Abstract
Flavopiridol is active against chronic lymphocytic leukemia (CLL) cells in vitro and in the treatment of advanced stage disease, but the mechanisms of these actions remain unclear. Originally developed as a general cyclin-dependent kinase inhibitor, flavopiridol is a potent transcriptional suppressor through the inhibition of positive transcription elongation factor b (P-TEFb; CDK9/cyclin T). P-TEFb phosphorylates the C-terminal domain (CTD) of RNA polymerase II to promote transcriptional elongation. Because most CLL cells are not actively cycling, and their viability is dependent upon the continuous expression of antiapoptotic proteins, we hypothesized that flavopiridol induces apoptosis in CLL cells through the transcriptional down-regulation of such proteins. This study demonstrated that flavopiridol inhibited the phosphorylation of the CTD of RNA polymerase II in primary CLL cells and reduced RNA synthesis. This was associated with a decline of the transcripts and the levels of short-lived antiapoptotic proteins such as myeloid cell leukemia 1 (Mcl-1), and resulted in the induction of apoptosis. The B-cell lymphoma 2 (Bcl-2) protein level remained stable, although its mRNA was consistently reduced, suggesting that the outcome of transcriptional inhibition by flavopiridol is governed by the intrinsic stability of the individual transcripts and proteins. The dependence of CLL-cell survival on short-lived oncoproteins may provide the biochemical basis for the therapeutic index in response to flavopiridol. (Blood. 2005;106:2513-2519)
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by the gradual accumulation of small mature B cells, most of which are nonproliferating cells that display the T-cell marker CD5 in addition to the typical B-cell surface marker CD19.1,2 High levels of the antiapoptotic B-cell lymphoma (Bcl-2) family proteins are expressed in most cases of CLL. This was correlated with resistance to therapy and may account for the prolonged survival of CLL cells.3,4 Reduction in their levels in model systems and in CLL cells by antisense oligodeoxynucleotides or small interfering RNA (siRNA) causes cell death.5-7
The purine nucleoside analog, fludarabine, is the most active single agent in the treatment of CLL. It induces higher response rates and longer progression-free survival than DNA alkylating agents such as chlorambucil.8 Nevertheless, CLL almost uniformly progresses to refractory disease given sufficient time.9 The underlying defects in apoptosis are likely to be the major contributors to therapeutic resistance. In addition, 40% to 50% of clinical resistance to fludarabine alone or in combination with alkylating agents is associated with mutation or deletion in the P53 gene.10,11 Therefore, novel agents or strategies are needed to abrogate blocks to apoptosis in CLL, particularly in the treatment of patients who become refractory to primary therapy.
Flavopiridol (NSC649890) is a semisynthetic flavonoid derived from an indigenous plant from India.12 It has shown potent cytotoxicity on CLL cells in vitro13-15 and promising activity in clinical trials.16 Importantly, this proapoptotic activity of flavopiridol is independent of p53 function,12,13 which makes it a potential drug to overcome resistance associated with p53 abnormalities. Flavopiridol inhibits cyclin-dependent kinases (CDKs) by competing with adenosine triphosphate (ATP) for the active site of each kinase. The activities of CDK1, CDK2, CDK4, CDK6, and CDK7 are inhibited with inhibitory concentration at 50% (IC50) values in the range of 20 to 300 nM.12 Consistent with this action, in growing cell populations flavopiridol blocks cell-cycle progression at the G1/S and G2/M boundaries. However, since most CLL cells are not actively cycling, the inhibition of cell-cycle-related CDKs is unlikely to be the major mechanism for its cytotoxicity. In seeking the mechanism of flavopiridol-induced apoptosis, Kitada et al observed that flavopiridol toxicity to CLL cells in vitro was associated with a decline of antiapoptotic proteins such as Bcl-2, myeloid-cell leukemia 1 (Mcl-1), X-linked inhibitor of apoptosis (XIAP), and Bcl-2-associated athanogene 1 (BAG-1).14 However, the molecular basis for the observed down-regulation by flavopiridol remains to be determined.
It is now known that flavopiridol decreases transcription by inhibiting CDK917-19 and CDK7,20 which are responsible for the phosphorylation of the C-terminal domain (CTD) of the largest subunit of RNA polymerase II, an activity essential for both transcriptional initiation and elongation.21,22 The human RNA polymerase II CTD contains 52 tandem repeats of the consensus heptapeptide sequence N-Tyr1-Ser2-Pro3-Thr4-Ser5-Pro6-Ser7-C. CDK9/cyclin T (positive transcription elongation factor b, P-TEFb) preferentially phosphorylates the Ser2 sites of this sequence to promote transcriptional elongation; CDK7/cyclin H, in the complex of transcription factor (TFIIH), preferentially phosphorylates Ser5, which facilitates promoter clearance and transcriptional initiation.21,22 Compared with 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (IC50 ∼ 1-3 μM)23 and other compounds, flavopiridol is the most potent CDK9 inhibitor known, with an inhibition constant (Ki) of 3 nM;17 CDK7 activity is inhibited by flavopiridol with an IC50 of 110 to 300 nM.20 These findings give rise to the possibility that transcriptional inhibition by flavopiridol may contribute to the mechanism of its cytotoxicity to CLL cells.
We hypothesized that the most sensitive therapeutic targets of transcriptional inhibitors, likely proteins that sustain the malignant phenotype, exhibit short half-lives in their mRNA and protein. Since the transcripts of Bcl-2, Mcl-1, and XIAP have rapid turnover rates,19,24-26 we postulated that flavopiridol might be selectively toxic to CLL by transcriptional suppression of such proteins. Our results demonstrated that flavopiridol inhibited the phosphorylation of the CTD of RNA polymerase II and decreased transcription in primary CLL cells. This was associated with a reduction in the transcripts of antiapoptotic genes and the consequent decrease in their protein products. As the survival of CLL cells is largely dependent upon the continuous expression of antiapoptotic proteins, their reduction was implicated as the mechanism of flavopiridol-induced apoptosis of CLL cells.
Patients, materials, and methods
Patients
Samples from 13 patients with CLL were used in this study. Their average white blood cell count was 95.8 × 109/L (range, 37.9-221 × 109/L) with an average lymphocyte percentage of 89% (range, 81%-97%). Complete studies were performed on the cells of 9 patients. Of these, 5 were untreated, 1 had received chlorambucil, and 2 of 3 fludarabine-treated patients, both of whom were diploid by conventional cytogenetic analysis, were resistant to therapy (Patients 2 and 8). In the 5 untreated patients, 2 were Rai stage I, and 3 were at Rai stage IV of the disease. The fludarabine-treated patients were at Rai stages I, II and IV, respectively; the chlorambucil-treated patient was at Rai stage I disease. This investigation was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board, and all patients agreed to participate and signed the informed consents for use of their cells for in vitro studies.
Isolation of CLL lymphocytes
Peripheral blood (10 mL) from the patients with CLL was collected in heparin vacutainer tubes and centrifuged at 430g (1500 rpm) for 15 minutes to separate the patient plasma. The plasma (upper layer) was removed and saved for cell culture. The lower layer was diluted with phosphate-buffered saline (PBS), and the mononuclear cells were isolated by Ficoll density-gradient centrifugation. The isolated CLL cells were cultured at 1 × 107 cells/mL in RPMI 1640 medium containing 10% of autologous plasma or autologous plasma supplemented with human blood type AB serum (Sigma-Aldrich, St Louis, MO) when the patient plasma was not adequate.
Materials
Flavopiridol was obtained from the Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute (Bethesda, MD). It was dissolved in dimethylsulfoxide (DMSO) at 10 mM, and stored at -70°C in small aliquots. [3H]uridine 1.85 TBq/mmol was purchased from Moravek Biochemical (Brea, CA).
Measuring the RNA synthesis
RNA synthesis was measured by quantitating incorporation of [3H]uridine into the perchloric acid-insoluble materials. Briefly, after incubation with flavopiridol, CLL cells were labeled for 30 minutes with [3H]uridine (0.37 MBq/mL). The cells were then washed twice with 10 mL of ice-cold PBS and then lysed with 1 mL 0.4 N perchloric acid. Following centrifugation, the pellet was washed once with 1 mL 0.4 N perchloric acid, dissolved in 1 mL H2O with 50 μL of 10 N KOH overnight. The liquids were then transferred to scintillation vials to count radioactivity.
Immunoblotting
CLL cells were lysed in buffer containing 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.5), 300 mM NaCl, 1.5 mM MgCl2, 0.5% sodium deoxycholate, 20 mM β-glycerophosphate, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 0.2 mM EDTA (ethylenediaminetetraacetic acid; pH 8), 0.5 mM dithriothritol (DTT), 1 mM sodium orthovanadate (pH 10), 1 mM phenylmethylsulfonyl fluoride, 20 μg/mL aprotinin, and 20 μg/mL leupeptin. Protein content in the lysate was determined using the Bio-Rad DC Protein Assay kit according to the manufacturer's instructions (Bio-Rad, Hercules, CA). Cell-lysate proteins (20 μg) were separated by SDS-polyacrylamide gel electrophoresis and then electrotransferred to a nitrocellulose membrane (GE Osmonics Labstore, Minnetonka, MN). The membranes were blocked for 1 hour in PBS containing 5% nonfat dried milk and then incubated with primary antibodies for 3 hours, followed by incubation with secondary antibodies conjugated to horseradish peroxidase for 1 hour. The blots were visualized by enhanced chemiluminescence according to the manufacturer's instructions (Pierce, Rockford, IL), exposed to X-ray films (Amersham Biosciences, Piscataway, NJ), and quantified by densitometry (Molecular Dynamics, Sunnyvale, CA). Multiple-film exposure was used to ensure that the quantitation was made within the linear range of each protein. The antibodies to Mcl-1 (S-19) and Bcl-2 (100) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies to poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) and BAG-1 were from BD Biosciences Pharmingen (San Diego, CA). Antibody to XIAP was purchased from Cell Signaling Technology (Beverly, MA). Antibodies for total RNA polymerase II (8WG16), phosphorylated CTD at Ser2 (H5) or Ser5 (H14) were purchased from Covance Research Products (Berkeley, CA). Anti-mouse immunoglobulin G (IgG) and anti-rabbit IgG horseradish peroxidase-conjugated antibody were obtained from Amersham Biosciences.
RNA isolation and real-time quantitative polymerase chain reaction
Total cellular RNA was isolated from the primary CLL cells by either extraction with RNAzol B (Tel-test, Friendswood, TX) or using the RNeasy mini kit (Qiagen, Valencia, CA) with DNase digestion to completely remove the genomic DNA. Total RNA (20-50 ng) was used for the 1-step real-time reverse transcription polymerase chain reaction (RT-PCR) reaction in the TaqMan One-Step RT-PCR Master Mix (Applied Biosystems, Foster City, CA). Each PCR reaction was carried out in a 25 μL volume on 96-well optic reaction plate for 30 minutes at 48°C for reverse transcription reaction, followed by 10 minutes at 95°C for initial denaturing, then followed by 40 cycles of 95°C for 15 seconds and 60°C for 2 minutes in the 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The relative gene expression was analyzed by the Comparative Ct method using 18s ribosomal RNA as endogenous control, after confirming that the efficiencies of the target and the endogenous control amplifications were approximately equal. The results were presented as the percentage of gene expression of the time matched controls incubated with dimethyl sulfoxide (DMSO). All the primers and probes and RT-PCR reaction buffers were purchased from Applied Biosystems. The Bcl-2 and 18s primers and probes were purchased from Applied Biosystems' TaqMan Pre-Developed Assay Reagents; the primers and probes for Mcl-1, BAG-1, and XIAP were purchased through Applied Biosystems' TaqMan Assays-on-Demand program.
Statistical analysis
The correlation studies were carried out by the Pearson test using the GraphPad Prism software (GraphPad Software, San Diego, CA). A P value less than .05 indicated that the correlation was significant.
Flavopiridol inhibited RNA synthesis in CLL cells. (A) The impact on the potency of flavopiridol was compared between 10% autologous patient plasma and fetal bovine serum in the culture medium. [3H]uridine incorporation was measured in the presence of 1 μM (♦) and 3 μM (•) flavopiridol in media with autologous plasma, or in the presence of 1 μM flavopiridol in fetal bovine serum (▪). Data are presented as percentages of time-matched controls (mean ± SE; n=4 patients each performed in triplicate). The disintegrations per minute (DPM) values for untreated samples in plasma or fetal bovine serum averaged 70 057 and 39 159, respectively. (B) Time and concentration dependence of inhibition on [3H]uridine incorporation by flavopiridol. Data are presented as percentages of time-matched controls (mean ± SE, triplicate samples) of results from CLL cells from 11 patients incubated with 0.1 (▪), 0.3 (▾), 1 (♦), and 3 μM (•) flavopiridol.
Flavopiridol inhibited RNA synthesis in CLL cells. (A) The impact on the potency of flavopiridol was compared between 10% autologous patient plasma and fetal bovine serum in the culture medium. [3H]uridine incorporation was measured in the presence of 1 μM (♦) and 3 μM (•) flavopiridol in media with autologous plasma, or in the presence of 1 μM flavopiridol in fetal bovine serum (▪). Data are presented as percentages of time-matched controls (mean ± SE; n=4 patients each performed in triplicate). The disintegrations per minute (DPM) values for untreated samples in plasma or fetal bovine serum averaged 70 057 and 39 159, respectively. (B) Time and concentration dependence of inhibition on [3H]uridine incorporation by flavopiridol. Data are presented as percentages of time-matched controls (mean ± SE, triplicate samples) of results from CLL cells from 11 patients incubated with 0.1 (▪), 0.3 (▾), 1 (♦), and 3 μM (•) flavopiridol.
Results
Flavopiridol inhibits RNA synthesis in CLL cells
It was previously reported that there is a high degree of protein binding of flavopiridol in human plasma.27 A higher flavopiridol concentration was necessary to cause cell death when CLL cells were incubated with human plasma versus fetal bovine serum. To verify if a similar effect would occur in the inhibition of RNA synthesis, we compared the RNA synthesis by CLL cells incubated with flavopiridol in medium supplemented with either 10% autologous human plasma or 10% fetal bovine serum. As indicated by [3H]uridine incorporation, RNA synthesis in CLL cells was inhibited 50% by 1 μM flavopiridol in fetal bovine serum after 4 hours (Figure 1A). RNA synthesis decreased to less than 25% of controls at 24 hours and 48 hours. However, in medium containing 10% autologous human plasma, 3 μM flavopiridol was required for similar inhibition during the time course; 1 μM flavopiridol was only half as inhibitory. These data are consistent with a higher affinity for flavopiridol to human plasma proteins compared with fetal bovine serum that is used in most cell cultures. Therefore, 10% autologous human plasma was used in the experiments reported in this study. In the single instance when autologous plasma was not adequate, human AB type serum supplemented the medium.
Incubation of the CLL cells with flavopiridol resulted in a time- and concentration-dependent decrease of RNA synthesis in every patient sample (Figure 1B). The [3H]uridine incorporation was inhibited by 50% at 3 μM flavopiridol after 3.3 hours of incubation. A concentration dependence was also observed; at 24 hours, 1 μM flavopiridol inhibited RNA synthesis by 45%, whereas 3 μM flavopiridol inhibited 77%. The IC50 was 1.3 μM after 24 hours of incubation.
Flavopiridol inhibits the phosphorylation of the CTD of RNA polymerase II
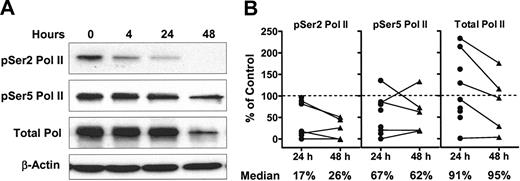
Phosphorylation of the CTD of RNA polymerase II by CDK7/cyclin H and CDK9/cyclin T is essential for both transcriptional initiation and elongation, respectively.21,22 As flavopiridol inhibits both CDK7 and CDK9 in cell-free systems,17,20 its effects on the phosphorylation status of RNA polymerase II CTD at both Ser2 and Ser5 sites were investigated. Ser2 phosphorylation was greatly diminished within 4 hours; phosphorylation of the Ser5 site was also decreased, albeit to a lesser extent (Figure 2A). Total protein level of RNA polymerase II was reduced at 24 hours as well as at 48 hours, as revealed by multiple film exposures (data not shown). The data of CLL cells from 9 patients are summarized in Figure 2B. After a 24-hour incubation with 3 μM flavopiridol, the median phosphorylation level of Ser2 was 17% (range, 0%-96%) of controls incubated with DMSO. The phosphorylation of Ser5 was 67% of controls (range, 0%-135%). There was no further change in the median phosphorylation level of either site by 48 hours. The median level of total RNA polymerase II remained unchanged (median, 91%; range, 1%-233% at 24 hours), although this decreased between 24 hours and 48 hours in 4 of the 5 longitudinal samples. Further, inhibition of RNA synthesis measured by [3H]uridine incorporation correlated significantly with the decrease in the phosphorylation of both Ser2 (n = 23, P = .002, r = 0.62) and Ser5 (n = 23, P = .029, r = 0.46). These relationships were even stronger when the samples incubated with lesser concentrations of flavopiridol (0.3 μM and 1 μM) were included in the analysis (n = 50; P < .001, r = 0.54 for Ser2 and P = .01, r = 0.35 for Ser5, respectively). These data indicated that inhibition of phosphorylation on both the Ser2 and Ser5 of the CTD contributed to the observed inhibition of RNA synthesis.
Flavopiridol inhibits the phosphorylation of RNA polymerase II CTD. CLL lymphocytes were incubated with 3 μM flavopiridol for 24 or 48 hours. The phosphorylation of RNA polymerase II was analyzed by immunoblotting, using antibodies towards the phosphorylated Ser2 or Ser5 sites of the CTD, as well as total RNA polymerase II. (A) Representative blot from patient no. 7. (B) Action of flavopiridol on the phosphorylation status of RNA polymerase II. The immunoblots were quantified by densitometry. Levels of phosphorylation were normalized to the loading control β-actin, and then expressed as percentage of controls incubated with DMSO. Five CLL samples were incubated with 3 μM flavopiridol for 48 hours as well, and 4 samples incubated with flavopiridol for 24 hours only are shown. • indicates 24 hours; ▴, 48 hours.
Flavopiridol inhibits the phosphorylation of RNA polymerase II CTD. CLL lymphocytes were incubated with 3 μM flavopiridol for 24 or 48 hours. The phosphorylation of RNA polymerase II was analyzed by immunoblotting, using antibodies towards the phosphorylated Ser2 or Ser5 sites of the CTD, as well as total RNA polymerase II. (A) Representative blot from patient no. 7. (B) Action of flavopiridol on the phosphorylation status of RNA polymerase II. The immunoblots were quantified by densitometry. Levels of phosphorylation were normalized to the loading control β-actin, and then expressed as percentage of controls incubated with DMSO. Five CLL samples were incubated with 3 μM flavopiridol for 48 hours as well, and 4 samples incubated with flavopiridol for 24 hours only are shown. • indicates 24 hours; ▴, 48 hours.
Flavopiridol down-regulates the mRNA and protein levels of antiapoptotic proteins and induces apoptosis
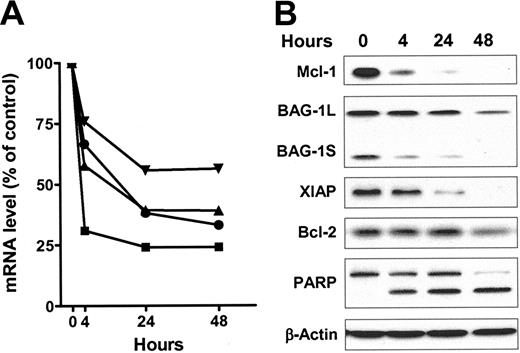
We investigated the action of flavopiridol on the levels of transcripts and proteins of antiapoptotic genes to examine the hypothesis that inhibition of transcription will selectively reduce the concentrations of those mRNAs and proteins with rapid turnover rates. Incubation with 3 μM flavopiridol substantially decreased the mRNA of Mcl-1, XIAP, and BAG-1 by between 25% and 50% after 4 hours (Figure 3A). Bcl-2 mRNA was most affected, decreasing by 70% within this time. There was a lesser rate of decrease of all transcripts over the next 20 hours, and little change thereafter. The proteins of Mcl-1, BAG-1L (long), BAG-1S (short), and XIAP decreased significantly within 4 hours of incubation, and continued to decrease by 24 and 48 hours (Figure 3B). Bcl-2 protein was stable until 48 hours, although its mRNA was largely reduced by the same treatment. The signal for another BAG-1 mRNA-encoded protein, BAG-1M (medium), was weak and therefore was not quantified. Apoptosis was induced as early as 4 hours, as indicated by the cleavage of PARP protein.
Flavopiridol reduced the mRNA and protein levels of antiapoptotic proteins. The total RNA and protein of CLL cells from patient no. 7 were isolated after 4, 24, and 48 hours of incubation with or without 3 μM of flavopiridol. (A) The mRNA levels of Mcl-1 (▴), Bcl-2 (▪), BAG-1 (▾), and XIAP (•) were measured by real-time RT-PCR, each performed in duplicate. After normalizing to 18s ribosomal RNA, the relative level expressed as percentage of time-matched controls incubated in DMSO were calculated for each mRNA. (B) Immunoblots of Mcl-1, BAG-1, XIAP, and Bcl-2 and PARP from the same samples described in panel A.
Flavopiridol reduced the mRNA and protein levels of antiapoptotic proteins. The total RNA and protein of CLL cells from patient no. 7 were isolated after 4, 24, and 48 hours of incubation with or without 3 μM of flavopiridol. (A) The mRNA levels of Mcl-1 (▴), Bcl-2 (▪), BAG-1 (▾), and XIAP (•) were measured by real-time RT-PCR, each performed in duplicate. After normalizing to 18s ribosomal RNA, the relative level expressed as percentage of time-matched controls incubated in DMSO were calculated for each mRNA. (B) Immunoblots of Mcl-1, BAG-1, XIAP, and Bcl-2 and PARP from the same samples described in panel A.
The median values of transcripts and proteins, expressed as percentages of time-matched controls, were compared with each patient sample to evaluate the overall effect of flavopiridol on antiapoptotic protein expression (Table 1). After 24 hours incubation with 3 μM flavopiridol there was a marked trend for the mRNA of Mcl-1 to decrease (median, 47% of control, n = 9) whereas its protein was reduced even more (median, 22% of control). This pattern appeared consistent among the samples, and was also evident in samples from the same patients after 48 hours incubation, where the Mcl-1 protein continued to decrease to a median of less than 5% of controls. Bcl-2 mRNA was also consistently reduced in all samples at 24 hours, but, in contrast to Mcl-1, the Bcl-2 protein remained unchanged. This is consistent with the reported short half-life of Bcl-2 mRNA (2.5-4 hours) and longer half-life of Bcl-2 protein (10-24 hours).28,29 Although heterogeneity exists among patients, the mRNA and protein of XIAP and BAG-1 decreased within the first 24 hours in the majority of the samples. The reported half-lives of XIAP are 3 to 5 hours for mRNA26 and about 6.5 hours30 for its protein. The mRNA half-life for BAG-1 is 8 to 10 hours.19 The median levels of XIAP mRNA of were 58% and 43% of controls after 24 and 48 hours incubation with flavopiridol, and its protein levels were 30% and 11%. At 24 hours, the mRNA of BAG-1 was 51% of controls; the proteins of BAG-1L and BAG-1S were 68% and 58%, respectively.
Effect of flavopiridol on the expression of apoptotic genes 24 and 48 hours after incubation of CLL cells with 3 μM flavopiridol
. | Effect of flavopiridol on the expression of apoptotic genes, % of control . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Mcl-1 . | . | Bcl-2 . | . | XIAP . | . | BAG-1 . | . | . | ||||||||
| Pt no. by h . | mRNA . | Protein . | mRNA . | Protein . | mRNA . | Protein . | mRNA . | pBAG-1L . | pBAG-1S . | ||||||||
| 24 h | |||||||||||||||||
| 1 | 44 | 2 | 26 | 36 | 104 | 4 | 64 | 74 | 58 | ||||||||
| 2* | 62 | 33 | 54 | 82 | 216 | 59 | 101 | 50 | 8 | ||||||||
| 3 | 66 | 3 | 25 | 75 | 56 | 30 | 81 | 38 | 25 | ||||||||
| 4 | 53 | 14 | 58 | 104 | 93 | 123 | 70 | 29 | 21 | ||||||||
| 5 | 45 | 22 | 41 | 100 | 58 | 18 | 68 | 72 | 59 | ||||||||
| 6 | 53 | 43 | 68 | 116 | 91 | 25 | 66 | 152 | 124 | ||||||||
| 7 | 39 | 0 | 24 | 98 | 38 | 17 | 56 | 51 | 0 | ||||||||
| 8* | 10 | 22 | 14 | 138 | 17 | 63 | 57 | 17 | 95 | ||||||||
| 9 | 47 | 24 | 47 | 100 | 52 | 69 | 131 | 102 | 82 | ||||||||
| Median | 47 | 22 | 41 | 100 | 58 | 30 | 68 | 51 | 58 | ||||||||
| 48 h | |||||||||||||||||
| 3 | 63 | 0 | 30 | 87 | 47 | 54 | 101 | 57 | 29 | ||||||||
| 5 | 27 | 11 | 17 | 100 | 21 | 11 | 39 | 86 | 44 | ||||||||
| 6 | 92 | 2 | 183 | 145 | 132 | 0 | 197 | 130 | 78 | ||||||||
| 7 | 39 | 0 | 24 | 76 | 33 | 5 | 56 | 17 | 0 | ||||||||
| 9 | 81 | 17 | 82 | 98 | 43 | 46 | 159 | 77 | 49 | ||||||||
| Median | 63 | 2 | 30 | 98 | 43 | 11 | 101 | 77 | 44 | ||||||||
. | Effect of flavopiridol on the expression of apoptotic genes, % of control . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Mcl-1 . | . | Bcl-2 . | . | XIAP . | . | BAG-1 . | . | . | ||||||||
| Pt no. by h . | mRNA . | Protein . | mRNA . | Protein . | mRNA . | Protein . | mRNA . | pBAG-1L . | pBAG-1S . | ||||||||
| 24 h | |||||||||||||||||
| 1 | 44 | 2 | 26 | 36 | 104 | 4 | 64 | 74 | 58 | ||||||||
| 2* | 62 | 33 | 54 | 82 | 216 | 59 | 101 | 50 | 8 | ||||||||
| 3 | 66 | 3 | 25 | 75 | 56 | 30 | 81 | 38 | 25 | ||||||||
| 4 | 53 | 14 | 58 | 104 | 93 | 123 | 70 | 29 | 21 | ||||||||
| 5 | 45 | 22 | 41 | 100 | 58 | 18 | 68 | 72 | 59 | ||||||||
| 6 | 53 | 43 | 68 | 116 | 91 | 25 | 66 | 152 | 124 | ||||||||
| 7 | 39 | 0 | 24 | 98 | 38 | 17 | 56 | 51 | 0 | ||||||||
| 8* | 10 | 22 | 14 | 138 | 17 | 63 | 57 | 17 | 95 | ||||||||
| 9 | 47 | 24 | 47 | 100 | 52 | 69 | 131 | 102 | 82 | ||||||||
| Median | 47 | 22 | 41 | 100 | 58 | 30 | 68 | 51 | 58 | ||||||||
| 48 h | |||||||||||||||||
| 3 | 63 | 0 | 30 | 87 | 47 | 54 | 101 | 57 | 29 | ||||||||
| 5 | 27 | 11 | 17 | 100 | 21 | 11 | 39 | 86 | 44 | ||||||||
| 6 | 92 | 2 | 183 | 145 | 132 | 0 | 197 | 130 | 78 | ||||||||
| 7 | 39 | 0 | 24 | 76 | 33 | 5 | 56 | 17 | 0 | ||||||||
| 9 | 81 | 17 | 82 | 98 | 43 | 46 | 159 | 77 | 49 | ||||||||
| Median | 63 | 2 | 30 | 98 | 43 | 11 | 101 | 77 | 44 | ||||||||
Patients were refractory to fludarabine.
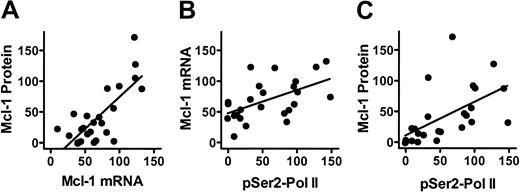
The consistency of patterns among individuals suggests that the effect of transcriptional inhibition by flavopiridol is governed by the intrinsic stability of the individual transcripts and proteins. Comparisons of the changes in Mcl-1 mRNA and protein levels in CLL cells during a 48-hour incubation with 3 μM flavopiridol demonstrated a significant correlation between these parameters, indicating that the decrease in Mcl-1 protein was related to the decrease of its mRNA (Figure 4A). Further, both the mRNA and protein of Mcl-1 were significantly associated with the reduced phosphorylation of the RNA polymerase II CTD at Ser2 (Figure 4B-C, respectively). In addition, the mRNA level of XIAP was associated with the phosphorylation of Ser2 (n = 25, P = .023, r = 0.45). The mRNA level of Mcl-1 was also correlated with the phosphorylation status of CTD at Ser5 (n = 25, P = .006, r = 0.54). These relationships were also observed when samples incubated with 0.3 or 1 μM of flavopiridol were included in the analysis (data not shown). Thus, these results suggest that flavopiridol inhibited the phosphorylation of the RNA polymerase II CTD, an action that reduced Mcl-1 and XIAP transcripts and diminished their proteins.
PARP cleavage is concentration and time dependent
Clinical trials of flavopiridol in CLL suggested that a micromolar concentration of flavopiridol in plasma is associated with achieving a clinical response.16,31 Therefore, we compared 1 μM and 3 μM flavopiridol on the time of induction of apoptosis, indicated by PARP cleavage (the appearance of the 84-kDa cleaved product, or the decrease of the 116-kDa full-length PARP protein compared with time-matched controls on immunoblots). In 8 of 9 CLL samples examined, PARP cleavage occurred within a 4-hour incubation with 3 μM flavopiridol (Table 2). In contrast, 1 μM flavopiridol induced PARP cleavage by 4 hours in only 1 of 9 samples. In fact, no PARP cleavage was detected until 24 hours in 4 samples, and was not detected in 4 other samples within the duration of experiments (1 24 hours, and 2 48 hours). These data, which demonstrate the concentration and time dependency of apoptosis induction by flavopiridol in CLL lymphocytes, suggest the need to achieve more than 1 μM flavopiridol in plasma during clinical administration to induce apoptosis during short infusions of the drug.
Comparison of 1 μM and 3 μM flavopiridol on the time of initiation of PARP cleavage in CLL cells
. | Time of initiation of PARP cleavage, h . | . | |
|---|---|---|---|
| Pt no. . | 1 μM . | 3 μM . | |
| 1 | 4 | 2 | |
| 2 | >24 | 4 | |
| 3 | 24 | 2 | |
| 4 | 24 | 24 | |
| 5 | 24 | 4 | |
| 6 | 48 | 4 | |
| 7 | >48 | 4 | |
| 8 | 24 | 4 | |
| 9 | >48 | 4 | |
. | Time of initiation of PARP cleavage, h . | . | |
|---|---|---|---|
| Pt no. . | 1 μM . | 3 μM . | |
| 1 | 4 | 2 | |
| 2 | >24 | 4 | |
| 3 | 24 | 2 | |
| 4 | 24 | 24 | |
| 5 | 24 | 4 | |
| 6 | 48 | 4 | |
| 7 | >48 | 4 | |
| 8 | 24 | 4 | |
| 9 | >48 | 4 | |
Relationship between Mcl-1 transcripts, proteins, and RNA polymerase II phosphorylation status. (A) Mcl-1 protein levels were related to Mcl-1 mRNA in CLL cells after incubation with 3 μM flavopiridol for 2, 4, 24, and 48 hours (n = 25, P < .001, r = 0.75). The mRNA and protein levels were expressed as percentages of controls incubated with DMSO. (B) Mcl-1 mRNA levels were related to the phosphorylation of RNA polymerase II CTD at Ser2 (pSer2-Pol II) in CLL cells after incubation with 3 μM flavopiridol for 2, 4, 24, and 48 hours (n = 25, P = .006, r = 0.54). (C) Mcl-1 protein levels were related to the phosphorylation of RNA polymerase II CTD at Ser2 (pSer2-Pol II) in CLL cells after incubation with 3 μM flavopiridol for 2, 4, 24, and 48 hours (n = 25, P = .004, r = 0.56). Solid line indicates linear regression of data points (•).
Relationship between Mcl-1 transcripts, proteins, and RNA polymerase II phosphorylation status. (A) Mcl-1 protein levels were related to Mcl-1 mRNA in CLL cells after incubation with 3 μM flavopiridol for 2, 4, 24, and 48 hours (n = 25, P < .001, r = 0.75). The mRNA and protein levels were expressed as percentages of controls incubated with DMSO. (B) Mcl-1 mRNA levels were related to the phosphorylation of RNA polymerase II CTD at Ser2 (pSer2-Pol II) in CLL cells after incubation with 3 μM flavopiridol for 2, 4, 24, and 48 hours (n = 25, P = .006, r = 0.54). (C) Mcl-1 protein levels were related to the phosphorylation of RNA polymerase II CTD at Ser2 (pSer2-Pol II) in CLL cells after incubation with 3 μM flavopiridol for 2, 4, 24, and 48 hours (n = 25, P = .004, r = 0.56). Solid line indicates linear regression of data points (•).
Discussion
Recent studies have established that P-TEFb (CDK9/cyclin T) phosphorylates the Ser2 of the heptapeptide repeats of the CTD of RNA polymerase II, inactivates transcriptional suppressors, and facilitates transcriptional elongation.22,32 As flavopiridol is the most potent CDK9 inhibitor known,17 this study investigated the hypothesis that transcriptional inhibition may be a component of the mechanism of flavopiridol-induced apoptosis in CLL cells. The present results demonstrated that flavopiridol inhibited the phosphorylation of the CTD of RNA polymerase II and decreased transcription in primary CLL cells. This was associated with a reduction of the transcripts of genes with antiapoptotic functions, and with the consequent decline of cellular levels of their proteins. As the survival of CLL cells is dependent upon the continuous expression of antiapoptotic proteins, their reduction by flavopiridol is the most likely cause of apoptosis. The quiescent nature of the peripheral blood CLL cells has served as an excellent model for studying the action of flavopiridol on transcription, as these cells do not require the cell-cycle—specific kinases. A recent study indicated that a small fraction of CLL cells in the lymphoid tissue proliferate at an appreciable rate.33 Those cells could also be targeted by flavopiridol through the inhibition of the CDKs that regulate cell-cycle progression.
In addition to inhibition of CDK9, flavopiridol also inhibits CDK7, albeit with less potency at an IC50 of 100 to 300 nM.20 CDK7 has dual functions; it phosphorylates CDK1, CDK2, CDK4, CDK5, and CDK6, and activates these kinases. This CDK activation activity is essential for cell-cycle progression.34 CDK7 also plays a central role in the regulation of mRNA synthesis by RNA polymerase II. CDK7 is part of the general transcription factor TFIIH, and in this complex it preferentially phosphorylates Ser5 of the heptapeptide repeats in CTD, which facilitates promoter clearance and initiation of transcription.34,35 In CLL samples, flavopiridol inhibited phosphorylation of Ser5 of the CTD of RNA polymerase II, although to a lesser extent compared with the inhibition of Ser2 phosphorylation (median is 67% of control for Ser5 compared with 17% of control for Ser2 after 24 hours incubation with 3 μM flavopiridol) (Table 1). Therefore, inhibition on CDK7 activity may also contribute, in part, to the transcriptional suppression by flavopiridol. In this regard, future studies may use siRNA or antisense to CDK9 or CDK7 to discriminate the multiple targets of flavopiridol in the cells.
A similar action of flavopiridol was reported in multiple myeloma cells, in which Mcl-1 also plays an important role in survival.36 The proapoptotic activity of flavopiridol was associated with transcriptional suppression and rapid turnover of Mcl-1 mRNA and protein. R-roscovitine (CYC202), another CDK inhibitor with activity against P-TEFb, also induced apoptosis in CLL cells independent of p53 gene status.37,38 R-roscovitine—induced apoptosis was preceded by the inhibition of RNA polymerase II phosphorylation, which was associated with the decrease of prosurvival proteins, most distinctively Mcl-1.38 While flavopiridol and R-roscovitine block transcription by inhibiting the CDKs, the adenosine analog, 8-chloro-adenosine (8-Cl-Ado), prematurely terminates transcription after being incorporated into mRNA.39 Despite this different mechanism of inhibiting RNA synthesis, incubation of CLL cells with 8-Cl-Ado resulted in a significant decline in the transcript and protein of Mcl-1, and the induction of apoptosis.40 Mcl-1 levels also decreased in CLL cells during exposure to fludarabine,4 which also prematurely terminates transcription.41
Consistent among all the samples, incubation with flavopiridol rapidly decreased the transcripts of both Mcl-1 and Bcl-2; however, the Mcl-1 protein declined rapidly whereas the Bcl-2 protein decreased slowly. Similarly, Mcl-1 and XIAP proteins were decreased in CLL cells after incubation with R-roscovitine, but the expression of Bcl-2 remained unchanged, although its mRNA was also reduced.38 This pattern suggested that the outcome of transcriptional inhibition by flavopiridol is governed by the intrinsic stability of the individual transcripts and proteins. Some specific motifs in the mRNA or protein sequences are signals for rapid degradation. The adenylate/uridylate-rich elements (AREs) in the 3′ untranslated region of many mRNAs target them for rapid and selective degradation.42,43 The ARE motifs in Mcl-1 and Bcl-2 transcripts may explain their short half-lives (∼1 hour for Mcl-1 and 2.5-4 hours for Bcl-2, respectively).19,24,25 In addition, the PEST regions, rich in proline (P), glutamic acid (E), serine (S), and threonine (T), target proteins for rapid destruction.44,45 It is likely that the 2 PEST regions in the primary protein structure of Mcl-1 directly contribute to its short half-life (t1/2 ∼0.5-1 hour).46 Bcl-2, which does not have a PEST region, is a long-lived protein, with a half-life of 10 to 24 hours.28,29 Induction of apoptosis by flavopiridol in the absence of changes in Bcl-2 protein levels suggests that Bcl-2 is not the dominant antiapoptotic protein in CLL. Although XIAP has a short turnover rate and both XIAP and BAG-1 transcripts decreased in the majority of the samples, no ARE motifs were found in their sequence according to the ARE database.47 Thus, some other mechanism may mediate their degradation. As CLL cells and other tumors appear to be dependent on Mcl-1 and XIAP for survival,48,49 they are excellent targets of flavopiridol for the induction of apoptosis. Once the apoptotic cascade is initiated, the cleavage of Mcl-1 and XIAP proteins by caspases likely serves to accelerate these processes.50-52
A substantial body of evidence now supports the oncogenic nature of the Mcl-1 protein, as it appears critical for the survival of CLL cells as well as other human tumors.48 In addition, small molecular inhibitors to XIAP facilitated caspase activities and induced apoptosis in tumor cell lines and CLL cells.49 Tumor cells that are dependent upon single proteins for their malignant phenotype and survival have been characterized as being “addicted” to the activity of the oncogene.53,54 This has provided a rational basis for the development of therapeutics that are directed at inhibiting the activity of the particular oncogene products. In such an approach, the biologic context of the dependency of the tumor on oncogene function provides a biologic basis for the therapeutic index. The present study demonstrates that this same goal may be achieved by a more general approach that takes advantage of the intrinsic instability of the oncoproteins. This action of flavopiridol is currently under clinical evaluation in patients with CLL.55
Flavopiridol was originally administered to patients in clinical trials by prolonged continuous infusions (24-72 hours), based on preclinical studies that suggested improved efficacy with longer drug exposure.56,57 A plasma concentration of 300 to 400 nM was achieved,27,57 which is sufficient to inhibit the CDKs in vitro. However, despite encouraging preclinical results, only marginal activity was demonstrated by this schedule.31,58 Pharmacokinetic factors, such as binding of the drug to plasma proteins,27 may have limited its activity. Later, it was discovered that shorter infusions, which could achieve micromolar plasma concentrations, are likely to be more effective.59 Indeed, the phase 1 clinical trial in CLL using a 30-minute intravenous loading bolus followed by a 4-hour infusion, which maintained the plasma flavopiridol concentration in the micromolar range for at least 4 hours, demonstrated a 43% overall response rate.16,55 Consistent with that, our data showed that more than 1 μM flavopiridol is required to induce apoptosis within a short incubation period (2-4 hours) in vitro (Table 2). Thus, flavopiridol dosage regimens designed to achieve and maintain such plasma concentrations may optimize clinical responses.
Prepublished online as Blood First Edition Paper, June 21, 2005; DOI 10.1182/blood-2005-04-1678.
Supported by grants CA81534 and CA100632 from the National Cancer Institute, Department of Health and Human Services and a Fellowship from the MD Anderson/Aventis-Translational Research Program (R.C.)
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are thankful to Min Du, Department of Experimental Therapeutics, and Yelena V. Krupnik and Susan Lerner, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, Houston, Texas, for collecting the CLL patient samples and providing the patient information.

![Figure 1. Flavopiridol inhibited RNA synthesis in CLL cells. (A) The impact on the potency of flavopiridol was compared between 10% autologous patient plasma and fetal bovine serum in the culture medium. [3H]uridine incorporation was measured in the presence of 1 μM (♦) and 3 μM (•) flavopiridol in media with autologous plasma, or in the presence of 1 μM flavopiridol in fetal bovine serum (▪). Data are presented as percentages of time-matched controls (mean ± SE; n=4 patients each performed in triplicate). The disintegrations per minute (DPM) values for untreated samples in plasma or fetal bovine serum averaged 70 057 and 39 159, respectively. (B) Time and concentration dependence of inhibition on [3H]uridine incorporation by flavopiridol. Data are presented as percentages of time-matched controls (mean ± SE, triplicate samples) of results from CLL cells from 11 patients incubated with 0.1 (▪), 0.3 (▾), 1 (♦), and 3 μM (•) flavopiridol.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/7/10.1182_blood-2005-04-1678/6/m_zh80190584920001.jpeg?Expires=1767709711&Signature=pRSMc038Hk8S1-dESiCQUQt2Xn38l10N7PhxWeBo9KgYaMriy6JsS0diNWx3aNGACcLtbMU6yQ4~tMrOfiVQH4A7Yf95mW3QcdKAtNt63Xq5vwe6ymL9daaoj6x6BvqiuhL62Syl8flsSSU-bE571b~tqz6Qqow~cg9ejL83cWdVYh5bC3~u7Nk5Kd2qblHx410cAvL2fhlen9SG58YWDDW156RRV8VKIm3KxWR92-w8EF0N9c4oUHG-XFEqhzFSkZ1TcuKTA3xKREep2VoC2zGUmaitLLjPAdlA5laIkXmutD8WvI1oIyLuC0r-EoYn2FceG0Q3SfXfdfHOaOxgrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal