Abstract
Oncogenic rearrangements of the tyrosine kinase receptor anaplastic lymphoma kinase (ALK), most commonly represented by the nucleophosmin/ALK fusion protein (NPM/ALK), are involved in the pathogenesis of anaplastic large-cell lymphomas (ALCLs). In an effort to identify new intracellular transducers operative in ALK-positive malignancies, we have investigated the potential involvement of diacylglycerol kinase (DGK). Here we show that αDGK is constitutively activated in the NPM/ALK-positive ALCL-derived cell line Karpas 299 and in NPM/ALK-infected 32D hematopoietic cells. These results were further validated in fibroblastic NIH-3T3 cells expressing a previously described chimeric epidermal growth factor receptor (EGFR)/ALK molecule that allows dissection of ALK enzymatic function under conditions of controlled ligand-induced activation. In this cell system, we also show that ALK-mediated αDGK activation is dependent on p60src tyrosine kinase, with which αDGK forms a complex. The specific inhibition of αDGK, obtained by cell treatment with R59949, significantly reduced cellular growth in all cell lines. This result was further confirmed in Karpas 299 cells following specific down-regulation of αDGK by RNA interference. Overall, our data indicate that αDGK activation is involved in the control of ALK-mediated mitogenic properties. (Blood. 2005;106: 2175-2182)
Introduction
The anaplastic lymphoma kinase (ALK) gene, localized on chromosome 2p23, encodes for a large, glycosylated 200-kDa membrane-spanning tyrosine kinase receptor whose expression is highly tissue specific, being restricted to components of the nervous system; structural homology studies place ALK within the family of insulin receptor tyrosine kinases (RTKs), with the highest homology to the leukocyte tyrosine kinase (LTK).1,2 Involvement of ALK in the pathogenesis of hematopoietic malignancies derives from the observation that constitutively active forms of ALK are detected with a high frequency in anaplastic large-cell lymphomas (ALCLs), a subgroup of non-Hodgkin lymphomas, predominantly of T or null type.3 The oncogenic forms of ALK are the result of somatic chromosome translocations that fuse the ALK cytoplasmic domain to the 5′ region from different partner genes. The most frequent oncogenic version of ALK is represented by nucleophosmin (NPM)/ALK, an 80-kDa hybrid protein created by the t(2;5)(p23; q35) rearrangement.4,5 The tumorigenic properties of NPM/ALK have been demonstrated in vitro in different cell systems6-9 and confirmed in vivo by the generation of NPM/ALK-mediated tumor models.10-13
Despite several studies, the pathogenic mechanisms leading to ALK-mediated transformation remain still poorly defined. Thus far, several signaling molecules have been identified that associate and/or are activated by ALK, including growth factor receptor-bound protein 2 (Grb2), Src homology and collagen (Shc), insulin receptor substrate-1 (IRS-1), phospholipase C-γ (PLC-γ), p60src, and phosphatidylinositol 3-kinase (PI3-K), although only a few have been shown to be strictly specific for ALK's transforming potential.3 In particular, several lines of investigation have indicated that both PLC-γ and PI3-K are critical transducers of NPM/ALK-mediated oncogenesis through activation of mitogenic and/or survival signals,8,14,15 while the exact role of the ras/mitogen activated protein kinase (MAPK) cascade needs further evaluation.3 The contribution of p60src has been recently evaluated in NPM/ALK-positive cell lines and demonstrated through the effects of p60src down-regulation and pharmacologic inhibition on cellular proliferative rate.16 Additional relevant effectors of NPM/ALK-mediated lymphomagenesis are represented by signal transducer and activator of transcription 3 (Stat3) and Stat5.17
Obviously, further investigations are needed to identify other pathways that are operative in ALK-positive malignancies and that could represent intracellular targets for a more appropriated therapeutic intervention in ALCL treatment.
Diacylglycerol kinase (DGK) has attracted much attention in recent years since growing evidence indicates its direct involvement in the regulation of signal transduction processes. The family of mammalian DGKs comprises 9 isoenzymes classified in 5 distinct groups on the basis of their primary structure.18 In particular, type I DGKs include α-, β-, and γ-isoforms. αDGK is abundant in the cytosol of T lymphocytes,19 but it is also expressed in endothelial and epithelial cells, fibroblasts, and oligodendrocytes.20-22
It has been reported that αDGK activity is required for interleukin-2 (IL-2)-induced G1- to S-phase transition in T cells.23,24 In addition, more recent studies in nonlymphoid cells have highlighted the importance of αDGK activation for the transduction of migratory signals mediated by the hepatocyte growth factor receptor (HGFR)21 and for vascular endothelial growth factor receptor-2 (VEGFR-2)-induced angiogenesis.22 In these cell systems, it has also been demonstrated that receptor-mediated αDGK activation takes place through a p60src-dependent mechanism, since it requires p60src-tyrosine kinase activity and involves the formation of a p60src/αDGK complex.21,22
On the basis of these data and on the requirement of p60src activation in NPM/ALK-induced signaling,16 we sought to analyze the role of αDGK in ALK-mediated mitogenic properties. Here we report that the αDGK catalytic function is up-regulated by activated ALK through a p60src-dependent mechanism and is critical for the proliferation of NPM/ALK-positive cell lines. These data indicate that αDGK is a downstream target of ALK signaling and suggest that the inhibition of its activity can interfere with NPM/ALK function in ALCL cells.
Materials and methods
Retroviral construct and infection
The NPM/ALK retroviral construct was obtained by cloning the open-reading frame (ORF) of pcDNA3-NPM/ALK into the PINCO vector encoding the enhanced green fluorescent protein (GFP), as previously described.25 Recombinant polymerase chain reaction (PCR) was used to amplify the entire NPM/ALK sequence4 using primers designed to yield the NPM/ALK cDNA flagged by unique EcoRI site at both ends of PCR products. The sequence of the primers used was 5′ primer GAATTCATGGAAGATTCGATGGACATGG and 3′ primer GAATTCCTCAGGGCCCAGGCTGG. Following EcoRI digestion, the PCR fragment was cloned into the EcoRI site of the PINCO retroviral vector. To infect 32D cells, the cells were cultured for 3 hours in the presence of 0.45 μM filtered viral supernatant collected from Phoenix cells 2 days after transfection with PINCO or PINCO-NPM/ALK vectors.25 Two infection cycles were performed in the presence of 5 μg/mL polybrene (Sigma, St Louis, MO). GFP-positive infected cells were sorted following standard procedures by a fluorescence-activated cell sorter (FACS) scan (FACS Vantage; Becton Dickinson, Omaha, CA) with an excitation wavelength of 488 nm.
Cell lines and transfections
The murine growth factor-dependent 32D parental cell line and 32D cells infected with empty virus were maintained in RPMI 1640 containing 10% fetal bovine serum (FBS; Invitrogen, Paisley, United Kingdom) and 10% WEHI-conditioned medium as a source of IL-3. NPM/ALK-infected 32D cells, the human NPM/ALK-negative T lymphoblastoid CEM cell line, the human NPM/ALK-positive Karpas 299 (kindly provided by B. Falini, University of Perugia, Italy), and TS (kindly provided by R. Piva, University of Turin, Italy) cell lines were cultured in RPMI 1640 containing 10% FBS. Genetically engineered NIH-3T3 cells overexpressing the chimeric epidermal growth factor receptor (EGFR)-ALK receptor (NIH-EGFR/ALK) were described previously26 and maintained in Dulbecco modified Eagle medium (D-MEM; Invitrogen) containing 10% FBS. For epidermal growth factor (EGF) triggering experiments, subconfluent NIH-EGFR/ALK cell monolayers, grown in poly-l-lysine-coated dishes, were incubated for 18 hours in D-MEM medium supplemented with transferrin (5 μg/mL; Becton Dickinson, Franklin Lakes, NJ) and Na2SeO3 (10-8 M; Sigma) in the absence of serum (starvation conditions) before EGF treatment (Upstate Biotechnology, Lake Placid, NY). When indicated, 1 to 10 μM R59949 or 1 μM PP2 ([4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-di] pyrimidine]; both from Alexis Biochemicals, San Diego, CA) were added to the culture medium 30 minutes to 1 hour before the addition of EGF and maintained at the same concentration throughout the experiments. Stock solutions of the inhibitors were prepared in dimethyl sulfoxide (DMSO) and diluted so that the final concentration in culture medium never exceeded 0.02%.
In some experiments, NIH-EGFR/ALK cells were transiently transfected with pMT2-myc-αDGK expression vector21 by the calciumphosphate precipitation technique. Thirty-six hours from transfection, cells were cultured overnight under starvation conditions and then EGF stimulated.
Cell lysis and protein analysis
Total cell lysates were prepared at 4°C by using 1% Triton X-100 lysis buffer as previously described,26 unless otherwise specified. For immunoprecipitation procedures, cellular lysates were incubated with the indicated antibody for at least 2 hours at 4°C and immunocomplexes were recovered by adsorption to Protein-G Sepharose beads (Amersham Biosciences, Piscataway, NJ). Lysates or immunocomplexes were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose filters, and immunoblotted according to previously described procedures.26,27 Bound proteins were visualized with [125I]-labeled anti-mouse immunoglobulin G (IgG, 0.2 μCi [0.0074 MBq]/mL; Amersham Biosciences) or by enhanced chemiluminescence (ECL) following incubation with the appropriate horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences).
The monoclonal antibody recognizing the intracellular domain of ALK28 was kindly provided by B. Falini (University of Perugia, Italy) and P. G. Pellicci (European Institute of Oncology, Milan, Italy). A commercially available anti-p60src monoclonal antibody (clone GD11; Upstate Biotechnology) was used. The mouse monoclonal antibody, clone 9E10, recognizing the c-myc carboxy terminal domain (residues 408-439) was purchased either from Sigma or from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-αDGK antibodies were a mixture of monoclonal antibodies obtained as described.29 The mouse monoclonal anti-α-tubulin (clone B-5-1-1) was from Sigma. Ab-1, the monoclonal antibody directed against the extracellular domain of EGFR, was purchased from Calbiochem (San Diego, CA).
The pGEX-p60src/Src homology 2 (SH2) vector, used for the in vitro association experiments described in Figure 5A, was kindly provided by R. M. Melillo (Department of Cellular and Molecular Biology and Pathology, University Federico II, Naples, Italy) and G. Superti-Furga (Cellzome, Heidelberg, Germany). Glutathione S-transferase (GST) fusion proteins were induced in log-phase growing bacteria upon treatment with 1 mM isopropyl-beta-d-thiogalactopyranoside (IPTG) for 4 hours. Bacteria were recovered by centrifugation, resuspended in 1/100 volume lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 8.0, 500 mM NaCl, 0.5% nonidet P-40 [NP-40], 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM dithiothreitol [DTT], 2 mg/mL lysozyme, 2 mM phenylmethylsulfonylfluoride [PMSF], and 50 μg/mL aprotinin) and lysed on ice by sonication. Lysates were clarified by centrifugation and the resulting supernatant was incubated with glutathione-agarose (Amersham Biosciences) overnight at 4°C with gentle rotation. The resin was washed with excess phosphate-buffered saline (PBS) and then resuspended in lysis buffer before storing at -70°C. For in vitro binding assays, cell lysates (2 mg) were incubated with 2 μg recombinant protein immobilized onto glutathione-agarose for 1.5 hours at 4°C under gentle rotation. After several washes, proteins were eluted in SDS-PAGE sample buffer and analyzed by immunoblotting.
p60src kinase assay
Following in vivo treatment with 100 ng/mL EGF for the indicated time, NIH-EGFR/ALK cells were lysed with Staph A buffer (10 mM sodium phosphate, pH 7.5, 100 mM NaCl, 1% Triton X100, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM PMSF, and 50 μg/mL aprotinin) and immunoprecipitated with the indicated antibody. Half of each sample was analyzed directly by Western blot; the other was subjected to in vitro kinase assay. To this end, immunocomplexes were resuspended in 50 μL kinase buffer (0.1% Triton X-100, 6 mM MnCl2, 50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid], pH 7.5) containing 10 μCi (0.37 MBq) [γ-32P] adenosine triphosphate (ATP, ∼ 3000 Ci [111 000 GBq]/nmol; Amersham Biosciences) and 5 μg acid-denaturated rabbit muscle enolase (Sigma)30 for 10 minutes at room temperature. Kinase reactions were stopped by adding SDS-PAGE sample buffer; samples were then analyzed directly by SDS-PAGE and autoradiography. Spots were quantified using a PhosphorImager apparatus (Molecular Dynamics, Sunnyvale, CA), and background was subtracted from each sample using the local median algorithm from the Image Quant program (version 1.2; Molecular Dynamics).
DGK assay
Immunocomplexes, obtained as described, were washed once with buffer containing 0.1% Triton X-100, 20 mM HEPES (pH 7.5), 10% glycerol, and 150 mM NaCl; once with 0.5 M LiCl and 25 mM Tris, pH 8; and twice with TNE (25 mM Tris, 150 mM NaCl and 1 mM EDTA, pH 8). Immunocomplexes or cellular extracts (10 μg) were incubated for 10 minutes at 30°C with 0.45 μg/μL diolein (Sigma), 1 mM ATP, 30 μCi (1.110 MBq) [γ-32P]-ATP (Amersham Biosciences), 10 mM MgCl2, 1 mM ZnCl2, and 1 mM Na3VO4 in 25 mM HEPES, pH 8. Reactions were stopped and lipids extracted by adding 1 N HCl and chloroform-methanol (1:1). Phosphatidic acid (PA) was separated by thin-layer chromatography (TLC) in chloroform-methanol-water-25% ammonium hydroxide (60:47:6:6), followed by exposure to autoradiographic film (Kodak, Rochester, NY). [32P]-PA was identified by comigration with nonradioactive PA standards (Sigma) stained by incubation in a iodine chamber. PA spots were quantified using a PhosphorImager apparatus (Molecular Dynamics). Background was subtracted from each sample as indicated in the previous section.
Cell proliferation assay
[3H]-thymidine incorporation assay was performed as previously described.26 For MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) proliferation assay, cell lines were seeded into 96-well plates at a concentration of 2 × 104 cells/well in 100 μL RPMI supplemented with 0.1% FCS and 0.2 mg/mL bovine serum albumin (BSA) in the presence of 10 μM R59949 or DMSO as control. After 24 or 48 hours, a volume of 15 μL MTT dye (Sigma) was added to each culture. Reaction was stopped following 3 hours of incubation at 37°C by adding to each well 150 μL of a solution containing 50% dimethyl formamide and 10% SDS. The absorbance of converted water-insoluble MTT-formazan was measured at a wavelength of 540 nm. Bromodeoxyuridine (BrdU) incorporation was measured by the Cell Proliferation ELISA Biotrak System (Amersham Biosciences) according to the manufacturer's directions.
RNA interference
Karpas 299 cells were transfected by electroporation with siPORT siRNA Electroporation Buffer (Ambion, Austin, TX) using a Gene pulser apparatus (Bio-Rad Laboratories, Hercules, CA) as suggested by the manufacturer. siRNAs used were chemically synthesized as double-strand RNA (Ambion). An interfering RNA, which has been previously shown to efficiently down-regulate the expression of human αDGK in endothelial cells,22 was used. Sequences were as follows: sense, GGAUUUAGAGAUGAGUAAATT and antisense, UUUACUCAUCUCUAAAUCCTT (target AAGGATTTAGAGATGAGTAAA); a scramble siRNA was used as negative control.
Results
Coupling of ALK with DG kinase activity
In vitro DGK activity was initially evaluated in whole-cell lysates obtained from the human NPM/ALK+ ALCL-derived cell line Karpas 299. DGK activity was determined by quantifying the in vitro phosphorylation of exogenously added diacylglycerol (DG) to generate [32P]-PA. As shown in Figure 1A (left panel), this cell line revealed a high constitutive DGK activity. Similar results were observed in the murine hematopoietic 32D cell line infected with a retrovirus expressing a GFP-NPM/ALK fusion protein (32D-NPM/ALK). 32D is a committed-undifferentiated cell line, absolutely dependent for growth and survival on IL-3. As it has been previously shown for other growth factor-dependent hematopoietic cell lines,8 the expression of NPM/ALK in 32D cells is sufficient to induce a growth factor-independent phenotype, a marker of cellular transformation (data not shown). In 32D-NPM/ALK cells, the total DGK activity was significantly higher compared with that measured in the parental 32D cells infected with the empty vector, indicating that a DGK activity could be directly related to the expression of NPM/ALK (Figure 1A).
In addition, pretreatment of both cell lines with 10 μM R59949, a specific inhibitor of DGK type I,31 resulted in a dramatic decrease of radioactive PA (approximately 70% of the control value), suggesting that the observed DGK activity was mainly due to the enzymatic action of the α-isoform (Figure 1A). In order to demonstrate more directly the activation of the α-isoform, the in vitro DGK assay was performed in anti-αDGK immunocomplexes obtained from Karpas 299 cells or CEM, a NPM/ALK-negative human T-lymphoblast cell line. Both cell lines express αDGK at similar levels, but DGK activity in anti-αDGK immunoprecipitates from Karpas 299 was considerably higher than from CEM cells (Figure 1B, left panel). Similar results were observed in an additional human NPM/ALK-positive cell line derived from a different patient32 (Figure 1B, right panel).
αDGK activity in NPM/ALK-positive cells. Cell lines were cultured for 30 minutes to 1 hour at 37°C in the absence (-) or presence (+) of either 10 μM R59949 or 1 μM PP2 and lysed thereafter. Total cell lysates (A) or anti-αDGK immunocomplexes (B, upper panel) were analyzed for in vitro DGK enzymatic activity in the presence of [γ-32P] ATP and diolein as exogen substrate; lipid phase was separated by TLC. An aliquot from each anti-αDGK immunoprecipitate was analyzed by SDS-PAGE to ensure that equal amounts of αDGK were present during in vitro kinase assay (B, bottom panel). PA indicates phosphatidic acid.
αDGK activity in NPM/ALK-positive cells. Cell lines were cultured for 30 minutes to 1 hour at 37°C in the absence (-) or presence (+) of either 10 μM R59949 or 1 μM PP2 and lysed thereafter. Total cell lysates (A) or anti-αDGK immunocomplexes (B, upper panel) were analyzed for in vitro DGK enzymatic activity in the presence of [γ-32P] ATP and diolein as exogen substrate; lipid phase was separated by TLC. An aliquot from each anti-αDGK immunoprecipitate was analyzed by SDS-PAGE to ensure that equal amounts of αDGK were present during in vitro kinase assay (B, bottom panel). PA indicates phosphatidic acid.
To further validate our findings, we took advantage of a previously described model system represented by a chimeric molecule encompassing the extracellular and transmembrane domain of the EGFR and the entire cytoplasmic portion of ALK. We have previously shown that in this chimera the biochemical and biologic properties of ALK are controlled by EGF stimulation, thus making it possible to dissect ALK enzymatic function under condition of controlled ligand-induced activation.26 Therefore, fibroblastic NIH-3T3 cells expressing the chimera EGFR/ALK (NIH-EGFR/ALK) were cultured at 37°C for different time periods in the absence or presence of EGF prior to lysis and subsequent in vitro DGK assay on total homogenate. As shown in Figure 2, an increase in total DGK activity was observed after EGF treatment, further supporting a direct correlation between ALK activation and stimulation of a cellular DGK enzymatic function. The effect was rapid, reached a maximum in 10 minutes (about 2-fold over control), and went back to the basal level within 30 minutes.
Similarly to Karpas 299 and 32D-NPM/ALK cells, the DGK activity observed in EGF-treated NIH-EGFR/ALK cells was very sensitive to R59949 treatment, again suggesting the involvement of the α isoenzyme in ALK-mediated signal transduction (data not shown).
Limitations of the anti-αDGK antibodies used in this study do not allow efficient immunoprecipitation of the murine isoform. Therefore, to assess our results more directly and provide further support to the hypothesis that αDGK activity is stimulated upon activation of ALK tyrosine kinase, we have transiently transfected NIH-EGFR/ALK cells with a myc-tagged αDGK construct and determined αDGK activity in anti-myc immunoprecipitates obtained from lysates of either untreated or EGF-stimulated cell cultures. As shown in Figure 3 (upper panel), αDGK activity was significantly higher (around 3-fold over control) in immunocomplexes obtained from EGF-stimulated NIH-EGFR/ALK cell lysates, despite a comparable amount of immunoprecipitated myc-αDGK (lower panel).
These results indicate a specific activation of αDGK by ALK under conditions in which this molecule promotes mitogenic and transforming signals.
ALK-induced αDGK activation is mediated by a p60src-dependent mechanism
Evidence is increasing that members of the Src family of tyrosine kinases provide regulatory signals relevant for DGK activation. Indeed recent studies obtained in both lymphoid and non-lymphoid cells have indicated the requirement of p60src activity for αDGK21,22,33 and ζDGK34 activation.
DGK activity in NIH-EGFR/ALK cells following EGF stimulation. NIH-EGFR/ALK cells were serum-starved for 18 hours and then either mock-treated or treated with 100 ng/mL EGF for the indicated time at 37°C. (A) Cell lysates were analyzed for in vitro DGK enzymatic activity as described in Figure 1. (B) PA spots were quantified by densitometric scanning of the autoradiograms and results are expressed as percent of control (signal obtained from medium-treated cells). Results are representative of 3 independent experiments.
DGK activity in NIH-EGFR/ALK cells following EGF stimulation. NIH-EGFR/ALK cells were serum-starved for 18 hours and then either mock-treated or treated with 100 ng/mL EGF for the indicated time at 37°C. (A) Cell lysates were analyzed for in vitro DGK enzymatic activity as described in Figure 1. (B) PA spots were quantified by densitometric scanning of the autoradiograms and results are expressed as percent of control (signal obtained from medium-treated cells). Results are representative of 3 independent experiments.
In Karpas 299 and TS cells, we have initially observed that αDGK activity was strongly reduced by 1 hour of cell pretreatment with the specific Src inhibitor PP2 (Figure 1B), suggesting that the elevated αDGK activity in these NPM/ALK-positive cells is maintained through Src tyrosine kinase activity. Similar results have been observed in 32D-NPM/ALK cells (data not shown).
EGF-induced αDGK activation in NIH-EGFR/ALK cells. NIH-EGFR/ALK cell line was transiently transfected with the expression vector pMT2-myc-αDGK according to the calcium-phosphate technique. Cells were serum-starved for 18 hours and then either mock-treated (-) or stimulated (+) for 10 minutes at 37°C with 100 ng/mL EGF and lysed thereafter. Lysates were immunoprecipitates with appropriate concentrations of agarose-conjugated anti-c-myc antibody. (A) Immunocomplexes were subjected to in vitro kinase assay as described in Figure 1. (B) An aliquot of each sample was analyzed by Western blot to control that equal amounts of myc-αDGK were present during in vitro kinase assay.
EGF-induced αDGK activation in NIH-EGFR/ALK cells. NIH-EGFR/ALK cell line was transiently transfected with the expression vector pMT2-myc-αDGK according to the calcium-phosphate technique. Cells were serum-starved for 18 hours and then either mock-treated (-) or stimulated (+) for 10 minutes at 37°C with 100 ng/mL EGF and lysed thereafter. Lysates were immunoprecipitates with appropriate concentrations of agarose-conjugated anti-c-myc antibody. (A) Immunocomplexes were subjected to in vitro kinase assay as described in Figure 1. (B) An aliquot of each sample was analyzed by Western blot to control that equal amounts of myc-αDGK were present during in vitro kinase assay.
p60src kinase activity in NIH-EGFR/ALK cells following EGF stimulation. Quiescent NIH-EGFR/ALK cells were either mock-treated or treated with 100 ng/mL EGF for the indicated time at 37°C. Cell lysates were immunoprecipitated with an anti-p60src antibody. (A) An aliquot from each immunoprecipitate was directly analyzed by immunoblotting to evaluate p60src content. (B) The remainder of each immunoprecipitate was subjected to in vitro kinase assay in the presence of [γ-32P] ATP and acid-denaturated enolase. Samples were then analyzed by SDS-PAGE and autoradiography. (C) The 32P content of bands corresponding to enolase shown in panel B was quantified by densitometric scanning of the autoradiograms and results are expressed as percent of control (signal obtained from medium-treated cells).
p60src kinase activity in NIH-EGFR/ALK cells following EGF stimulation. Quiescent NIH-EGFR/ALK cells were either mock-treated or treated with 100 ng/mL EGF for the indicated time at 37°C. Cell lysates were immunoprecipitated with an anti-p60src antibody. (A) An aliquot from each immunoprecipitate was directly analyzed by immunoblotting to evaluate p60src content. (B) The remainder of each immunoprecipitate was subjected to in vitro kinase assay in the presence of [γ-32P] ATP and acid-denaturated enolase. Samples were then analyzed by SDS-PAGE and autoradiography. (C) The 32P content of bands corresponding to enolase shown in panel B was quantified by densitometric scanning of the autoradiograms and results are expressed as percent of control (signal obtained from medium-treated cells).
This possibility was further tested in NIH-EGFR/ALK cells. To this end, we have first analyzed p60src kinase activity following in vivo stimulation of NIH-EGFR/ALK cells with EGF for different time periods. Lysates were immunoprecipitated with a p60src-specific antibody, and kinase assay was performed in the presence of [γ-32P]ATP and 5 μg acid-denaturated enolase added as exogenous substrate. Under our experimental conditions, p60src autophosphorylation was increased within 2 minutes of ligand exposure, reached a maximum in 5 minutes (about 2.2-fold stimulation), and returned to basal levels after 20 minutes (Figure 4B). The increased autokinase activity of p60src was accompanied by an increase in phosphorylation of the exogenous substrate enolase (Figure 4B-C). These data indicate that p60src kinase activity is stimulated by ALK activation, according to a recent report that describes the association/activation of p60src with NPM/ALK.16 In vivo association of p60src with the cytoplasmic domain of activated ALK was also confirmed in our model system by coimmunoprecipitation (data not shown) and further validated in vitro by pull-down experiments using a bacterially expressed GST-p60src/SH2 fusion protein (Figure 5A).
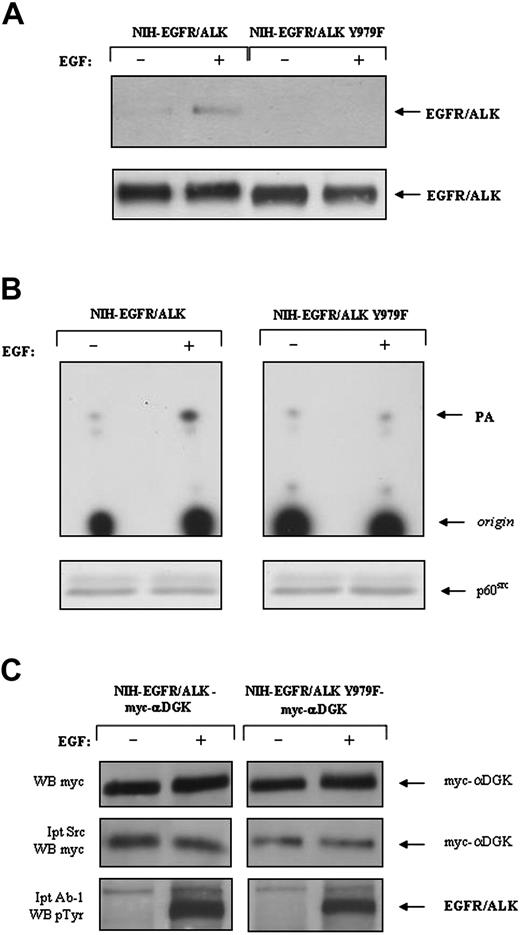
Association of αDGK protein and activity with p60src in NIH-EGFR/ALK and NIH-EGFR/ALK-Y979F cells. (A-B) NIH-EGFR/ALK and NIH-EGFR/ALK-Y979F cells, expressing comparable levels of receptors, were serum-starved for 18 hours and then either mock-treated or stimulated for 5 minutes at 37°C with 100 ng/mL EGF and lysed thereafter. (A) A 2-mg aliquot of total cellular proteins from each lysate was incubated with GST-p60src/SH2 (10-7 M) immobilized onto glutathione-agarose for 1.5 hours at 4°C. After extensive washing, proteins were electrophoresed on SDS-PAGE and immunoblotted with the anti-ALK monoclonal antibody (top panel). The same lysate (100 μg) was directly analyzed by immunoblot with the anti-ALK antibody (bottom panel). (B) Total cellular proteins (3 mg) were immunoprecipitated with an anti-p60src-specific antibody. Two thirds of each immunoprecipitate was subjected to in vitro DGK enzymatic activity as described in Figure 1 (top panel). The remainder of each immunoprecipitate was analyzed by immunoblot with p60src antibody (bottom panel). Comparable results were obtained in 3 different experiments. (C) NIH-EGFR/ALK and NIH-EGFR/ALK-Y979F cells were transiently transfected with the expression vector pMT2-myc-αDGK. Cell treatment and lysis were performed as described in Figure 3. Control (top panel) and p60src immunoprecipitates (middle panel) were analyzed for myc-αDGK protein by Western blot with anti-myc antibodies. Each lysate was also analyzed for proper receptor activation by immunoprecipitation with Ab-1 antibody, which recognizes the EGFR extracellular domain and subsequent Western blot with anti-phospho-Tyr antibodies (bottom panel). WB indicates Western blot.
Association of αDGK protein and activity with p60src in NIH-EGFR/ALK and NIH-EGFR/ALK-Y979F cells. (A-B) NIH-EGFR/ALK and NIH-EGFR/ALK-Y979F cells, expressing comparable levels of receptors, were serum-starved for 18 hours and then either mock-treated or stimulated for 5 minutes at 37°C with 100 ng/mL EGF and lysed thereafter. (A) A 2-mg aliquot of total cellular proteins from each lysate was incubated with GST-p60src/SH2 (10-7 M) immobilized onto glutathione-agarose for 1.5 hours at 4°C. After extensive washing, proteins were electrophoresed on SDS-PAGE and immunoblotted with the anti-ALK monoclonal antibody (top panel). The same lysate (100 μg) was directly analyzed by immunoblot with the anti-ALK antibody (bottom panel). (B) Total cellular proteins (3 mg) were immunoprecipitated with an anti-p60src-specific antibody. Two thirds of each immunoprecipitate was subjected to in vitro DGK enzymatic activity as described in Figure 1 (top panel). The remainder of each immunoprecipitate was analyzed by immunoblot with p60src antibody (bottom panel). Comparable results were obtained in 3 different experiments. (C) NIH-EGFR/ALK and NIH-EGFR/ALK-Y979F cells were transiently transfected with the expression vector pMT2-myc-αDGK. Cell treatment and lysis were performed as described in Figure 3. Control (top panel) and p60src immunoprecipitates (middle panel) were analyzed for myc-αDGK protein by Western blot with anti-myc antibodies. Each lysate was also analyzed for proper receptor activation by immunoprecipitation with Ab-1 antibody, which recognizes the EGFR extracellular domain and subsequent Western blot with anti-phospho-Tyr antibodies (bottom panel). WB indicates Western blot.
Tyrosine 418 of NPM/ALK (numbered according to Morris et al4 ) has been identified as the residue responsible for NPM/ALK-p60src interaction.16 Indeed, tyrosine to phenylalanine substitution at the corresponding position in the EGFR/ALK sequence (EGFR/ALK-Y979F, according to Piccinini et al26 ) completely abolished p60src-EGFR/ALK binding, even when lysates were prepared from EGF-stimulated cells (Figure 5A). Although the Y979F mutation did not alter the in vivo autophosphorylation activity of the EGFR/ALK chimera (Figure 5C), the mutation impaired significantly the chimera's ability to trigger an efficient proliferative response to EGF (data not shown), supporting the importance of p60src activation for ALK-mediated mitogenicity.16
In NIH-EGFR/ALK cells, the time course of DGK activation following EGF stimulation correlates with the period of p60src activity, in line with the possibility of a tight regulation of DGK activity by p60src. Therefore, to evaluate whether the catalytic activation of αDGK was dependent on ALK-induced p60src activation, we have analyzed DGK activity copurified with p60src from either control or EGF-treated NIH-EGFR/ALK cells. As shown in Figure 5B (left panel), DGK activity in anti-p60src immunocomplexes from treated cells was significantly higher than control cells. p60src-associated DGK activity was completely abrogated by pretreatment of NIH-EGFR/ALK with 1 μM R59949 (data not shown). Furthermore, in EGF-treated NIH-3T3 cells expressing the EGFR/ALKY979F mutant (NIH-EGFR/ALKY979F), the amount of DGK activity coprecipitated in complex with p60src remained at the basal level (Figure 5B, right panel). Taken together, these data are consistent with the existence of a signaling pathway in which ALK activation modulates the αDGK catalytic function through a p60src-dependent mechanism.
Results shown in Figure 5B suggest a physical interaction in vivo between αDGK and p60src. However, under our experimental conditions, only endogenous DGK activity but not endogenous αDGK protein could be detected in p60src immunocomplexes obtained from NIH-EGFR/ALK, because of the poor recognition of the murine protein by the anti-human αDGK antibodies. Therefore, to provide further evidence for an in vivo p60src/αDGK association and to establish whether this complex is induced by EGF cell treatment, coimmunoprecipitation experiments were performed in NIH-EGFR/ALK cells transiently transfected with the myc-tagged αDGK construct previously described (Figure 3). Under these conditions, αDGK was easily detected in anti-p60src immunoprecipitates; however, the amount of recovered αDGK was not dependent on EGF cell stimulation (Figure 5C), suggesting that the p60src/αDGK interaction does not require p60src activation. Indeed, similar results were observed in NIH-3T3 cells expressing the EGFR/ALKY979F mutant in which p60src signaling is specifically abrogated (Figure 5C).
Role of αDGK in ALK-mediated signal transduction
To establish the importance of αDGK in ALK-mediated signaling, we first examined the effect of R59949 treatment on EGF-induced proliferation of NIH-EGFR/ALK cells. As shown in Figure 6A, 10 μM R59949 markedly inhibited the EGF responsiveness of NIH-EGFR/ALK cells (maximal effect, about 40% inhibition). Notably, in the same cells, αDGK inhibition by R59949 did not influence the mitogenic response of EGFR/ALK to 1% FBS (Figure 6B), indicating that αDGK is involved in a pathway that is specifically required for ALK signaling but dispensable for serum-induced mitogenesis. The presence of 10 μM R59949 also resulted in a significant inhibition of 32D-NPM/ALK cell growth, determined at 24 (data not shown) and 48 (Figure 6C) hours using the MTT proliferation assay. Similar results have been obtained in Karpas 299 cells (Figure 6D). We have observed no toxic effect on all cell lines maintained for 96 hours in the presence of 10 μM R59949 as measured by trypan blue exclusion.
Effect of the R59949 inhibitor on NIH-EGFR/ALK, 32D-NPM/ALK, and Karpas 299 cell growth. (A-B) NIH-EGFR/ALK cells were serum starved for 72 hours and then pretreated for 30 minutes with 1 μM R59949 (▪) or DMSO (▦) as control, before stimulation with the indicated dose of EGF (A) or 1% FBS (B) in the presence of 4 μCi (0.148 MBq) [methyl-3H] thymidine/well for 22 hours. Results are expressed as percent of control (radioactive incorporation obtained from medium + DMSO-treated cells). (C-D) 32D NPM/ALK and Karpas 299 cells were cultured for 48 hours in medium containing FBS 0.1% and BSA0.2 mg/mL in the presence of 10 μM R59949 (▪) or DMSO 0.02% (▦) as control. Cell proliferation was determined using the MTT proliferation assay. The values represent the means ± SD (n = 3). OD indicates optical density.
Effect of the R59949 inhibitor on NIH-EGFR/ALK, 32D-NPM/ALK, and Karpas 299 cell growth. (A-B) NIH-EGFR/ALK cells were serum starved for 72 hours and then pretreated for 30 minutes with 1 μM R59949 (▪) or DMSO (▦) as control, before stimulation with the indicated dose of EGF (A) or 1% FBS (B) in the presence of 4 μCi (0.148 MBq) [methyl-3H] thymidine/well for 22 hours. Results are expressed as percent of control (radioactive incorporation obtained from medium + DMSO-treated cells). (C-D) 32D NPM/ALK and Karpas 299 cells were cultured for 48 hours in medium containing FBS 0.1% and BSA0.2 mg/mL in the presence of 10 μM R59949 (▪) or DMSO 0.02% (▦) as control. Cell proliferation was determined using the MTT proliferation assay. The values represent the means ± SD (n = 3). OD indicates optical density.
Biologic effects following αDGK-specific RNA interference
To obtain further evidence that αDGK is involved in NPM/ALK mitogenic activities, we have down-regulated αDGK expression by RNA interference in Karpas 299 cell line. Transfection with a specific interfering RNA lowered but did not abolish the expression of αDGK, as detected by Western blot on whole-cell lysates (Figure 7A). BrdU incorporation, carried out 72 hours after transfection with this siRNA, showed a marked inhibitory effect on cell proliferation (Figure 7B), comparable with that obtained following treatment with 10 μM R59949 (Figure 6D). On the contrary, transfection with control siRNA did not result in any cell growth alteration. In conclusion, these data indicate that either inhibition of αDGK catalytic activity or down-regulation of its expression severely impair the ability of NPM/ALK-expressing cells to proliferate, providing evidence that the catalytic function of αDGK is required for the transduction of mitogenic and transforming signal promoted by ALK.
Down-regulation of αDGK by RNA interference on Karpas 299 cell proliferation. (A) siRNA-transfected Karpas 299 cells were lysed 72 hours from transfection, and their αDGK content was assayed by Western blot with anti-αDGK antibodies (top panel). As an internal control, the same blot was reprobed with an anti-α-tubulin antibody without prior stripping (bottom panel). (B) BrdU incorporation of siRNA-transfected Karpas 299, 72 hours following transfection. Results are expressed as percent of control (BrdU incorporation obtained from untransfected cells). Upon our experimental conditions, 60% to 70% of Karpas 299 cells resulted transiently permeabilized, as measured by trypan blue staining immediately after electroporation. The values represent the means ± SD (n = 3).
Down-regulation of αDGK by RNA interference on Karpas 299 cell proliferation. (A) siRNA-transfected Karpas 299 cells were lysed 72 hours from transfection, and their αDGK content was assayed by Western blot with anti-αDGK antibodies (top panel). As an internal control, the same blot was reprobed with an anti-α-tubulin antibody without prior stripping (bottom panel). (B) BrdU incorporation of siRNA-transfected Karpas 299, 72 hours following transfection. Results are expressed as percent of control (BrdU incorporation obtained from untransfected cells). Upon our experimental conditions, 60% to 70% of Karpas 299 cells resulted transiently permeabilized, as measured by trypan blue staining immediately after electroporation. The values represent the means ± SD (n = 3).
Discussion
Clonal abnormalities of the ALK receptor are typically associated with ALCLs and most frequently represented by the t(2;5) translocation.3 This chromosomal rearrangement leads to the expression of the NPM/ALK fusion protein, which possesses a high constitutive tyrosine kinase activity and can potently transform in vitro a wide array of different cell types.6-9 The oncogenic properties of NPM/ALK in vivo have been initially supported by a murine bone marrow retroviral transduction/transplantation-based approach10 and more recently confirmed in transgenic murine models.11-13
Efforts aimed at the molecular characterization of the signaling pathways relevant for NPM/ALK-mediated oncogenesis have led to the identification of different intracellular effectors. These include PLC-γ, PI3-K, Stat3-5, and p60src.8,14-17 However, current knowledge of the signaling events promoted by ALK are still preliminary, and therefore further studies are required to identify additional critical transducers of NPM/ALK-mediated transformation. In this study, we have investigated the potential involvement of αDGK, since cumulative evidence indicates a central role for this enzyme family as mediators of various cellular responses.35
For this purpose, we have used 2 different NPM/ALK-positive cell systems represented by the murine NPM/ALK-transformed 32D cell line and the human ALCL-derived Karpas 299. However, a comparison of the biologic and biochemical properties of the activated versus a ligand-inducible ALK receptor is necessary to gain insight into its function as a transforming gene. Although pleiotrophin and midkine have been proposed as potential high-affinity ligands for human ALK,36,37 the physiologic relevance of these receptor/ligand pairs has not been undoubtedly demonstrated and is presently under discussion.3,38 Moreover, these data have not found correspondence in Drosophila, where the ALK homolog binds to and is activated by jelly belly (Jeb), a protein structurally unrelated to both pleiotrophin and midkine.39 Therefore, to analyze the different aspects of ALK-mediated mitogenic signal under conditions of controlled ligand-induced activation, we made use of a previously described EGFR/ALK chimera in which the ALK enzymatic function could be modulated by EGF stimulation.26
In the current study, we demonstrate that activation of ALK correlates with an up-regulation of αDGK catalytic function. The identification of αDGK as the isoform mainly involved in ALK-mediated signaling is based on (1) sensitivity to R59949, a class I αDGK-specific inhibitor; (2) immunoprecipitation of endogenous αDGK with specific antibodies in Karpas 299 cells; and (3) immunoprecipitation of myc-tagged αDGK transfected in NIH-EGFR/ALK cells.
Our data also indicate that ALK-induced activation of αDGK takes place through a p60src tyrosine kinase-dependent mechanism. Indeed, R59949-sensitive DGK activity copurified with p60src in anti-p60src immunoprecipitates was obtained from EGF-stimulated but not from unstimulated NIH-EGFR/ALK cells. Furthermore, only basal levels of DGK activity were revealed in anti-p60src immunocomplexes obtained from EGF-treated NIH-EGFR/ALKY979F cells, in which p60src signaling is specifically abrogated. The inhibition of radioactive PA formation from exogenous DG in NPM/ALK-positive cells by the PP2 p60src inhibitor is in agreement with these results and with previous data obtained in other receptor signaling systems in both lymphoid and nonlymphoid cells.21,22,33
Surprisingly, in NIH-3T3 fibroblasts the complex between p60src and myc-αDGK is preformed, as the 2 proteins efficiently coprecipitate from both unstimulated or EGF-treated NIH-EGFR/ALK and NIH-EGFR/ALKY979F cells. This finding contrasts with previous reports that indicated ligand-induced p60src/αDGK association in COS and PAE cells expressing HGFR and VEGFR-2, respectively.21,22 However, when EGFR/ALK and αDGK were coexpressed in COS cells, p60src/αDGK protein complex formation was dependent on receptor and p60src activation (data not shown). Taken together, these data suggest that while activation of αDGK is tightly regulated by p60src tyrosine kinase activity, its association in a complex with p60src might be either dependent on or independent of receptor-induced p60src activation, according to different cell types. This observation might reflect different status of basal p60src activity, which is also regulated by cell adhesion in different cells. Alternatively, it is possible to speculate that αDGK might associate with p60src through multiple interactions, either dependent on or independent of p60src activation.
The molecular details of ALK-mediated αDGK activation and the functional relevance of αDGK/p60src association in living cells are still unclear. Since different p60src interactors, such as p130Cas, are tyrosine phosphorylated by p60src upon binding to its SH3 domain and given the requirement of p60src catalytic function for αDGK activation, the more obvious possibility is that αDGK acts as a substrate of p60src. Indeed, 2 potential Src phosphorylation sites are present on αDGK amino acid sequence, and in vivo αDGK tyrosine phosphorylation has been observed following its transient coexpression with p60src in COS cells or upon cell treatment with pervanadate.21 However, at present, no evidence exists for αDGK tyrosine phosphorylation under more physiologic experimental conditions, and we also were unable to reveal any in vivo αDGK tyrosine phosphorylation in the cell systems used in this study. Although it cannot be excluded that tyrosine phosphorylation of αDGK occurs at very low stoichiometry or under the specific control of a tyrosine phosphatase, alternative possibilities should be taken into consideration and further studies are required to elucidate this issue.
Finally, in the present study we have demonstrated the importance of αDGK in ALK-mediated signaling. Indeed, we have shown that pharmacologic inhibition of αDGK significantly impairs the mitogenic responsiveness of NIH-EGFR/ALK to EGF, as well as the growth rate of NPM/ALK-positive cells. The importance of αDGK in ALK-mediated mitogenic activity has been further validated in Karpas 299 cells by using a siRNA approach.
By converting DG to PA, DGKs modulate the intracellular levels of 2 important second messengers and thus can participate in the regulation of different intracellular events. Because there is evidence of multiple DGK isoforms within the same cell type, it is currently believed that the different isoenzymes perform specialized roles, probably by recognizing DG pools generated in response to different stimuli and that are localized in specific membrane compartments. Indeed, membrane translocation appears to be an effective process for regulating DGK activity and subtype-specific functions.40,41 Up-regulation of these enzymes results in a transient increase of the intracellular amount of PA. The precise role of αDGK-generated PA has not been elucidated, although several studies linked PA to the modulation of different molecules known to control cell proliferation, including protein kinase Cϵ (PKCϵ)42 and PKCζ,43 c-Raf,44 and phosphatases.45,46 The potential role of DGK-generated PA in the mammalian target of rapamycin (mTOR) pathway has been analyzed in a recent study and indicates that mTOR activity interacts with and is regulated by ζ-rather than the α-DGK.47
Intriguingly, ALK-mediated mitogenic properties involve activation of p60src,16 which activates αDGK and PLC-γ,8 which provides the lipid substrate to it and PI3-K,14,15 for which a direct role in regulating αDGK activity has recently been described in lymphocytes.33 It is therefore becoming evident that the turnover of bioactive lipids plays a pivotal role in ALK-mediated signal transduction, and further studies are needed to explore in greater detail how the lipid enzymes involved in their generation are functionally linked upon ALK activation to extend our knowledge into the oncogenic mechanisms involved in NPM/ALK-positive ALCL.
In conclusion, our data indicate that αDGK activation is involved in ALK-mediated mitogenic signaling and suggest that the inhibition of this activity can interfere with the pathogenic NPM/ALK function in ALCL cells.
Prepublished online as Blood First Edition Paper, May 31, 2005; DOI 10.1182/blood-2005-01-0316.
Supported by a grant from the Associazione Italiana Ricerca sul Cancro (F.F. and A.G.), by the (Fondo Investimenti Ricerca di Base) postgenomic program (A.G. and G.B.), and by Regione Piemonte (A.G). C. C. is supported by a Fellowship from Fondazione Italiana Ricerca Cancro.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to R. Cellerino for his continuous support during the course of this work. We also thank B. Falini (University of Perugia, Italy) and P. G. Pellicci (European Institute of Oncology, Milan, Italy) for providing the anti-ALK monoclonal antibody and Karpas 299 cells. We are also grateful to R. M. Melillo (University Federico II, Naples, Italy) and G. Superti-Furga (Cellzome, Heidelberg, Germany) for providing the GST-p60src/SH2 fusion protein; R. Piva (University of Turin, Italy) for providing TS and CEM cell lines; and G. Piccinini, M. Serresi, C. Vivani, E. Pierpaoli, and S. Baldassarri for helpful discussions.

![Figure 1. αDGK activity in NPM/ALK-positive cells. Cell lines were cultured for 30 minutes to 1 hour at 37°C in the absence (-) or presence (+) of either 10 μM R59949 or 1 μM PP2 and lysed thereafter. Total cell lysates (A) or anti-αDGK immunocomplexes (B, upper panel) were analyzed for in vitro DGK enzymatic activity in the presence of [γ-32P] ATP and diolein as exogen substrate; lipid phase was separated by TLC. An aliquot from each anti-αDGK immunoprecipitate was analyzed by SDS-PAGE to ensure that equal amounts of αDGK were present during in vitro kinase assay (B, bottom panel). PA indicates phosphatidic acid.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-01-0316/6/m_zh80180583880001.jpeg?Expires=1767785209&Signature=x6ga6MbSjlLINsxhPasjtnFeEcXZ0FWEpKJwXToHi5AQfOPWhciHx~cRhsFWWM815DLFsh6QI-5Jq0Du0HHO4ZQynRXuVJ6-YOVGFCSbulkzRrSEcbj~DeKzWKTEFTfFgs5dVt2BQvKwJKcOgCgmcS7TW8sK7QkyW8XrTWfa5S2zFQL-3kUmDr~wZ6gAHhzBk8Tl3uHNnPM3HxA2F7QjGiMLBmJbyGEl~37mjgqwt~6KVnjfk8Sz-Nh46UqJrXJ6DRCDYOIkq2w4iyWuGvaTIDPwqj06HRK46IVVwEog4S~DBGNsVuccH6-OUAwYqHaBy9BmyHZaqE434tMzRnJwZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 4. p60src kinase activity in NIH-EGFR/ALK cells following EGF stimulation. Quiescent NIH-EGFR/ALK cells were either mock-treated or treated with 100 ng/mL EGF for the indicated time at 37°C. Cell lysates were immunoprecipitated with an anti-p60src antibody. (A) An aliquot from each immunoprecipitate was directly analyzed by immunoblotting to evaluate p60src content. (B) The remainder of each immunoprecipitate was subjected to in vitro kinase assay in the presence of [γ-32P] ATP and acid-denaturated enolase. Samples were then analyzed by SDS-PAGE and autoradiography. (C) The 32P content of bands corresponding to enolase shown in panel B was quantified by densitometric scanning of the autoradiograms and results are expressed as percent of control (signal obtained from medium-treated cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-01-0316/6/m_zh80180583880004.jpeg?Expires=1767785209&Signature=Jn-yIgr5GMbJ6t4~FQ9jkBTjhhrNUvuU1rcBddYowKw35G9VzgpOlIfWS6c-34w6JIuHeM1ON-ZiD0p8eviNyhRYJOJyJS~p52-pAHGavPDCZUXjg9F41TJ91ZO4Pjp3kdaX6wQzXyijkG3AhjS4svX2uR82NJZW-ecj0-oEf4tPWYzHhpnI8-O13sHxetxHXB~vMYdgwvBAnHFsRsII1GQV0BLgHQSVjohbVZwhjPlyL3tpf4ElQxFfNk1nJe8ji55e4Zu3nGUMR1fNVzRE1aJ2C3bAP2~LoXRpobeUA1ahVXK99lVM-scqyCBmLhcclMVmxwxBNvYWs4vtEHFE3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Effect of the R59949 inhibitor on NIH-EGFR/ALK, 32D-NPM/ALK, and Karpas 299 cell growth. (A-B) NIH-EGFR/ALK cells were serum starved for 72 hours and then pretreated for 30 minutes with 1 μM R59949 (▪) or DMSO (▦) as control, before stimulation with the indicated dose of EGF (A) or 1% FBS (B) in the presence of 4 μCi (0.148 MBq) [methyl-3H] thymidine/well for 22 hours. Results are expressed as percent of control (radioactive incorporation obtained from medium + DMSO-treated cells). (C-D) 32D NPM/ALK and Karpas 299 cells were cultured for 48 hours in medium containing FBS 0.1% and BSA0.2 mg/mL in the presence of 10 μM R59949 (▪) or DMSO 0.02% (▦) as control. Cell proliferation was determined using the MTT proliferation assay. The values represent the means ± SD (n = 3). OD indicates optical density.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/6/10.1182_blood-2005-01-0316/6/m_zh80180583880006.jpeg?Expires=1767785209&Signature=QOSXgPteAfxaQvx2yW2d~Uq2LOkBB6~EfSV051hVFhs3tUb7X0s1anNlKvI2tNrXZwbcde4qGkIrKcz3szO5jBwJvKvUpTGAT~PKV8Qh3WZ7oA0EA7tXvvckKkBpSTj5Dh0VjRZ2ogK8tev89f~HQhnmH-K0Qweb5oBICQKc4Lm~d7YvvjsY2JfCa~p2pM2tQtdX~sqeQApfnwMnGJcgpq380uxjqvm3yQuPLFzOqoz6ayiUCVIQUblh87bhhjrD1xNxSVfV58A9Vow72wo~PAOvdzXMoCJEvWK7GFJ2L50SigGy~dklszygbWFqQQpbVsNzH-JS4ZmlZu6sIzf1iw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal