | 117 | - | 86.9 | Skin disorder | ||||||
| 18 | M | 32 | 16 | PC | 62 | 66.4 | 111 | 26 | 103 | - | 16.9 | LIP | ||||||
| 19 | F | 37 | 23 | PC | 175 | 65.1 | 65 | 17 | 77 | - | 60.2 | Splenomegaly, skin disorder | ||||||
| 20 | M | 57 | 2 | PC | 48 | 40.3 | 116 | 28 | 138 | - | 16.2 | LIP | ||||||
| 21 | M | 63 | 7 | PC | 29 | 27.9 | 96 | 25 | 165 | - | 18.8 | Splenomegaly, LIP, skin disorder, SA | ||||||
| 22 | F | 27 | 3 | PC | 53 | 41.2 | 99 | 30 | 129 | - | 7.3 | LIP, skin disorder | ||||||
| 23 | M | 39 | 5 | PC | 108 | 50.7 | 51 | 34 | 119 | - | 170.0 | LIP | ||||||
| 24 | M | 38 | 5 | PC | 117 | 60.6 | 94 | 27 | 106 | - | 44.7 | None | ||||||
| 25 | M | 49 | 5 | PC | 127 | 84.7 | 62 | 20 | 77 | + | 28.7 | LIP | ||||||
| 26 | M | 36 | 3 | PC | 25 | 42.0 | 137 | 36 | 125 | - | 10.5 | Splenomegaly, hepatomegaly, LIP | ||||||
| 27 | M | 37 | 4 | PC | 31 | 28.3 | 68 | 25 | 88 | - | 15.5 | LIP, skin disorder, SA | ||||||
| 28 | F | 43 | 3 | PC | 114 | 68.0 | 72 | 23 | 118 | - | 42.7 | None | ||||||
. | . | . | . | . | Laboratory data at baseline . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex . | Age at entry into trial, y . | Duration of disease, y . | Histologic diagnosis . | CRP, mg/L . | IgG, g/L . | Hb, g/L . | Alb, g/L . | T-cho, mg/dL . | HHV-8 . | IL-6, pg/mL* . | Manifestation/complication . | ||||||
| 1 | F | 33 | 9 | PC | 100 | 41.0 | 77 | 27 | 52 | + | 31.9 | Splenomegaly, skin disorder | ||||||
| 2 | F | 36 | 4 | PC | 122 | 48.8 | 68 | 28 | 89 | - | 56.2 | Splenomegaly, hepatomegaly, LIP, skin disorder | ||||||
| 3 | M | 64 | 1 | PC | 24 | 58.9 | 113 | 29 | 148 | - | 7.5 | Splenomegaly, LIP | ||||||
| 4 | M | 53 | 11 | PC | 92 | 48.5 | 102 | 24 | 89 | - | 25.2 | LIP, skin disorder | ||||||
| 5 | M | 37 | 1 | PC | 70 | 39.9 | 126 | 34 | 131 | - | 16.5 | LIP | ||||||
| 6 | M | 66 | 2 | PC | 24 | 11.5 | 113 | 34 | 231 | - | 15.9 | Skin disorder, CMMoL | ||||||
| 7 | M | 23 | 3 | PC | 135 | 48.3 | 101 | 23 | 116 | - | 36.8 | Skin disorder | ||||||
| 8 | M | 55 | 11 | PC | 110 | 42.6 | 108 | 31 | 91 | - | 30.6 | Splenomegaly, hepatomegaly | ||||||
| 9 | F | 27 | 2 | PC | 136 | 73.7 | 93 | 30 | 94 | - | 44.8 | Splenomegaly, hepatomegaly, LIP, skin disorder | ||||||
| 10 | F | 37 | 5 | PC | 89 | 33.6 | 115 | 31 | 169 | - | 10.7 | Splenomegaly, LIP | ||||||
| 11 | F | 51 | 9 | PC | 50 | 102.0 | 73 | 17 | 80 | - | 21.4 | None | ||||||
| 12 | M | 27 | 3 | PC | 107 | 74.0 | 112 | 26 | 97 | - | 19.8 | LIP | ||||||
| 13 | F | 50 | 14 | PC | 215 | 66.5 | 59 | 21 | 101 | - | 90.6 | Splenomegaly, hepatomegaly, LIP | ||||||
| 14 | F | 35 | 14 | PC | 27 | 24.6 | 112 | 36 | 144 | - | 7.0 | Splenomegaly, skin disorder | ||||||
| 15 | M | 60 | 4 | PC | 24 | 65.2 | 72 | 32 | 85 | - | 5.9 | LIP | ||||||
| 16 | F | 53 | 3 | PC | 104 | 48.8 | 71 | 22 | 111 | - | 35.8 | LIP | ||||||
| 17 | M | 49 | 4 | PC | 120 | 58.5 | 99 | 25 | 117 | - | 86.9 | Skin disorder | ||||||
| 18 | M | 32 | 16 | PC | 62 | 66.4 | 111 | 26 | 103 | - | 16.9 | LIP | ||||||
| 19 | F | 37 | 23 | PC | 175 | 65.1 | 65 | 17 | 77 | - | 60.2 | Splenomegaly, skin disorder | ||||||
| 20 | M | 57 | 2 | PC | 48 | 40.3 | 116 | 28 | 138 | - | 16.2 | LIP | ||||||
| 21 | M | 63 | 7 | PC | 29 | 27.9 | 96 | 25 | 165 | - | 18.8 | Splenomegaly, LIP, skin disorder, SA | ||||||
| 22 | F | 27 | 3 | PC | 53 | 41.2 | 99 | 30 | 129 | - | 7.3 | LIP, skin disorder | ||||||
| 23 | M | 39 | 5 | PC | 108 | 50.7 | 51 | 34 | 119 | - | 170.0 | LIP | ||||||
| 24 | M | 38 | 5 | PC | 117 | 60.6 | 94 | 27 | 106 | - | 44.7 | None | ||||||
| 25 | M | 49 | 5 | PC | 127 | 84.7 | 62 | 20 | 77 | + | 28.7 | LIP | ||||||
| 26 | M | 36 | 3 | PC | 25 | 42.0 | 137 | 36 | 125 | - | 10.5 | Splenomegaly, hepatomegaly, LIP | ||||||
| 27 | M | 37 | 4 | PC | 31 | 28.3 | 68 | 25 | 88 | - | 15.5 | LIP, skin disorder, SA | ||||||
| 28 | F | 43 | 3 | PC | 114 | 68.0 | 72 | 23 | 118 | - | 42.7 | None | ||||||
To convert total cholesterol from milligrams per deciliter to millimoles per liter, multiply milligrams per deciliter by 0.02586.
T-cho indicates total cholesterol; F, female; M, male; PC, plasma-cell type; LIP, lymphocytic interstitial pneumonia; SA, secondary amyloidosis.
Normal range of serum IL-6 is less than 4 pg/mL.
Efficacy
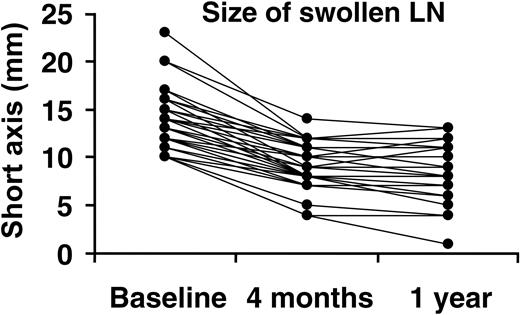
Figure 1 shows the size changes in the swollen lymph nodes with MRA treatment. MRA remarkably improved lymphadenopathy. At baseline, 23 patients had affected lymph nodes that were 10.0 mm or larger on average. Mean short-axis length of these specified swollen lymph nodes was 13.5 mm (SD = 2.9). After 16 weeks of treatment with MRA, the mean short-axis length had decreased from baseline to 9.1 mm (SD = 2.2), and after 1 year of treatment it decreased to 8.6 mm (SD = 2.7). These results demonstrated a 30% reduction in the mean short axis of the specified lymph nodes (P < .001; paired t test vs baseline) after 1 year of treatment. Lymph nodes had decreased to less than 10 mm after 16 weeks of MRA treatment in 10 (43.4%) of 23 patients and in 12 (52.2%) of 23 patients after 1 year of treatment.
Changes in short-axis length of swollen lymph nodes after 4 months and 1 year of treatment with MRA. Size changes of specified lymph nodes (LN) whose short axes were larger than 10 mm at baseline were examined using computed tomography.
Changes in short-axis length of swollen lymph nodes after 4 months and 1 year of treatment with MRA. Size changes of specified lymph nodes (LN) whose short axes were larger than 10 mm at baseline were examined using computed tomography.
Mean changes in laboratory markers with MRA treatment are shown in Figure 2 and Table 2. Abnormally high values of inflammatory parameters, such as CRP, fibrinogen, serum amyloid A protein (SAA), and erythrocyte sedimentation rate (ESR), as is usually noted in patients with MCD, were observed in all patients at baseline. At week 6, treatment with MRA resulted in significant improvement in these parameters compared with baseline (P < .001; paired t test). CRP and fibrinogen were completely normalized in 18 (64.3%) patients and in 20 (71.4%) patients, respectively, at the week 16 evaluation.
Mean values for laboratory tests at baseline and at weeks 6, 16, and 60
Measure . | Baseline . | Week 6 . | Week 16 . | Week 60* . |
|---|---|---|---|---|
| Fibrinogen level, mg/dL | 639 ± 36 | 357 ± 28† | 317 ± 28† | 317 ± 22† |
| ESR, mm/h | 114 ± 7 | 63 ± 7† | 48 ± 8† | 40 ± 7† |
| HDL cholesterol level, mg/dL | 36.1 ± 2.4 | 52.0 ± 4.6† | 52.7 ± 5.3† | 49.4 ± 3.0† |
| LDL cholesterol level, mg/dL | 71.4 ± 4.6 | 96.8 ± 5.4† | 106.5 ± 4.9† | 109.5 ± 5.9† |
| Triglyceride level, mg/dL | 54 ± 4 | 105 ± 11† | 121 ± 15† | 138 ± 19† |
| Body weight, kg | 56.3 ± 2.0 | 58.1 ± 2.0 | 60.1 ± 2.2 | 61.0 ± 2.3 |
| BMI | 21.6 ± 0.6 | 22.3 ± 0.6† | 23.1 ± 0.6† | 23.4 ± 0.6† |
| General fatigue, mm | 29.9 ± 4.3 | 17.4 ± 3.3‡ | 17.7 ± 3.2§ | 20.4 ± 3.8 |
| IgA, g/L | 3.19 ± 0.31 | 2.58 ± 0.28† | 2.47 ± 0.29† | 2.26 ± 0.21† |
| IgM, g/L | 7.13 ± 0.73 | 3.82 ± 0.38† | 3.44 ± 0.38† | 3.30 ± 0.33† |
| IgE, mg/L | 5.03 ± 1.05 | 3.49 ± 0.85† | 4.64 ± 1.62 | 3.99 ± 0.92† |
| Neutrophil count, × 109/L | 5.2 ± 0.7 | 4.5 ± 0.5† | 4.3 ± 0.6† | 4.3 ± 0.4† |
Measure . | Baseline . | Week 6 . | Week 16 . | Week 60* . |
|---|---|---|---|---|
| Fibrinogen level, mg/dL | 639 ± 36 | 357 ± 28† | 317 ± 28† | 317 ± 22† |
| ESR, mm/h | 114 ± 7 | 63 ± 7† | 48 ± 8† | 40 ± 7† |
| HDL cholesterol level, mg/dL | 36.1 ± 2.4 | 52.0 ± 4.6† | 52.7 ± 5.3† | 49.4 ± 3.0† |
| LDL cholesterol level, mg/dL | 71.4 ± 4.6 | 96.8 ± 5.4† | 106.5 ± 4.9† | 109.5 ± 5.9† |
| Triglyceride level, mg/dL | 54 ± 4 | 105 ± 11† | 121 ± 15† | 138 ± 19† |
| Body weight, kg | 56.3 ± 2.0 | 58.1 ± 2.0 | 60.1 ± 2.2 | 61.0 ± 2.3 |
| BMI | 21.6 ± 0.6 | 22.3 ± 0.6† | 23.1 ± 0.6† | 23.4 ± 0.6† |
| General fatigue, mm | 29.9 ± 4.3 | 17.4 ± 3.3‡ | 17.7 ± 3.2§ | 20.4 ± 3.8 |
| IgA, g/L | 3.19 ± 0.31 | 2.58 ± 0.28† | 2.47 ± 0.29† | 2.26 ± 0.21† |
| IgM, g/L | 7.13 ± 0.73 | 3.82 ± 0.38† | 3.44 ± 0.38† | 3.30 ± 0.33† |
| IgE, mg/L | 5.03 ± 1.05 | 3.49 ± 0.85† | 4.64 ± 1.62 | 3.99 ± 0.92† |
| Neutrophil count, × 109/L | 5.2 ± 0.7 | 4.5 ± 0.5† | 4.3 ± 0.6† | 4.3 ± 0.4† |
Values are expressed as mean ± standard error. To convert fibrinogen from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 0.0249. To convert HDL and LDL cholesterol from milligrams per deciliter to millimoles per liter, multiply milligrams per deciliter by 0.02586. To convert triglyceride from milligrams per deciliter to millimoles per liter, multiply milligrams per deciliter by 0.0113.
ESR indicates erythrocyte sedimentation rate; LDL, low-density lipoprotein; BMI, body mass index.
Patients treated with MRA up to 16 weeks (n = 28) and up to 60 weeks (n = 27).
P < .001; paired t test compared with baseline.
P = .01; paired t test compared with baseline.
P = .008; paired t test compared with baseline.
Most patients exhibited severe anemia at baseline, and the mean Hb level was 92 g/L (SD = 23). MRA significantly improved anemia in these patients, and the Hb level was increased to 120 g/L (SD = 21) at week 16 (P < .001; paired t test vs baseline). No patient required transfusion during MRA administration.
MRA also improved Alb values and IgG values (P < .001; paired t test; week 16 vs baseline). Fatigue measured by VAS also showed significant improvement at week 16 (P = .008; paired t test vs baseline).
Nutritional status demonstrated by total cholesterol, high-density lipoprotein (HDL)–cholesterol, triglycerides, Alb, body weight, and body mass index (BMI) improved significantly in all patients during treatment with MRA. In particular, as shown in Table 3, 5 (17.8%) patients had BMIs below 18.5 and were considered underweight at baseline. These patients also had severe hypoalbuminemia and hypocholesterolemia. MRA improved this severe nutritional status. Weight gain and improvement in nutritional status markers were observed with MRA treatment in all these patients, with 4 of 5 patients achieving BMIs up to 18.5.
Body weight, BMI, albumin, total cholesterol, and HDL-cholesterol improvement of underweight patients with MRA treatment
. | Body weight, kg . | . | . | BMI . | . | . | Alb, g/L . | . | . | Total cholesterol, mg/dL . | . | . | HDL cholesterol, mg/dL . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | ||||||||||
| 2 | 43.9 | 53.3 | 55.2 | 16.1 | 19.6 | 20.3 | 28 | 40 | 45 | 89 | 158 | 171 | 19 | 46 | 44 | ||||||||||
| 13 | 43.0 | 55.0 | 55.0 | 16.4 | 21.0 | 21.0 | 21 | 39 | 42 | 101 | 186 | 183 | 33 | 59 | 59 | ||||||||||
| 15 | 43.6 | 45.2 | 48.0 | 17.5 | 18.1 | 19.2 | 32 | 31 | 34 | 85 | 135 | 134 | 30 | 35 | 41 | ||||||||||
| 17 | 51.6 | 56.3 | 60.1 | 18.5 | 20.1 | 21.7 | 25 | 35 | 39 | 117 | 139 | 185 | 29 | 43 | 60 | ||||||||||
| 28 | 40.8 | 41.7 | 43.8 | 17.1 | 17.5 | 18.4 | 23 | 39 | 41 | 118 | 298 | 217 | 53 | 160 | 98 | ||||||||||
. | Body weight, kg . | . | . | BMI . | . | . | Alb, g/L . | . | . | Total cholesterol, mg/dL . | . | . | HDL cholesterol, mg/dL . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | 0 wk . | 16 wk . | 60 wk . | ||||||||||
| 2 | 43.9 | 53.3 | 55.2 | 16.1 | 19.6 | 20.3 | 28 | 40 | 45 | 89 | 158 | 171 | 19 | 46 | 44 | ||||||||||
| 13 | 43.0 | 55.0 | 55.0 | 16.4 | 21.0 | 21.0 | 21 | 39 | 42 | 101 | 186 | 183 | 33 | 59 | 59 | ||||||||||
| 15 | 43.6 | 45.2 | 48.0 | 17.5 | 18.1 | 19.2 | 32 | 31 | 34 | 85 | 135 | 134 | 30 | 35 | 41 | ||||||||||
| 17 | 51.6 | 56.3 | 60.1 | 18.5 | 20.1 | 21.7 | 25 | 35 | 39 | 117 | 139 | 185 | 29 | 43 | 60 | ||||||||||
| 28 | 40.8 | 41.7 | 43.8 | 17.1 | 17.5 | 18.4 | 23 | 39 | 41 | 118 | 298 | 217 | 53 | 160 | 98 | ||||||||||
To convert total and HDL cholesterol from milligrams per deciliter to millimoles per liter, multiply milligrams per deciliter by 0.02586.
Change of serum CRP, SAA, Hb, Alb, IgG, and total cholesterol levels. Points and vertical bars indicate means and SEs, respectively. Patients treated with MRA up to 48 weeks (n = 28) and up to 60 weeks (n = 27). *P < .001, paired t test, compared with baseline. To convert total cholesterol from milligrams per deciliter to millimoles per liter, multiply milligrams per deciliter by 0.02586.
Change of serum CRP, SAA, Hb, Alb, IgG, and total cholesterol levels. Points and vertical bars indicate means and SEs, respectively. Patients treated with MRA up to 48 weeks (n = 28) and up to 60 weeks (n = 27). *P < .001, paired t test, compared with baseline. To convert total cholesterol from milligrams per deciliter to millimoles per liter, multiply milligrams per deciliter by 0.02586.
At baseline, 17 (60.7%) patients were positive for antinuclear antibodies; 9 (52.9%) of these patients became negative for antinuclear antibodies at week 60. Antibodies to double-stranded DNA were also normalized with 60-week treatment in 19 (86.4%) of 22 patients whose antibodies to double-stranded DNA were high at baseline. Neither systemic lupus erythematosus nor another autoimmune disease developed in any patient. Furthermore, 6 (37.5%) of 16 patients who had positive findings on direct Coombs test at baseline had negative findings after 60 weeks of MRA treatment.
MRA also improved hepatosplenomegaly and skin disorders associated with Castleman disease. At baseline, 11 patients had hepatosplenomegaly; 4 (36.4%) of these patients experienced improvement after 16 weeks of treatment. Eight of 12 patients with skin disorders experienced improvement after 16 weeks of treatment.
During the extension phase, 15 (53.6%) patients continuously received 8 mg/kg MRA every 2 weeks; 7 (25%) patients received treatment once every 4 weeks, and 1 patient received treatment once every 3 weeks. Five (17.9%) patients received 8 mg/kg MRA at intervals of less than 2 weeks (the average interval of these 5 patients was 10 days to enhance the therapeutic efficacy).
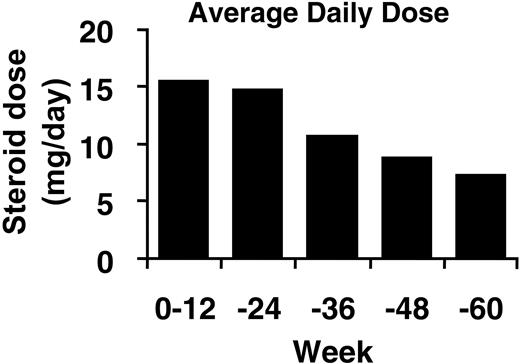
At baseline, 15 (53.6%) patients received oral corticosteroid treatment (average daily dose, 15.5 mg/body per day [SD = 3.5] prednisolone). During treatment with MRA, 11 (73.3%) of these 15 patients were able to decrease the dose or discontinue corticosteroid therapy without exacerbation (Figure 3). Two KSHV/HHV-8–seropositive patients were also successfully treated with MRA.
Safety
MRA was generally well tolerated in patients with MCD. Median duration of MRA treatment was 65 weeks (range, 55-76 weeks). Of 28 patients enrolled in the study, 27 (96.4%) patients have continued to receive MRA treatment for more than 3 years (as of December 2004).
One patient (patient 6; Table 1) was withdrawn from the study at 40 weeks because of aggravation of a concurrent disease. This patient was a 66-year-old man with chronic myelomonocytic leukemia (CMMoL). After his withdrawal from the study, he received MRA treatment continuously as compassionate use of the drug. In total, he received MRA treatment at the same regimen for 55 weeks without any aggravation of Castleman disease. Acute leukemia developed, however, and he died at 57 weeks. Given that this patient had had CMMoL for approximately 4 years before entry in the study, it was considered that the development of acute leukemia most likely occurred in the natural course of CMMoL. No other patient experienced malignancies or aggravation of a secondary disease, such as amyloidosis, for the first 60 weeks.
Table 4 shows the frequently observed adverse events possibly related to the study drug (ie, adverse reactions). Adverse reactions were reported in 27 of 28 patients. All adverse reactions were transient and resolved spontaneously or were treated with medication. No severe adverse reactions were reported. There was no evidence of an increase in the frequency of adverse reactions with long-term treatment. The most frequent adverse reaction was the common cold (16 of 28 patients; 57.1%) and symptoms related to the common cold, such as cough, rhinorrhea, and pharyngitis. The symptom of malaise was transient and improved during MRA treatment.
Adverse reactions observed at least 4 patients
. | No. patients (%) . | . | . | ||
|---|---|---|---|---|---|
| Adverse reaction . | Total . | Mild . | Moderate . | ||
| Common cold | 16 (57.1) | 0 (0) | 16 (57.1) | ||
| Pruritus | 6 (21.4) | 3 (10.7) | 3 (10.7) | ||
| Malaise | 6 (21.4) | 4 (14.3) | 2 (7.1) | ||
| Pharyngitis | 5 (17.9) | 0 (0) | 5 (17.9) | ||
| Diarrhea | 5 (17.9) | 2 (7.1) | 3 (10.7) | ||
| Rash | 5 (17.9) | 2 (7.1) | 3 (10.7) | ||
| Eczema | 5 (17.9) | 0 (0) | 5 (17.9) | ||
| Low-grade fever | 5 (17.9) | 3 (10.7) | 2 (7.1) | ||
| Urinary tract infection | 4 (14.3) | 0 (0) | 4 (14.3) | ||
| Chest pain | 4 (14.3) | 3 (10.7) | 1 (3.6) | ||
| Aphthous stomatitis | 4 (14.3) | 0 (0) | 4 (14.3) | ||
. | No. patients (%) . | . | . | ||
|---|---|---|---|---|---|
| Adverse reaction . | Total . | Mild . | Moderate . | ||
| Common cold | 16 (57.1) | 0 (0) | 16 (57.1) | ||
| Pruritus | 6 (21.4) | 3 (10.7) | 3 (10.7) | ||
| Malaise | 6 (21.4) | 4 (14.3) | 2 (7.1) | ||
| Pharyngitis | 5 (17.9) | 0 (0) | 5 (17.9) | ||
| Diarrhea | 5 (17.9) | 2 (7.1) | 3 (10.7) | ||
| Rash | 5 (17.9) | 2 (7.1) | 3 (10.7) | ||
| Eczema | 5 (17.9) | 0 (0) | 5 (17.9) | ||
| Low-grade fever | 5 (17.9) | 3 (10.7) | 2 (7.1) | ||
| Urinary tract infection | 4 (14.3) | 0 (0) | 4 (14.3) | ||
| Chest pain | 4 (14.3) | 3 (10.7) | 1 (3.6) | ||
| Aphthous stomatitis | 4 (14.3) | 0 (0) | 4 (14.3) | ||
Twenty-eight patients were enrolled in this study. The severity of an adverse reaction was graded as mild (the event did not interfere with the patient's usual activity), moderate (the event interfered with the patient's usual activity), or severe (the event prevented the patient from undertaking some usual activity and necessitated therapeutic cessation of the study drug).
Twenty-one infusion-related reactions were reported in 14 patients. All infusion-related reactions were transient and mild. The most frequent infusion-related reaction was low-grade fever (maximum body temperature, 37.4°C). Neither anti-MRA antibodies nor tuberculosis developed during the course of the study.
A serious adverse event, cellulitis, occurred in 2 patients, and a causal relationship with MRA could not be ruled out. Both patients required prolongation of hospital admission; the cellulitis resolved with antibiotic treatment. Both patients continued the study after resolution of the event.
Most laboratory abnormalities were mild. A transient, mild decrease in leukocyte counts to the low normal range was observed a few days after MRA administration (grade 1 in 9 [32.1%] patients and grade 2 in 3 [10.7%] patients), but they returned to normal without any treatment.
Transient liver function disorders were observed in 9 (32.1%) patients. An increase in alanine aminotransferase to grades 2 and 3 was observed in each of 2 patients; the other 5 patients had grade 1. An increase in aspartate aminotransferase to grade 2 was observed in 2 patients; 7 others showed grade 1. No continuous aggravation was observed by repeated treatment with MRA. No patients acquired systemic lupus erythematosus or any other autoimmune disease.
Discussion
Castleman disease, especially MCD, is rare. Previous studies have reported that blockade of the IL-6 signal was effective in treating Castleman disease.10,14 However, these findings were based on a small number of patients. Here we report findings from the first prospective study to recruit a substantial number of patients (almost one fifth of the total number of MCD patients reported in the past 10 years in Japan) to examine the efficacy and safety of MRA, a potential therapeutic agent for this disease. All patients enrolled in this study had the characteristic features of affected nodes, constitutional symptoms, and laboratory abnormalities associated with MCD.
Average daily dose of prednisolone over time. The average corticosteroid dose converted to prednisolone dose is shown.
Average daily dose of prednisolone over time. The average corticosteroid dose converted to prednisolone dose is shown.
MRA is a humanized anti–human IL-6 receptor monoclonal antibody that blocks the activity of IL-6, alleviating symptoms and biochemical abnormalities in patients with Castleman disease. Because MRA treatment improved lymphadenopathy, IL-6 produced by the lymph nodes might have affected the hyperplasia of lymph nodes in an autocrine manner in patients with MCD. Most of the MCD patients in this study had very low levels of serum albumin, total cholesterol, and HDL-cholesterol at baseline, which were markedly improved through MRA treatment. Gains in body weight and BMI, together with improved nutritional status, were observed in patients with severe wasting at baseline. These findings further support the hypothesis that overproduction of IL-6 may be responsible for the poor nutritional status observed in MCD patients, and they may also suggest a role for this cytokine in cachexia and chronic inflammatory diseases.18 MRA may also prevent the development of secondary amyloidosis associated with MCD19 because treatment markedly decreased the levels of SAA, a causative factor of this disorder.
Another clinical benefit of MRA treatment arises from the corticosteroid-sparing effect of this antibody. Corticosteroids are commonly used to treat MCD but are associated with dose-related toxicity, which limits their use for long-term treatment. Patients receiving MRA were able to receive significantly reduced doses of corticosteroids, suggesting that treatment with MRA could decrease the use of these drugs in the future treatment of MCD.
Regarding antibody-based treatments of chronic disease, there was no evidence of anti-MRA antibody development or attenuation of MRA efficacy during 1 year of treatment. These favorable features are likely to be attributable to the humanization of the monoclonal antibody, which reduces the content of foreign protein and thus the potential for the development of neutralizing antibodies.
Two patients were seropositive for KSHV/HHV-8, a known etiologic factor in MCD; this incidence (2 of 28 patients) was much lower than that reported in Western countries,6 but we do not know why.
KSHV/HHV-8 encodes a human IL-6 homolog, called viral IL-6 (vIL-6). vIL-6 shares functional properties with human IL-6. vIL-6, without formation of the vIL-6–IL-6 receptor complex, can bind to glycoprotein (gp) 130, an actual signal transducer of the IL-6 receptor system, and it activates the gp130-related signal transduction system. Thus, MRA cannot inhibit vIL-6–related gp130 activation. However, treatment with MRA improved the symptoms and laboratory parameters of patients positive for KSHV/HHV-8. Therefore, although vIL-6 may be expressed in these patients, human IL-6 is still thought to be responsible for the systemic manifestations of KSHV/HHV-8–seropositive MCD.
MRA treatment was well tolerated over the long term. A mild and transient decrease in leukocyte counts was sometimes observed after MRA administration. However, a similar phenomenon was reported in clinical studies with other biologic agents such as anti-CD2020 antibody and anti–tumor necrosis factor–TNF-alpha antibody.21 Therefore, this phenomenon is not specific to MRA treatment.
In conclusion, MRA improves the symptoms and biochemical abnormalities associated with MCD. The efficacy observed was sustained or improved over the course of 1 year. The safety profile is acceptable relative to the clinical benefit provided. A follow-up study is ongoing to determine the long-term prognosis of patients treated with MRA.
Appendix
Kuniaki Meguro, Junichi Kameoka, and Osamu Sasaki (Tohoku University, Miyagi, Japan); Shinichiro Okamoto, Michito Hirakata, Takaki Nojima, Shinji Sato, and Yoshitaka Miyakawa (Keio University, Tokyo, Japan); Kazuya Minatohara, Tetsuya Higuchi, and Yasuhiro Miyazaki (Tokyo Medical and Dental University, Tokyo, Japan); Atsushi Sato (Aichi Medical University, Aichi, Japan); Yukihiko Saeki, Toshio Tanaka, Satoru Tsukada, Tetsuji Naka, Masao Mizuki, Yoshihito Shima, Masashi Narasaki, Toru Mima, Hiroyuki Sugawara, Hiroshi Fujiwara, and Shoji Hashimoto (Osaka University, Osaka, Japan); Masahiro Koshiba and Tomoko Nakamura (Kobe University, Hyogo, Japan); Hiromasa Niimi and Akiko Nomura (Hiroshima Red Cross Hospital and Atomic-Bomb Survivors Hospital, Hiroshima, Japan); Yoshinobu Maeda and Yuichiro Nawa (Ehime Prefectural Central Hospital, Ehime, Japan); Kazuto Togitani and Seisho Takeuchi (Kochi Medical School, Kochi, Japan); Shuji Nakano and Kenji Mitsugi (Kyushu University, Fukuoka, Japan).
Prepublished online as Blood First Edition Paper, July 5, 2005; DOI 10.1182/blood-2004-12-4602.
A list of all members of the Japanese MRA study group on MCD who are not listed in this article's byline appears in the “Appendix.”
Supported by grants from Chugai Pharmaceutical Co, Ltd, Roche Group.
N. Nishimoto has declared a financial interest in Chugai Pharmaceutical Co, Ltd, whose product was studied in the present work.
Y. Kanakura and N. Nishimoto contributed to the conception and design of the study, analysis and interpretation of the data, and preparation of the manuscript. T. Kishimoto and K. Yoshizaki contributed to the conception of the study and worked as coordinators of the clinical trial. K.A. made a histologic diagnosis of each patient's lymph node, which was essential for the trial, and reviewed and approved the paper. T.J. contributed to the conception of the study and analyzed lymph node sizes by computed tomography. M.N. contributed to the acquisition of data, reviewed and approved the paper, and analyzed lymph nodes sizes by computed tomography. Y.I., T.S., H.T., S.K., Y. Kimura, K.N., N. Nakamura, S.N., N. Nakano, M.H., Y. Kato, H.A., F.K., and K.H. contributed to the acquisition of data and reviewed and approved the paper.
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 7, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Paul Langman for his valuable assistance with the preparation of the English version of this paper.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal