Comment on Fang et al, page 2671
The platelet integrin receptor, αIIbβ3, is central to the platelet's role in hemostasis and its absence results in the human bleeding disorder, Glanzmann thrombasthenia (GT). In this issue of Blood, Fang and colleagues describe ex vivo viral transduction of mouse hematopoietic stem cells and determine the in vivo efficiency required to rescue the GT phenotype in a mouse model of the disorder.
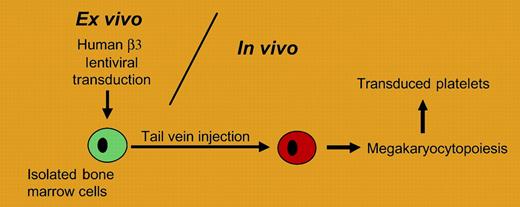
The treatment of inherited bleeding disorders via some form of gene therapy remains a key goal in the clinical management of coagulation. Although relatively straightforward in design, the pilot studies have often encountered unanticipated problems that have diminished the effectiveness or safety of a particular strategy. Recent gene therapy successes in large animals continue to look promising,1 but a successful application to a human disorder is still lacking. In the platelet field, a number of rare bleeding disorders exist that could greatly benefit from such a therapy, but many issues must be addressed. For example, how does one direct lineage specificity of a given gene product to the megakaryocyte or platelet? Will the gene integration remain stable and will the new gene product be tolerated by the host's immune system?FIG1
A gene therapy strategy for transducing platelets is presented. Bone marrow cells are transduced ex vivo and infused into animals following bone marrow ablation. The approach is applied to a mouse model of Glanzmann thrombasthenia by Fang and colleagues.
A gene therapy strategy for transducing platelets is presented. Bone marrow cells are transduced ex vivo and infused into animals following bone marrow ablation. The approach is applied to a mouse model of Glanzmann thrombasthenia by Fang and colleagues.
Some insight into these questions is addressed in the report by Fang and colleagues. Using a viral approach, the β3 subunit of the platelet integrin receptor, αIIbβ3, is delivered to mouse platelets in animals deficient in β3 and mimicking Glanzmann thrombasthenia (see figure). A relatively small fragment, approximately 900 nucleotides in length, of the megakaryocytic-specific human αIIb promoter was used to generate a lentivirus transfer vector capable of expressing human β3 coding sequence. The authors characterize platelets expressing a chimeric αIIbβ3 membrane-expressed receptor composed of a murine αIIb subunit and the transgenic viral product, human β3. When levels of expressed receptor exceeded 7% of the normal αIIbβ3 level, a shortening of the mouse bleeding time was observed along with restored platelet function in the aggregometer. When an antibody response to αIIbβ3 was observed, intravenous immunoglobulin treatment reduced the associated platelet clearance consistent with results obtained with immunoglobulin treatment in humans. These preliminary findings address major issues of sustained lineage specificity and immune response.
The clinical application of such a strategy is obvious but the work also presents unique opportunities for scientific investigations in platelet biology. The inability to manipulate megakaryocytes and platelets with standard DNA technologies has remained a technical problem that has led to a significant lag in the understanding of megakaryocytopoiesis. The report by Fang et al continues to expand possibilities for experimental manipulation of both megakaryocytes and platelets, a strategy that can be applied to recently developed models of platelet dysfunction.2
Does gene therapy for platelet disorders hold promise? Certainly, the identification of small megakaryocytic promoter fragments and the ability to transduce bone marrow cells in an ex vivo setting suggests the potential exists. The use of the lentivirus, while promising, does present safety issues that require further address. Lentivirus does offer some unique properties owing to its ability to infect the nondividing cell. The work presented by Fang et al establishes feasibility for such a strategy and provides a model for future investigations in both platelet biology and in the clinical management of bleeding disorders. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal