Abstract
Mice lacking all 3 Vav proteins fail to produce significant numbers of recirculating follicular or marginal zone B cells. Those B cells that do mature have shortened lifespans. The constitutive nuclear factor-kappaB (NF-κB) activity of resting naive B cells required Vav function and expression of cellular reticuloendotheliosis (c-Rel). Rel-A was reduced in Vav-deficient B cells. Furthermore, expression of the NF-κB-regulated antiapoptotic genes A1 and Bcl-2 was reduced in mature Vav-deficient B cells. Overexpression of Bcl-2 restored the number of mature follicular B cells in the spleens of Vav-deficient mice. When activated by B-cell receptor (BCR) cross-linking, Vav-deficient B cells failed to activate NF-κB. Vav proteins thus regulate an NF-κB-dependent survival signal in naive B cells and are required for NF-κB function after BCR cross-linking.
Introduction
Signal transduction pathways initiated by the B-cell antigen receptor and the related pre-B-cell receptor regulate the developmental progress, survival, and activation of B cells.1 During B-cell development in the bone marrow, successful immunoglobulin heavy (IgH) chain rearrangement leads to the assembly of a functional pre-B-cell receptor (pre-BCR) that additionally contains the V-pre-B and λ5 proteins. This receptor signals both cellular proliferation and allelic exclusion of further IgH rearrangement. Subsequent productive rearrangement of κ or λ light chain, and successful pairing with heavy chain, lead to the expression of membrane IgM and define the immature B cell. B-cell development beyond the immature B-cell stage is crucially dependent on BCR signaling. Immature B cells are negatively selected after encounter with self-antigen but also require IgM-mediated signal transduction to undergo maturation into long-lived recirculating B cells.2-4 This process, which has been termed positive selection, gives rise to follicular entry and increased longevity of mature B cells. The mechanisms that mediate B-cell-positive selection remain unclear. It is established that expression of the BCR is required to protect mature B cells from apoptosis.5,6 Furthermore, immunoreceptor tyrosine-based activation motif (ITAM)-deficient mutants of CD79a, which allow BCR assembly and expression, reveal an essential role for this BCR-associated signal transducer in the generation of the survival signal.6 Moreover, B cells deficient in the spleen tyrosine kinase (Syk) are unable to undergo positive selection.7 In addition, the mutation of other components of the BCR signal transduction apparatus display defects in positive selection, though these are less severe than those reported for Syk mutants.8 Competition between newly formed and mature recirculating B cells for limiting resources9 plays an important role in controlling entry into the mature pool and may be regulated by interactions between B cells and stromal cells.
In mouse splenic B cells and in B-cell lines, members of the nuclear factor-kappaB (NF-κB) family of transcription factors are constitutively active and located in the nucleus. This contrasts with most cell types in which NF-κB is found as an inactive complex in the cytoplasm.10 Gene-targeting experiments have demonstrated roles for NF-κB family members in the regulation of B-cell survival and activation.11 Splenic B cells constitutively express nuclear NF-κB primarily in the form of p50/cellular reticuloendotheliosis (c-Rel) and p52/RelB complexes. P52 is generated by the so-called alternative pathway of NF-κB activation that is regulated by the survival cytokine B-cell activation factor (BAFF).12,13 Maintenance of p50/c-Rel activity is mediated through a novel Ca2+-dependent, but proteasome- and ubiquitin ligase-independent, degradation of the inhibitor protein IκB.14-16 This novel pathway is still only partly understood, but it demonstrates a requirement for calmodulin and not calcineurin.17
The Vav family of proteins is composed of 3 multidomain molecules with the capacity to act as guanine nucleotide exchange factors (GEFs) for the Rho family of guanosine triphosphatases (GTPases).18 All 3 Vav proteins are phosphorylated in response to antigen receptor cross-linking of B and T cells. Gene-targeting studies have established roles for Vav proteins in T- and B-cell development and function. Vav-1 is required for normal T-cell and B1 B-cell subset development. By contrast, lymphocyte development appears normal in mice lacking Vav-2 and Vav-3.19 In mice lacking both Vav-1 and Vav-2, the maturation of immature B cells into recirculating B cells is impaired,20,21 though the mechanism by which this occurs has yet to be identified. Mice lacking Vav-1 and Vav-3 display substantial further quantitative defects in T-cell development above those seen in Vav-1 mutant mice.19 In mice lacking all 3 Vav proteins, T-cell development was profoundly impaired, primarily at the level of β-selection in the thymus, and B-cell development was impaired beyond the immature stage.19 Analysis of the signaling pathways activated in Vav-deficient B lymphocytes after antigen receptor cross-linking has demonstrated the importance of Vav proteins in the control of intracellular calcium.19,22 However, the mechanisms by which Vav proteins regulate calcium are still poorly understood.18
We studied peripheral B-cell maturation in mice lacking Vav proteins. Vav function is dispensable for the accumulation of T1 transitional cells in the spleen and for the developmentally regulated changes in the expression of CXC motif chemokine receptor type 5 (CXCR5) and B-cell lymphoma apoptosis regulator XL (Bcl-XL). However, those Vav-deficient B cells that develop beyond the T1 stage turn over faster in vivo and die more rapidly in vitro. In this report, we show that Vav proteins are required for the proper expression of c-Rel and Rel-A and for the accumulation of nuclear NF-κB complexes in resting mature B cells. Moreover, the expression of the NF-κB target genes A1 and Bcl-2 by resting mature B cells requires Vav function. Mature follicular (FO) B-cell numbers were recovered in Vav-deficient mice after the overexpression of Bcl-2. In addition to controlling the level of NF-κB activity in resting B cells, the ability of Vav-deficient B cells to activate NF-κB after high-avidity cross-linking of the BCR was impaired. We, therefore, suggest Vav proteins regulate the expression and function of components of the NF-κB pathway that are required for the survival of splenic naive B cells.
Materials and methods
Mice
Mutant mice harboring null mutations in Vav-1,23 Vav-2,20 and Vav-319 have been described and were backcrossed to B10.BR for at least 5 generations before they were intercrossed to generate Vav double- and triple-deficient mice. Vav triple-deficient mice were crossed to mice carrying an Eμ-BCL2-36 transgene.24 Rag γc-deficient mice were previously described.25 All mice were maintained according to United Kingdom Home Office guidelines under project license 80/1263.
In vivo BrdU incorporation
Adult wild-type or Vav-deficient mice were administered 1 mg/mL 5-bromo-2′-deoxyuridine (BrdU) continuously in the drinking water for the indicated times. Spleen cells were stained for CD93, CD23, and IgM and subsequently with anti-BrdU using the BrdU flow kit (Becton Dickinson, San Jose, CA) according to the manufacturer's instructions.
Data were analyzed using analysis of variance (ANOVA). Factors in the analyses were the time points in days (2.5, 4.5, 6.5), the mouse genotype, and the interaction of these 2 effects. Analyses were carried out for T3 and mature B cells.
Flow cytometry
Single-cell suspensions from bone marrow and spleen were surface-stained using various combinations of antibodies unconjugated or conjugated to biotin, fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), allophycocyanin (APC), or Cy5. Staining with biotinylated antibodies was revealed by streptavidin Quantum-Red (Sigma, Chicago, IL). Cells were analyzed using a FACScalibur flow cytometer with CellQuest (Becton Dickinson) or FlowJo (Treestar,Ashland, OR) software. The following antibodies were used: anti-CD25 (7D4), anti-B220 (RA3-6B2), anti-CD21 (7G6), anti-CD23 (B3B4), anti-CXCR5 (2G8) (all from Becton Dickinson); anti-IgD (11-26; Southern Biotechnology, Birmingham,AL); polyclonal anti-IgM, and anti-rat IgG (Jackson ImmunoResearch, West Grove, PA). AA4.1 conjugated to PE and APC was provided by D. Allman (University of Pennsylvania School of Medicine, Philadelphia, PA). For cytoplasmic staining of intracellular proteins, cells were first stained with surface markers, fixed with Cytofix/Cytoperm (Becton Dickinson) for 20 minutes on ice, and washed twice with phosphate-buffered saline (PBS) containing 0.5% (wt/vol) bovine serum albumin (BSA), 0.01% (wt/vol) NaN3, and 0.03% saponin. Subsequently, cells were stained with anti-Bcl-2 PE (Caltag, Burlingame, CA), anti-Bcl-XL PE (Southern Biotechnology), or anti-c-Rel (Santa Cruz Biotechnology, Santa Cruz, CA) biotinylated antibodies diluted in PBS/BSA/NaN3/0.03% saponin for 1 hour at 4°C. Biotinylated antibodies were revealed by staining with streptavidin Quantum-Red (Sigma) in PBS/0.5%BSA/NaN3/0.03% saponin for 20 minutes at 4°C. Finally, cells were washed twice in PBS/0.5%BSA/NaN3/0.03% saponin and resuspended in PBS/0.5%/BSA/NaN3.
Cell sorting and culture
Splenocytes were stained with anti-CD23, AA4.1, and Fab′ fragments of goat anti-IgM (Jackson ImmunoResearch) to avoid BCR cross-linking. Mature cells were sorted on a cell sorter (DIVA or Aria; Becton Dickinson). Reanalysis of sorted mature B cells revealed more than 97% purity. Purified mature B cells were cultured at 1 × 106 cells/mL in RPMI supplemented with 10% fetal calf serum (FCS), 50 μM β-mercaptoethanol, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in the presence or absence of 10 μg/mL anti-IgM.
Analysis of gene expression
RNA was extracted from sorted mature B cells using TRIzol reagent (Invitrogen, Carlsbad, CA) and was used to make cDNA for real-time polymerase chain reaction (PCR) with a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Real-time PCR reactions used Taq-man Universal Master Mix (Applied Biosystems) and an ABI Prism 7700 sequence detection system. RNA concentrations were calculated relative to hypoxanthine phosphoribosyltransferase (HPRT) expression. Primers and FAM-labeled probes were obtained from Applied Biosystems (Taqman assays on demand) except for A1. The sequence of the primers and probe used for A1 were as follows: A1 forward, CAGGAGAATGGATACGGCAGA; A1 reverse, CAGATCTGTCCTGTCATCTGCAG; probe (MGB), TCTTCCCAACCTCCA.
Western blotting and nuclear c-Rel levels
B cells were purified after complement lysis of T cells, as previously described.20 Lysates from 3 × 106 splenic B cells per lane were analyzed by immunoblot with anti-c-Rel, anti-Rel-A (Santa Cruz), or antitubulin antibodies (Sigma), as previously described.26 Nuclear extracts were prepared from splenic B cells using Nuclear Extract kit (Active Motif, Carlsbad, CA) according to the manufacturer's instruction, and 5 μg was assessed for active c-Rel using TransAM NF-κB family kit (Active Motif).
Generation of chimeric mice
Bone marrow cells from 6-week-old mice of the indicated genotype were injected into the tail veins of irradiated (6 Gy) Rag γc-deficient mice (3 × 106cells/mouse). Six weeks later B-cell development was analyzed.
Results
Vav proteins are required for peripheral B-cell maturation
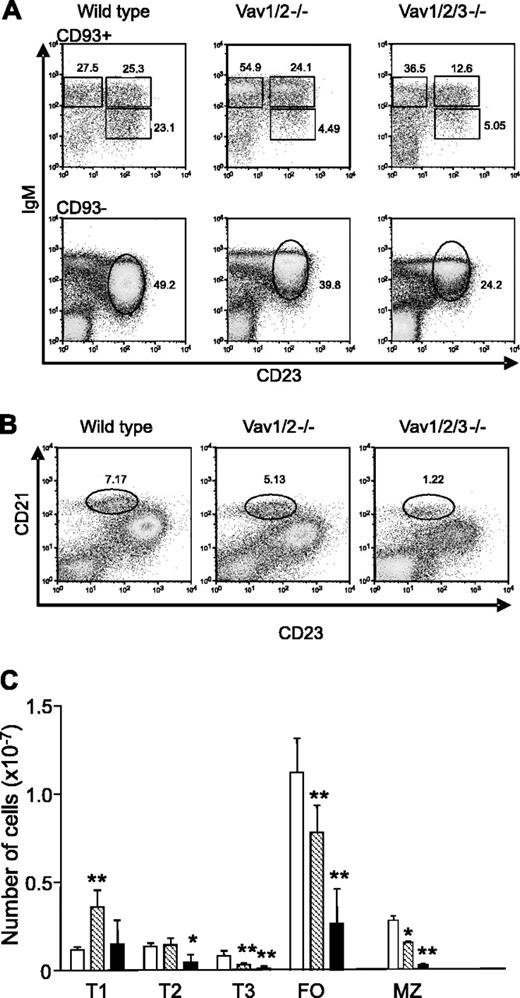
Mice lacking different combinations of Vav proteins have normal numbers of pre-B cells and immature bone marrow B cells (Fujikawa et al19 and data not shown). However, mice deficient in Vav-1 and Vav-2 or mice lacking all 3 Vav proteins (hereafter referred to as Vav-deficient mice) have reduced numbers of mature B cells.19-21 To more precisely define the stage at which peripheral B-cell development was blocked in Vav-deficient mice, we used a recently described approach that resolves immature splenic B cells into 3 nonproliferative subsets.27 Antibody AA4.1 recognizes C1qRp, designated CD93, which is expressed on newly formed B cells in the spleen. These immature B cells can be further distinguished by expression of CD23 and IgM. IgM levels decrease as B cells mature, whereas CD23 levels increase27 (Figure 1A). The numbers of immature B cells in the spleens of B10.BR mice were similar to those previously described for BALB/c and A/J mice (Figure 1C).27,28 The T1 subset was increased in the Vav-1/Vav-2 double-deficient mice, whereas the T2 subset was normal. However, the number of T3 cells was significantly reduced (Figure 1A-C). In the spleens of Vav-deficient mice, the numbers of T1 cells were normal. However, T2-cell numbers were significantly reduced, and the numbers of T3 cells were much lower than in the Vav-1/Vav-2 mutant mice (Figure 1C). The splenic CD93- population of normal mice includes FO and marginal zone (MZ) B cells, which can be distinguished on the basis of IgM and CD23 expression. In the Vav-deficient mice, mature FO B-cell numbers were greatly reduced (Figure 1A-C). However, we observed defective down-regulation of IgM levels on B cells that otherwise displayed an FO phenotype in Vav-1/Vav-2 double-mutant mice, an effect that was exacerbated on cells from Vav-deficient mice (Figure 1A). The definition of MZ B cells as IgMhigh and CD23low thus yielded a mixed population, which was readily apparent from the lack of uniformly high staining for CD1d, a marker of MZ B cells (data not shown). Therefore, we counted MZ B cells as CD93-, CD21high, CD23low (Figure 1B). As shown in Figure 1B-C, in the absence of Vav-1 and Vav-2, the numbers of MZ B cells were decreased, and further significant reductions were observed in the Vav-deficient mice. It is also evident from Figure 1B that the levels of CD21 were lower on B cells from the Vav-deficient mice. These results are consistent with the existence of multiple selection points during peripheral B-cell maturation.27 The T1-T2 transition is thought to correspond to selection for follicular entry and is impaired in the absence of all 3 Vav proteins but not in the Vav-1/Vav-2 double mutant. By contrast, the loss of Vav function profoundly impairs the T2-T3 transition, indicating an important role for Vav proteins at this stage of selection. The absence of Vav proteins does not appear to selectively block differentiation of immature B cells into MZ or FO subsets.
Peripheral B-cell maturation in Vav-deficient mice. Flow cytometric analysis of splenocytes gated for lymphocytes by forward and side scatter. (A) Cells from the indicated genotype were stained for CD93, CD23, and IgM. CD93+ cells were further analyzed by the expression of IgM and CD23. Gates indicate T1 (IgMhigh/CD23low), T2 (IgMhigh/CD23high), and T3 (IgMlow/CD23high) B cells. Numbers indicate the percentage of cells falling into each gate. CD93- cells were further subdivided into FO mature (CD23high, IgM+). Percentages of cells falling in the mature gate are shown. (B) Cells from the indicated genotypes were stained with anti-CD21 and anti-CD23 antibodies. Percentages refer to cells in the MZ B-cell gate (CD21high/CD23low). (C) Absolute numbers of T1, T2, T3, mature FO, and MZ (defined as CD21high, CD23low) cells. Results are presented as mean ± SD, respectively, from wild-type (10 mice; □), Vav-1/Vav-2 (10 mice; ▧), and Vav-deficient mice (4 mice; ▪). Significant differences between wild-type and Vav-1/Vav-2 mutant mice or wild-type and Vav-deficient mice are indicated. *P < .02; **P < .0001. Statistical analysis was performed using the Student t test.
Peripheral B-cell maturation in Vav-deficient mice. Flow cytometric analysis of splenocytes gated for lymphocytes by forward and side scatter. (A) Cells from the indicated genotype were stained for CD93, CD23, and IgM. CD93+ cells were further analyzed by the expression of IgM and CD23. Gates indicate T1 (IgMhigh/CD23low), T2 (IgMhigh/CD23high), and T3 (IgMlow/CD23high) B cells. Numbers indicate the percentage of cells falling into each gate. CD93- cells were further subdivided into FO mature (CD23high, IgM+). Percentages of cells falling in the mature gate are shown. (B) Cells from the indicated genotypes were stained with anti-CD21 and anti-CD23 antibodies. Percentages refer to cells in the MZ B-cell gate (CD21high/CD23low). (C) Absolute numbers of T1, T2, T3, mature FO, and MZ (defined as CD21high, CD23low) cells. Results are presented as mean ± SD, respectively, from wild-type (10 mice; □), Vav-1/Vav-2 (10 mice; ▧), and Vav-deficient mice (4 mice; ▪). Significant differences between wild-type and Vav-1/Vav-2 mutant mice or wild-type and Vav-deficient mice are indicated. *P < .02; **P < .0001. Statistical analysis was performed using the Student t test.
Vav-deficient B cells are short-lived
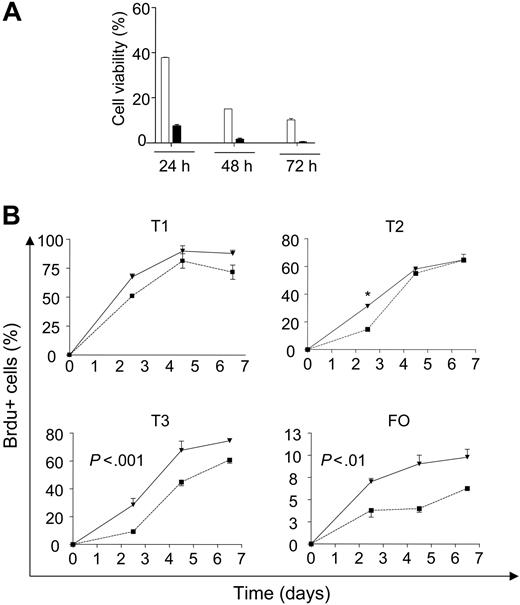
We observed a significantly higher rate of death on in vitro culture of Vav-deficient splenic B cells (data not shown), an observation that was recapitulated on culture of sorted mature B cells (Figure 2A). To determine whether Vav-deficient B cells were turning over faster in vivo, we measured the percentage of BrdU-labeled cells in the spleens after administration of BrdU in the drinking water. Previous studies of transitional B-cell populations have revealed sequential labeling of T1-T3 subsets. Consistent with previous observations,27 we observed sequential labeling of T1-T3 followed by mature B cells in wild-type mice (Figure 2B). In Vav-deficient mice, the labeling of the T1 population or its bone marrow precursors was not significantly different (Figure 2B and data not shown). By contrast, a significantly greater fraction of T2 cells was labeled at the earliest time point in the Vav-deficient mice. At the later time points, all T2 cells were labeled equivalently among the different groups of mice, whereas the T3 and mature FO populations were labeled more efficiently at all time points in the Vav-deficient mice (Figure 2B). The increased numbers of BrdU-positive cells in Vav-deficient mice could indicate faster transition from T2 to T3 or T3 to mature cells. Alternatively, this can be interpreted as reduced survival of Vav-deficient cells. Taken together with the in vitro increased death rate of Vav-deficient mature B cells, we favored the hypothesis that the T2, T3, and mature populations died at an accelerated rate in the spleens of Vav-deficient mice.
Reduced c-Rel and Rel-A levels in Vav-deficient B cells
To determine the consequences of Vav deficiency for constitutive NF-κB activity in resting B cells, we analyzed the expression of nuclear c-Rel containing DNA-binding complexes using a transcription factor enzyme-linked immunosorbent assay (ELISA). This assay uses plate-bound oligonucleotides to capture DNA-binding proteins, which are then revealed by antibody binding. Analysis of nuclear extracts from Vav-deficient B cells demonstrated NF-κB complexes containing c-Rel were reduced when compared with the levels found in wild-type B cells (Figure 3A).
Increased turnover of B cells from Vav-deficient mice. (A) Sorted mature B cells (CD93-, CD23+, IgM+) from wild-type and Vav-deficient mice were incubated in culture medium for the indicated time points. Viability was assessed by propidium iodide (PI) exclusion. □ indicates wild type; ▪, Vav-deficient. (B) Wild-type or Vav-deficient mice were continuously administered BrdU and analyzed using antibodies for CD93, IgM, CD23, and BrdU at the indicated time points. CD93+ and CD93- cells were subdivided using IgM and CD23 levels, as shown in Figure 1A-B, and the percentage of BrdU-positive cells was calculated within each gate. Each data point represents the mean ± SD of measurements from 3 mice. (▪) Wild-type mice. (▾) Vav-deficient mice. Statistical analysis for T2 cells was performed using the Student t test; *P < .001. Two-way ANOVA of T3 subset produced the following results: wild-type mean, 38.3; Vav-/- mean, 57.1; SE, 1.31; P < .001. For mature FO B cells: wild-type mean, 4.7; Vav-/- mean, 10.5; SE, 1.34; P < .01. Details of the application of ANOVA are described in “Materials and methods.”
Increased turnover of B cells from Vav-deficient mice. (A) Sorted mature B cells (CD93-, CD23+, IgM+) from wild-type and Vav-deficient mice were incubated in culture medium for the indicated time points. Viability was assessed by propidium iodide (PI) exclusion. □ indicates wild type; ▪, Vav-deficient. (B) Wild-type or Vav-deficient mice were continuously administered BrdU and analyzed using antibodies for CD93, IgM, CD23, and BrdU at the indicated time points. CD93+ and CD93- cells were subdivided using IgM and CD23 levels, as shown in Figure 1A-B, and the percentage of BrdU-positive cells was calculated within each gate. Each data point represents the mean ± SD of measurements from 3 mice. (▪) Wild-type mice. (▾) Vav-deficient mice. Statistical analysis for T2 cells was performed using the Student t test; *P < .001. Two-way ANOVA of T3 subset produced the following results: wild-type mean, 38.3; Vav-/- mean, 57.1; SE, 1.31; P < .001. For mature FO B cells: wild-type mean, 4.7; Vav-/- mean, 10.5; SE, 1.34; P < .01. Details of the application of ANOVA are described in “Materials and methods.”
To establish whether Vav proteins were required for normal expression of NF-κB subunits, which are themselves autoregulated by NF-κB, we measured mRNA levels in sorted mature B cells by real-time PCR. Levels of c-Rel, Rel-A, IκBα, and NF-κB1 mRNA were decreased in Vav-deficient mature B cells (Figure 3B). However, the levels of CXCR5 and NF-κB2 mRNA were unchanged. Furthermore, levels of c-Rel and Rel-A protein were reduced in lysates of unfractionated B cells from Vav-deficient mice (Figure 3C). In agreement with this, the levels of c-Rel were lower in Vav-deficient B cells at all stages of development (Figure 3D). These data indicate that Vav proteins are required for the maintenance of normal levels of c-Rel-containing DNA-binding complexes, a major component of the constitutive NF-κB activity in resting B cells.29-32
Vav proteins are required for normal expression of Bcl-2 and A1 in B cells
Bcl-2 family members play an essential role in maintaining cell survival during B-cell development. Bcl-2 expression is very low in immature IgMhigh IgDlow B cells but much higher in IgMlow IgDhigh mature B cells.33-35 Similarly, A1 levels are much higher in mature B cells, defined as HSAlow B220high, when compared with immature splenic B cells (HSAhigh B220low IgD-).36 In addition, deficiency of Mcl-1 compromises peripheral B-cell survival.37 Therefore, we assessed the levels of Bcl-2, A1, and Mcl-1 mRNA in mature B cells from wild-type and Vav-deficient mice. Vav-deficient mature B cells showed reduced expression of Bcl-2 and A1 mRNA (Figure 4A). Mcl-1 and CXCR5 levels, by contrast, were normal (Figure 4A). We more precisely defined the developmental stage at which Bcl-2 was increased by measuring the levels of Bcl-2 protein in transitional B-cell subsets. This analysis was restricted to Bcl-2 because of the lack of reagents for measuring A1 and Mcl-1 by flow cytometry. Bcl-2 levels were very low in T1 cells but were greatly increased in T2, T3, and FO B-cell subsets (Figure 4B). Bcl-2 levels in T1 cells from Vav-deficient mice were as low as in the wild-type mice. However, the increased expression of Bcl-2 that accompanied further maturation was greatly reduced in the Vav-deficient B cells (Figure 4B). Thus, Vav function was required for normal Bcl-2 expression during B-cell maturation.
Bcl-XL, another antiapoptotic member of the Bcl-2 family, is expressed at high levels in immature bone marrow B cells and at lower levels in naive FO B cells.34,38 Using intracellular staining, we found that the highest levels of Bcl-XL were in T1 cells (Figure 4B). Lower levels of Bcl-XL were found in T2, T3, and mature FO B cells of wild-type mice, indicating that Bcl-XL levels were decreased on the T1-T2 transition. We detected no differences in the levels of Bcl-XL between wild-type and Vav-deficient B cells at any stage of development (Figure 4B). The high levels of Bcl-XL expression in T1 may contribute to the survival of Vav-deficient T1 cells. We also measured levels of the chemokine receptor CXCR5 on transitional and mature B-cell subsets. Figure 4B shows that CXCR5 was expressed at low levels on T1 cells but was increased on T2 and FO cells, which reside within B-cell follicles.39,40 The levels of CXCR5 were similar on wild-type and Vav-deficient B cells. Taken together, these data indicate that Vav deficiency selectively impairs the developmental increase in Bcl-2 and A1 expression but does not globally affect the changes in gene expression that accompany peripheral B-cell maturation.
NF-κB activity is affected in the absence of Vav proteins. (A) c-Rel abundance in nuclear extracts from wild-type (□) or Vav-deficient (▪) B cells. Results are expressed relative to the levels found in wild-type cells and correspond to the mean ± SD of B cells from 3 independent mice. (B) mRNA levels of NF-κB subunits from sorted wild-type (□) and Vav-deficient (▪) mature B cells were compared by real-time PCR. CXCR5 expression was included as control because it is expressed at similar levels in wild-type and Vav-deficient cells (Figure 4B). (C) Purified splenic B cells from wild-type or Vav-deficient cells were blotted with antibodies against c-Rel, Rel-A, or tubulin (loading control). (D) Spleen cells were stained with antibodies against c-Rel, CD93, IgM, and CD23, and subsets are defined as indicated in Figure 1A-B. In the histogram overlays, the solid line represents Vav-deficient cells, the broken line represents wild-type cells, and the shaded histograms correspond to the isotype control. Data are representative of 5 mice from each group.
NF-κB activity is affected in the absence of Vav proteins. (A) c-Rel abundance in nuclear extracts from wild-type (□) or Vav-deficient (▪) B cells. Results are expressed relative to the levels found in wild-type cells and correspond to the mean ± SD of B cells from 3 independent mice. (B) mRNA levels of NF-κB subunits from sorted wild-type (□) and Vav-deficient (▪) mature B cells were compared by real-time PCR. CXCR5 expression was included as control because it is expressed at similar levels in wild-type and Vav-deficient cells (Figure 4B). (C) Purified splenic B cells from wild-type or Vav-deficient cells were blotted with antibodies against c-Rel, Rel-A, or tubulin (loading control). (D) Spleen cells were stained with antibodies against c-Rel, CD93, IgM, and CD23, and subsets are defined as indicated in Figure 1A-B. In the histogram overlays, the solid line represents Vav-deficient cells, the broken line represents wild-type cells, and the shaded histograms correspond to the isotype control. Data are representative of 5 mice from each group.
Bcl-2 and A1 levels are reduced in Vav-deficient B cells. (A) (▪) Levels of A1, Bcl-2, Mcl-1, and CXCR5 mRNA in Vav-deficient mature B cells. (□) Wild-type levels. (B) Protein levels expressed by B-cell subsets. Spleen cells from wild-type or Vav-deficient mice were stained for Bcl-2, Bcl-XL, or CXCR5 in combination with anti-CD93, anti-CD23, and anti-IgM. Bar graphs show the mean fluorescence intensity (MFI) (± SD) of Bcl-2, Bcl-XL, and CXCR5 levels from the splenic B-cell subsets for 4 mice of each genotype. Protein levels were calculated subtracting the MFI of the isotype control from the MFI of the protein of interest. Protein expression in the spleen is represented as follows: T1 (open columns); T2 (stippled columns); T3 (gray columns); mature FO (hatched columns). Significant differences between wild-type and Vav-deficient mice are indicated by asterisks (*P < .01). Statistical analysis was performed using the Student t test.
Bcl-2 and A1 levels are reduced in Vav-deficient B cells. (A) (▪) Levels of A1, Bcl-2, Mcl-1, and CXCR5 mRNA in Vav-deficient mature B cells. (□) Wild-type levels. (B) Protein levels expressed by B-cell subsets. Spleen cells from wild-type or Vav-deficient mice were stained for Bcl-2, Bcl-XL, or CXCR5 in combination with anti-CD93, anti-CD23, and anti-IgM. Bar graphs show the mean fluorescence intensity (MFI) (± SD) of Bcl-2, Bcl-XL, and CXCR5 levels from the splenic B-cell subsets for 4 mice of each genotype. Protein levels were calculated subtracting the MFI of the isotype control from the MFI of the protein of interest. Protein expression in the spleen is represented as follows: T1 (open columns); T2 (stippled columns); T3 (gray columns); mature FO (hatched columns). Significant differences between wild-type and Vav-deficient mice are indicated by asterisks (*P < .01). Statistical analysis was performed using the Student t test.
Expression of Bcl-2 partially restores B-cell development in Vav-deficient mice
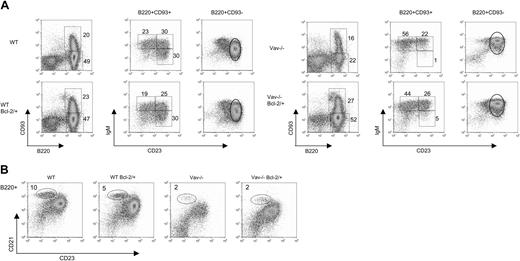
We next determined whether enforced expression of Bcl-2 restored the developmental defect observed in Vav-deficient mice. To this end, Vav-deficient bone marrow progenitor cells expressing or not expressing transgenic Bcl-2 were used for reconstitution of Rag γc nonlymphoid mice. Analysis of splenic B cells from the radiation chimeras reconstituted with Vav-deficient stem cells indicates that the defects observed in the numbers of T2, T3, mature follicular, and marginal zone Vav-deficient B cells are intrinsic to hematopoietic cells (compare Figure 1 with Figures 5 and 6).
The BCL-2 transgene increased the number of B cells in wild-type and Vav-deficient mice to similar levels (Figure 6). However, the overexpression of Bcl-2 led to slightly different effects in wild-type or Vav-deficient cells. Bcl-2 transgenic wild-type mice had increased levels of T2, T3, and mature FO B cells, but normal numbers of T1 and MZ B cells (Figure 6). The proportion of mature to immature B cells was not affected in Bcl-2 transgenic wild-type cells (Figure 5). In contrast, the overexpression of Bcl-2 in Vav-deficient cells promoted the maturation of B cells as the ratio of mature to immature B cells reached the level of wild-type cells (Figure 5A). Consequently, the number of mature FO B cells dramatically rose to levels similar to those of Bcl-2 transgenic wild-type cells (Figure 6). We also observed recovery in the number of MZ B cells, though not to the levels observed in wild-type cells. Overexpression of Bcl-2 also affected the number of B cells of the transitional subsets. The proportion of T1 cells in Bcl-2 transgenic Vav-deficient mice was still higher than it was in wild-type cells (Figure 5), which led to an accumulation of T1 cells, an effect that was not observed in the wild-type counterparts (Figure 6). The number of T2 cells was also elevated, but no changes were observed in the T3 subset. We conclude that Bcl-2 overexpression drives the maturation of Vav-deficient B cells, as judged by decreased levels of CD93. However, this process is still defective, as indicated by the inability of mature Vav-deficient cells to down-regulate IgM levels (Figure 5).
Overexpression of Bcl-2 partially rescues B-cell development of Vav-deficient cells. Recipient mice were reconstituted with bone marrow cells from Vav-deficient mice with or without expressing transgenic Bcl-2 (7 and 8 mice, respectively). Wild-type mice carrying or not carrying the Bcl-2 transgene were used as controls (5 mice each). Spleen cells were analyzed at 6 weeks after reconstitution. (A) B cells of the indicated genotype were stained and analyzed as described in Figure 1. (B) B cells identified as B220+ were further analyzed by staining using antibodies against CD23 and CD21. Numbers shown represent the percentages of B cells falling within the regions illustrated.
Overexpression of Bcl-2 partially rescues B-cell development of Vav-deficient cells. Recipient mice were reconstituted with bone marrow cells from Vav-deficient mice with or without expressing transgenic Bcl-2 (7 and 8 mice, respectively). Wild-type mice carrying or not carrying the Bcl-2 transgene were used as controls (5 mice each). Spleen cells were analyzed at 6 weeks after reconstitution. (A) B cells of the indicated genotype were stained and analyzed as described in Figure 1. (B) B cells identified as B220+ were further analyzed by staining using antibodies against CD23 and CD21. Numbers shown represent the percentages of B cells falling within the regions illustrated.
Increased numbers of B cells in Bcl-2 transgenic Vav-deficient mice. Scatter plots show the absolute numbers of B, T1, T2, T3, mature FO, and MZ B cells based on the data presented in Figure 5. Mice genotypes are represented as follows: wild-type (▪, 5 mice), Bcl-2 transgenic wild-type (□, 5 mice), Vav-deficient (▾, 7 mice), and Bcl-2 transgenic Vav-deficient (▿, 8 mice). Cell numbers were calculated using the regions illustrated in Figure 5. Horizontal bars indicate the mean for each group of observations. Statistical analysis was performed using ANOVA. The hypothesis of normal distribution was confirmed for all the samples using the Kolmogorov-Smirnov test. *P < .05; **P < .01; ***P < .001. n.s. indicates not significant.
Increased numbers of B cells in Bcl-2 transgenic Vav-deficient mice. Scatter plots show the absolute numbers of B, T1, T2, T3, mature FO, and MZ B cells based on the data presented in Figure 5. Mice genotypes are represented as follows: wild-type (▪, 5 mice), Bcl-2 transgenic wild-type (□, 5 mice), Vav-deficient (▾, 7 mice), and Bcl-2 transgenic Vav-deficient (▿, 8 mice). Cell numbers were calculated using the regions illustrated in Figure 5. Horizontal bars indicate the mean for each group of observations. Statistical analysis was performed using ANOVA. The hypothesis of normal distribution was confirmed for all the samples using the Kolmogorov-Smirnov test. *P < .05; **P < .01; ***P < .001. n.s. indicates not significant.
Vav proteins regulate NF-κB activation in response to BCR signaling
In addition to the developmental defect observed in the absence of Vav proteins, calcium responses after BCR cross-linking or BCR and CD19 co-ligation are impaired in Vav-deficient B cells.19,22 We, therefore, assessed whether Vav proteins were required for NF-κB induction in response to BCR cross-linking. The increase in c-Rel expression that followed BCR cross-linking in mature B cells was defective in Vav-deficient mature B cells (Figure 7A). Furthermore, BCR-stimulated accumulation of c-Rel-containing nuclear complexes was defective in Vav-deficient B cells (Figure 7B). We next assessed Iκ-Bα phosphorylation using a phosphospecific antibody. BCR cross-linking stimulated IκBα phosphorylation in B cells from wild-type and Vav-1 or Vav-2 mutant mice (Figure 7C). Vav-deficient mice were not used in this experiment because of technical limitations arising from the low number of B cells recovered. Instead, we used B cells from Vav-1- and Vav-2-deficient mice that show a similar but less severe phenotype19 (Figure 1). BCR cross-linking failed to stimulate significant IκBα phosphorylation in Vav-1 and Vav-2 B cells (Figure 7C). Taken together, these data suggest that BCR stimulation of Vav-deficient B cells fails to properly activate the NF-κB pathway.
Discussion
Our results show that peripheral B-cell maturation in the mouse requires Vav function. Deficiency in all 3 Vav proteins does not affect the expansion of pre-B cells or the generation of immature B cells in the bone marrow. Instead, the number of immature B cells in the periphery is affected. Previous analysis of Vav-deficient mice using the combination of CD21, CD23, IgM, and IgD staining defined the stage at which peripheral B-cell development was blocked as the CD21high, IgDhigh, IgMhigh stage,19 which corresponds to transitional 2B cells according to Loder et al.41 However, those cells include cycling cells41 and cells that respond to BCR cross-linking by undergoing proliferation.42,43 Such cells may represent partially activated B cells. The reduction in this population could, therefore, be a reflection of a well-documented role of Vav proteins in antigen receptor-mediated proliferation.18 The use of AA4.1 to identify CD93+ B cells in the spleen allowed us to separate 3 nonproliferating, immature, peripheral B-cell subsets from activated, MZ, and FO subsets, whose presence complicates analysis by other staining strategies. Numbers of T1 cells were not reduced in the absence of Vav, suggesting that Vav proteins are dispensable for the egress of immature B cells from the bone marrow and their homing to the spleen. A recent study44 has demonstrated that B cells lacking Rac1 and Rac2 have severely impaired peripheral B-cell maturation in the spleen. Although the authors have used a different method for enumerating peripheral B cells, Rac1/2 mutant mice, unlike Vav-deficient mice, have greatly reduced numbers of T1 cells. These data suggest Rac proteins exert their crucial effects at an earlier developmental stage than Vav proteins. Rac deficiency would uncouple multiple Rac GEFs from their downstream effectors in B-lymphocytes, some of which have been shown to influence B-cell development and responses.45-49
The spleens of Vav-1/Vav-2 mutant mice contain normal numbers of T2 cells, whereas deficiency in all 3 Vav proteins led to significantly reduced numbers of T2 cells. Taken together with our BrdU findings, these data indicate that the T2-cell losses caused by the absence of the 3 Vav proteins were caused by a survival defect. We have excluded deficiencies in migration into the B-cell follicles because increased expression of CXCR5, which correlates with follicular entry, occurred normally in the absence of Vav. Moreover, we could readily identify B-cell follicles by histologic analysis (Sarah Bell, E.V., and M.T., unpublished data, September 2003). The numbers of T3, mature FO, and MZ B cells are reduced in Vav-1/Vav-2 double-mutant mice and are further reduced in the absence of all 3 Vav proteins. Thus, Vav proteins are critical for the T2-T3 transition and appear to function at the level of survival. Because Vav-1 deficiency leads to a dramatic reduction in B1 cells, we conclude Vav proteins are required by all B-cell subsets from T2 onward. Vav proteins appear to participate in the generation of a signal required to promote B-cell survival.
NF-κB activity induced by BCR ligation is impaired in the absence of Vav proteins. (A) Splenocytes from wild-type and Vav-deficient mice (3 mice per group) were incubated with or without 10 μg/mL Fab2 α-IgM for 16 hours. Subsequently, mature B cells were identified as HSAlow, CD21+, or CD23+, and c-Rel levels were analyzed by flow cytometry. (□) Cells incubated in culture medium. (▪) Stimulated cells. (B) B cells from wild-type mice (□) or Vav-deficient (▪) were stimulated as in panel A. Subsequently, nuclear extracts were prepared, and c-Rel nuclear complexes were measured and represented as indicated in Figure 3D. (C) Splenic B cells from wild-type, Vav-1-, Vav-2-, or Vav-1/2-deficient mice were stimulated with 10 μg/mL Fab2 α-IgM for the indicated time points. Phosphorylation status of IκBα (p-IκBα) was analyzed by Western blot. Stripping and reprobing with α-IκBα antibodies was used as loading control. Error bars correspond to standard deviation.
NF-κB activity induced by BCR ligation is impaired in the absence of Vav proteins. (A) Splenocytes from wild-type and Vav-deficient mice (3 mice per group) were incubated with or without 10 μg/mL Fab2 α-IgM for 16 hours. Subsequently, mature B cells were identified as HSAlow, CD21+, or CD23+, and c-Rel levels were analyzed by flow cytometry. (□) Cells incubated in culture medium. (▪) Stimulated cells. (B) B cells from wild-type mice (□) or Vav-deficient (▪) were stimulated as in panel A. Subsequently, nuclear extracts were prepared, and c-Rel nuclear complexes were measured and represented as indicated in Figure 3D. (C) Splenic B cells from wild-type, Vav-1-, Vav-2-, or Vav-1/2-deficient mice were stimulated with 10 μg/mL Fab2 α-IgM for the indicated time points. Phosphorylation status of IκBα (p-IκBα) was analyzed by Western blot. Stripping and reprobing with α-IκBα antibodies was used as loading control. Error bars correspond to standard deviation.
Substantial levels of NF-κB activity are found in naive B cells. This NF-κB activity is important for B-cell survival because Iκ-B kinase function is required for the survival of mature B cells.50 Vav-deficient B cells have decreased expression of mRNA for NF-κB subunits and decreased c-Rel and Rel-A protein levels. Defective expression of c-Rel and Rel-A have previously been associated with defective B-cell development and survival.35,51,52 Moreover, as in Vav-deficient B cells, Bcl-2 and CD21 levels are reduced in B cells lacking c-Rel and Rel-A.35 Given that NF-κB has been reported to regulate Bcl-253-56 and A1 expression,57 it is likely that the abnormal NF-κB levels contribute to defective Bcl-2 and A1 gene expression in naive Vav-deficient B cells. It is notable that the phenotype of B cells from c-Rel/Rel-A double-mutant mice is similar to the phenotype of Vav-deficient, PLCγ2-/-, BCAP-/-, or PU1+/-SpiB-/- B cells.35,51,52,58-60 The molecular basis for the reduction in the levels of c-Rel or Rel-A may reflect a dependence on Vav for the generation of constitutive levels of NF-κB in resting naive B cells. In resting B cells, this is maintained by a proteasome-independent, Ca2+-mediated pathway.16 Because c-Rel is itself an NF-κB-responsive gene,61 the reduced expression we have observed is likely a reflection of low constitutive NF-κB activity. Consistent with this, the levels of other NF-κB-regulated genes were also reduced. Given that Vav proteins are well established in the control of calcium signaling after acute ligation of the antigen receptor, we suggest that in resting naive B cells, Vav transduces a BCR signal by calcium to NF-κB. Such a hypothesis is supported by the observation that NF-κB responds to transient calcium oscillations separated by at least 30 minutes.62 Such a hypothesis is also consistent with the calcium dependence of the constitutive NF-κB activity,16 though it remains possible that Vav may regulate other aspects of NF-κB signaling—for example, through association with IκB kinase (IKK).63 The defect in NF-κB activity in the absence of Vav is not limited to resting naive B cells because we observed defective IκBα phosphorylation, c-Rel induction, and translocation of NF-κB complexes to the nucleus after ligation of the BCR. These defects most likely underlie the impaired proliferative and immune responses of Vav-deficient B cells.19
It is well established that the expression of Bcl-2 family members is essential for the survival of immature and mature B cells. During the maturation of B cells, Bcl-XL protein levels decreased at the T1-T2 transition while Bcl-2 expression increased. Vav-deficient B cells down-regulated Bcl-XL to levels equivalent to those of wild-type B cells but failed to express normal levels of Bcl-2. The significant cell losses observed in the absence of Vav proteins occurred after the T1-T2 transition, suggesting Bcl-XL levels may be sufficient to protect Vav-deficient T1 but not subsequent developmental stages, which may depend more greatly on Bcl-2. Such a suggestion is consistent with the existence of a tonic BCR signal required for peripheral B-cell survival.5 Moreover, Bcl-2 overexpression in Vav-deficient B cells partially rescued B-cell development of Vav-deficient cells. We observed a partial recovery of the numbers of MZ B cells. More strikingly, we found a significant increase in the number of mature FO B cells to levels similar to those of Bcl-2 transgenic wild-type mice. Cell recovery driven by the overexpression of Bcl-2 correlated with increased in vitro survival of Bcl-2 transgenic cells. Despite the profound effect of Bcl-2 on the recovery of cell numbers, mature cells from Bcl-2 transgenic Vav-deficient mice still expressed high levels of surface IgM, suggesting additional Vav-dependent signaling pathways for the down-regulation of surface IgM. Taken together, our results suggest Vav proteins contribute to the propagation of a constitutive, or tonic, survival signal generated by the BCR. This signal acts through NF-κB and leads to the expression of the antiapoptotic molecules Bcl-2 and A1.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2004-12-4894.
Supported by a Senior Fellowship from the Medical Research Council (M.T.) and a Biotechnology and Biological Sciences Research Council project grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs David Allman and Victor Tybulewicz for generously providing reagents, the animal facility staff, Helen Reynolds and Katie Welch for technical support, Geoff Morgan for expert assistance with flow cytometry, Eurof Walters for advice on statistical analysis, and members of the laboratory for advice and comments on the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal