Abstract
The association between tumor Epstein-Barr virus (EBV) status and clinical outcome in Hodgkin lymphoma (HL) is controversial. This population-based study assessed the impact of EBV status on survival in age-stratified cohorts of adults with classic HL (cHL). Data from 437 cases were analyzed with a median follow-up of 93 months. Overall survival (OS) was significantly better for EBV-negative compared with EBV-positive patients (P < .001), with 5-year survival rates of 81% and 66%, respectively; disease-specific survival (DSS) was also greater for EBV-negative patients (P = .03). The impact of EBV status varied with age at diagnosis. In patients aged 16 to 34 years, EBV-associated cases had a survival advantage compared with EBV-negative cases, but differences were not statistically significant (P = .21). Among patients 50 years or older, EBV positivity was associated with a significantly poorer outcome (P = .003). Excess deaths occurred in EBV-positive patients with both early- and advanced-stage disease. In multivariate analysis of OS in the older patients, EBV status retained statistical significance after adjusting for the effects of sex, stage, and B symptoms (P = .01). Impaired immune status may contribute to the development of EBV-positive cHL in older patients, and strategies aimed at boosting the immune response should be investigated in the treatment of these patients. (Blood. 2005;106:2444-2451)
Introduction
The Epstein-Barr virus (EBV) is consistently associated with a proportion of cases of Hodgkin lymphoma (HL) and this association is believed to be causal.1 In these cases, there is a clonal expansion of EBV-infected cells, and viral gene products are detectable in Hodgkin and Reed-Sternberg (HRS) cells, the tumor cells in HL. In western countries, approximately one third of adult HL cases are EBV associated, but this proportion varies in different geographic locales.1,2 In addition, EBV-association rates vary with age, sex, and histologic subtype.1-3
The impact of the tumor-cell EBV status on the prognosis of patients with HL remains controversial. Most previous studies have not reported a significant association between EBV status and outcome,4-11 while others have found that EBV-associated cases have either a more or less favorable prognosis12-18 (Table 1). Some of the interstudy variation may be attributable to the different epidemiologic features of the disease in different geographic settings and some may be related to case selection. Three previous population-based studies have demonstrated that EBV-associated cases have a poorer prognosis: one was relatively small,11 and although EBV-positive cases were found to have a poorer outcome than patients with EBV-negative disease, this difference did not reach statistical significance in this small study; one was confined to adults 60 years or older18 ; and the third was confined to women.17 In the latter cohort,17 the adverse effect of EBV association was confined to patients aged 45 to 79 years.
Summary of previous studies that have examined the effect of EBV status on clinical outcome in Hodgkin lymphoma
Reference . | Population-based . | No. of Patients . | Age range, y . | Median follow-up, mo . | NLPHL included . | %EBV-associated . | Prognostic significance of EBV-association on OS . |
|---|---|---|---|---|---|---|---|
| Armstrong et al5 | No | 59 | Not stated | Not stated | Yes | 36 | None |
| Axdorph et al6 | No | 95 | 14-77 | 145 | No | 33 | None |
| Enblad et al11 | Yes | 117 | 11-87 | 130 | Yes | 27 | None |
| Flavell et al8 | No | 273 | ≥15 | 60 | Yes | 29 | None |
| Murray et al7 | No | 190 | 22-49 | 86 | Yes | 27 | None |
| Vestlev et al4 | No | 66 | 9-78 | 22 | Yes | 41 | None |
| Krugmann et al9 | No | 119 | 14-83 | 122 | No | 26 | None |
| Herling et al10 | No | 303 | Adults | 65 | No | 21 | None |
| Vassalo et al15 | No | 78 | 15-75 | Not stated | No | 64 | Favorable (assessed by LMP1 expression) |
| Glavina-Durdov et al16 | No | 100 | 13-84 | 60 | Yes | 26 | None. Positive impact on DFS in those ≤30 y |
| Engel et al13 | No | 47 | ≤14 | Not stated | No | 68 | Favorable |
| Morente et al12 | No | 140 | 5-83 | >24 | Yes | 51 | Favorable |
| Naresh et al14 | No | 110 | 4-61 | 57 | No | 78 | Favorable |
| Stark et al18 | Yes | 102 | ≥60 | 62 | Yes | 34 | Unfavorable |
| Clarke et al17 | Yes | 311 | 19-79 | 73 | Yes | 17 | Unfavorable in those ≥45 y |
Reference . | Population-based . | No. of Patients . | Age range, y . | Median follow-up, mo . | NLPHL included . | %EBV-associated . | Prognostic significance of EBV-association on OS . |
|---|---|---|---|---|---|---|---|
| Armstrong et al5 | No | 59 | Not stated | Not stated | Yes | 36 | None |
| Axdorph et al6 | No | 95 | 14-77 | 145 | No | 33 | None |
| Enblad et al11 | Yes | 117 | 11-87 | 130 | Yes | 27 | None |
| Flavell et al8 | No | 273 | ≥15 | 60 | Yes | 29 | None |
| Murray et al7 | No | 190 | 22-49 | 86 | Yes | 27 | None |
| Vestlev et al4 | No | 66 | 9-78 | 22 | Yes | 41 | None |
| Krugmann et al9 | No | 119 | 14-83 | 122 | No | 26 | None |
| Herling et al10 | No | 303 | Adults | 65 | No | 21 | None |
| Vassalo et al15 | No | 78 | 15-75 | Not stated | No | 64 | Favorable (assessed by LMP1 expression) |
| Glavina-Durdov et al16 | No | 100 | 13-84 | 60 | Yes | 26 | None. Positive impact on DFS in those ≤30 y |
| Engel et al13 | No | 47 | ≤14 | Not stated | No | 68 | Favorable |
| Morente et al12 | No | 140 | 5-83 | >24 | Yes | 51 | Favorable |
| Naresh et al14 | No | 110 | 4-61 | 57 | No | 78 | Favorable |
| Stark et al18 | Yes | 102 | ≥60 | 62 | Yes | 34 | Unfavorable |
| Clarke et al17 | Yes | 311 | 19-79 | 73 | Yes | 17 | Unfavorable in those ≥45 y |
NLPHL indicates nodular lymphocyte predominant Hodgkin lymphoma; EBV, Epstein Barr virus; OS, overall survival; LMP1, Epstein-Barr virus latent membrane protein 1; and DFS, disease-free survival.
Although many characteristics of EBV-associated and nonassociated HL appear similar, the demographic features of these disease subgroups are different. We have proposed a 4-disease model of HL, which divides the disease into 4 subgroups on the basis of age, EBV association, and age of exposure to EBV.5 The model recognizes a single EBV-negative group of cases, which accounts for the young adult peak in disease incidence seen in developed countries, and 3 EBV-positive subgroups. The latter included a childhood group, accounting for almost all cases of HL in early childhood; a young adult group, which our data suggest is associated with delayed exposure to EBV19,20 ; and an older adult group, which we speculate results from loss of the normal balance between latent EBV infection and host immunity. The distinct epidemiologic features of EBV-associated and nonassociated HL in different age groups highlight the need to investigate these variables in any analysis of HL.
The Scotland and Newcastle Epidemiological study of Hodgkin Disease (SNEHD) is a case-control, population-based study that was established with the aim of identifying risk factor profiles for HL development, with stratification of cases by EBV status. The association between EBV positivity and age, sex, and histologic subtype in SNEHD has been described previously.21 Briefly, EBV-positive and -negative cases showed distinct age-specific incidence patterns, with EBV-negative cases predominating in the young-adult age group and EBV-positive cases accounting for the majority in older adult years. Consistent with many other studies, males were overrepresented among the EBV-associated patients, and cases of mixed cellularity HL (MCHL) were significantly more likely to be EBV associated than nodular sclerosis HL (NSHL) cases. A further analysis showed a specific association between EBV-associated HL in young adults and previous infectious mononucleosis (IM).20
The present study investigates the impact of EBV status on survival of patients included in SNEHD with particular emphasis on age-related differences. Age stratifications are those suggested by the “4-disease” model.2 Since patients 60 years or older are often unable to tolerate the more intensive 8 drug therapies that have led to improved survival in younger patients, this group was analyzed separately with regard to outcome.22
Patients, materials, and methods
SNEHD was designed as a case-control study; however only cases are included in the present study. Patients aged 16 to 74 years were eligible for entry into the SNEHD study if they were diagnosed with HL between January 1, 1993, and July 31, 1997; were normally resident in Scotland (excluding Dumfries, Galloway, and the Western Isles) or the former Northern Health Region of England; and were born in the United Kingdom. Patients were identified from the Scotland and Newcastle Lymphoma Group (SNLG) Registry and annual cross-checks were made with the relevant cancer registries.21 A study questionnaire was completed at face-to-face interview with subjects (or relatives of deceased patients) who gave informed consent. A history of shingles (varicella zoster virus reactivation) occurring more than 1 year prior to the diagnosis of HL was obtained from the questionnaire data. The Carstairs index of social deprivation23 was calculated using the address at diagnosis, which was obtained from questionnaires. Details of previous or concurrent diagnoses of cancer were obtained from questionnaires or from the SNLG database.
Clinical information, including stage at presentation, presence or absence of B symptoms, first-line treatment modality, and outcome data were collected from the SNLG database. Clinical stage was defined using the Ann Arbor staging system.24 Ann Arbor stages IA and IIA were defined as early-stage disease; all other cases were defined as advanced-stage disease for the purpose of this study. Choice of treatment was at the discretion of the supervising physician. Annual follow-up was performed and provided additional data including date last seen, details of subsequent cancers, and date and cause of death. Follow-up data regarding attainment of remission, subsequent cancers, date of death, and cause of death were obtained either by statements from the physicians in charge of treatment or by review of the medical records by the study team. The study protocol was approved by the local ethics review committee of each participating hospital; all patients in the study gave their informed consent for participation in accordance with the Declaration of Helsinki.
Pathology
The pathologic review process, including a description of the antibodies used in immunohistochemical studies, has been described previously.21 The procedure of histopathologic review was designed so that the reviewing histopathologists (A.S.K., B.A.) did not review cases in which they had made the original diagnosis. Cases were initially classified under the revised European-American lymphoma (REAL) classification,25 but the more recent World Health Organisation (WHO) nomenclature26 is used in the present report. Only patients confirmed to have HL are included in the present study. EBV status was determined using EBV-encoded RNA (EBER) in situ hybridization27 ; cases were designated as EBV associated or EBV positive if HRS cells were positive in this assay. Patients with nodular lymphocyte predominant HL (NLPHL), which is now believed to be a low-grade B-cell neoplasm,28 are excluded from this report, which is confined to patients with classic HL (cHL).
Overall survival (OS) was measured from the date of diagnosis until the date of death. For disease-specific survival (DSS), patients were censored at the time of death if this was from an HL-unrelated cause. Deaths from treatment-related causes were classified as death from HL. Follow-up was to September 2003 or the date that the patient was last known to be alive.
Statistical analysis
Data were analyzed using Prism 3.0 Software (GraphPad, San Diego, CA) or SPSS version 11.5 (Chicago, IL). Analyses of the effect of EBV status were performed for “all ages,” young adults (16-34 years), and older adults (≥ 50 years); selected analyses were performed on patients in the intermediate age group (35-49 years) and patients 60 years or older. The association between EBV status and clinical parameters was analyzed using logistic regression with results expressed as odds ratios (ORs) and 95% confidence intervals (CIs). In the latter analyses, stage was analyzed as a trend across stages and also as a categoric variable using stage III as the reference category. Actuarial survival curves were compiled using the Kaplan-Meier method29 and log-rank tests were used to compare curves. Cox regression was used for the multivariate analysis of outcome; analyses of all ages were adjusted for age group, and all analyses were adjusted for sex. In addition, all analyses were adjusted for the effect of either stage (as a linear trend across stages) and B symptoms or early/advanced disease. Results are expressed as risk ratios (RRs) and 95% CIs. Chi-squared analysis was used to test for associations between EBV status and previous shingles, prior cancer, and deprivation status. Deprivation status was analyzed as a trend across 5 deprivation categories (depcats), with depcat 1 including the most affluent cases (Carstairs categories 1 and 2) and depcat 5 the most deprived (Carstairs categories 6 and 7).
Results
Case selection
Of the 531 patients with confirmed cHL in the SNEHD study, tumor EBV status was available for 461 patients (87%), who were therefore eligible for this study (Table 2). The distribution of these cases with respect to age group, sex, and histologic subtype was not significantly different from that of the total SNEHD series. The requirement for histologic material suitable for evaluation of EBV status, however, resulted in slightly smaller proportions of older cases and cases with a diagnosis of HL not otherwise specified (Table 2). Clinical outcome data were available for 95% of cases eligible for the study. Depcat status and self-reported shingles occurring more than 1 year prior to diagnosis were available for 312 and 315 patients, respectively. Since these data were derived from questionnaires, which were completed at interview, they were less likely to be available from patients who died soon after diagnosis. As a result, OS was significantly poorer for patients from whom a history of shingles and/or depcat status was not available (P < .01). Since older patients had a poorer prognosis, they were also underrepresented in this group, but this effect was not statistically significant (Table 2).
Case selection
. | SNEHD no. . | Eligible for current study, no. (%*) . | Outcome data available, no. (%†) . | Depcat available, no. (%†) . |
|---|---|---|---|---|
| All cases | 531 | 461 (87*) | 437 (95†) | 312 (68†) |
| Age group | ||||
| 16 to 34 y | 259 | 236 (91) | 227 (96) | 171 (72) |
| 35 to 49 y | 106 | 88 (83) | 83 (94) | 59 (67) |
| 50 y or older | 166 | 137 (83) | 127 (93) | 82 (60) |
| Sex | ||||
| Male | 296 | 254 (86) | 240 (94) | 163 (64) |
| Female | 235 | 207 (88) | 197 (95) | 149 (72) |
| Histologic subtype | ||||
| MCHL | 125 | 105 (84) | 101 (96) | 68 (65) |
| LDHL | 5 | 3 (60) | 2 (67) | 2 (67) |
| NSHL | 358 | 320 (89) | 305 (95) | 227 (71) |
| LRCHL | 16 | 16 (100) | 15 (94) | 5 (31) |
| cHL NOS | 27 | 17 (63) | 14 (82) | 10 (59) |
| EBV status‡ | ||||
| EBV positive | NA | 154 | 145 (94) | 101 (66) |
| EBV negative | NA | 307 | 292 (95) | 211 (69) |
. | SNEHD no. . | Eligible for current study, no. (%*) . | Outcome data available, no. (%†) . | Depcat available, no. (%†) . |
|---|---|---|---|---|
| All cases | 531 | 461 (87*) | 437 (95†) | 312 (68†) |
| Age group | ||||
| 16 to 34 y | 259 | 236 (91) | 227 (96) | 171 (72) |
| 35 to 49 y | 106 | 88 (83) | 83 (94) | 59 (67) |
| 50 y or older | 166 | 137 (83) | 127 (93) | 82 (60) |
| Sex | ||||
| Male | 296 | 254 (86) | 240 (94) | 163 (64) |
| Female | 235 | 207 (88) | 197 (95) | 149 (72) |
| Histologic subtype | ||||
| MCHL | 125 | 105 (84) | 101 (96) | 68 (65) |
| LDHL | 5 | 3 (60) | 2 (67) | 2 (67) |
| NSHL | 358 | 320 (89) | 305 (95) | 227 (71) |
| LRCHL | 16 | 16 (100) | 15 (94) | 5 (31) |
| cHL NOS | 27 | 17 (63) | 14 (82) | 10 (59) |
| EBV status‡ | ||||
| EBV positive | NA | 154 | 145 (94) | 101 (66) |
| EBV negative | NA | 307 | 292 (95) | 211 (69) |
SNEHD indicates Scotland and Newcastle Epidemiological study of Hodgkin's Disease; MCHL, mixed cellularity Hodgkin lymphoma; LDHL, lymphocyte depleted Hodgkin lymphoma; NSHL, nodular sclerosis Hodgkin lymphoma; LRCHL, lymphocyte-rich classic Hodgkin lymphoma; NOS, not otherwise specified; and NA, not applicable.
Percent of SNEHD case series.
Percent of eligible cases.
EBV status of Hodgkin and Reed-Sternberg cells.
EBV status and outcome
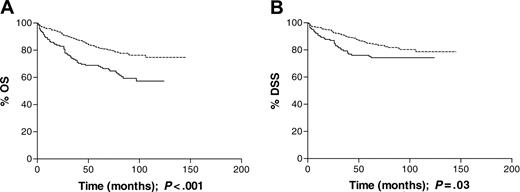
All ages. In the cohort as a whole, EBV-positive HL was not associated with advanced-versus early-stage disease or with B symptoms. There was no significant association between EBV status and disease stage when analyzed as a trend across all stages. Using stage III disease as a reference category, however, EBV-positive cases were significantly more likely to be stage I and less likely to be stage II than EBV-negative cases (Table 3). With a median follow-up time of 93 months, EBV positivity was associated with inferior OS (P < .001; Figure 1A) and DSS (P = .03; Figure 1B). The 5-year survival rate for EBV-associated patients was 66% compared with 81% for the non-EBV-associated patients. The effect of EBV status on DSS did not remain significant following adjustment for the effects of age group, sex, stage, and B symptoms (RR = 1.29; CIs, 0.83-2.01), while that for OS approached statistical significance (RR = 1.45; CIs, 1.00-2.11; Table 4). Patients with EBV-positive HL were more likely to have had reactivation of varicella zoster virus more than 1 year prior to diagnosis than EBV-negative patients (P = .01; Table 5), but there was no evidence of an association between EBV status and either depcat or prior cancer in the complete data set. Previous history of shingles or malignancy and depcat status did not have a significant effect on OS.
Clinical Features of cases by EBV status
. | All cases, no. (%) . | EBV negative, no. (%) . | EBV positive, no (%) . | OR (95% CI) . |
|---|---|---|---|---|
| All ages, adjusted for sex and age group | ||||
| Stage* | ||||
| I | 78 (17.8) | 34 (11.6) | 44 (30.3) | 2.61 (1.40-4.92) |
| II | 182 (41.6) | 144 (49.3) | 38 (26.2) | 0.58 (0.33-1.01) |
| III | 102 (23.3) | 68 (23.3) | 34 (23.4) | 1.00 |
| IV | 75 (17.2) | 46 (15.6) | 29 (20.0) | 1.16 (0.61-2.22) |
| B symptoms | ||||
| No | 243 (56.0) | 166 (56.8) | 77 (54.2) | 1.00 |
| Yes | 191 (44.0) | 126 (43.2) | 65 (45.8) | 0.67 (0.59-1.40) |
| Disease stage | ||||
| Early | 180 (41.2) | 125 (42.8) | 55 (37.9) | 1.00 |
| Advanced | 257 (58.8) | 167 (57.1) | 90 (62.1) | 1.08 (0.7-1.65) |
| Young adults, 15 to 34 years, adjusted for sex | ||||
| Stage* | ||||
| I | 36 (15.9) | 12 (6.9) | 24 (45.3) | 7.70 (2.90-20.44) |
| II | 99 (43.6) | 88 (50.6) | 11 (20.8) | 0.54 (0.21-1.36) |
| III | 55 (24.2) | 44 (25.3) | 11 (20.8) | 1.00 |
| IV | 37 (16.3) | 30 (17.2) | 7 (13.2) | 0.80 (0.28-2.35) |
| B symptoms | ||||
| No | 136 (59.9) | 100 (57.5) | 36 (67.9) | 1.00 |
| Yes | 91 (40.1) | 74 (42.5) | 17 (32.1) | 0.56 (0.29-1.10) |
| Disease stage | ||||
| Early | 98 (43.2) | 70 (40.2) | 28 (52.8) | 1.00 |
| Advanced | 129 (56.8) | 104 (59.8) | 25 (47.2) | 0.53 (0.28-1.00) |
| Older adults, 50 y or older, adjusted for sex | ||||
| Stage† | ||||
| I | 26 (20.5) | 14 (22.2) | 12 (18.9) | 0.88 (0.31-2.49) |
| II | 44 (34.6) | 26 (41.3) | 18 (28.1) | 0.71 (0.28-1.77) |
| III | 33 (26.0) | 17 (27.0) | 16 (25.0) | 1.00 |
| IV | 24 (18.9) | 6 (9.5) | 18 (28.1) | 3.38 (1.06-10.82) |
| B symptoms | ||||
| No | 64 (51.2) | 36 (57.1) | 28 (45.2) | 1.00 |
| Yes | 61 (48.8) | 27 (42.9) | 34 (55.8) | 1.68 (0.81-3.48) |
| Disease stage | ||||
| Early | 48 (37.8) | 29 (46.0) | 19 (29.7) | 1.00 |
| Advanced | 79 (62.2) | 34 (54.0) | 45 (70.3) | 2.04 (0.98-4.26) |
. | All cases, no. (%) . | EBV negative, no. (%) . | EBV positive, no (%) . | OR (95% CI) . |
|---|---|---|---|---|
| All ages, adjusted for sex and age group | ||||
| Stage* | ||||
| I | 78 (17.8) | 34 (11.6) | 44 (30.3) | 2.61 (1.40-4.92) |
| II | 182 (41.6) | 144 (49.3) | 38 (26.2) | 0.58 (0.33-1.01) |
| III | 102 (23.3) | 68 (23.3) | 34 (23.4) | 1.00 |
| IV | 75 (17.2) | 46 (15.6) | 29 (20.0) | 1.16 (0.61-2.22) |
| B symptoms | ||||
| No | 243 (56.0) | 166 (56.8) | 77 (54.2) | 1.00 |
| Yes | 191 (44.0) | 126 (43.2) | 65 (45.8) | 0.67 (0.59-1.40) |
| Disease stage | ||||
| Early | 180 (41.2) | 125 (42.8) | 55 (37.9) | 1.00 |
| Advanced | 257 (58.8) | 167 (57.1) | 90 (62.1) | 1.08 (0.7-1.65) |
| Young adults, 15 to 34 years, adjusted for sex | ||||
| Stage* | ||||
| I | 36 (15.9) | 12 (6.9) | 24 (45.3) | 7.70 (2.90-20.44) |
| II | 99 (43.6) | 88 (50.6) | 11 (20.8) | 0.54 (0.21-1.36) |
| III | 55 (24.2) | 44 (25.3) | 11 (20.8) | 1.00 |
| IV | 37 (16.3) | 30 (17.2) | 7 (13.2) | 0.80 (0.28-2.35) |
| B symptoms | ||||
| No | 136 (59.9) | 100 (57.5) | 36 (67.9) | 1.00 |
| Yes | 91 (40.1) | 74 (42.5) | 17 (32.1) | 0.56 (0.29-1.10) |
| Disease stage | ||||
| Early | 98 (43.2) | 70 (40.2) | 28 (52.8) | 1.00 |
| Advanced | 129 (56.8) | 104 (59.8) | 25 (47.2) | 0.53 (0.28-1.00) |
| Older adults, 50 y or older, adjusted for sex | ||||
| Stage† | ||||
| I | 26 (20.5) | 14 (22.2) | 12 (18.9) | 0.88 (0.31-2.49) |
| II | 44 (34.6) | 26 (41.3) | 18 (28.1) | 0.71 (0.28-1.77) |
| III | 33 (26.0) | 17 (27.0) | 16 (25.0) | 1.00 |
| IV | 24 (18.9) | 6 (9.5) | 18 (28.1) | 3.38 (1.06-10.82) |
| B symptoms | ||||
| No | 64 (51.2) | 36 (57.1) | 28 (45.2) | 1.00 |
| Yes | 61 (48.8) | 27 (42.9) | 34 (55.8) | 1.68 (0.81-3.48) |
| Disease stage | ||||
| Early | 48 (37.8) | 29 (46.0) | 19 (29.7) | 1.00 |
| Advanced | 79 (62.2) | 34 (54.0) | 45 (70.3) | 2.04 (0.98-4.26) |
EBV indicates Epstein Barr virus; OR, odds ratio for EBV-positive compared with EBV-negative using stage III as the reference group; and CI, confidence inverval.
P value for heterogeneity <.001.
P value for heterogeneity = .06.
Multivariate analysis of overall survival
. | All ages, adjusted for age and sex . | Young adults, adjusted for sex . | Older adults, adjusted for sex . |
|---|---|---|---|
| EBV status, adjusted for stage and B symptoms | 1.45 (1.00−2.11) | 0.60 (0.20−1.76) | 1.88 (1.14−3.12)* |
| EBV status, adjusted for early versus advanced stage disease | 1.39 (0.97−2.01) | 0.55 (0.19−1.62) | 1.96 (1.19−3.22)* |
| B symptoms, adjusted for EBV and stage | 1.49 (1.01−2.19)* | 1.70 (0.77−3.74) | 1.49 (0.90−2.47) |
| Early/advanced, adjusted for EBV | 2.05 (1.38−3.06)* | 2.87 (1.17−7.06)* | 1.62 (0.96−2.75) |
| Stage, adjusted for EBV status and B symptoms | 1.44 (1.19−1.73)* | 1.55 (1.02−2.38)* | 1.29 (1.01−1.66)* |
. | All ages, adjusted for age and sex . | Young adults, adjusted for sex . | Older adults, adjusted for sex . |
|---|---|---|---|
| EBV status, adjusted for stage and B symptoms | 1.45 (1.00−2.11) | 0.60 (0.20−1.76) | 1.88 (1.14−3.12)* |
| EBV status, adjusted for early versus advanced stage disease | 1.39 (0.97−2.01) | 0.55 (0.19−1.62) | 1.96 (1.19−3.22)* |
| B symptoms, adjusted for EBV and stage | 1.49 (1.01−2.19)* | 1.70 (0.77−3.74) | 1.49 (0.90−2.47) |
| Early/advanced, adjusted for EBV | 2.05 (1.38−3.06)* | 2.87 (1.17−7.06)* | 1.62 (0.96−2.75) |
| Stage, adjusted for EBV status and B symptoms | 1.44 (1.19−1.73)* | 1.55 (1.02−2.38)* | 1.29 (1.01−1.66)* |
Data are presented as risk ratio (95% confidence interval).
Risk ratios (RRs) are for EBV-positive relative to EBV-negative cases, presence or absence of B symptoms, and advanced-stage relative to early-stage disease; stage has been modeled as a trend across all stages and RRs are an estimate of increased risk for each increase in stage (eg, stage III relative to stage II disease).
RR indicates risk ratio; CI, confidence interval; and EBV, Epstein-Barr virus.
Significant values.
Association between EBV status and previous shingles, prior malignancy, and depcat score
. | All cases, no. (%) . | EBV negative, no. (%) . | EBV positive, no. (%) . | P* . |
|---|---|---|---|---|
| All ages | ||||
| Shingles | .01‡ | |||
| Yes | 26 (8.3) | 12 (5.6) | 14 (14) | |
| No | 289 (91.7) | 203 (94.4) | 86 (86) | |
| Prior cancer | .21 | |||
| Yes | 12 (2.7) | 6 (2.1) | 6 (4.1) | |
| No | 425 (97.3) | 28 (97.9) | 139 (95.9) | |
| Depcat§ | .33 | |||
| 1 | 47 (15.1) | 34 (16.1) | 13 (12.9) | |
| 2 | 52 (16.7) | 38 (18.0) | 14 (13.9) | |
| 3 | 58 (18.6) | 39 (8.5) | 19 (18.8) | |
| 4 | 71 (22.8) | 41 (19.4) | 30 (29.7) | |
| 5 | 84 (26.9) | 59 (28.0) | 25 (24.8) | |
| Young adults, 15 to 34 years | ||||
| Shingles† | .20 | |||
| Yes | 13 (7.4) | 8 (6.0) | 5 (11.9) | |
| No | 162 (92.6) | 125 (94.0) | 37 (88.1) | |
| Prior cancer | .20 | |||
| Yes | 4 (1.8) | 2 (1.1) | 2 (3.8) | |
| No | 223 (98.2) | 172 (98.9) | 51 (96.2) | |
| Depcat§ | .56 | |||
| 1 | 24 (14.0) | 18 (13.7) | 6 (15.0) | |
| 2 | 29 (17.0) | 24 (18.3) | 5 (12.5) | |
| 3 | 37 (21.6) | 29 (22.1) | 8 (20.0) | |
| 4 | 36 (21.1) | 24 (18.3) | 12 (30.0) | |
| 5 | 45 (26.3) | 36 (27.5) | 9 (22.5) | |
| Older adults, 50 y or older | ||||
| Shinglest† | .27 | |||
| Yes | 9 (11.4) | 3 (7.5) | 6 (15.4) | |
| No | 70 (88.6) | 37 (92.5) | 33 (84.6) | |
| Prior cancer | .713 | |||
| Yes | 7 (5.5) | 3 (4.8) | 4 (6.3) | |
| No | 120 (94.5) | 60 (95.2) | 60 (93.8) | |
| Depcat§ | .04‡ | |||
| 1 | 13 (15.9) | 9 (22.0) | 4 (9.8) | |
| 2 | 13 (15.9) | 7 (17.1) | 6 (14.6) | |
| 3 | 9 (11.0) | 3 (7.3) | 6 (14.6) | |
| 4 | 20 (24.4) | 5 (12.2) | 15 (36.6) | |
| 5 | 27 (32.9) | 17 (41.5) | 10 (24.4) |
. | All cases, no. (%) . | EBV negative, no. (%) . | EBV positive, no. (%) . | P* . |
|---|---|---|---|---|
| All ages | ||||
| Shingles | .01‡ | |||
| Yes | 26 (8.3) | 12 (5.6) | 14 (14) | |
| No | 289 (91.7) | 203 (94.4) | 86 (86) | |
| Prior cancer | .21 | |||
| Yes | 12 (2.7) | 6 (2.1) | 6 (4.1) | |
| No | 425 (97.3) | 28 (97.9) | 139 (95.9) | |
| Depcat§ | .33 | |||
| 1 | 47 (15.1) | 34 (16.1) | 13 (12.9) | |
| 2 | 52 (16.7) | 38 (18.0) | 14 (13.9) | |
| 3 | 58 (18.6) | 39 (8.5) | 19 (18.8) | |
| 4 | 71 (22.8) | 41 (19.4) | 30 (29.7) | |
| 5 | 84 (26.9) | 59 (28.0) | 25 (24.8) | |
| Young adults, 15 to 34 years | ||||
| Shingles† | .20 | |||
| Yes | 13 (7.4) | 8 (6.0) | 5 (11.9) | |
| No | 162 (92.6) | 125 (94.0) | 37 (88.1) | |
| Prior cancer | .20 | |||
| Yes | 4 (1.8) | 2 (1.1) | 2 (3.8) | |
| No | 223 (98.2) | 172 (98.9) | 51 (96.2) | |
| Depcat§ | .56 | |||
| 1 | 24 (14.0) | 18 (13.7) | 6 (15.0) | |
| 2 | 29 (17.0) | 24 (18.3) | 5 (12.5) | |
| 3 | 37 (21.6) | 29 (22.1) | 8 (20.0) | |
| 4 | 36 (21.1) | 24 (18.3) | 12 (30.0) | |
| 5 | 45 (26.3) | 36 (27.5) | 9 (22.5) | |
| Older adults, 50 y or older | ||||
| Shinglest† | .27 | |||
| Yes | 9 (11.4) | 3 (7.5) | 6 (15.4) | |
| No | 70 (88.6) | 37 (92.5) | 33 (84.6) | |
| Prior cancer | .713 | |||
| Yes | 7 (5.5) | 3 (4.8) | 4 (6.3) | |
| No | 120 (94.5) | 60 (95.2) | 60 (93.8) | |
| Depcat§ | .04‡ | |||
| 1 | 13 (15.9) | 9 (22.0) | 4 (9.8) | |
| 2 | 13 (15.9) | 7 (17.1) | 6 (14.6) | |
| 3 | 9 (11.0) | 3 (7.3) | 6 (14.6) | |
| 4 | 20 (24.4) | 5 (12.2) | 15 (36.6) | |
| 5 | 27 (32.9) | 17 (41.5) | 10 (24.4) |
EBV indicates Epstein Barr virus.
P value for chi-squared analysis.
Shingles (herpes zoster reactivation) diagnosed more than 1 year before diagnosis of Hodgkin lymphoma.
Significant values.
Depcat score using the Carstairs index analyzed as a trend across depcat categories; Carstairs categories 1 and 2 (most affluent) are grouped as depcat 1, and Carstairs categories 6 and 7 are grouped as depcat 5 (least affluent).
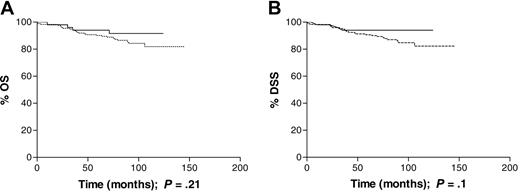
Young adults (16 to 34 years). This age group included 123 male and 105 female patients; 53 (24%) of the cases had EBV-associated disease. EBV status was not significantly associated with B symptoms or early versus advanced disease, as defined in “Patients, materials, and methods.” When stage was analyzed as a trend, however, the EBV-negative cases were more likely than EBV-positive cases to have higher stage disease (P = .001). Using stage III disease as the reference category, EBV-associated cases were significantly more likely to be stage I at presentation than EBV-negative cases (Table 3). Patients with EBV-positive HL had improved survival compared with patients with EBV-negative disease (5-year OS of 94% versus 89% and 5-year DSS of 94% versus 90%). Differences in OS and DSS, during the follow-up period, did not attain statistical significance (P = .21 and P = .24, respectively; Figure 2A-B). Only 1 of the 29 deaths in this age group was not HL related; 1 patient died of acute myeloid leukemia (AML) that was diagnosed within 2 months of finishing initial treatment and was thought to be an incidental occurrence. EBV status was not significantly associated with previous history of shingles, prior cancer, or depcat status (Table 5). Previous cancer was associated with a poorer OS (RR = 10.41; CIs, 1.61-67.44), but previous shingles and depcat status did not have a significant effect on OS.
Thirty-five to 49 years. Of the 83 patients in this age group, 34 were female and 49 were male; 28 (34%) had EBV-associated disease. There was no significant association between EBV status and stage at presentation, B symptoms, OS (P = .7), or DSS (P = .8). The 5-year OS was 71% versus 75%, and DSS was 74% versus 78% for EBV-positive and -negative cases, respectively. There were 5 deaths that were not HL related. The causes were as follows: epileptic fit (1), pulmonary embolism (1), respiratory disease (1), and sarcoma (1), and one from septicemia 6 years from diagnosis in a patient who was in remission. The proportion of HL-unrelated deaths was evenly distributed between EBV-positive and -negative patients.
Survival data for all patients analyzed by tumor EBV status at presentation. (A) Overall survival (OS) was significantly longer (P < .001) for EBV-negative patients (n = 292) than EBV-positive patients (n = 145). (B) Disease-specific survival (DSS) was also significantly longer, in the same cohort, among EBV-negative patients; P = .03. Dotted line indicates EBV-negative; solid line, EBV-positive.
Survival data for all patients analyzed by tumor EBV status at presentation. (A) Overall survival (OS) was significantly longer (P < .001) for EBV-negative patients (n = 292) than EBV-positive patients (n = 145). (B) Disease-specific survival (DSS) was also significantly longer, in the same cohort, among EBV-negative patients; P = .03. Dotted line indicates EBV-negative; solid line, EBV-positive.
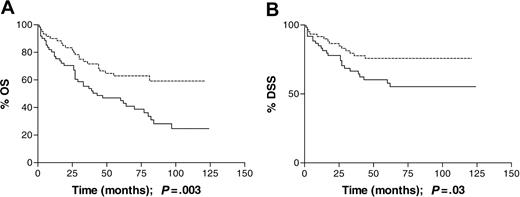
Older adults (≥ 50 years). There were 127 patients in this age group, including 69 males and 58 females; 64 (50%) of the cases had EBV-associated disease. There was no significant association between EBV status and early versus advanced disease, B symptoms, and stage when analyzed as a trend. When stage was analyzed as a categoric variable, there was some indication that EBV-positive cases were more likely to have stage IV disease at presentation (Table 3). In this age group, patients with EBV-associated disease experienced significantly poorer OS (P = .003; Figure 3A) and DSS (P = .03; Figure 3B) than patients with EBV-negative HL: 5-year OS was 43% for EBV-positive cases compared with 62% for EBV-negative cases, and DSS was 55% for EBV-positive cases compared with 73% for EBV-negative cases. Inferior 5-year DSS was even more marked when patients 60 years or older were analyzed separately (26% versus 48%). Excess deaths were found in EBV-associated cases with both early- and advanced-stage disease, and the effect of EBV status on OS remained significant after adjusting for the effects of sex, B symptoms, and stage (RR = 1.88; CIs, 1.14-3.12; Table 4).
The effect of EBV status on DSS did not remain significant in the multivariate analysis (RR = 1.70; CIs, 0.90-3.20). There was a higher proportion of deaths from HL-unrelated causes among the EBV-associated cases, 17 of 64 versus 11 of 63 for EBV-positive and EBV-negative cases, respectively, although differences by EBV status were not statistically significant (P = .22). The causes of death that were unrelated to HL in the EBV-associated cases were as follows: cerebrovascular accident (CVA, 2), bladder carcinoma (1), gastric carcinoma (2), bronchial carcinoma (2), pancreatic carcinoma (1), colorectal carcinoma (1), pneumonia (1), ruptured aortic aneurysm (1), neurologic event otherwise undefined (1), congestive cardiac failure (1), myocardial infarction (1), and 3 deaths in remission from unknown cause. The 11 HL-unrelated deaths in the EBV-negative cases comprised the following: CVA (1), non-Hodgkin lymphoma (2), myocardial infarction (1), emphysema (1), bronchial carcinoma (2), AML (1), ischemic heart disease (1), pneumonia (1), and one remission death, cause unknown.
Among older adults, there was no significant association between EBV status and previous shingles or prior cancer. EBV-associated cases were significantly less likely to have a low depcat score (P = .04), indicating more deprivation, but this effect was not linear across all depcat categories (Table 5). History of shingles, prior malignancy, and depcat status were not significant in the multivariate analysis of OS, but the effect of EBV status remained significant after adjustment for these variables (RR = 2.07; CIs, 1.24-3.46).
Discussion
This population-based study demonstrates an age-dependent relationship between tumor EBV status and clinical outcome in cHL. In the cohort as a whole, patients with EBV-positive HL had a poorer outcome than those with EBV-negative disease, with respect to both OS and DSS (Figure 1), but this did not remain significant following adjustment for the effects of age group, sex, stage, and B symptoms. In adults aged 16 to 49 years, EBV status had no significant impact on survival, but there was a suggestion that EBV-positive patients in the 16- to 34-year age group had a better OS and DSS (Figure 2). It was only in the oldest age group (≥ 50 years) that EBV positivity was significantly associated with an unfavorable OS and DSS (Figure 3). Although older adult cases were underrepresented in the present study compared with the original SNEHD series, there was no evidence for selection bias by EBV status (Table 2). Among older adult patients, EBV-associated cases were more likely to have stage IV disease, but the excess deaths occurred in patients with both early- and advanced-stage disease and EBV status remained significant in the multivariate analysis (Table 4). Deaths were both HL related and unrelated with a slightly greater number of deaths from other cancers occurring in the EBV-positive compared with EBV-negative cases. An age-dependent relationship between stage at presentation and EBV status was also observed; among young adults the EBV-positive patients were more likely than the EBV-negative patients to have stage I disease, whereas among older adults the EBV-positive patients were more likely to have stage IV disease. These data provide further evidence for differences in the natural history of EBV-associated HL in younger and older adult age groups.
Survival of patients aged 16 to 34 years at diagnosis analyzed by tumor EBV status. (A) Overall survival (OS) was similar among EBV-positive (n = 53) and EBV-negative (n = 175) patients; P = .21. (B) Disease-specific survival (DSS) was similar, in the same cohort, among EBV-positive and -negative patients; P = .1. Dotted line indicates EBV-negative; solid line, EBV-positive.
Survival of patients aged 16 to 34 years at diagnosis analyzed by tumor EBV status. (A) Overall survival (OS) was similar among EBV-positive (n = 53) and EBV-negative (n = 175) patients; P = .21. (B) Disease-specific survival (DSS) was similar, in the same cohort, among EBV-positive and -negative patients; P = .1. Dotted line indicates EBV-negative; solid line, EBV-positive.
Previous reports describing clinical outcome in relation to EBV status are conflicting (Table 1). Differences between published results may be attributable to small study size, differences in the age and sex distribution of the cases under investigation, sample selection, and inclusion of NLPHL cases. Several studies have not found a significant relationship between HL tumor-cell EBV status and prognosis. In the majority of these, the number of cases investigated was relatively small4-6 and the patients were not stratified by age.4,6,7,10 If the impact of EBV status in HL is indeed variable and age dependent, as suggested by this study, a single analysis of patients of all ages would mask the impact of EBV status on the results. Such a masking effect could also apply to multivariate analysis if performed over the whole age spectrum.5,10 In the dataset described by Armstrong et al,5 there was indeed evidence that EBV-positive cases had an improved survival in the young adult age group but not as good an outcome in the older age group, although differences in EBV status did not attain significance.
Three studies have demonstrated improved survival in relation to EBV and HL.12,14,15 In the study of patients from India reported by Naresh et al,14 the patients were younger than in the present study, with 45% in the pediatric age group; overall, there was a high proportion of EBV-positive cases and these were younger than the EBV-negative cases. Thus the age distribution of the EBV-positive cases may explain their favorable outcome. In a study of adult patients from Brazil, Vassalo et al15 noted a favorable outcome in cases in which HRS cells expressed EBV latent membrane protein 1 (LMP1). EBV EBER expression, however, which is generally considered the most robust method to define EBV status, did not influence survival. Morente et al12 also observed a favorable impact of EBV tumor-cell positivity on OS in a study of 210 patients recruited from 11 Spanish centers. Only patients who received adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD)30 or equivalent regimens were included in the analysis; this clearly represents a relatively selected population. Case selection could, therefore, account for the difference in results between this study and our own, as could the inclusion of pediatric cases.
Glavina-Durdov et al16 reported findings similar to those reported here in a 2-center study. This group found that HL tumor-cell EBV status had no impact on OS or disease-free survival (DFS) when their population was analyzed as a whole. Among patients younger than 35 years, DFS was significantly longer in patients with EBV-positive HL. In contrast, in patients older than 50 years, DFS was shorter among those with EBV-positive than those with EBV-negative disease, with median survivals of 22.6 and 36.6 months, respectively. The authors considered, however, that the small size of this older patient cohort (n = 10) precluded statistical analysis.
Survival of patients 50 years or older at diagnosis analyzed by tumor EBV status. (A) Overall survival (OS) was significantly shorter for EBV-positive (n = 64) than EBV-negative (n = 62) patients; P = .003. (B) Disease-specific survival (DSS) was also significantly shorter, in the same cohort, among EBV-positive rather than -negative patients; P = .03. Dotted line indicates EBV-negative; solid line, EBV-positive.
Survival of patients 50 years or older at diagnosis analyzed by tumor EBV status. (A) Overall survival (OS) was significantly shorter for EBV-positive (n = 64) than EBV-negative (n = 62) patients; P = .003. (B) Disease-specific survival (DSS) was also significantly shorter, in the same cohort, among EBV-positive rather than -negative patients; P = .03. Dotted line indicates EBV-negative; solid line, EBV-positive.
Of the studies in this field, it seems likely that other population-based studies would be most comparable with our own. In a population-based study of 311 women in California, Clarke et al17 demonstrated no difference in OS for patients with EBV-positive and EBV-negative HL when their entire cohort was analyzed. Among patients 45 years or older, however, OS was reduced in those with EBV-associated disease. Similarly, another population-based study focusing on patients 60 years or older18 demonstrated reduced OS among patients with EBV-associated disease. It should be noted, however, that EBV status was assessable for only 70 of 102 patients studied; unassessable patients were significantly older than assessable patients in this study. Enblad et al11 found that patients with EBV-positive disease had lower DFS and OS than EBV-negative patients, although differences did not reach statistical significance (P = .17 and .11, respectively). This group noted, however, that these differences by EBV status were essentially attributable to the 36 patients older than 60 years in their study. Overall, there is therefore a body of evidence, largely from population-based studies, indicating that (a) the effect of EBV status on clinical outcome is age dependent and (b) older adult patients with EBV-positive HL have a particularly poor prognosis.
Patterns of general ill health in the elderly suggest that immune responses decline in older people,31-33 and a decline in cytotoxic T-cell responses with increasing age has been observed.34,35 These findings, coupled with the excess deaths from unrelated causes observed in the older adult EBV-positive HL patients, led us to speculate that an age-related decrease in immunity may be contributing to the observed age effects. In order to investigate this, we first examined the relationship between EBV status and previous history of shingles, prior malignancy, and depcat status. Shingles, which is caused by reactivation of varicella zoster virus, is often related to immunosuppression,36 and depcat status has frequently been used as an indirect indicator of general health and immunity. Shingles, occurring more than 1 year before HL diagnosis, was significantly more common in EBV-positive cases, but this effect was not restricted to older cases (Table 5). Younger and older EBV-positive cases were also more likely than EBV-negative cases to have had a previous malignancy, although differences by EBV status were not statistically significant (Table 5). Older, but not younger, EBV-positive cases were more likely to be of higher depcat status (more deprived) than EBV-negative cases. This effect, however, was not linear across the range of depcat categories analyzed and is therefore difficult to evaluate. In addition, the selection bias in our data that associates good prognosis with availability of depcat and zoster data must lead to caution in interpreting these data; these data do, however, provide interesting indications of possible biologic pathways for confirmation elsewhere. Taken together, these findings provide some support for the idea that decreased immune function may contribute to the development of EBV-associated HL. Reduced immunity to EBV could have a role at 2 levels. First, decreased immunosurveillance may result in an increase in the number of circulating EBV-infected B cells, and this may lead to an increased risk of EBV-associated HL development. In support of this, we have evidence that EBV-positive HL cases have a greater frequency of circulating EBV-infected cells, at the time of diagnosis, when compared with EBV-negative cases.37 Secondly, decreased immunity to LMP1 and LMP2, EBV latent proteins that are expressed by HRS cells, may contribute to disease pathogenesis.
The effect of decreased immunity and general ill health on disease outcome was more difficult to evaluate using the available data. In the multivariate analysis of survival, a history of shingles was associated with poorer outcome in the older age group, but this effect was not statistically significant and was not confined to EBV-associated cases. Prior malignancy was associated with a less favorable prognosis in both younger and older adults, and for young adults this effect was statistically significant; risk was increased for both EBV-positive and -negative cases in both younger and older adult age groups. Material deprivation was not associated with inferior outcome in the overall dataset or following stratification by EBV status or age group. These data do not therefore provide evidence that poor general health at, or prior to, the time of diagnosis of HL is contributing to the less favorable outcome of the EBV-positive, older adult patients. However, deaths from HL-unrelated causes in this group of cases suggest that comorbidity is an important determinant of outcome.
In conclusion, this study demonstrates that older patients with EBV-positive HL have a poor prognosis. EBV-positive disease comprises the majority of HL cases in the elderly, and these patients, in general, tolerate current treatment regimens less well than young adults.22 Strategies aimed at boosting the immune response to EBV may therefore play a valuable role in treatment of older patients with HL.
Appendix
The members of the SNLG Study Group are: Dr M Abela, Dr T L Allan, Dr B Angus, Dr A D J Birch, Dr D T Bowen, Dr R Brooke Hogg, Dr N M Browning, Dr P Cachia, Dr R Campbell Tait, Dr B Cantwell, Dr P J Carey, Dr J E Chandler, Dr P T Clarke, Dr D Culligan, Dr R Dang, Dr S N Das, Dr M Dewar, Dr J M Davies, Dr D J Dunlop, Dr A Dykes, Dr M H Elia, Dr D Ellis, Dr J G Erskine, Dr LA Evan-Wong, Dr P Eynaud, Dr E J Fitzsimons, Dr P Forsyth, Dr M J Galloway, Dr K Gelly, Dr D Goff, Dr J Goodlad, Professor M Greaves, Dr P J Hamilton, Dr A Hendrick, Dr A Hung, Dr A Iqbal, Dr P R E Johnson, Dr R Jones, Dr E J Junor, Dr F M Keenan, Dr P Kesteven, Dr M Leach, Dr A L Lennard, Dr J Lewis, Dr N P Lucie, Dr H Lucraft, Professor C A Ludlam, Dr C J Lush, Dr M J Mackie, Dr L Manson, Dr L M Matheson, Dr Z Maung, Dr C J McCallum, Dr M McColl, Dr P McKay, Dr G McQuaker, Dr D Meiklejohn, Dr A Morrison, Dr P Mounter, Dr J Murphy, Dr I Neilly, Dr A J Neylon, Dr H O'Brien, Dr N O'Rourke, Dr J P Owen, Dr A Parker, Professor M J Pippard, Dr D Plews, Dr A Raafat, Dr D Ramsay, Dr M Ratcliffe, Dr A Rathmell, Dr M Robertson, Dr S Y Rogers, Dr M Ronghe, Dr L Samuel, Dr F Scott, Dr R A Sharp, Dr P Shepherd, Dr I O Singer, Dr R Soutar, Dr A N Stark, Dr G L Stark, Dr G P Summerfield, Dr R P Symonds, Dr P Tansey, Dr J Tighe, Dr H Tinegate, Dr C Tiplady, Dr J P Wallis, Dr W Watson, Dr N West, Dr D Whillis, Dr B Wilkins, Dr P J Williamson, Dr A Wood, Dr K Wood, Dr H M Yosef, Dr A Youart, and Dr F Yuille.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2004-09-3759.
A complete list of the members of the Scotland and Newcastle Epidemiology of Hodgkin Disease (SNLG) Study Group appears in the “Appendix.”
Supported by the Leukaemia Research Fund and the Kay Kendall Leukaemia Fund. P.R.A.T. is supported by a grant from Yorkshire and Northern Regional R&D.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank all the members of the Scotland and Newcastle Lymphoma Group for allowing us to study their patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal