Comment on de Goër de Herve et al, page 2806
Janus, the god of gates and doorways, was depicted with 2 faces looking in opposite directions. In the paper by de Goër de Herve and colleagues, they suggest a mechanism whereby anti-CD40 could potentially fulfill such a two-faced role in both immune activation and immunosuppression.
The CD40 cell-surface receptor is required for normal function of the immune system and is known to be a positive regulator of cell survival for normal B-lymphocytes. However, there is emerging evidence to support both pro- and antiapoptotic functions for CD40 in malignant disease. The CD154-CD40 ligand pair interaction plays a central role in both induction of the immune response and in immune effector functions against tumors, including T-cell responses. Several groups have previously found that ligation of CD40 on dendritic cells (DCs) is a potent method of improving the antigen (Ag)–presenting function of these cells and of empowering them to present Ag to naive CD8 cells and to activate them.1
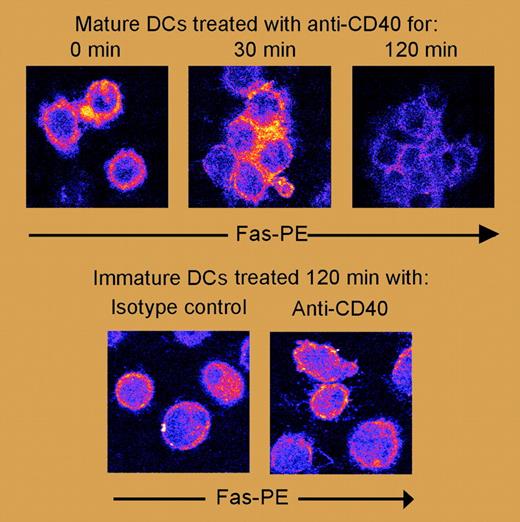
Furthermore, CD40 monoclonal antibody (mAb) signaling in immature DCs is known to provide an antiapoptotic environment that protects them from subsequent apoptotic signals. De Goër de Herve and colleagues have confirmed that anti-CD40, in combination with T-lymphocyte receptor (TLR) agonists, functions to mature DCs and leads to increased longevity and survival. In contrast, however, they demonstrate that anti-CD40 induces apoptosis of mature DCs directly, via Fas activation (see figure). This activation subsequently leads to Fas-associating protein with death domain (FADD) recruitment, followed by rapid cleavage and activation of caspases 8 and 3, but without major involvement of the mitochondrial pathway of apoptosis.
Of interest and potential importance is the finding that anti-CD40 mAb–matured DCs were resistant to anti-CD40–mediated apoptosis in a way that DCs matured with TLR agonists and proinflamatory cytokines were not. This intriguing observation of a differential effect on DCs matured by proinflammatory cytokines with anti-CD40 may provide us with a potential explanation as to how anti-CD40 can inhibit some chronic autoimmune disorders such as rheumatoid arthritis.2 In the setting of established autoimmune disease it is possible to postulate that by shortening the lifespan of mature DCs, anti-CD40 could reduce their capacity to generate effector and memory T cells directed against immunogenic antigens, including self-antigens that DCs have captured.FIG1
Fas internalization in mature DCs treated with anti-CD40. See the complete figure in the article beginning on page 2806.
Fas internalization in mature DCs treated with anti-CD40. See the complete figure in the article beginning on page 2806.
The findings of a differential effect of anti-CD40 on immature and mature DCs may also have important implications in using anti-CD40–based approaches in cancer therapy. Dendritic cells have been shown, depending on their activation state, to orchestrate the outcome of cytotoxic T lymphocyte (CTL) immunity against tumors leading either to an ineffective immune response or potent antitumor immunity. Compelling preclinical data have emerged in recent years that anti-CD40 mAb can stimulate potent CTL responses against a range of tumor models. Furthermore, these responses leave the hosts resistant to rechallenge and bypass the need for T-cell help.3,4 Recently, the antitumor response elicited by anti-CD40 mAb has been shown to operate via in vivo activation of DCs by anti-CD40, resulting in gain-of-effector function of tumor-specific CTLs and eradication of established tumors.5
A true “Janus” role for anti-CD40 mAb therapy, therefore, seems less likely than an important biologic immune regulation applied to immature and mature DCs. Further probing of the mechanisms involved in the interaction of DCs and CD40/CD154 as well as an increased understanding of the markers of “conditioning” within the different settings of malignant disease and autoimmunity are required to truly unlock the potential of these powerful immune regulators. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal