Abstract
We report the development of a mouse B cell-depleting immunoconjugate (anti-CD22 monoclonal antibody [mAb] conjugated to calicheamicin) and its in vivo use to characterize the kinetics of CD22+ B-cell depletion and reconstitution in murine primary and secondary lymphoid tissues. The effect of B-cell depletion was further studied in a murine collagen-induced arthritis (CIA) model and a respiratory syncytial virus (RSV) vaccination model. Our results show that (1) the immunoconjugate has B-cell-specific in vitro and in vivo cytotoxicity; (2) B-cell reconstitution starts in the bone marrow and spleen around day 30 after depletion and is completed in all tissues tested by day 50; (3) B-cell depletion inhibits the development of clinical and histologic arthritis in the CIA model; (4) depletion of type II collagen antibody levels is not necessary for clinical and histologic prevention of CIA; and (5) B-cell depletion does not adversely affect memory antibody responses after challenge nor clearance of infectious virus from lungs in the RSV vaccination model. These results demonstrate for the first time that only B-cell reduction but not type II collagen antibody levels correlate with the prevention of arthritis and represent key insights into the role of CD22-targeted B-cell depletion in mouse autoimmunity and vaccination models.
Introduction
The pathogenesis of autoimmune diseases involves a complicated network of tissue-damaging mechanisms that are governed primarily by recognition of self-antigens and an imbalance in cytokine production.1,2 In rheumatoid arthritis (RA), major cell types responsible for chronic inflammation and subsequent cartilage destruction and bone erosion in the joints are macrophages, synovial fibroblasts, neutrophils, and lymphocytes. Recent studies demonstrated that T and B lymphocytes that infiltrate inflamed synovial tissues are often organized into structures that resemble lymphoid follicles.3-7 Normally, in T-dependent immune responses antigen-activated B cells migrate into lymphoid follicles of lymphoid organs and form germinal centers. Within the unique milieu of the germinal center, hypermutation leads to diversification and selection of the B-cell repertoire for high affinity and differentiation into antibody-secreting plasma cells or memory B cells.8-10 Molecular analysis of B cells isolated from synovial follicular structures during rheumatoid arthritis demonstrated the importance of B cells in local antigen-driven specific immune responses and increased production of rheumatoid factor (RF), the high-affinity antibodies with self-reactivity.5 Positivity for RF is associated with more aggressive articular disease and higher frequency of extra-articular manifestations.11 Studies using RA synovium-severe combined immunodeficiency (SCID) mouse chimeras showed that activation of synovial germinal center CD4+ T cells required the presence of B cells, but not macrophages and dendritic cells, raising the possibility that B cells provided critical function in T-cell activation or harbored the relevant antigens.12 The significance of extranodal lymphoid follicle formation in inflamed RA joints is unknown, and it remains to be defined whether the reaction is a bystander effect induced by the local chronic inflammation or results from an autoimmune reaction directly related to the pathogenesis of the disease.
Intriguing evidence on the pathogenic contribution of B cells in the development of arthritis was recently provided using K/BxN T-cell receptor (TCR) transgenic mice, which spontaneously develop joint disorders with many of the clinical, histologic, and immunologic characteristics of human RA.13 The articular manifestations are caused by arthritogenic antibodies directed against glucose-6-phosphate isomerase, which develop at high titers in K/BxN transgenic mice.14-16 More convincing evidence regarding the pathogenicity of B cells in RA was recently obtained from clinical trials in patients with refractory disease by using B-cell ablation with rituximab (Rituxan), a human chimeric anti-CD20 monoclonal antibody (mAb).17,18 Expression of CD20, a B-cell-specific transmembrane glycoprotein with similar patterns of expression and function in humans and in mice, emerges on late pre-B cells and is extinguished on plasma cells.19,20 Anti-CD20 therapy has also been tested in several other autoimmune disorders, including idiopathic thrombocytopenic purpura (ITP) and systemic lupus erythematosus (SLE), providing, in the process, novel insights into the role of B cells in autoimmunity.21-24 Thus, numerous studies have demonstrated a beneficial effect of B-cell depletion in patients with autoimmune disorders.20,25 At present, the mechanism by which removal of pathogenic B cells and their precursors, but not antibody-secreting plasma cells, leads to clinical improvement remains elusive.20 Given that B cells exist as lymphoid aggregates within the synovium of patients with RA, it is reasonable to assume that B-cell functions other than antibody production (eg, cytokine production, antigen presentation, provision of costimulatory signals to T cells) might also play important roles in disease pathogenesis.6,12,20,26,27
In human clinical trials with rituximab, the extent of B-cell depletion has only been assessed in peripheral-blood samples. In addition, one concern regarding B-cell ablative therapies is the potential for an increased risk of infections by major or opportunistic pathogens. To date, no clinical data support this scenario.20 The use of preclinical models would facilitate a more thorough evaluation of B-cell depletion and subsequent reconstitution in immune tissues and accompanying risk for infectious disease. However, this approach has been hampered by the lack of a reagent to deplete B cells from adult mice with fully developed immune systems. Previous studies have relied on neonatal depletion of B cells with anti-IgM, which does not accurately model the therapeutic conditions in RA.28 Most recently, a new mouse model for anti-CD20 immunotherapy has been reported. In this model, a panel of mouse anti-mouse CD20 mAbs deplete B cells through FcγR-dependent pathways.29 In this report, we developed for the first time a mouse B-cell-targeted cytotoxic immunoconjugate (anti-CD22 mAb conjugated to calicheamicin) and studied by flow cytometric analysis the kinetics of B-cell depletion and recovery in peripheral blood (PB), spleen, bone marrow (BM), and lymph node (LN) samples from naïve mice. Furthermore, we evaluated the effect of B-cell depletion on antibody responses against type II collagen and the development of clinical and histologic arthritis in a mouse collagen-induced arthritis (CIA) model and on humoral responses in the mouse model of respiratory syncytial virus (RSV) infection.
Materials and methods
Mice
Female and male C57BL/6 (B6) and female interferon γ-negative (IFNγ-/-) in B6 background (B6-IFNγ-KO), 6 to 8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were kept at the animal facility of Wyeth Research in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council. Study protocols were reviewed by the Institutional Animal Care and Use Committee (IACUC) at Wyeth Research, Cambridge, MA.
Anti-CD22/calicheamicin immunoconjugate
The immunoconjugate (referred here as CD22/cal) is a conjugate of an anti-mouse CD22 mAb and N-acetyl-γ-calicheamicin dimethyl acid, a member of the enediyne antitumor antibiotics. Anti-mouse CD22 is a mouse IgG1 mAb purified from Cy34.1 hybridoma (American Type Culture Collection [ATCC], Rockville, MD). The synthesis of antibody/calicheamicin conjugates has been previously described.30 CD22/cal has an average loading of 17 to 30 μg calicheamicin/mg antibody protein (1.2-2.6 moles calicheamicin/mol antibody). On binding to CD22-expressing mouse B cells, the conjugate is internalized and exhibits potent dose-dependent cytotoxicity due to DNA damage caused by calicheamicin.31,32 A mouse IgG1 anti-rat very late antigen 4 (VLA-4) mAb (does not bind on mouse cells) conjugated to calicheamicin (GG5/cal) was used as a control in the in vitro and in vivo cytotoxicity studies. Mouse A20 B-cell lymphoma cells (ATCC) were used for flow cytometry studies on binding of Cy34.1 and CD22/cal on mouse CD22.
In vitro B- and T-cell cytotoxicity studies
Primary mouse B and T cells from male B6 mice were purified from single splenocyte suspensions using CD19 and CD3 Microbeads (Miltenyi Biotec, Auburn, CA), respectively, and cultured in a 96-well plate (105 cells/well) with various concentrations of the conjugate. For in vitro cytotoxicity (50% inhibitory concentration [IC50]) studies, B-cell proliferation was determined 48 hours after stimulation with 50 μg/mL lipopolysaccharide (LPS; Escherichia coli 026:B6, L 2762; Sigma-Aldrich, St Louis, MO). T-cell proliferation was assessed 48 hours after culture with a suboptimal (500 ng/mL) concentration of soluble anti-CD3 mAb (145-2C11; PharMingen, San Diego, CA) plus 1 μg/mL anti-CD28 mAb (clone 37.51; PharMingen). 3H-thymidine (1 μCi/well [0.037 MBq] Perkin Elmer Life Sciences, Boston, MA) was added during the last 6 hours of culture. 3H-thymidine incorporation was determined by liquid scintillation counting.
In vivo B-cell cytotoxicity studies
Male B6 mice were used for the establishment of the in vivo protocol and the characterization of B-cell depletion and recovery. Five different CD22/cal dosing schedules (Table 1) were tested in groups of 3 mice. The most effective (schedule 5) consisted of 2 intraperitoneal injections at a calicheamicin dose of 160 μg/kg/injection, 5 days apart. Male B6 mice received 2 (day 0 and 5) intraperitoneal injections with the conjugate (160 μg/kg/injection). B-cell depletion was monitored in individual mice by flow cytometry in BM, spleen, LN, and PB samples on days 12, 20, 30, 35, 40, and 50 after the first injection. Three mice per time point were studied and the numbers presented in Table 2 are mean percentages ± SD.
CD22/cal dosing regimens and relative percentage of whole BM cells staining for CD22 in CD22/cal-treated C57BL/6 mice
Dose/injection, μ/g . | No. injections . | Frequency . | CD22+ cells, %* . |
|---|---|---|---|
| No CD22/cal | – | – | 27.9 ± 2.2 |
| 1. 40 | 2 | 5 d apart | 19.3 ± 1.5 |
| 2. 40 | 3 | Every 4 d | 14.3 ± 2 |
| 3. 80 | 2 | 5 d apart | 13.3 ± 2.5 |
| 4. 80 | 3 | Every 4 d | 9.3 ± 1.5 |
| 5. 160 | 2 | 5 d apart | 1.7 ± 1.25 |
Dose/injection, μ/g . | No. injections . | Frequency . | CD22+ cells, %* . |
|---|---|---|---|
| No CD22/cal | – | – | 27.9 ± 2.2 |
| 1. 40 | 2 | 5 d apart | 19.3 ± 1.5 |
| 2. 40 | 3 | Every 4 d | 14.3 ± 2 |
| 3. 80 | 2 | 5 d apart | 13.3 ± 2.5 |
| 4. 80 | 3 | Every 4 d | 9.3 ± 1.5 |
| 5. 160 | 2 | 5 d apart | 1.7 ± 1.25 |
Samples were stained 4 to 5 d after the last CD22/cal injection.
– indicates not applicable.
The numbers are the mean percentages ± SD of 3 mice per group
Kinetics of CD22+ B-cell recovery in B6 mice after injections of CD22/cal
. | Relative percentage of whole sample cells staining for CD22* . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue . | Day 1 . | Day 12 . | Day 20 . | Day 30 . | Day 35 . | Day 40 . | Day 50 . | ||||||
| PB | 42.9 ± 3.3 | 0.8 ± 0.5 | 1.1 ± 0.3 | 1.3 ± 0.9 | 4.5 ± 0.4 | 16.1 ± 1.4 | 35.6 ± 2.1 | ||||||
| Spleen | 44.5 ± 2.2 | 0.9 ± 0.4 | 2.1 ± 0.2 | 13.5 ± 0.6 | 24.9 ± 2.5 | 42.2 ± 1.9 | 53.3 ± 1.9 | ||||||
| BM | 26.7 ± 1.6 | 2.0 ± 0.3 | 1.6 ± 0.6 | 15.1 ± 1.0 | 19.1 ± 2.7 | 20.8 ± 1.5 | 27.3 ± 1.1 | ||||||
| LN | 41 ± 2.5 | 0.9 ± 0.3 | 1.1 ± 0.5 | 1.4 ± 0.4 | 4.9 ± 1.3 | 15.8 ± 1.5 | 43.8 ± 1.7 | ||||||
. | Relative percentage of whole sample cells staining for CD22* . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue . | Day 1 . | Day 12 . | Day 20 . | Day 30 . | Day 35 . | Day 40 . | Day 50 . | ||||||
| PB | 42.9 ± 3.3 | 0.8 ± 0.5 | 1.1 ± 0.3 | 1.3 ± 0.9 | 4.5 ± 0.4 | 16.1 ± 1.4 | 35.6 ± 2.1 | ||||||
| Spleen | 44.5 ± 2.2 | 0.9 ± 0.4 | 2.1 ± 0.2 | 13.5 ± 0.6 | 24.9 ± 2.5 | 42.2 ± 1.9 | 53.3 ± 1.9 | ||||||
| BM | 26.7 ± 1.6 | 2.0 ± 0.3 | 1.6 ± 0.6 | 15.1 ± 1.0 | 19.1 ± 2.7 | 20.8 ± 1.5 | 27.3 ± 1.1 | ||||||
| LN | 41 ± 2.5 | 0.9 ± 0.3 | 1.1 ± 0.5 | 1.4 ± 0.4 | 4.9 ± 1.3 | 15.8 ± 1.5 | 43.8 ± 1.7 | ||||||
Mice were given injections on days 0 and 5 with CD22/cal (160 μg/kg/ injection).
Numbers are the mean percentages (± SD) of 3 mice/time point
Flow cytometry
The following fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies directed to mouse cell-surface antigens were from BD PharMingen (San Jose, CA): CD3e (145-2C11) CD19 (1D3), CD22.2 (Cy34.1), CD45R/B220 (RA3-6B2), Gr-1 (RB6-8C5), and Mac-3 (M3/84). For staining of A20 cells with unconjugated Cy34.1 or Cy34.1/calicheamicin (CD22/cal), anti-mouse IgG1-biotin and streptavidin PE polyclonal antibody were used. Cells were analyzed by flow cytometry using FACSCalibur and CellQuest software package (BD PharMingen).
B-cell depletion in the CIA model
CIA was induced in male B6 IFN-γ-KO mice by one (day 0) intradermal immunization in the base of the tail with 100 μg bovine type II collagen (CII; Chondrex, Redmond, WA) in complete Freund adjuvant (CFA; Difco Laboratory, Detroit, MI), containing 5 mg/mL killed Mycobacterium tuberculosis (H37Ra).33 CII-immunized mice received 2 intraperitoneal injections (days 5 and 10) with CD22/cal or control GG5/cal (160 μg/kg/injection). The paws were evaluated for clinical arthritis and each paw was individually scored using a 4-point scale: 0, normal paw; 1, minimal swelling or redness; 2, redness and swelling involving the entire forepaw; 3, redness and swelling involving the entire limp; 4, joint deformity or ankylosis or both.
Anti-type II collagen antibody ELISA
IgG2b antibody levels against type II collagen were measured by standard enzyme-linked immunosorbent assay (ELISA) methodology using peroxidase-conjugated secondary anti-IgG2b antibody and substrate 2;2′-azimobis(3-ethylbenzthiazoline-6-sulfonic acid [ABTS]). Serum dilutions, 1:1000, were chosen after preliminary assays. The optic density was measured at 405 nm using a Spectramax Plus 384 plate reader (Molecular Devices, Sunnyvale, CA). The anti-type II collagen concentrations were determined by reference to standard curves generated from 1:2 serial dilutions of a standard CIA serum to calculate the antibody content (in arbitrary units/mL).
Histology
Paws were collected for histologic analysis 25 or 75 days after immunization, were fixed in 10% neutral-buffered formalin, and decalcified in Cal-Ex II (Fisher Scientific, Hampton, NH) for 10 days. Decalcified paws were routinely processed and then embedded in paraffin blocks. Specimens were sectioned at 6 μm and stained with hematoxylin and eosin according to the manufacturer's protocol (Sigma-Aldrich). The sections were microscopically evaluated for the degree of inflammatory-cell infiltration, cartilage degeneration and erosion, synovial hyperplasia and pannus formation, and bone degeneration and remodeling. The arthritis severity of the disease was graded using a scoring system from 0 to 4: 0, within normal limits; 1, slight/mild; 2, moderate; 3, marked; 4, severe. The score assigned to each paw reflected the overall extent and severity of involvement of the many joints represented on each slide. All hematoxylin-and-eosin images were analyzed using a Nikon Eclipse E400 microscope equipped with 2×, 4×, 10×, 20×, 40×, and 60× objective lenses with numerical apertures ranging from 0.1 to 0.95 (Nikon, Burlingame, CA). Images were photographed with a Spot Insight QE camera (Diagnostic Instruments, Sterling Heights, MI) using C-view Digital Camera Viewer and Controller software (Digital Video Camera Company, Austin, TX). All images were processed with Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA).
B-cell depletion in the RSV vaccination model
Female B6 mice (aged 7-9 weeks) were given vaccine and CD22/cal according to the protocol depicted in Table 3. Mice from groups 1 and 2 (F/AlPO) were immunized intramuscularly with the RSV fusion (F) protein (1 μg/dose) adsorbed to aluminum phosphate (AlPO) adjuvant (100 μg/dose) on weeks 0 and 2. Natural F protein was purified as previously described34 from Vero cells (ATCC no. CCL 81) infected with the A2 strain of RSV. On weeks 4 and 4 plus 5 days mice in groups 1 and 3 given intraperitoneal injections of the CD22/cal (160 μg/kg). Control mice were given injections of phosphate-buffered saline (PBS). Flow cytometric analysis was performed on PB samples collected prior to and 9 days after secondary treatment with the conjugate. On week 12 plus 4 days all mice were challenged intranasally with about 106 plaque-forming units RSV (A2 strain). Sera were collected at weeks 0, 2, 4, 8, 12, 14, 25 and ELISAs were performed to ascertain serum anti-F protein IgG and IgM titers.
The protocol for immunization and treatment of C57BI/6 mice with F/AIPO and CD22/cal
. | Week . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | 0 . | 2 . | 4 . | 4 + 5 d . | 6 . | 8 . | 12 . | 12 + 4 d . | 14 + 4 d . | 25 . | |||||||||
| 1. F/AIPO | B/V | B/V | B/CD22 | CD22 | B | B | B | RSV | B | B/RSV | |||||||||
| 2. F/AIPO | B/V | B/V | B/PBS | PBS | B | B | B | RSV | B | B/RSV | |||||||||
| 3. PBS | B | – | B/CD22 | CD22 | B | B | B | RSV | B | B/RSV | |||||||||
| 4. PBS | B | – | B/PBS | PBS | B | B | B | RSV | B | B/RSV | |||||||||
. | Week . | . | . | . | . | . | . | . | . | . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group . | 0 . | 2 . | 4 . | 4 + 5 d . | 6 . | 8 . | 12 . | 12 + 4 d . | 14 + 4 d . | 25 . | |||||||||
| 1. F/AIPO | B/V | B/V | B/CD22 | CD22 | B | B | B | RSV | B | B/RSV | |||||||||
| 2. F/AIPO | B/V | B/V | B/PBS | PBS | B | B | B | RSV | B | B/RSV | |||||||||
| 3. PBS | B | – | B/CD22 | CD22 | B | B | B | RSV | B | B/RSV | |||||||||
| 4. PBS | B | – | B/PBS | PBS | B | B | B | RSV | B | B/RSV | |||||||||
B indicates bleed (for serum or flow cytometry); V vaccination (F/AIPO); CD22, CD22/cal; PBS, hosphate-buffered saline; RSV, challenge with RSF; and –, not applicable.
Determination of RSV infectivity
The detection of infectious virus in the lungs after challenge on week 25 was assessed in a plaque assay as previously described.34 Briefly, the lungs were removed 4 days after challenge, homogenized, clarified, snap frozen, and stored at -70°C until assayed on HEp-2 cell monolayers.
Serum antibody determinations
The geometric mean serum anti-F protein IgM and IgG titers were determined by end-point ELISA as previously described.34 Significant differences (P < .05) were determined after log transformation by Tukey-Kramer honestly significant difference (HSD) multiple comparison using JMP statistical software (SAS Institute, Cary, NC). The data are expressed ± 1 standard deviation (SD).
Statistical analysis
The data on clinical scores and serum IgG2b levels against type II collagen (Figure 4A-B) were analyzed with the Student t test and are presented as the mean ± SEM. P < .05 was considered significant.
Results
Anti-CD22/calicheamicin has in vitro B-cell-specific antiproliferative effect
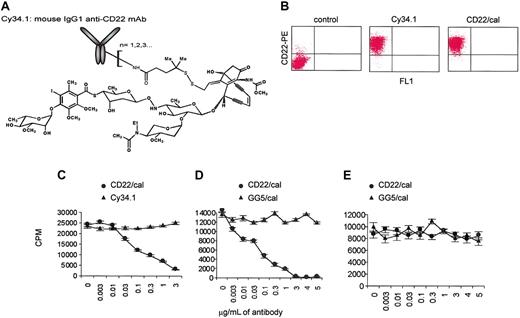
Cy34.1 mAb binds to CD22 expressed on the surface of mouse primary B cells and B-cell lines. We conjugated this antibody to calicheamicin (Figure 1A), a DNA-binding antibiotic that induces double-stranded DNA breaks in cells after internalization, resulting in cell-cycle arrest and apoptosis.31 Biochemical conjugation to calicheamicin (CD22/cal) had no effect on the binding properties of Cy34.1 mAb. On staining, both Cy34.1 and CD22/cal bound similarly to CD22 on A20 B-cell lymphoma cells (Figure 1B). To test in vitro cytotoxicity, purified B cells from B6 mice were cultured with various concentrations of CD22/cal and proliferation in response to stimulation with LPS was studied after 48 hours of culture. Whereas unconjugated Cy34.1 had no effect, 3 μg/mL CD22/cal completely inhibited B-cell proliferation (Figure 1C). The IC50 of CD22/cal was 0.02 μg/mL, whereas the control GG5/cal immunoconjugate had no effect (Figure 1D). The cytotoxicity of CD22/cal was B-cell specific because the compound had no effect in the in vitro T-cell proliferation assays (Figure 1E).
CD22/cal has B-cell-specific in vitro cytotoxicity. (A) Cy34.1 mouse IgG1 mAb is conjugated to N-acetyl-γ-calicheamicin dimethyl acid. CD22/cal has an average loading of 17 to 30 μg calicheamicin/mg antibody protein. (B) Biochemical conjugation of Cy34.1 mAb to calicheamicin (CD22/cal) had no effect on the binding properties of Cy34.1. On staining, both Cy34.1 and CD22/cal bound similarly to CD22 on A20 cells. (C) 3H-thymidine incorporation by primary mouse CD19+ B cells stimulated with LPS and incubated for 48 hours with increasing concentrations of Cy34.1 or CD22/cal. Unconjugated Cy34.1 had no effect, 3 μg/mL CD22/cal completely inhibited B-cell proliferation. (D) 3H-thymidine incorporation by primary mouse B cells stimulated with LPS and incubated for 48 hours with increasing concentrations of CD22/cal or control GG5/cal antibody. GG5/cal had no cytotoxicity against B cells. (E) 3H-thymidine incorporation by primary mouse CD3+ T cells after TCR costimulation and incubation for 48 hours with increasing concentrations of CD22/cal or GG5/cal. CD22/cal had no cytotoxicity against T cells. 3H-thymidine incorporation results are representative of 2 independent experiments and are shown as the mean and SD of triplicate culture.
CD22/cal has B-cell-specific in vitro cytotoxicity. (A) Cy34.1 mouse IgG1 mAb is conjugated to N-acetyl-γ-calicheamicin dimethyl acid. CD22/cal has an average loading of 17 to 30 μg calicheamicin/mg antibody protein. (B) Biochemical conjugation of Cy34.1 mAb to calicheamicin (CD22/cal) had no effect on the binding properties of Cy34.1. On staining, both Cy34.1 and CD22/cal bound similarly to CD22 on A20 cells. (C) 3H-thymidine incorporation by primary mouse CD19+ B cells stimulated with LPS and incubated for 48 hours with increasing concentrations of Cy34.1 or CD22/cal. Unconjugated Cy34.1 had no effect, 3 μg/mL CD22/cal completely inhibited B-cell proliferation. (D) 3H-thymidine incorporation by primary mouse B cells stimulated with LPS and incubated for 48 hours with increasing concentrations of CD22/cal or control GG5/cal antibody. GG5/cal had no cytotoxicity against B cells. (E) 3H-thymidine incorporation by primary mouse CD3+ T cells after TCR costimulation and incubation for 48 hours with increasing concentrations of CD22/cal or GG5/cal. CD22/cal had no cytotoxicity against T cells. 3H-thymidine incorporation results are representative of 2 independent experiments and are shown as the mean and SD of triplicate culture.
CD22/cal has in vivo B-cell-specific cytotoxicity
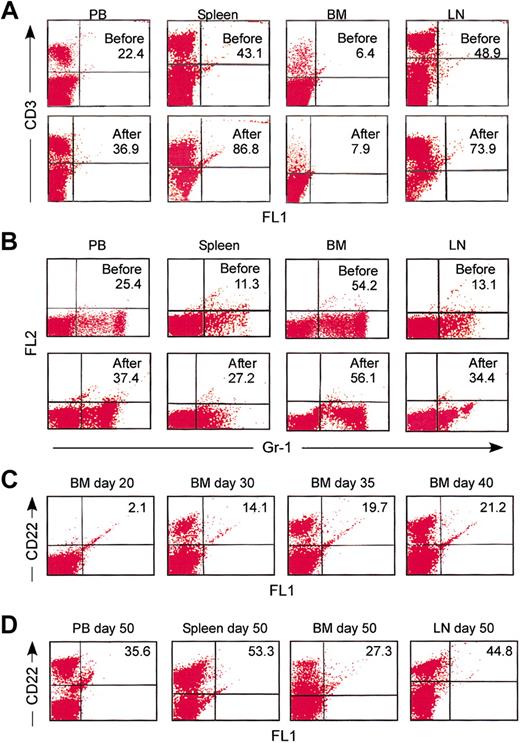
When B6 mice were treated with schedule 5 on days 0 and 5, CD22+ B cells were almost completely absent from PB samples as early as day 8 after the first CD22/cal injection (data not shown). Flow cytometric analysis on day 12 further revealed that the percentage of CD22+ B cells was decreased from 42.9% ± 3.3% to 0.8% ± 0.5% in PB, 44.5% ± 2.2% to 0.9% ± 0.4% in spleen, 26.7% ± 1.6% to 2% ± 0.3% in BM, and 41% ± 2.5% to 0.9% ± 0.3% in LNs (Table 2; Figure 2A). Similar results were obtained when cells were stained with either B220 (data not shown) or anti-CD19 mAb (Figure 2B). As shown, in all tissues tested, the same population of B cells in naive B6 mice expressed both CD22 and CD19 (Figure 2C). Interestingly, when the less efficacious dosing schedules 1 to 4 were used, the level of B-cell depletion was reproducibly higher in PB and LNs as compared to BM and spleen (data not shown). Collectively, these results demonstrate that 2 intraperitoneal injections with 160 μg/CD22/cal/kg, 5 days apart, have very potent cytotoxic activity against B cells in vivo.
Characteristics of B-cell depletion and kinetics of B-cell recovery
The in vivo cytotoxicity of CD22/cal against T cells and myeloid cells was also examined by flow cytometry using mAbs specific for CD3 (T cell) and Gr-1 (myeloid). On day 12, the percentages of CD3+ and Gr-1+ cells were increased in PB, spleen, and LN samples, presumably due to the depletion of CD22+ B cells, whereas they showed insignificant changes in BM samples (Figure 3A-B). The latter observation can be explained by the expression of CD22 on the cell surface as B cells mature to become IgD+ and enter the circulation. On day 20, the percentages of CD22+ cells were similar to those on day 12 (Table 2). By day 30, the percentage of CD22+ cells was increased in the BM and spleen but not in PB and LNs (Table 2). By day 35, the percentages of CD22+ B cells were significantly higher (but below normal levels) in BM and spleen samples and remained less than 5% in PB and LN samples (Table 2). Of interest was the observation that 5% to 8% of CD19+ cells in day 30 and 35 BM and spleen samples were negative for CD22+ expression (data not shown). The numbers of CD22+ B cells were within normal ranges in all tissues tested on day 50 of the experiment (Table 2; Figure 3D). Collectively, these results demonstrate that CD22/cal has B-cell specific in vivo cytotoxicity. In addition, repopulation of the BM and spleen with B cells begins approximately 30 days after the first CD22/cal injection and is completed by day 50.
B-cell depletion inhibits the development of clinical CIAs
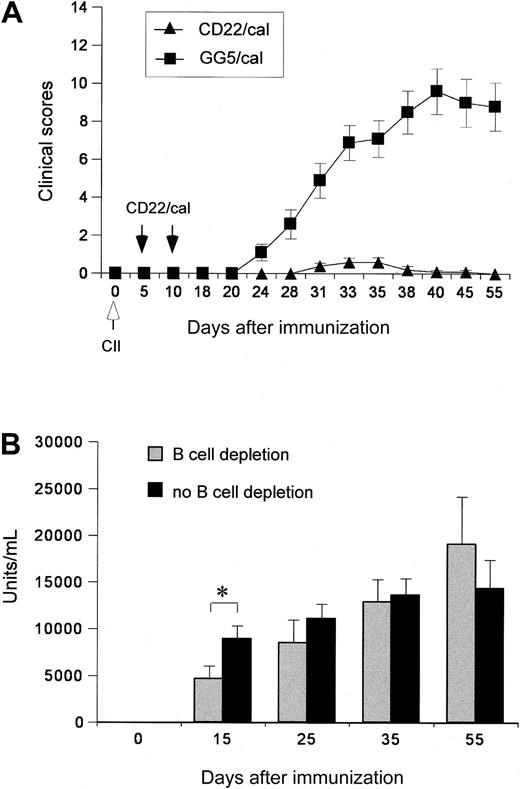
The reactivity of the Cy34.1 antibody (reacts with CD22 on strains having the Lyb-8.2 alloantigen, eg, C57BL/6, but not DBA) restricted the use of the susceptible DBA/136 CIA model in this study. Recent reports, however, demonstrated that CIA could be induced in the resistant C57BL/6 (B6) mice if IFN-γ signaling was abolished.37 Further characterization of the B6 IFN-γ-KO model revealed that 60% to 80% of mice developed progressive arthritis that was similar to classic CIA observed in susceptible DBA/1 mice.33 B6 IFN-γ KO mice, in addition, produced significantly higher levels of IgG2b and IgG1 autoantibodies against murine collagen II compared with B6 mice.33 The B6 IFN-γ-KO CIA model was used in our CIA studies. Depletion was verified by flow cytometric analysis of blood samples 6 to 8 days after the second CD22/cal injection. The percentage of CD22+ and CD19+ B cells was less than 2% (data not shown). The paws were evaluated for clinical signs of arthritis. Whereas 90% of immunized mice that were given injections with GG5/cal developed arthritis by day 33, injections with CD22/cal almost completely inhibited the development of clinical arthritis (Figure 4A). Mice given CD22/cal injections remained free of clinical arthritis beyond day 50, at which time full B-cell pool recovery had occurred in PB samples (data not shown). To investigate if the inhibition of CIA development in the CD22/cal-treated mice was due to the lack of an antibody response to type II collagen, the anti-CII-specific levels of IgG2a in the serum were determined at various time points of the CIA experiment. Anti-CII antibody levels were significantly lower in B-cell-depleted mice compared to control mice on day 15 after immunization, but no statistically significant difference was observed between the 2 groups on days 25, 35, and 55 (Figure 4B). These results suggest that B cells are indispensable for the pathology of the disease, whereas serum anti-CII antibody levels do not appear to have a role during the development of clinical and histologic arthritis in this CIA model.
CD22/cal has B-cell-specific in vivo cytotoxicity. Male B6 mice received 2 (days 0 and 5) intraperitoneal injections with the conjugate at a calicheamicin dose of 160 μg/kg/injection. B-cell depletion was monitored in individual mice by flow cytometry in BM, spleen, LN, and PB samples on days 12, 20, 30, 35, 40, and 50 after the first injection. Three mice per time point were studied and the results (mean percentages ± SD) are listed in Table 2. (A) Flow cytometry dot plot representation of the data listed in Table 2. Numbers are the percentages of CD22+ B cells in PB, spleen, BM, and LNs before and 12 days after 2 injections with CD22/cal. Representative data from one mouse are shown. (B) Day 12 samples were also stained for CD19 expression. (C) Flow cytometric analysis demonstrating that the same population of B cells stained positive for CD22 and CD19 expression in PB, spleen, BM, and LN tissue samples in wild-type B6 mice. Representative data from one mouse are shown.
CD22/cal has B-cell-specific in vivo cytotoxicity. Male B6 mice received 2 (days 0 and 5) intraperitoneal injections with the conjugate at a calicheamicin dose of 160 μg/kg/injection. B-cell depletion was monitored in individual mice by flow cytometry in BM, spleen, LN, and PB samples on days 12, 20, 30, 35, 40, and 50 after the first injection. Three mice per time point were studied and the results (mean percentages ± SD) are listed in Table 2. (A) Flow cytometry dot plot representation of the data listed in Table 2. Numbers are the percentages of CD22+ B cells in PB, spleen, BM, and LNs before and 12 days after 2 injections with CD22/cal. Representative data from one mouse are shown. (B) Day 12 samples were also stained for CD19 expression. (C) Flow cytometric analysis demonstrating that the same population of B cells stained positive for CD22 and CD19 expression in PB, spleen, BM, and LN tissue samples in wild-type B6 mice. Representative data from one mouse are shown.
B-cell depletion inhibits the development of histologic CIA
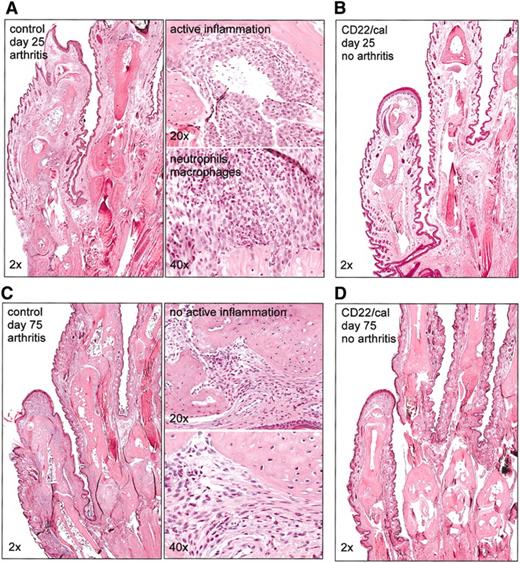
Paws were collected from 2 different experiments on days 25 or 75 after immunization for histologic evaluation. At day 25, paws from immunized control mice were infiltrated by large numbers of neutrophils and macrophages that surrounded and infiltrated the joints and associated connective tissues consistent with active inflammation (Figure 5A). In contrast, paws from CD22/cal-treated mice had normal joint architecture and no inflammatory infiltrates consistent with a lack of previous or ongoing arthritis (Figure 5B). We then compared the paws collected on day 75. At this time, the percentages of CD22+ cells were within normal ranges in PB, LN, and spleen samples (data not shown). Paws from immunized control mice had remodeling and destruction of the joints and adjacent structures consistent with chronic arthritis, although the lack of neutrophils and edema indicated that there was no longer active inflammation (Figure 5C). In contrast, paws from immunized and B cell-depleted mice had microscopically normal joints, which is consistent with there never having been an arthritic reaction in these paws (Figure 5D). Collectively, these results demonstrate that B-cell depletion inhibits the development of CIA that persists in efficacy on recovery of the CD22+ B-cell pool.
Characteristics of B-cell depletion and kinetics of B-cell recovery. Flow cytometric analysis of PB, spleen, BM, and LN before and 12 days after 2 injections with CD22/cal demonstrating that CD22/cal is not cytotoxic for (A) CD3+ T cells and (B) Gr-1+ myeloid cells. (C) Flow cytometric analysis showing the kinetics of CD22+ B-cell recovery in BM samples. (D) Flow cytometric analysis demonstrating that there is complete recovery of CD22+ B cells in PB, spleen, BM, and LNs 50 days after the first injection with CD22/cal. Three mice per group were analyzed; representative data from one mouse are shown.
Characteristics of B-cell depletion and kinetics of B-cell recovery. Flow cytometric analysis of PB, spleen, BM, and LN before and 12 days after 2 injections with CD22/cal demonstrating that CD22/cal is not cytotoxic for (A) CD3+ T cells and (B) Gr-1+ myeloid cells. (C) Flow cytometric analysis showing the kinetics of CD22+ B-cell recovery in BM samples. (D) Flow cytometric analysis demonstrating that there is complete recovery of CD22+ B cells in PB, spleen, BM, and LNs 50 days after the first injection with CD22/cal. Three mice per group were analyzed; representative data from one mouse are shown.
The effect of B-cell depletion with CD22/cal on the clinical scores and antibody responses in CIA. (A) Clinical arthritis scores of B6 IFN-γ-KO mice injected with CD22/cal or GG5/cal. Mice were immunized on day 0 with collagen II in CFA and given intraperitoneal injections on days 5 and 10 with CD22/cal or GG5/cal. The paws were evaluated for clinical signs of arthritis and each paw was individually scored using a 4-point scale. The results represent the mean values ± SEM for 10 mice in each experimental group. CIA was almost completely inhibited in the CD22/cal group and severe CIA was observed in the GG5/cal group (P < .001). (B) Serum IgG2b antibody levels during the course of CIA against type II collagen were measured by standard ELISA as described in “Materials and methods.” The anti-type II collagen concentrations were determined by reference to standard curves generated from 1:2 serial dilutions of a standard CIA serum to calculate the antibody content (in arbitrary units/mL). The values shown are the mean ± SEM for 10 mice in the B-cell depletion group and 15 mice in the no B-cell depletion group (10 mice injected with GG5/cal and 5 mice that had received no injections). The asterisk indicates a significant difference (P < .05) between the mean of the groups on day 15.
The effect of B-cell depletion with CD22/cal on the clinical scores and antibody responses in CIA. (A) Clinical arthritis scores of B6 IFN-γ-KO mice injected with CD22/cal or GG5/cal. Mice were immunized on day 0 with collagen II in CFA and given intraperitoneal injections on days 5 and 10 with CD22/cal or GG5/cal. The paws were evaluated for clinical signs of arthritis and each paw was individually scored using a 4-point scale. The results represent the mean values ± SEM for 10 mice in each experimental group. CIA was almost completely inhibited in the CD22/cal group and severe CIA was observed in the GG5/cal group (P < .001). (B) Serum IgG2b antibody levels during the course of CIA against type II collagen were measured by standard ELISA as described in “Materials and methods.” The anti-type II collagen concentrations were determined by reference to standard curves generated from 1:2 serial dilutions of a standard CIA serum to calculate the antibody content (in arbitrary units/mL). The values shown are the mean ± SEM for 10 mice in the B-cell depletion group and 15 mice in the no B-cell depletion group (10 mice injected with GG5/cal and 5 mice that had received no injections). The asterisk indicates a significant difference (P < .05) between the mean of the groups on day 15.
B-cell depletion does not affect antibody responses against the F protein of RSV
The effect of B-cell depletion on memory antibody-secreting cells was studied in a murine RSV vaccination model using a protocol depicted in Table 3. Flow cytometric analysis of PB samples showed that the percentages of CD22+ cells were reduced from 34.4% ± 6.3% to 2.0% ± 0.7% in the immunized group and from 38.6% ± 4.1% to 2.5% ± %0.6 in the PBS group (data not shown). CD3+ and Gr-1+ cells were unaffected (data not shown). Mice vaccinated with F antigen prior to B-cell depletion had robust IgM and IgG responses to F protein, exhibiting no differences in serum IgM (Figure 6A) and IgG (Figure 6B) titers as compared with control (PBS) mice. In addition, infection of B-cell-depleted naive mice with the A2 strain of RSV on week 12 (8 weeks after B-cell depletion) resulted in comparable levels of anti-F protein IgM (Figure 6A) and IgG (Figure 6B) serum titers, as in control (PBS) naïve mice, indicating that the B cells that have reconstituted by the time of challenge are fully functional. Furthermore, infection of mice with the A2 strain of RSV resulted in comparable anti-F protein IgM and IgG responses in the serum at weeks 14 and 25 (Figure 6A-B), indicating that the memory B-cell pool for protein F was not affected by treatment with CD22/cal. Finally, infectious virus was not detected in the lungs of mice 4 days after challenge on week 25 of the experiment (data not shown).
Discussion
In this report, we demonstrate for the first time that a B-cell-depleting protocol consisting of 2 in vivo injections with CD22/cal is very efficacious in a CIA model while only transiently significantly reducing anti-type II collagen antibody responses and that the same protocol does not have an unfavorable effect on memory responses and clearance of virus after challenge in an RSV model. We also show that CD22/cal has B-cell-specific in vitro and in vivo cytotoxicity, leading to depletion of only CD22+, but not CD3+ T cells and Gr-1+ myeloid cells. Mice that have almost undetectable levels of CD22+ B cells in BM and spleen start repopulating these organs around day 30 to 35 and have complete CD22+ B-cell-pool reconstitution 50 days after CD22/cal injections.
CD22/cal binds to CD22, a member of the immunoglobulin superfamily that serves as an adhesion receptor for sialic acid-bearing ligands.38 Mouse CD22 (mCD22) is detected in the cytoplasm early in B-cell development, is absent from the surface of newly emerging IgM+ B cells, is present at a low density on the immature B220lo IgMhi B cells, and is fully expressed by mature B220hi IgD+ B cells of the BM.39,40 In the periphery, mCD22 is expressed at high levels on all B-cell subsets including follicular and marginal zone B cells and peritoneal B1 cells. However, a minor subset of immature B cells in the spleen recently derived from BM, expresses low density CD22.41 CD22 is constitutively endocytosed and degraded with a relatively short half-life on the cell surface.42 On binding to anti-CD22 mAb, CD22 is rapidly internalized,42 and this property makes it a suitable target for calicheamicin-mediated B-cell cytotoxicity. Indeed, in our studies CD22/cal conferred B cell-specific in vitro and in vivo cytotoxicity. The pattern and kinetics of CD22 expression on B cells may explain our observation that B-cell depletion with CD22/cal was less effective in the BM and spleen, as compared to PB and LNs, when schedules with doses lower than 160 μg/kg/injection were used. Given that a rapid turnover of newly emerging IgM+ B cells constantly occurs in the BM and the spleen, it is reasonable to assume that the concentrations that can effectively deplete B cells in these 2 organs are higher than the concentrations needed for the PB and LNs. Of note, the absence of CD22+ cells in the PB did not mirror the level of depletion in the BM and spleen. This observation is important, particularly in relation to B-cell-ablative therapies in the clinic, and indicates that caution should be taken when clinical responses in these patients are correlated to the level of B-cell depletion because the evaluation of the latter one is based on analysis of PB samples.
In a clinical study involving 22 patients with RA treated with B-cell depletion, PB B-lymphocyte counts fell to undetectable levels in all cases and remained below normal for at least 6 months.17 In our mouse studies, CD22+ B cells were severely depleted from BM and spleen for about a period of 4 weeks. Evidence of repopulation was observed in both tissues between days 30 to 35, whereas B-cell numbers remained low in LN and blood samples. Interestingly, prior to depletion the same population of B cells expressed both CD22 and CD19 (Figure 2C). However, on days 30 and 35, more CD19+ cells were detected than CD22+ cells. At present, the mechanisms that explain the presence of these CD22-CD19+B220+ cells are not apparent. It is unlikely that the explanation may be attributed to CD22/calrelated cytotoxicity or to masking of the CD22 epitope on B cells by unconjugated anti-CD22 antibody, because the period after the last injection exceeded by far the expected half-life of a mouse IgG antibody.
B-cell depletion with CD22/cal inhibits the development of histologic CIA. Mice (n = 10/group) were immunized on day 0 with collagen II in CFA and injected on days 5 and 10 with PBS or CD22/cal. Paws were collected on days 25 or 75 for histologic evaluation. (A) Histopathology of hematoxylin and eosin-stained joints of front paws of control PBS mice collected on day 25. The paws were infiltrated by neutrophils and macrophages consistent with active inflammation. (B) Histopathology of joints of front paws of CD22/cal-treated mice (collected on day 25) showing normal joint architecture. Joint histopathology of (C) control and (D) of B-cell-depleted mice on day 75 after immunization with collagen II. Paws from control mice had remodeling and destruction of the joints consistent with chronic arthritis; paws from B-cell-eted mice had normal joints.
B-cell depletion with CD22/cal inhibits the development of histologic CIA. Mice (n = 10/group) were immunized on day 0 with collagen II in CFA and injected on days 5 and 10 with PBS or CD22/cal. Paws were collected on days 25 or 75 for histologic evaluation. (A) Histopathology of hematoxylin and eosin-stained joints of front paws of control PBS mice collected on day 25. The paws were infiltrated by neutrophils and macrophages consistent with active inflammation. (B) Histopathology of joints of front paws of CD22/cal-treated mice (collected on day 25) showing normal joint architecture. Joint histopathology of (C) control and (D) of B-cell-depleted mice on day 75 after immunization with collagen II. Paws from control mice had remodeling and destruction of the joints consistent with chronic arthritis; paws from B-cell-eted mice had normal joints.
Our CD22/cal studies in the B6 IFN-γ-KO CIA model provide strong evidence for the pathogenic role of B cells in the development of arthritis. This is supported by prior observations, linking B-cell function with disease in CIA. Cross-breeding CBA/N xid43 mice onto the highly susceptible CIA DBA/1 mice resulted in a strain that was resistant to induction of CIA and did not develop an antibody response to type II collagen.44 In addition, mice lacking B cells due to the deletion of the IgM heavy chain gene (muMT) are resistant to CIA.45 In these models, however, B cells were either reduced and defective (xid) or completely absent (muMT) at the time of immunization with collagen. The development of the in vivo CD22/cal protocol using B6 IFN-γ-KO mice enabled us to evaluate the role of B-cell depletion on CIA initiated during priming and effector responses of T and B cells to the injected collagen II. Of note, we currently have only preliminary data showing that incomplete B-cell depletion is less protective in the CIA model (K.D-J., unpublished observation, June 2003), and more studies are needed to determine the level of B-cell depletion required for inhibition of arthritis in this model. In this study, B6 IFN-γ-KO mice treated with schedule 5 not only remained free of clinical and histologic signs of arthritis during the CD22+ B-cell depletion period, but also after complete reconstitution of the CD22+ B-cell pool, as demonstrated by day 75 paw histology. Intriguing was the observation that antibody responses against type II collagen were significantly affected only on day 15 and returned back to normal levels in the absence of clinical and histologic arthritis and of an apparent B-cell recovery, as measured by the numbers of CD22+ cells in the PB. Although more studies are needed for the localization and characterization of antibody-producing cells in this B-cell depletion CIA model, it is tempting to speculate that a very small number of residual CD22+ B cells or CD22- plasma cells that escape CD22/cal-mediated cytotoxicity are sufficient for initiation and subsequent quick growth of the anti-type II collagen antibody responses. The fact that B cells but not antibody responses against type II collagen were indispensable in the pathology of CIA in this model suggests that CD22/cal may have reduced pathogenic B cells to a level that, even after complete reconstitution of the B-cell pool, remained insufficient for the generation of inflammatory mechanisms leading to clinical or histologic arthritis. In this context, CD22/cal may have eliminated B cells that exhibit diverse functions and display pathogenic characteristics, other than autoantibody production.27,46 Thus, it is likely that there are additional mechanisms by which B-cell-lineage depletion modulates autoimmune diseases.20,47,48 This is supported by reports showing that in patients with RA with lymphoid aggregates within the synovium, B cells function as antigen-presenting cells and provide costimulatory signals that promote expansion of effector T cells.49 B cells within the synovium may also secrete proinflammatory cytokines and contribute to inflammation.6,27,50 Future mechanistic studies will address how CD22/cal affects immune responses other than anti-type II collagen antibody production in the CIA model.
B-cell depletion of F protein-educated mice with CD22/cal does not affect antibody responses against the F protein of RSV. (A) Serum IgM and (B) serum IgG titers in B6 mice immunized on week 0 and 2 (black arrows) with F protein (F/AlPO). Control mice (PBS) were not immunized. On weeks 4 and 4 plus 5 days (white arrows) F/AlPO and PBS mice received CD22/cal or PBS alone. All mice were administered infectious RSV on week 12 (⋆). The geometric mean end point titers were determined by ELISA on serum samples of 5 mice per group. Significant differences between the groups were not observed. In A and B ▪ indicates F/AIPO/CD22/cal; •, F/AIPO; ▴, PBS/CD22/cal; X, PBS.
B-cell depletion of F protein-educated mice with CD22/cal does not affect antibody responses against the F protein of RSV. (A) Serum IgM and (B) serum IgG titers in B6 mice immunized on week 0 and 2 (black arrows) with F protein (F/AlPO). Control mice (PBS) were not immunized. On weeks 4 and 4 plus 5 days (white arrows) F/AlPO and PBS mice received CD22/cal or PBS alone. All mice were administered infectious RSV on week 12 (⋆). The geometric mean end point titers were determined by ELISA on serum samples of 5 mice per group. Significant differences between the groups were not observed. In A and B ▪ indicates F/AIPO/CD22/cal; •, F/AIPO; ▴, PBS/CD22/cal; X, PBS.
The effect of CD22/cal was also examined in the B6 mice immunized with the F protein of RSV. The purpose of these studies was to evaluate whether B-cell depletion of F protein-educated mice with CD22/cal would adversely affect anti-F protein IgM and IgG responses and clearance of virus after challenge. Our interest was motivated by concerns that B-cell ablation might significantly diminish, if not abolish, most of the B-cell pool, including previously educated “memory” B cells, and thus cause hypogammaglobulinemia and humoral immunodeficiency. Our results demonstrate that serum immunoglobulin levels remained within normal ranges in mice that were treated with the CD22/cal protocol and that these mice were able to exhibit normal immunoglobulin responses and clearance of virus after challenge. This observation is in agreement with data obtained from a clinical trial with anti-CD20, showing that a significant drop in autoantibodies could be achieved without a concomitant loss in specific IgG antibodies against tetanus toxoid and pneumococcal capsular polysaccharides.51 This selective effect observed in the clinic can be extrapolated to our observations in the CIA and RSV models, showing that CD22/cal is effective in the CIA model of autoimmunity, in the absence of an unfavorable effect in the RSV vaccination model. Most importantly, depletion of B cells in the RSV model allowed for the preservation of preexisting memory antibody responses, suggesting that long-lived plasma cells are involved in humoral memory. Equally important, B-cell depletion allowed the generation of antibody responses to new antigens on reconstitution of the B-cell compartment.
Autoimmunity is a complex process involving the interaction of multiple immune cell types. Whereas B cells are considered key components in the pathogenesis of diseases such as ITP and SLE, their role in RA has been controversial. The clinical efficacy of emerging B cell-directed therapies in patients with RA provide solid evidence for the key contribution of B cells to the immunopathogenesis of RA. The results presented herein extend the preclinical validation of B-cell depletion as a therapy in RA and allow for further exploration of the effects of B cell-depleting regimens. More detailed preclinical analyses of immune function after B-cell depletion and extension of this concept into additional animal models of autoimmunity will give us a better understanding of the potential application of B cell-depleting therapies for autoimmune and inflammatory diseases.
Prepublished online as Blood First Edition Paper, June 9, 2005; DOI 10.1182/blood-2004-11-4547.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank S. Jaison, K. Heers-Dack, and K. S. Pryharski for technical assistance, and B. Carreno for critically reviewing the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal