Abstract
Hepcidin is the principal iron regulatory hormone and its overproduction contributes to anemia of inflammation (AI). In vitro, hepcidin binds to and induces the degradation of the exclusive iron exporter ferroportin. We explored the effects and distribution of synthetic hepcidin in the mouse. A single intraperitoneal injection of hepcidin caused a rapid fall of serum iron in a dose-dependent manner, with a 50-μg dose resulting in iron levels 80% lower than in control mice. The full effect was seen within only 1 hour, consistent with a blockade of iron export from tissue stores and from macrophages involved in iron recycling. Serum iron remained suppressed for more than 48 hours after injection. Using radiolabeled hepcidin, we demonstrated that the serum concentration of hepcidin at the 50-μg dose was 1.4 μM, consistent with the inhibitory concentration of 50% (IC50) of hepcidin measured in vitro. Radiolabeled hepcidin accumulated in the ferroportin-rich organs, liver, spleen, and proximal duodenum. Our study highlights the central role of the hepcidin-ferroportin interaction in iron homeostasis. The rapid and sustained action of a single dose of hepcidin makes it an appealing agent for the prevention of iron accumulation in hereditary hemochromatosis. (Blood. 2005;106:2196-2199)
Introduction
Recent studies indicate that hepcidin is the principal iron regulatory hormone. The involvement of hepcidin in iron metabolism was initially suggested by the increase in hepatic hepcidin mRNA during iron overload.1 The key role of hepcidin was demonstrated by observations that genetic hepcidin deficiency due to the disruption of an adjacent gene resulted in hemochromatosis in mice2 and that homozygous disruption of the hepcidin gene in humans caused juvenile hemochromatosis.3 At the opposite extreme, excessive hepcidin production resulted in severely iron-restricted erythropoiesis in both mice and humans.4,5 Even less severe hepcidin excess was shown to contribute to anemia with hypoferremia, in the face of preserved tissue iron stores.6 In mouse models, the genetic absence of hepcidin or ablation of interleukin-6 (IL-6), a cytokine that normally induces hepcidin, prevented the development of hypoferremia in response to inflammation.2,7
Hepcidin acts by causing the internalization and degradation of the sole vertebrate iron export protein, ferroportin (IREG1, MTP1, SCL11A3).8 Ferroportin is present on the basal surface of intestinal enterocytes, as well as in hepatocytes, macrophages, and placental cells.9 Homeostatic regulation of iron concentrations in plasma and extracellular fluid could therefore be explained by a feedback loop where ingestion of iron increases plasma iron concentration leading to increased hepcidin release from the liver and a rise in plasma hepcidin. Increased hepcidin concentrations then cause increased ferroportin degradation, thus blocking iron release from absorptive enterocytes and iron-storing hepatocytes and macrophages. Continuous utilization of plasma iron by erythropoiesis and other processes then rapidly returns iron concentrations toward their normal values. In anemia of inflammation where hepcidin synthesis is stimulated by cytokines,7,10-12 iron mobilization from stores and iron absorption are prevented, leading to the characteristic hypoferremia with relatively preserved tissue iron stores.
The pharmacodynamics of hepcidin action has not been studied. The reported assays of serum prohepcidin in clinical specimens13 do not measure the active form of hepcidin and yield values much higher than those which effectively inhibited iron transport in vitro.8,14
We determined the effects of a single injection of hepcidin on serum iron concentration in vivo. We performed dose-response and pharmacokinetic studies in mice and determined the effective serum concentration of exogenously administered hepcidin. Finally, we explored the distribution of radiolabeled hepcidin in vivo and showed that hepcidin is preferentially taken up in ferroportin-rich tissues.
Materials and methods
Animals
C57/Bl6J mice were obtained from Charles River Laboratories (Wilmington, MA) or bred in our facility. Mice (equal numbers of both sexes, 8 weeks old, weighing 20 to 25 g) were maintained on 1 of 2 normal iron diets: NIH 31 rodent diet (333 parts per million [ppm] iron; Harlan Teklad, Indianapolis, IN) or Prolab RMH breeder diet (440 ppm iron; PMI International, Brentwood, MO). In order to suppress endogenous hepcidin, mice were switched to a diet containing less than 4 ppm iron (Harlan Teklad) for 2 weeks. We had previously observed that this duration of iron deprivation leads to suppression of endogenous hepcidin without substantial hypoferremia. All studies were approved by the animal research committee at UCLA.
Hepcidin causes a dose-dependent fall in serum iron. Mice received a single dose of hepcidin by intraperitoneal injection. Four hours later, serum iron was determined. There is a strong correlation between serum iron and the dose of hepcidin administered (R = -0.929, P < .001). Four mice were used for every dose. Horizontal lines with P values indicate comparisons by Student t test.
Hepcidin causes a dose-dependent fall in serum iron. Mice received a single dose of hepcidin by intraperitoneal injection. Four hours later, serum iron was determined. There is a strong correlation between serum iron and the dose of hepcidin administered (R = -0.929, P < .001). Four mice were used for every dose. Horizontal lines with P values indicate comparisons by Student t test.
Hypoferremia is induced by hHep-25 but not its truncated variants. Mice received a single 50 μg injection of hHep-25, diluent, hHep-22, or hHep-20. Only the full-length, 25-amino acid hHep induced hypoferremia at 2 hours (n = 4 mice for every treatment). Means and standard deviations are shown, and comparisons indicated by solid lines, and P values were tested by Student t test.
Hypoferremia is induced by hHep-25 but not its truncated variants. Mice received a single 50 μg injection of hHep-25, diluent, hHep-22, or hHep-20. Only the full-length, 25-amino acid hHep induced hypoferremia at 2 hours (n = 4 mice for every treatment). Means and standard deviations are shown, and comparisons indicated by solid lines, and P values were tested by Student t test.
Animal model for testing the effect of human hepcidin in vivo
Human hepcidin-25 (hHep-25, the naturally occurring secreted form of hepcidin), and the N-terminally truncated forms of hepcidin, hepcidin-20 (hHep-20, lacking the first 5 amino acids of hHep-25) and hepcidin-22 (hHep-22, lacking the first 3 amino acids), were synthesized, refolded, and purified by reverse phase-high performance liquid chromatography (HPLC) as previously described.15 Purified hepcidin was dissolved in 0.016% HCl. The hepcidin stock was diluted to the appropriate concentration in phosphate-buffered saline (PBS; prepared as 2.7 mM potassium chloride, 1.1 mM potassium phosphate, 137 mM sodium chloride, and 8.1 mM sodium phosphate; pH 7.4). Depending on the concentration of hepcidin used, the final concentration of HCl was either 0.005% or 0.01%. Mice received a single intraperitoneal injection of 100 μL of this solution. Control mice received diluent only.
Iron measurements
Mice were killed with isoflurane. Blood was collected by cardiac puncture for serum iron determination using a commercial colorimetric kit as instructed by the manufacturer (Diagnostic Chemicals Limited, Oxford, CT).
Radiolabeled human hepcidin
Radioiodinated hepcidin was prepared as previously described.8 Hepcidin-25 substituted at position 21 with a tyrosine residue was iodinated with iodine 125 (125I) and IODO-BEADS (Pierce, Rockford, IL) as described by the manufacturer. The 125I-hepcidin (specific activity of more than 107 counts per minute [cpm]/μg) was purified by HPLC. Each mouse received a single 100-μL injection containing 50 μg nonradioactive hepcidin-25 trace-labeled with 2.5 × 105 cpm/μL 125I-hepcidin, as determined by a gamma counter (Beckman, Fullerton, CA). Thus, for determinations of the concentration of hepcidin in mouse blood or tissues, 1 μg hepcidin corresponded to 5000 cpm.
Mice were killed at 1, 2, and 24 hours after injection of radiolabeled hepcidin. The mice killed at 1 hour also had blood drawn at 30 minutes after injection. Immediately prior to killing, urine was collected by stimulating the bladder with a Petri dish. Blood was then collected from the retro-orbital sinus under isoflurane anesthesia. Mice were killed with isoflurane and the thorax was opened. The right atrium was punctured with a needle and 50 mL PBS was infused through the circulation to wash out as much blood as possible. Organs were then harvested and their radioactivity was measured with a gamma counter.
Statistics
Sigma Stat version 3.0 was used for statistical analyses (Systat Software, Point Richmond, CA). Data are presented as means plus or minus standard deviation. The Student t test is used to compare groups of parametric data. Nonparametric or ordinal data are compared using the Mann-Whitney rank sum test.
Results
In a dose titration study, mice maintained on an iron-deficient diet to suppress endogenous hepcidin received a single intraperitoneal dose of hepcidin (0, 5, 15.8, and 50 μg/mouse, 4 mice per dose) and their serum iron was analyzed 4 hours later. Increasing doses of hepcidin resulted in progressively decreased serum iron concentrations (Figure 1; Spearman correlation coefficient = -0.929, P < .001), with a 50-μg dose causing an 80% drop in serum iron levels compared with control mice (6.4 ± 1.2 μM and 32 ± 8 μM, respectively). To examine the specificity of hepcidin's effect, we also injected mice with N-terminally truncated hepcidins (hHep-20 lacking 5 amino acids8 or hHep-22 lacking 3 amino acids), which were shown in vitro to be inactive (hHep-20) or had greatly reduced activity (hHep-22). Only the full-length 25-amino acid form of hHep induced significant hypoferremia at the 50-μg dose (Figure 2).
The onset of the hypoferremic effect after a single 50-μg dose was quite rapid. Between 30 minutes and 1 hour after the injection of hHep-25, serum iron fell to its nadir value (Figure 3). In mice kept on a low-iron diet for 2 weeks, the pretreatment average serum iron concentration was 32.9 ± 8.1 μM, but 1 hour after injection, the serum iron concentration fell to 7.7 ± 4.8 μM (P < .001 by t test; Figure 3). The hypoferremic effect lasted at least 48 hours, but resolved by 96 hours (Figure 3).
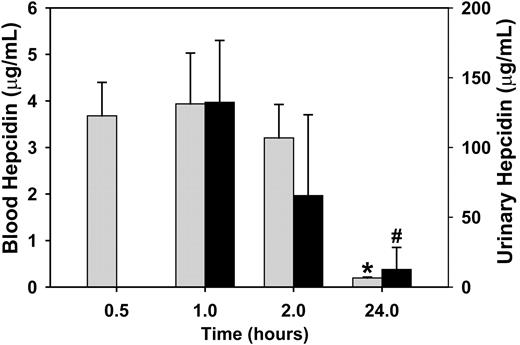
To determine whether the prolonged effect on serum iron levels was due to the long half-life of hepcidin in blood, we studied the kinetics of hepcidin in blood circulation. Mice were injected with a single dose of trace-labeled hepcidin and blood was collected at 0.5, 1, 2, and 24 hours. Essentially all hepcidin disappeared from blood by 24 hours (Figure 4). At the time of the initial decrease in serum iron at 1 hour, the concentration of hepcidin in blood was 3.9 ± 1.1 μg/mL (1.4 μM), consistent with the concentration of hepcidin that resulted in 50% binding to ferroportin in vitro (0.7 μM).8
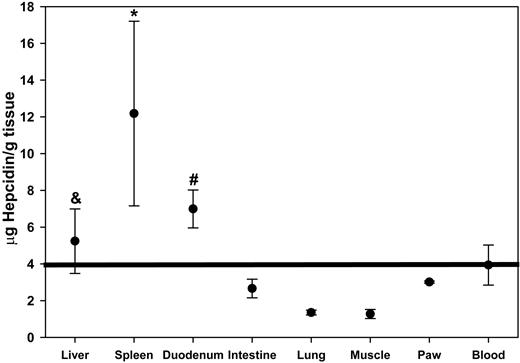
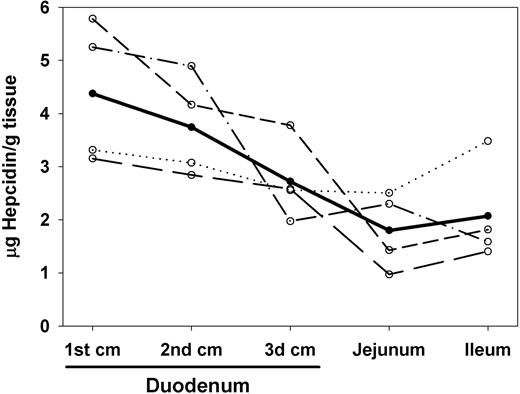
To test whether hepcidin preferentially accumulated in organs known to express ferroportin, we measured the tissue concentration of radiolabeled hepcidin 1 hour after injection. To decrease background from blood in tissues, we perfused the terminally anesthetized mouse with saline. The spleen and the duodenum, 2 of the organs with the highest ferroportin expression,9 had the highest concentration of radiolabeled hepcidin when compared with blood or other organs not expressing ferroportin (Figure 5). In addition, when the duodenum was divided into three 1-cm segments, hepcidin concentration was highest in the most proximal portion of the duodenum where iron uptake and ferroportin concentrations are also the highest (Figure 6).16 Hepcidin content in the liver, another site of ferroportin expression, was lower than in the spleen and duodenum and not significantly higher than in blood, but it was higher than in all other organs measured—the intestine, lung, muscle, and paw (Figure 5). By inspection, the washout of blood from the liver was highly effective, so it is unlikely that the high hepcidin concentrations in this organ were due to background blood. A similar pattern was seen 2 hours after hepcidin injection (data not shown).
Hepcidin causes a rapid, sustained fall in serum iron. Mice received a single 50 μg intraperitoneal injection of hHep. Between 30 and 60 minutes after injection, serum iron achieved its nadir value. This effect was sustained for at least 48 hours. Four to 5 mice were used for each time point.
Hepcidin causes a rapid, sustained fall in serum iron. Mice received a single 50 μg intraperitoneal injection of hHep. Between 30 and 60 minutes after injection, serum iron achieved its nadir value. This effect was sustained for at least 48 hours. Four to 5 mice were used for each time point.
Hepcidin is cleared from the circulation within 24 hours. Mice received a single dose of radiolabeled hepcidin at time 0. ▦ represents blood hepcidin; ▪, the corresponding urinary hepcidin. The 0.5-hour and 1-hour time points are from the same group of 4 mice. The 2- and 24-hour time points were obtained from 2 independent groups of 4 mice. Means and standard deviations are shown. *P < .001 compared with 0.5-hour values, #P = .004 compared with 1-hour values, both by Student t test.
Hepcidin is cleared from the circulation within 24 hours. Mice received a single dose of radiolabeled hepcidin at time 0. ▦ represents blood hepcidin; ▪, the corresponding urinary hepcidin. The 0.5-hour and 1-hour time points are from the same group of 4 mice. The 2- and 24-hour time points were obtained from 2 independent groups of 4 mice. Means and standard deviations are shown. *P < .001 compared with 0.5-hour values, #P = .004 compared with 1-hour values, both by Student t test.
Hepcidin is taken up by ferroportin-rich organs. Mice received a single dose of radiolabeled hepcidin. One hour later, mice were perfused with PBS to remove blood, and organs were collected. *P = .03 by rank-sum and #P = .007 by t test compared with blood; &P < .04 by t test compared with intestine, lung, muscle, or paw.
Hepcidin is taken up by ferroportin-rich organs. Mice received a single dose of radiolabeled hepcidin. One hour later, mice were perfused with PBS to remove blood, and organs were collected. *P = .03 by rank-sum and #P = .007 by t test compared with blood; &P < .04 by t test compared with intestine, lung, muscle, or paw.
Hepcidin concentration declines from the proximal to distal small intestine. Mice (n = 4, each interrupted line representing an individual mouse) received a single dose of radiolabeled hepcidin, were killed 2 hours later, and their organs were perfused with PBS to remove blood. Hepcidin concentration (average shown as a bold line) decreased from proximal to distal position in the intestine (Spearman R = -0.929, P < .001, n = 4).
Hepcidin concentration declines from the proximal to distal small intestine. Mice (n = 4, each interrupted line representing an individual mouse) received a single dose of radiolabeled hepcidin, were killed 2 hours later, and their organs were perfused with PBS to remove blood. Hepcidin concentration (average shown as a bold line) decreased from proximal to distal position in the intestine (Spearman R = -0.929, P < .001, n = 4).
Discussion
This is the first study to show a direct systemic effect of hepcidin peptide in vivo. Although numerous studies have shown the association between hepcidin increase and hypoferremia,7,10,17,18 they did not directly implicate the hepcidin peptide as the causative substance. We demonstrated that the parenteral administration of the full-length 25-amino acid form of hepcidin causes a dose-dependent fall in serum iron. The 25-amino acid form is also the abundant natural form of the peptide in human plasma and urine,15,19 and the less abundant and much less active N-terminally truncated forms probably represent degradation products. The biological role of the circulating proform1,20 remains to be established.
The pharmacokinetics of the hepcidin effect is intriguing. Hepcidin acts within 1 hour, which is consistent with the previously observed rapid response of plasma iron to hepcidin increase after IL-6 administration.2,7 The fast “on” response is less surprising when one considers that serum iron turns over very rapidly due to the small size of the plasma iron pool in comparison to the iron flux necessary for erythropoiesis. The plasma volume is approximately 51 mL/kg,21 which in the average 25-g mouse would be approximately 1.3 mL. The serum iron levels in our mice on a low-iron diet were 32.9 and 7.7 μM before and 1 hour after hepcidin treatment, which corresponds to the total amount of iron in the plasma of only 43 nmol before and 10 nmol after hepcidin treatment. In contrast, using an average hemoglobin value of 15.8 g/dL22 and a blood volume of 2.3 mL,21 the red blood cell pool contains approximately 23 000 nmol iron. Since the erythrocyte half-life in mice is approximately 13 days,22 36 nmol of iron are needed every hour to make new hemoglobin. Normally, this iron would be supplied mostly by macrophages recycling senescent erythrocytes. However, if the export of recycled iron from macrophages is blocked, iron would be expected to disappear from the plasma on a time scale of less than 1 hour.
In contrast to the fast onset of hypoferremia, the recovery was much slower and serum iron did not return to normal levels until 96 hours after injection. Considering that after hepcidin-binding ferroportin is destroyed rather than recycled,8 slow resynthesis of membrane ferroportin could account for the observed delay. The rate of ferroportin synthesis, however, might also be subject to regulation by cellular iron and other stimuli. In our study, the hypoferremic effect of a single dose of hepcidin in mice lasted at least 48 hours, raising the possibility that relatively infrequent dosing of hepcidin could be used to prevent iron accumulation in hereditary hemochromatosis, either before significant iron overload develops or after iron depletion induced by phlebotomy or other measures.
Accumulation of radiolabeled hepcidin in the proximal duodenum and the spleen reflects the high expression of ferroportin in duodenal enterocytes and macrophages.16 The lower accumulation of hepcidin in the liver is probably due to the lower ferroportin expression in numerically dominant hepatocytes compared with hepatic macrophages. If so, the relatively lower ferroportin activity in hepatocytes could help explain why these cells (rather than the macrophages) preferentially accumulate iron in most iron overload diseases.
Based on the blood levels of hepcidin 1 hour after the administration of 50 μg intraperitoneally and the dose response relationship between the hepcidin dose and serum iron (Figure 1), we estimate that hepcidin exerts its hypoferremic activity at blood concentrations in the 0.1 to 1 μM range. This estimate is consistent with the in vitro studies of hepcidin-ferroportin interactions (effective concentration for 50% inhibition [EC50] = 0.7 μM).8 Moreover, exogenous hepcidin is concentrated in tissues that express ferroportin, providing further support for the key role of hepcidin-ferroportin interactions in the regulation of iron transport. Through its effect on ferroportin, hepcidin may fulfill the dual role of maintaining a stable milieu of extracellular iron concentration in support of erythropoiesis and other iron-dependent processes, and acting as a host-defense mediator by rapidly lowering extracellular iron concentrations in response to infections.
Prepublished online as Blood First Edition Paper, June 2, 2005; DOI 10.1182/blood-2005-04-1766.
Supported by National Institutes of Health grant DK065029 to T.G.
S.R. designed and performed the research, analyzed data, and wrote the paper; E.N. contributed vital new reagents and wrote the paper; V.G., M.A.L., and D.F. performed the research; T.G. designed the research, analyzed the data, and wrote the paper.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal