Abstract
Indoleamine 2,3-dioxygenase (IDO) is the rate-limiting enzyme in the kynurenine pathway of tryptophan metabolism. IDO activity is linked with immunosuppression by its ability to inhibit lymphocyte proliferation, and with neurotoxicity through the generation of quinolinic acid and other toxins. IDO is induced in macrophages by HIV-1 infection, and it is up regulated in macrophages in human brain tissue with HIV-1 encephalitis (HIVE). Using a model of HIVE, we investigated whether IDO inhibitor 1-methyl-d-tryptophan (1-MT) could affect the generation of cytotoxic T lymphocytes (CTLs) and clearance of virus-infected macrophages from the brain. Severe combined immunodeficient mice were reconstituted with human peripheral blood lymphocytes, and encephalitis was induced by intracranial injection of autologous HIV-1-infected monocyte-derived macrophages (MDMs). Animals treated with 1-MT demonstrated increased numbers of human CD3+, CD8+, CD8+/interferon-γ+ T cells, and HIV-1gag/pol-specific CTLs in peripheral blood compared with controls. At week 2 after MDM injection in the basal ganglia, mice treated with 1-MT showed a 2-fold increase in CD8+ T lymphocytes in the areas of the brain containing HIV-1-infected MDMs compared with untreated controls. By week 3, 1-MT-treated mice showed 89% reduction in HIV-infected MDMs in brain as compared with controls. Thus, manipulation of immunosuppressive IDO activity in HIVE may enhance the generation of HIV-1-specific CTLs, leading to elimination of HIV-1-infected macrophages in brain.
Introduction
The pathogenesis of HIV-1 infection is linked to dysfunction and depletion of CD4+ T lymphocytes.1-3 The virus persists and disseminates over years, despite an apparently intact host immune response. The inability to eliminate HIV-1 suggests that negative-regulatory (tolerogenic) signals may shield HIV-1 from adaptive immune clearance.4,5 However, the specific mechanisms by which the virus might protect itself from clearance remain unresolved.
HIV-1 is known to persist at low levels within the central nervous system (CNS) during most of the disease course.6 Significant productive HIV-1 replication occurs in brain mononuclear phagocytes (MPs; perivascular macrophages and microglia) during late stages of infection only in a subset of individuals with severe immune suppression and high peripheral viral loads. Secretory viral and cellular products from HIV-1-infected and immune competent brain MPs are known to induce neuronal dysfunction and injury.7-9 These include virotoxins such as Tat, Nef, and gp120 and cellular toxins such as proinflammatory cytokines, chemokines, arachidonic acid and its metabolites, platelet activating factor, nitric oxide, and quinolinic acid. Indoleamine 2,3-dioxygenase (IDO) is the first and the rate-limiting enzyme in the generation of quinolinic acid from tryptophan via the kynurenine pathway.10 An increase of functional IDO enzymatic activity in the brain could lead to enhanced production of neurotoxins, resulting in neurocognitive dysfunction and HIV-1-associated dementia (HAD).11-13 Signs of increased IDO activity correlate with tryptophan depletion, progression of systemic and brain HIV-1 infection, and HAD.14,15 Accumulating evidence suggests that IDO serves immunoregulatory and tolerogenic functions.16-21 It appears that certain antigen presenting cells (APCs) may regulate T-cell responses through the expression of IDO.22 A number of studies indicate that IDO overexpression by APCs may result in immune suppression and reduced T-cell responses.17,18,20,23-25 Therefore, HIV-induced IDO activity in the brain may participate not only in local neurotoxicity, but also in the failure of the immune system to clear HIV from this reservoir.
A strong association between HAD and profound immunodeficiency supports the notion that a lack of effective adaptive immune responses is associated with ongoing viral replication in the brain. One plausible explanation is that circulating HIV-1-specific CD8+ cells could be partially anergic and may be unable to eliminate HIV-1-infected cells in vivo in the setting of functionally impaired helper CD4+ T cells during late stages of infection.26,27
Based on these observations, we hypothesize that IDO-expressing APCs (specifically, HIV-1-infected macrophages) might help to create a protected reservoir for HIV-1 persistence in the brain. To test this idea we used a mouse model of HIV-1 encephalitis (HIVE) in which nonobese diabetic-severe combined immunodeficient (NOD/SCID) mice are reconstituted with human peripheral-blood lymphocytes (hu-PBL-NOD/SCID) and intracranially injected with HIV-1-infected monocyte-derived macrophages (MDMs) to induce viral encephalitis. This model recapitulates the cellular immune responses against HIV-1-infected brain macrophages that occur in humans during progressive disease.28,29 Using this model we tested whether pharmacologic inhibition of IDO activity would improve immune responses and promote clearance of virus from the “immunologic reservoir” formed by these tolerogenic APCs. To test this idea, we used humanized NOD/SCID mice treated with 1-methyl-D-tryptophan (1-MT), a competitive inhibitor of IDO.30 We demonstrate that effective elimination of HIV-1-infected MDMs by adaptive immune mechanisms (CTLs) parallels inhibition of IDO. These observations provide new insights toward novel therapeutic strategies for HAD.
Materials and methods
Cell isolation and viral infection
Monocytes and PBLs were obtained by countercurrent centrifugal elutriation of leukopheresis packs from HIV-1-, -2-, and hepatitis B-seronegative donors. Monocytes were cultivated in suspension culture using Teflon flasks in Dulbecco modified Eagle medium (DMEM; Sigma, Saint Louis, MO) supplemented with 10% heat-inactivated pooled human serum, 1% glutamine, 50 μg/mL gentamicin, 10 μg/mL ciprofloxacin (Sigma), and 1000 U/mL highly purified recombinant human macrophage colony stimulating factor (a generous gift from Genetics Institute, Cambridge, MA). After 7 days in culture, MDMs were infected with HIV-1ADA at a multiplicity of infection of 0.01.31
Hu-PBL-NOD/SCID HIVE mice
Four-week-old male NOD/C.B-17 SCID mice were purchased from Jackson Laboratory, Bar Harbor, ME. Animals were maintained in sterile microisolator cages under pathogen-free conditions in accordance with ethical guidelines for care of laboratory animals at the University of Nebraska Medical Center and the National Institutes of Health (NIH). All animals were injected intraperitoneally with rat anti-CD122 (0.25 mg/mouse) 3 days before PBL transplantation and twice with rabbit asialo-GM1 antibodies (0.2 mg/mouse; Wako, Richmond, VA) one day before and 3 days after PBL injection (20 × 106 cells/mouse) in order to deplete mouse natural killer (NK) cells and to facilitate proper engraftment of human lymphocytes. HIV-1ADA-infected MDMs (3 × 105 cells in 10 mL) were injected intracranially (intracerebrally)32 8 days after PBL reconstitution, generating hu-PBL-NOD/SCID HIVE mice. Immediately following intracerebral injection of HIV-1-infected MDMs, the hu-PBL-NOD/SCID HIVE mice were subcutaneously implanted with control (vehicle) or 1-MT pellets (28-day slow release; Innovative Research, Southfield, MI). Two initial experiments (n = 42, Table 1) were designed to confirm the induction of virus-specific CTLs in the hu-PBL-NOD/SCID HIVE animals treated with 1-MT. This was confirmed by tetramer staining and neuropathologic analyses of MDM elimination from the brain tissue. The third experiment (n = 30) was design to analyze human lymphocyte reconstitution, humoral immune responses, and neuropathologic alterations. In these experiments, animals were bled on day 7 and killed at 14 and 21 days after intracerebral injection of human MDMs. Blood collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes was used for flow cytometry and plasma was used for detection of HIV-1 p24 using enzyme-linked immunosorbent assay (ELISA). HIV-1-specific antibodies were detected by Western blot tests according to the manufacturer's instructions (Cambridge Biotech HIV-1 Western blot kit; Calypte Biomedical, Rockville, MD). Similar amounts of virus-specific antibodies were detected in control and 1-MT-treated animals (data not shown). 1-MT concentrations were detected in the brain tissue extracts by high-performance liquid chromatography as described.19 The average 1-MT peak value that could be detectable was 19.5 μM ± 2.23 μM. The concentrations are 30% of those seen in the plasma of mice treated with 1-MT at a similar dose. A total of 3 independent experiments were performed using 3 different human leukocyte donors.
Study design for 1-MT testing in hu-PBL-NOD/SCID HIVE mice
. | Week of killing, n . | . | . | ||
|---|---|---|---|---|---|
| Treatment group . | Week 1 . | Week 2 . | Week 3 . | ||
| Control | 7 | 17 (7)* | 8 (6) | ||
| 1-MT | 8 | 17 (7) | 15 (8) | ||
. | Week of killing, n . | . | . | ||
|---|---|---|---|---|---|
| Treatment group . | Week 1 . | Week 2 . | Week 3 . | ||
| Control | 7 | 17 (7)* | 8 (6) | ||
| 1-MT | 8 | 17 (7) | 15 (8) | ||
Three independent experiments were performed.
The number of mice in the third experiment is shown in parentheses
FACS of peripheral blood and spleen in hu-PBL-NOD/SCID HIVE mice and tetramer staining
Two-color fluorescence-activated cell sorting (FACS) analysis was performed on peripheral blood at weeks 1 to 3 and splenocytes at weeks 2 and 3 after intracerebral injection of human MDMs. Cells were incubated with fluorochrome-conjugated monoclonal antibodies (mAbs) to human CD4, CD8, CD56, CD3, and interferon gamma (IFN-γ; eBioscience, San Diego, CA) for 30 minutes at 4°C. To evaluate the cellular immune response, IFN-γ intracellular staining was performed in combination with anti-human CD8 and fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD45 to exclude murine cells. To determine the antigen (Ag)-specific CTLs, allophycocyanin-conjugated tetramer staining for HIV-1gag (p17 [aa77-85] SLYNTVATL, SL-9) and HIV-1pol ([aa476-485] ILKEPVHGV, IL-9) was performed on phytohemaglutinin/interleukin-2 (PHA/IL-2)-stimulated splenocytes. Single-cell suspensions were activated with PHA-P (1 μg/mL) in RPMI 1640 supplemented with 10% IL-2 (Advanced Biotechnologies, Columbia, MD). On day 11 after stimulation, cells were stained and free of any mouse CD45+ (leukocyte common Ag, Ly-5) contamination. Staining was carried out as per the recommendation of the NIH/National Institute of Allergy and Infections Disease, National Tetramer Core Facilities (Atlanta, GA). Data were analyzed with a FACS Calibur using CellQuest software (Becton Dickinson Immunocytometry System, San Jose, CA).
Histopathology and image analyses
Brain tissue was collected at days 14 and 21 after intracerebral injection of MDMs, fixed in 4% phosphate-buffered paraformaldehyde, and embedded in paraffin or frozen at -80°C for later use. Coronal sections from the embedded blocks were cut in order to identify the injection site. For each mouse, 30 to 100 5-μm-thick serial sections were cut from the human MDM injection site and 3 to 7 slides (10 sections apart) were analyzed. Brain sections were deparaffinized with xylene and hydrated in gradient alcohols. Immunohistochemical staining followed a basic indirect protocol, using antigen retrieval by heating to 95°C in 0.01 M citrate buffer for 30 minutes for antigen retrieval. To identify human cells in mouse brains, mAb to vimentin (1:50, clone 3B4; Dako, Carpenteria, CA), which identifies all human leukocytes, was used.29 Human MDMs and CD8+ lymphocytes were detected with CD68 (1:50 dilution, clone KP 1) and CD8 (1:50 dilution, clone 144B) antibodies, respectively. Virus-infected cells were labeled with mAb to HIV-1 p24 (1:10, clone Kal-1; all from Dako). Reactive murine microglial cells were detected with Iba-1 antibody (1:500; Wako, Richmond, VA). Expression of human IDO (huIDO) was visualized with Abs obtained from the Department of Cell Pharmacology, Central Research Institute, Sapporo, Japan. Primary antibodies were detected with the appropriate biotinylated secondary antibodies and visualized with avidin-biotin complexes (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) or horseradish peroxidase (HRP)-coupled dextran polymer (EnVision; Dako). Immunostained sections were counterstained with Mayer hematoxylin. Sections from which primary antibody was deleted or irrelevant IgG isotype was incorporated served as controls. Two independent observers in a blinded fashion counted the numbers of CD8+ lymphocytes, CD68+ MDMs, and HIV-1 p24+ in each section from each mouse. Light microscopic examination was performed with a Nikon Eclipse 800 microscope (Nikon Instruments, Melville, NY) equipped with a 20 ×/0.5 objective lens. Images were acquired with a digital CCD ColorView II camera (Soft Imaging Systems, Lakewood, CO). Semiquantitative analysis for ionized calcium-binding adapter molecule 1 (Iba1) (percentage of area occupied by immunostaining) was carried out by computer-assisted image analysis (Image-Pro Plus; Media Cybernetics, Silver Spring, MD) as previously described.33
Human brain tissue
Frontal cortex and basal ganglia specimens (frozen and paraffin-embedded) derived from 8 HIVE cases of different severity (6 severe and 2 mild according to previously described criteria34 ), 6 HIV-1-positive brains obtained from subjects without HIVE, and 5 seronegative age-matched controls were used and provided by the National NeuroAIDS Consortium (Washington, DC) and the brain bank within the Center for Neurovirology and Neurodegenerative Disorders (CNND). Approval was obtained from the University of Nebraska Medical Center institutional review board for these studies. Serial paraffin sections (5-μm thickness) from basal ganglia and frontal cortex were immunostained for CD68, HIV-1 p24, CD8, and IDO as previously described. Double staining for CD8/IDO, CD68/IDO, and CD68/HIV-1 p24 was performed as described.29 Numbers of CD8+ T lymphocytes were counted in blinded fashion in 10 consecutive fields (20× objective) in each brain section (basal ganglia and white matter of the frontal cortex).
Western blot assays
Western blot assays were performed on whole-cell protein extracts of human brain homogenates (basal ganglia) and MDM cell extracts using primary Abs against CD68 (Dako), hu-IDO, or α-actin. Briefly, 10 μg lysate protein was loaded onto 1.5-mm-thick 4% to 15% gradient polyacrylamide precast gels (Bio-Rad, Hercules, CA) and electrophoresed under denaturing nonreducing conditions. Proteins from the gels were transblotted onto nitrocellulose membranes (0.45 μm pore size) at 60 volts for 1 hour at room temperature. The membranes were blocked with superblock (Bio-Rad) containing 2% nonfat dry milk for 40 minutes at room temperature. Blots were incubated for 1 hour at room temperature with respective primary antibody diluted 1:10 in superblock solution in 20 mM phosphate-buffered saline, pH 7.4, containing 0.1% Tween-20 (PBST). Primary antibody-reacted blots were washed in 3 5-minute washes of PBST. Immunoreactive bands were detected by luminol detection kit (Pierce, Rockford, IL) after exposure to Kodak x-ray film. The bands were quantified densitometrically on GelExpert as arbitrary volume integration units using the Molecular Dynamics ImageQuant software (Sunnyvale, CA). Results were expressed as ratios of intensities for target proteins and α-actin internal standard.
Statistical analysis
Data were analyzed using Prism (Graph Pad) with Student t test for comparisons and analysis of variance (ANOVA). P values less than .05 were considered significant. All results are presented as means plus or minus standard error of the mean (SEM).
Results
Accumulation and distribution of lymphocytes in HIVE brain tissue
CD8+ T lymphocytes were readily observed infiltrating the brain parenchyma adjacent to HIV-1-infected brain MPs in brain tissues obtained from autopsies of patients who died of HIVE. There were significantly more CD8+ T lymphocytes in brain tissues from patients with HIVE (n = 8) than in brains of HIV-1-seropositive patients without evidence of encephalitis (n = 6), or to seronegative controls (n = 5). Human brain tissue from HIV-1-seropositive patients without evidence of HIVE showed CD8+ T lymphocytes (purple) only in areas of perivascular cuffing (Figure 1A, insert). Mild cases of HIVE (Figure 1A) showed higher numbers of CD8+ T lymphocytes (purple) both in perivascular spaces and within the neuropil surrounding HIV-1 p24+ MPs (brown). More extensive infiltration of CD8+ T lymphocytes into neuropil was observed in areas containing virus-infected macrophages and microglia (microglial nodules) in severe HIVE (Figure 1B-C). Rare CD8+ T lymphocytes were found in control (seronegative) brain tissues (data not shown). Semiquantitative assessment of CD8+ T lymphocyte numbers (10 consecutive ×20 power fields) revealed a significantly higher number of CD8+ T lymphocytes (P < .05) in the frontal cortex and basal ganglia of HIVE tissue as compared with HIV-seropositive (without HIVE) and -seronegative controls (Figure 1D). These results suggest that infiltration of CD8+ T lymphocytes parallels the presence of HIV-1-infected brain MPs in HIVE and correlates with the intensity of HIVE. Infiltrating CD8+ T cells are thought to be the source of activation stimuli for brain macrophages, and the inflammatory cytokines and cytotoxic molecules released by CD8+ T lymphocytes may contribute to CNS injury initiated by virus-infected brain MPs.
Accumulation of CD8+ T lymphocytes in the brain during HIVE. Increased number of CD8+ lymphocytes within the neuropil in HIVE brains (A) as compared with the seropositive patients without evidence of encephalitis (insert). Infiltrating CD8+ lymphocytes (purple) migrate toward HIV-1+ MPs (brown) of microglial nodule (B,C). The inset in panel A shows lymphocytes contained within the perivascular cuff in the HIV+ case. Human brain sections were double-immunostained with antibodies to CD8+ (purple, VIP substrate) and HIV-1 p24 (brown, DAB substrate). Original magnifications: panels A and B: ×100; panel C: ×200. (D) CD8+ lymphocytes were counted in basal ganglia and frontal cortex (white matter) of human brains (average of 10 consecutive 20 × fields). *P < .05 compared with HIV-seropositive and -seronegative controls. Error bars indicate standard error of the mean (SEM).
Accumulation of CD8+ T lymphocytes in the brain during HIVE. Increased number of CD8+ lymphocytes within the neuropil in HIVE brains (A) as compared with the seropositive patients without evidence of encephalitis (insert). Infiltrating CD8+ lymphocytes (purple) migrate toward HIV-1+ MPs (brown) of microglial nodule (B,C). The inset in panel A shows lymphocytes contained within the perivascular cuff in the HIV+ case. Human brain sections were double-immunostained with antibodies to CD8+ (purple, VIP substrate) and HIV-1 p24 (brown, DAB substrate). Original magnifications: panels A and B: ×100; panel C: ×200. (D) CD8+ lymphocytes were counted in basal ganglia and frontal cortex (white matter) of human brains (average of 10 consecutive 20 × fields). *P < .05 compared with HIV-seropositive and -seronegative controls. Error bars indicate standard error of the mean (SEM).
Induction of IDO in HIVE correlates with numbers of infiltrating CD8+ T lymphocytes
In order to determine what cells express IDO and whether induction of IDO is associated with enhanced infiltration of CD8+ T lymphocytes, serial human brain sections were stained for CD68, HIV-1 p24, CD8, and/or double stained for IDO. IDO expression was detected in CD68+ MPs surrounded by infiltrating CD8+ T lymphocytes (Figure 2A). IDO-expressing cells were predominantly CD68+ macrophages (Figure 2B) and microglia, and many of these cells were HIV-1 p24 antigen-positive as seen in the microglial nodule (Figure 2C) and perivascular macrophages (Figure 2D). In addition, occasional IDO-expressing astrocytes were seen in the most profound HIVE cases. Numbers of IDO-positive macrophages and microglia paralleled the numbers of CD8+ T lymphocytes and the severity of HIVE. In contrast, IDO expression was not detected in brain sections of HIV-1-seronegative cases with rare CD8+ T lymphocytes in perivascular spaces (Figure 2E) and without evidence of microgliosis (Figure 2F). Western blot analysis for IDO and CD68 was performed in tissue samples (cortex and basal ganglia) derived from the patients analyzed by immunohistochemistry (HIVE, n = 8; HIV+, n = 6; and seronegative, n = 5). As demonstrated in Figure 2G, IDO and CD68 protein levels in HIVE were significantly higher (P < .02) than in HIV-1-seropositive cases without HIVE and in control seronegative brain tissues. Up-regulation of IDO by HIV-1 infection was previously shown in macrophages,35 and IFN-γ is the most potent inducer of IDO in these cells.36 To test for an additive effect of IFN-γ on HIV-1 infection, we assessed IDO expression by Western blot in uninfected or infected MDMs on days 3 and 7 after infection in the presence or absence of IFN-γ stimulation. As shown in Figure 2H, virus-infected MDMs expressed higher levels of IDO protein as compared with uninfected cells, and IFN-γ further up-regulated IDO expression in MDMs. In summary, our results indicate that CD8+ T-lymphocyte infiltration in brain is a feature of HIVE. CD8+ cells migrate into neuropil and surround infected MP-expressing IDO, and IDO is up-regulated by HIV-1 infection following IFN-γ stimulation/activation.
IDO expression in human brain tissue with and without HIVE. Double immunostaining demonstrates accumulation of CD8+ T cells (purple) around IDO-positive cells (brown) in the microglial nodule in HIVE (A). CD68+ macrophages/microglia (brown) in the microglial nodule correspond to IDO-positive cells on serial section (B). IDO (brown) is mainly localized in HIVp24+ cells (purple) in microglia nodule (C) and perivascular cells (D). Occasional IDO-expressing astrocytes were seen in HIVE cases (arrows). Note the absence of purple stain in the cytoplasm of astrocytes. Fewer CD8+ T cells (E) and CD68+ cells (F) and lack of cells staining for IDO (E) are shown in HIV-negative control. Human brain sections were double-immunostained with antibodies to CD8+ and HIVp24 (purple, VIP substrate) and IDO (brown, DAB substrate;A,C-E) or CD68 (brown, DAB substrate; B,F). Original magnifications: panels A-D: ×200; inset, panels C and D: ×600. Western blot analysis for IDO and CD68 expression in brain homogenates from control (HIV-), HIV+, and HIVE cases. Representative immunoblots of CD68, IDO, and internal standard α actin, and ratios of IDO/α actin and CD68/α actin are shown (G). IDO expression detected by Western blot in uninfected or HIV-1-infected MDMs in vitro with or without IFN-γ stimulation (H).
IDO expression in human brain tissue with and without HIVE. Double immunostaining demonstrates accumulation of CD8+ T cells (purple) around IDO-positive cells (brown) in the microglial nodule in HIVE (A). CD68+ macrophages/microglia (brown) in the microglial nodule correspond to IDO-positive cells on serial section (B). IDO (brown) is mainly localized in HIVp24+ cells (purple) in microglia nodule (C) and perivascular cells (D). Occasional IDO-expressing astrocytes were seen in HIVE cases (arrows). Note the absence of purple stain in the cytoplasm of astrocytes. Fewer CD8+ T cells (E) and CD68+ cells (F) and lack of cells staining for IDO (E) are shown in HIV-negative control. Human brain sections were double-immunostained with antibodies to CD8+ and HIVp24 (purple, VIP substrate) and IDO (brown, DAB substrate;A,C-E) or CD68 (brown, DAB substrate; B,F). Original magnifications: panels A-D: ×200; inset, panels C and D: ×600. Western blot analysis for IDO and CD68 expression in brain homogenates from control (HIV-), HIV+, and HIVE cases. Representative immunoblots of CD68, IDO, and internal standard α actin, and ratios of IDO/α actin and CD68/α actin are shown (G). IDO expression detected by Western blot in uninfected or HIV-1-infected MDMs in vitro with or without IFN-γ stimulation (H).
Inhibition of IDO activity enhances HIV-1-specific T-cell responses in huPBL-NOD/SCID HIVE mice
Mice were reconstituted with human PBLs to generate hu-PBL-NOD/SCID animals. At 8 days after lymphocyte reconstitution, encephalitis was induced by stereotactic injection into the basal ganglia with HIV-1ADA-infected, autologous MDMs, thus generating hu-PBLNOD/SCID HIVE mice. To assess the level of human lymphocyte engraftment, flow cytometric analysis of peripheral blood (weeks 1-3) and spleen (weeks 2-3) was performed in vehicle and 1-MT-treated hu-PBL-NOD/SCID HIVE mice. Similar frequencies of human CD3+, CD4+, and CD8+ T lymphocytes were seen in spleens of control and 1-MT mice (weeks 2 and 3 after intracerebral MDM injection), indicating equal levels of engraftment in both groups (data not shown). Table 2 shows results of 2-color staining for human CD3+, CD4+, and CD8+ in peripheral blood samples of 1-MT- and vehicle-treated hu-PBL-NOD/SCID mice. At one week the percentages of CD8+ (P < .001; 1-MT versus vehicle) and CD3+ T lymphocytes (P < .05; 1-MT versus vehicle) in the 1-MT group were significantly higher than in the control group. In contrast, at week 2 this was reversed. The control mice showed more CD8+ and CD3+ cells as compared with 1-MT mice (P < .05). The percentage of CD4+ T lymphocytes in peripheral blood was similar in both groups of animals 1 and 2 weeks after MDM intracerebral injection. Similar levels of human lymphocytes (CD3+, CD4+, CD8+) were seen in peripheral blood at week 3 in both groups (Table 2). HIV-1 p24 levels in peripheral blood of 1-MT-treated mice were significantly lower than in the control animals (198 ± 22.5 pg/mL versus 103 pg/mL ± 18.3 pg/mL; P < .02) at week 2 (Table 2). This effect was not sustained at week 3 when similar levels of HIV-1 p24 were found in blood (320 pg/mL ± 48.3 pg/mL in control versus 328 pg/mL ± 63.22 pg/mL in 1-MT-treated animals).
Human cells and plasma levels of HIV-1 p24 in peripheral blood of hu-PBL-NOD/SCID mice with HIVE
Treatment group, by week* . | Lymphocyte population, % . | . | . | HIV-1 p24, mean pg/mL ± SEM . | ||
|---|---|---|---|---|---|---|
| . | CD3+ . | CD4+† . | CD8+ . | . | ||
| Week 1 | ||||||
| Control | 33.33 | 16.22 | 14.97 | 5.26 ± 3.25 | ||
| 1-MT | 46.36‡ | 19.56 | 22.25§ | 1.76 ± 0.66 | ||
| Week 2 | ||||||
| Control | 51.76 | 20.38 | 26.91 | 198.0 ± 22.5 | ||
| 1-MT | 39.80‡ | 17.92 | 19.63‡ | 103.0 ± 18.3∥ | ||
| Week 3 | ||||||
| Control | 44.48 | 21.78 | 21.10 | 320.0 ± 48.3 | ||
| 1-MT | 33.12 | 16.64 | 15.07 | 328.0 ± 63.22 | ||
Treatment group, by week* . | Lymphocyte population, % . | . | . | HIV-1 p24, mean pg/mL ± SEM . | ||
|---|---|---|---|---|---|---|
| . | CD3+ . | CD4+† . | CD8+ . | . | ||
| Week 1 | ||||||
| Control | 33.33 | 16.22 | 14.97 | 5.26 ± 3.25 | ||
| 1-MT | 46.36‡ | 19.56 | 22.25§ | 1.76 ± 0.66 | ||
| Week 2 | ||||||
| Control | 51.76 | 20.38 | 26.91 | 198.0 ± 22.5 | ||
| 1-MT | 39.80‡ | 17.92 | 19.63‡ | 103.0 ± 18.3∥ | ||
| Week 3 | ||||||
| Control | 44.48 | 21.78 | 21.10 | 320.0 ± 48.3 | ||
| 1-MT | 33.12 | 16.64 | 15.07 | 328.0 ± 63.22 | ||
Days after MDM injection
No significant difference in percentage of CD4+ cells between the groups
P < .05, compared with control
P < .001, compared with control
P < .02, compared with control
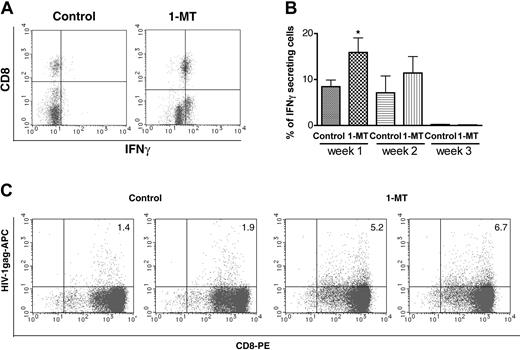
As a measure of enhanced immune responses after IDO inhibition by 1-MT, we used IFN-γ intracellular staining of CD8+ T lymphocytes by flow cytometric analysis. Figure 3A shows 2-color staining for CD8+ T lymphocyte versus IFN-γ expression in peripheral blood. The percentage of IFN-γ-positive cells significantly increased by 1-MT treatment (P < .05) when compared with controls at one week (Figure 3B). Hu-PBL-NOD/SCID HIVE mice treated with 1-MT demonstrated increased HIV-1pol (not shown) and HIV-1gag and specific CTLs detected by tetramer staining and FACS analyses of splenocytes (Figure 3C). Presence of HIV-1-specific CTLs in the splenocytes were detected as early as one week after MDM inoculation. Higher HIV-1-specific CTLs were found in 1-MT-treated animals as compared with controls that were persistent up to 3 weeks after MDM inoculation. These results suggest that inhibition of IDO activity enhances induction of virus-specific cellular immune response against HIV-1-infected MDMs.
Inhibition of IDO affects neuroinflammatory responses in hu-PBL-NOD/SCID HIVE mice
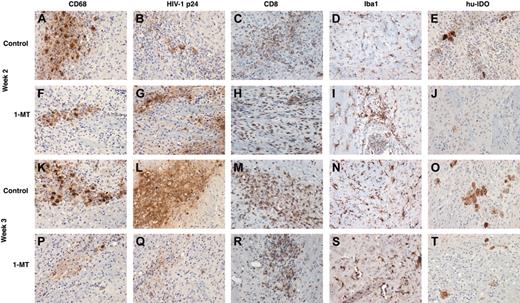
In order to analyze neuroinflammatory responses in the hu-PBL-NOD/SCID mice brains, sections were immunostained for human CD68 (specific for the human MDMs), HIV-1 p24 (viral antigen), human CD8 (T cells), and Iba1 (mouse microglial marker). Cells were counted in a blinded fashion by 2 investigators, and the number of positive cells averaged over the area covering the entire left hemisphere (inoculated with MDMs). At week 2 more infiltrating CD8+ T cells were seen in the 1-MT group when compared with control (Figure 4C versus 4H). However, the numbers of CD68+ MDMs (Figure 4A,F), HIV-1 p24+ MDMs (Figure 4B,G), and microglial cells (Figure 4G,I) were similar in 1-MT and controls groups.
By week 3, 1-MT-treated mice showed diminished numbers of human CD68+ MDMs compared with control mice (Figure 4K,P). More importantly, 1-MT-treated mice showed a dramatic reduction in MDMs that were HIV-1p24+ (Figure 4L versus 4Q). Figure 5A-B shows the injection site of a control and 1-MT mouse (week 3), with a localized collection of HIV-1 p24+ MDMs. The mice treated with 1-MT invariably showed significantly less HIV-1 p24+ MDMs than controls, reflecting more efficient clearance of HIV-1-infected cells.
Brain sections were also evaluated for expression of human IDO. Figure 4H,J compares the number of IDO-positive cells at week 2 in control and 1-MT mice. Cells expressing IDO were reduced in the 1-MT group at week 2. At week 3, differences in IDO-expressing cells in 1-MT and control-treated animals were significant (Figure 4O,T). This was consistent with the overall clearance of human CD68+ MDMs in the 1-MT group by 3 weeks in the hu-PBL-NOD/SCID HIVE mice.
CTL clearance of HIV-1-infected MDMs in 1-MT-treated hu-PBL-NOD/SCID HIVE mice
Our previous studies indicated development of virus-specific CTL responses and partial elimination of HIV-1-infected MDMs in the brains of hu-PBL-NOD/SCID mice with HIVE.29 In order to test the effect of IDO inhibition on eradication of virus-infected macrophages, CD68+, HIV-1 p24+, and CD8+ T lymphocytes were counted in brain tissue in blind fashion by 2 observers. Mean numbers of stained cells per section within the injected hemisphere were calculated for each mouse (3 sections/mouse) and mean numbers of cells for 6 to 8 mice per treatment group were evaluated at weeks 2 and 3 after MDM injection. Table 3 shows that similar numbers of MDMs were found in both groups at week 2. Although the average number of CD68+ MDMs present in the 1-MT group (9 ± 4.65) at week 3 after MDM inoculation was lower than the control group (44.2 ± 25.97), it failed to reach statistical significance (P = .07). The percentage of infected MDMs was similar in both groups, indicating that the level of infection was comparable between the groups and it was not influenced by 1-MT treatment. While comparable levels of HIV-1 p24+ MDMs were observed at week 2 (Figure 5C), a significant reduction in the number of MDMs was observed by week 3 in the 1-MT-treated group (4 ± 2.54; Figure 5C) as compared with controls (38.25 ± 23.47, P < .05; Figure 5C). Levels of microglial reaction (assessed by digital image analysis and expressed as percentage of the area occupied by Iba1 immunostaining) were similar in both groups at week 2 (4.65 ± 0.63 in 1-MT-treated versus 3.95 ± 0.46 in controls) and week 3 (5.07 ± 0.44 in 1-MT versus 4.52 ± 0.87 in controls) (Table 3).
Dynamics of elimination of autologus infected MDM and CD8+ cell infiltration in brains of 1-MT mice versus control mice
Treatment group, by week* . | Macrophages per section . | HIV-1 p24+ macrophages, % . | Iba1 area per section, % . | CD8+ T lymphocytes per section . |
|---|---|---|---|---|
| Week 2 | ||||
| Control | 70.83 ± 35.98 | 32.0 ± 9.0 | 3.95 ± 0.46 | 298.0 ± 91.22 |
| 1-MT | 68.66 ± 23.36 | 42.0 ± 10.77 | 4.65 ± 0.63 | 597.0 ± 109.76† |
| Week 3 | ||||
| Control | 44.2 ± 25.97 | 53.0 ± 2.51 | 4.52 ± 0.87 | 193.0 ± 106.65 |
| 1-MT | 9.0 ± 4.65‡ | 53.0 ± 11.32 | 5.07 ± 0.44 | 256.0 ± 45.6 |
Treatment group, by week* . | Macrophages per section . | HIV-1 p24+ macrophages, % . | Iba1 area per section, % . | CD8+ T lymphocytes per section . |
|---|---|---|---|---|
| Week 2 | ||||
| Control | 70.83 ± 35.98 | 32.0 ± 9.0 | 3.95 ± 0.46 | 298.0 ± 91.22 |
| 1-MT | 68.66 ± 23.36 | 42.0 ± 10.77 | 4.65 ± 0.63 | 597.0 ± 109.76† |
| Week 3 | ||||
| Control | 44.2 ± 25.97 | 53.0 ± 2.51 | 4.52 ± 0.87 | 193.0 ± 106.65 |
| 1-MT | 9.0 ± 4.65‡ | 53.0 ± 11.32 | 5.07 ± 0.44 | 256.0 ± 45.6 |
All values in table are mean ± SEM per 5-μm sections; 3 to 7 sections per mouse were analyzed.
Days after MDM injection
P < .03, compared with control
P = .07, compared with control
Effects of 1-MT on number of IFN-γ-secreting human CD8+ cells and HIV-1-specific CD8+ precursors. Number of IFN-γ-secreting cells in the peripheral blood one week after HIV-1 MDM injection (A,B). Representative histograms of human CD8/IFN-γ cells in control and 1-MT-treated groups are shown in panel A. Average percentage of IFN-γ-secreting cells (n = 7 and n = 8 for control and 1-MT mice, respectively) in peripheral blood (1-3 weeks) (B). Percentage of HIV-1gag (SL-9) tetramer-positive human CD8+ cells from spleen expanded by PHA/IL-2 stimulated in vitro 3 weeks after HIV-1 MDM injection (C). Two left panels are control animals, 2 right panels are 1-MT-treated mice. Error bars indicate SEM. *P < .05 (1-MT versus vehicle control).
Effects of 1-MT on number of IFN-γ-secreting human CD8+ cells and HIV-1-specific CD8+ precursors. Number of IFN-γ-secreting cells in the peripheral blood one week after HIV-1 MDM injection (A,B). Representative histograms of human CD8/IFN-γ cells in control and 1-MT-treated groups are shown in panel A. Average percentage of IFN-γ-secreting cells (n = 7 and n = 8 for control and 1-MT mice, respectively) in peripheral blood (1-3 weeks) (B). Percentage of HIV-1gag (SL-9) tetramer-positive human CD8+ cells from spleen expanded by PHA/IL-2 stimulated in vitro 3 weeks after HIV-1 MDM injection (C). Two left panels are control animals, 2 right panels are 1-MT-treated mice. Error bars indicate SEM. *P < .05 (1-MT versus vehicle control).
At week 2, mice treated with 1-MT demonstrated a 2-fold increase in infiltrating CD8+ T lymphocytes (597 ± 109.76) as compared with vehicle controls (298 ± 91.22, P < .03). At week 3, there was no difference between groups in CD8 cells. This increase in CD8+ T cells in the brain was inversely proportional to the number of CD8+ T lymphocytes present in the peripheral blood (Table 2) at week 2 in both groups. Thus, a 2-fold increase in CD8+ T lymphocyte infiltration (week 2) preceded significant reduction of HIV-1-infected MDMs at week 3 in the 1-MT group.
Neuroinflammatory responses in control and 1-MT-treated hu-PBL-NOD/SCID HIVE mice. Relationship between human macrophages (CD68), HIV p24 human lymphocytes (CD8), mouse microglial (Iba1), and human IDO (hu-IDO) expression are shown in weeks 2 and 3 after intracerebral injection of HIV-1-infected MDMs. Equal number of CD68+ and HIV-1 p24+ MDMs were found in the brains of control and 1-MT mice at week 2 (A,F, and B,G). Higher numbers of CD8+ lymphocytes were found in 1-MT versus control animals at this time point (H,C). Prominent decrease of MDMs (K and P) and especially HIV-1 p24+ MDMs (Q) were detected in 1-MT as compared with control mice at week 3 (L). Microglial reaction (detected by Iba1 staining) was not different between 2 groups at week 2 (D,I) or week 3 (N,S). Primary Abs were detected by Vectastain Elite Kit with DAB as a substrate. Original magnification of panels K-T: × 200.
Neuroinflammatory responses in control and 1-MT-treated hu-PBL-NOD/SCID HIVE mice. Relationship between human macrophages (CD68), HIV p24 human lymphocytes (CD8), mouse microglial (Iba1), and human IDO (hu-IDO) expression are shown in weeks 2 and 3 after intracerebral injection of HIV-1-infected MDMs. Equal number of CD68+ and HIV-1 p24+ MDMs were found in the brains of control and 1-MT mice at week 2 (A,F, and B,G). Higher numbers of CD8+ lymphocytes were found in 1-MT versus control animals at this time point (H,C). Prominent decrease of MDMs (K and P) and especially HIV-1 p24+ MDMs (Q) were detected in 1-MT as compared with control mice at week 3 (L). Microglial reaction (detected by Iba1 staining) was not different between 2 groups at week 2 (D,I) or week 3 (N,S). Primary Abs were detected by Vectastain Elite Kit with DAB as a substrate. Original magnification of panels K-T: × 200.
Elimination of HIV-1-infected MDMs from brains of 1-MT-treated hu-PBL-NOD/SCID HIVE mice. 1-MT-treated mice (B) contained fewer HIV-1 p24+ MDMs as compared with controls (A). HIV-1 p24+ cells were counted in brain tissue sections of control (C) and 1-MT-treated (D) at weeks 2 and 3 after intracerebral inoculation of HIV-1-infected MDMs. *P < .05 (1-MT versus vehicle control). Original magnification for panels A and B: × 10 (insets: × 200). Error bars indicate SEM.
Elimination of HIV-1-infected MDMs from brains of 1-MT-treated hu-PBL-NOD/SCID HIVE mice. 1-MT-treated mice (B) contained fewer HIV-1 p24+ MDMs as compared with controls (A). HIV-1 p24+ cells were counted in brain tissue sections of control (C) and 1-MT-treated (D) at weeks 2 and 3 after intracerebral inoculation of HIV-1-infected MDMs. *P < .05 (1-MT versus vehicle control). Original magnification for panels A and B: × 10 (insets: × 200). Error bars indicate SEM.
Discussion
Using an animal model for HIVE, we demonstrated that inhibition of IDO activity during HIV-1 CNS infection significantly enhances CD8+ CTL-mediated clearance of virus-infected macrophages in brain. Attenuation of the immunosuppressive and neurotoxic IDO activities leads to enhanced adaptive immune clearance of virus-infected cells. We assume that the following events occur after inhibition of IDO activity in hu-PBL-NOD/SCID HIVE mouse model. One week after MDM injection, 1-MT-treated mice show increased human IFN-γ-producing CD8+ T lymphocytes in peripheral blood as compared with vehicle control group. At this point, 1-MT animals demonstrated increased number of CD8+/IFN-γ cells in the peripheral blood as compared with controls. By week 2, there is an increase in CD8+ T lymphocytes in the brains of 1-MT animals compared with controls, whereas the numbers of HIV-1-infected MDMs remains constant in both groups. Increase of CD8+ T lymphocytes in the brain corresponds to the decreased number of CD8+ T lymphocytes in the peripheral blood in 1-MT animals. At week 3, 1-MT-treated mice enhanced cell trafficking of HIV-1-specific CTLs into the brain leads to more efficient elimination of virus-infected MDMs. Our data suggest that modulation of IDO activity can be used as a tool for immune manipulation in HIV-1 infection, and this may decrease the amount of virus-infected MDMs in HIVE. This is the first time, to our knowledge, that such a strategy was studied in an animal model of HIV-1 infection.
IDO serves as a negative regulator of the immune system. IDO activity in APCs is a potent mechanism for inducing immunosuppression and tolerance. Expression of IDO allows professional APCs (macrophages and dendritic cells) to inhibit T-lymphocyte activity in vitro.19,20,37 IDO contributes to T-cell tolerance of the fetus during gestation38 and suppresses autoimmunity in a variety of animal models.39-42 The IDO pathway is believed to confer a survival advantage for certain tumors and agents causing chronic infections that evade the immune system.43 In the case of infections, IDO was generally considered to function as a host-defense mechanism. In vitro studies show that IDO-mediated tryptophan deprivation protects cells by inhibiting the replication of a variety of microbial pathogens including viruses and bacteria [reviewed in Mellor and Munn43 ]. However, given that IDO is up-regulated in HIV-1-infected macrophages,35 we hypothesized that the in vivo immunosuppressive properties of IDO might confer an advantage to HIV-1 by allowing the virus to escape CTL clearance.
The antigen-specific CD8+ T lymphocytes serve a prominent role in the control of HIV-1 infection in both brain and peripheral tissues.44-46 In our prior studies using a murine model for HIVE, we demonstrated that viral infection of brain macrophages is itself a robust inducer of antiretroviral CTL activities and a powerful attractant for lymphocyte infiltration into the brain in the hu-PBL-NOD/SCID mouse model of HIVE.29 Similar mechanisms are likely functional during the course of virus infection in humans.9 Paradoxically, however, this infiltration of CD8+ T lymphocytes failed to eliminate infected macrophages from the affected mouse brain tissue (just as the antigen-specific CD8+ T lymphocytes in patients failed to clear systemic infection). Thus, it is plausible that IDO expression by HIV-infected macrophages can negatively affect clearance of infected cells. Our data support this idea by showing markedly enhanced clearance of HIV-1-infected macrophages from 1-MT-treated mice.
The current study presents clear indication of increased infiltration of CD8+ T lymphocytes in HIVE. Similar distribution of CD8+ T lymphocytes (followed by virus-infected cells in an antigen-specific manner) was recently described in simian immunodeficiency virus (SIV) encephalitis by Kim and colleagues.47 These observations are consistent with previous reports demonstrating that HIV-1-infected macrophages attract lymphocytes46 and serve as APCs.48,49 Two studies showed that IDO mRNA is high in peripheral lymphoid tissue in patients with AIDS, and the level of IDO expression decreases after improvement of immune function following initiation of antiretroviral therapy.50,51 The fact that IDO expression acts to prevent effective clearance of the infected cells is important as it serves to open up new pathways for treatments of chronic viral infections.
HIV-1-specific CTLs can also release soluble factors (including IFN-γ, tumor necrosis factor-α, and chemokines), which have diverse neurotoxic and immunologic activities.52-54 These proinflammatory mediators in combination with the direct effect of HIV-1 infection on the macrophages could be the principle inducers of IDO expression in HIVE.46,55 Thus, the CTL response may be ineffectual at clearing the virus from the protected reservoir of IDO-expressing macrophages, but nevertheless may be toxic to the brain—including possible neurotoxins generated by IDO activity.
The current study demonstrates the localization of IDO in human brain during HIVE and provides a clear correlation with the level of CD8+ lymphocyte infiltration and distribution of virus-infected macrophages in the brain. Macaques infected with SIV (an animal model for HIV-1 infection and HIVE) have demonstrated that multinucleated giant cells and macrophages are the main source of IDO production.56 Increase of IDO biochemical activity in the frontal cortex of the brain in HAD has been reported before.11 However, little is known about the source and sites of IDO expression in humans with HIVE. Our findings that perivascular macrophages and microglial nodules are the source of IDO production in the brains of patients with HIVE are in concordance with reports on the source of IDO expression in SIV-infected monkey brains.56,57 Our ex vivo human brain data are further supported by in vitro experiments demonstrating IDO up-regulation of HIV-1 infection and IFN-γ stimulation in human MDMs. CD8+ lymphocytes attracted by alpha-chemokines46 may be a source of chronic immune stimulation and a pathogenic force for HAD. IDO inhibition could also lead to decreased production of potent neurotoxins (like quinolinic acid and others) shown before to correlate with the development of HAD.8,13 Furthermore, down-regulation of IDO activity may preserve pool of tryptophan, precursor of the neurotransmitter serotonin.58 Depletion of serotonin was implicated in development of depression, one of initial clinical signs of HAD.59 Beneficial effects of IDO inhibitors in this regard during HIVE await further investigation.
Our hu-PBL-NOD/SCID HIVE mouse model offers a unique opportunity to study innate and antiviral immunity during ongoing neurologic disease caused by HIV-1. The ability to quantitatively evaluate both immune responses and neuropathologic events in these mice makes it a powerful model for testing both the fundamental pathogenesis and therapeutic strategies for HIVE. This mouse model was thus an excellent tool to test our present hypothesis. The addition of autologous PBLs to our previous macrophage-xenograft model is critically important,29 because T cells may contribute to the pathogenesis of HIVE. More importantly, as we show in the present study, these T cells are also capable of progressively clearing the HIV-1-infected macrophages from the brain, as long as IDO is inhibited.
HIV-1-infected MDMs may serve as a stable reservoir for virus in the brain. Based on our data and the growing literature, we hypothesize that this reservoir is accessible to the immune system and triggers an immune response, but it is resistant to clearance due to the immunosuppressive effects of IDO. This idea potentially has direct clinical relevance, because 1-MT is the pharmacologic inhibitor of IDO used to inhibit IDO activity in vivo,30,38 and it is now in preclinical development as an immunomodulatory drug. Interestingly, no toxic effect of increased CD8+ T-lymphocyte infiltration was seen in our study (as evidenced by quantitative assessment of microglial reaction).
Inhibition of IDO alone will certainly not be sufficient to clear all virus, or to improve all aspects of HIVE. However, our data provide an additional and previously unsuspected target for HIV-1 therapy, by inhibiting the immunosuppressive action of IDO. In combination with conventional neuroprotective and antiretroviral agents, this could provide an important new therapeutic agent, and may help target a protected reservoir of virus.
Prepublished online as Blood First Edition Paper, June 16, 2005; DOI 10.1182/blood-2005-04-1403.
Supported in part by grants from the National Institutes of Health 1P01 NS043 985-01 (H.E.G., Y.P.) and RO-1 MH65 151-01A1 (Y.P.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Robin Taylor for excellent editorial support and Dr R. Lee Mosley for critical reading of this manuscript. We would like to thank the NIH National NeuroAIDS Consortium and the CNND brain bank for the brain tissue specimens.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal