Abstract
We studied the role of multiple biomarkers in determining outcome in follicular lymphoma (FL), concentrating in particular on the role of benign macrophages. The study group consisted of uniformly staged and treated patients with FL enrolled in a phase 2 trial between 1987 and 1993. All patients were younger than 61 years of age, had advanced-stage FL, and were treated with a multiagent chemotherapy regimen, BP-VACOP (bleomycin, cisplatin, etoposide, doxorubicin, cyclophosphamide, vincristine, and prednisone), followed by involved region radiation. The median follow-up of living patients was 12.5 years, and the median survival was 16.3 years. The International Prognostic Index (IPI) was predictive of overall survival (OS) (P = .003). Biopsy specimens from all cases were stained with an anti-CD68 antibody. Of the 99 evaluable patients with FL, 87 had less than 15 CD68+ macrophages/high-power field (hpf) (median, 7; range, 1-14) and 12 had more than 15 CD68+ macrophages/hpf (median, 20; range, 16-25) with a median OS of 16.3 vs 5.0 years, respectively (P < .001). A multivariate Cox model that included the IPI score, the histologic grade, and the lymphoma-associated macrophage (LAM) score, showed IPI and LAM to be independent predictors of OS (P = .009 and P = .004, respectively). The LAM content of FL predicts survival, and these data support a prominent role for nonneoplastic immune cells in the biology of FL. (Blood. 2005;106:2169-2174)
Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma (NHL) subtype, surpassed only by diffuse large B-cell lymphoma (DLBCL).1 The typical survival curve in most studies attests to the marked clinical heterogeneity that characterizes FL. With median survivals of 8 to 10 years, approximately 15% of newly diagnosed patients will die of their disease in the first 2 years, while others are still alive more than 20 years later.2 In addition, interpretation of clinical trials of FL is made difficult by the fact that events unfold over many years.
A number of clinical prognostic variables have been described in FL but a recent international effort has culminated in a scoring system with a high likelihood of universal acceptance. The Follicular Lymphoma International Prognostic Index, or FLIPI,3 measures 5 variables including age, Ann Arbor stage, hemoglobin level, number of nodal sites, and serum lactate dehydrogenase (LDH) level. Although clinical variables, which are surrogate markers for underlying biology, can provide useful prognostic information, there is a clear need to develop biomarkers in FL that are predictive of outcome that could then be used to supplement indices such as the FLIPI index.4 However, few biologic markers have been developed that can be shown to have independent prognostic impact in patients with FL. A possible impact on survival has been described for several pathologic variables, although the results are inconsistent at best. The list of candidate biomarkers includes morphologic features (histologic grade, proliferation rate, presence of marginal zone differentiation, diffuse areas, small vessel content), molecular alterations (loss of p53, loss of p16, acquisition of CMYC alterations), cytogenetic abnormalities (del6q, -17p13, +18, +12q13-14) and altered protein expression (Bcl-2 and Bcl-6 proteins).5-28 Problems with patient selection and inclusion bias, lack of large patient cohorts treated with uniform therapy, inconsistent interobserver reproducibility of histologic/immunohistochemical features, and low frequency of several of these molecular events hamper the clinical translation of these biomarkers.
The presence of leukocytes in human tumors was first noted in neoplastic tissues by Rudolf Virchow in 1863.29,30 He suggested that the “lymphoreticular infiltrate” reflected an important interaction between cancer cells and cells involved in chronic inflammation. Over the past 10 years our understanding of the inflammatory microenvironment of tumors has provided support for Virchow's original hypothesis. Tumor-associated macrophages (TAMs) have been the subject of investigation in a number of different tumors, including breast, prostate, ovarian, and cervical cancers, where a clear correlation with inferior survival has been seen with increased TAM content.31 Curiously, in other tumors such as gastric and lung cancer, the results have been contradictory.32 These disparate findings may be due to specific functional characteristics of macrophages in some tumors and may be context dependent. Nonetheless, in the majority of studies increased TAM density has been associated with both metastatic spread and diminished survival. We are unaware of any previous literature implicating macrophages in the prognosis of FL.
Recent work by the Leukemia/Lymphoma Molecular Profiling Project (LLMPP) has shown that gene expression profiling can be used to establish a molecular subclassification of FL, and, importantly, was used to develop a powerful outcome predictor.33 In a study of 191 patients using pretreatment biopsies, it was revealed that nonneoplastic immune cells were predominantly responsible for the gene expression signatures contributing most to the outcome predictor. One signature, referred to as immune response-1 (IR-1), appeared to be derived from reactive T cells in the lymph node biopsies and conferred a favorable outcome. The other signature, referred to as immune response-2 (IR-2), revealed a gene expression pattern most reminiscent of macrophages and/or follicular dendritic cells. This IR-2 signature conferred an inferior survival on patients with FL. Treatment in this study was not uniform; nonetheless, these data allowed the development of a molecular predictor score with widely divergent survivals. Moreover, these results clearly demonstrated that molecular characteristics of the tumors that impact upon survival were present in original diagnostic biopsies, suggesting that ongoing stochastic events may not be as important for determining survival in FL. The character of the IR-2 gene list (eg, complement genes, toll-like receptors, Fc receptors, etc) suggested that macrophages might be playing an important role in the biology and survival of patients with FL. On the strength of these observations, we studied the clinical impact of the content and morphologic patterns of follicular dendritic cells (FDCs), T-cell subsets, and lymphoma-associated macrophage (LAM) content in patients with FL who had been uniformly staged and treated at our own institution.
Patients, materials, and methods
Patient characteristics
The British Columbia Cancer Agency (BCCA) is the primary referral center for patients diagnosed with lymphoid malignancies in the province of British Columbia, Canada. It serves a catchment area of approximately 4 million people. Consecutive patients seen at the BCCA between July 1987 and May 1993 were offered enrolment in the BP-VACOP program consisting of chemotherapy (bleomycin, cisplatin, etoposide, doxorubicin, cyclophosphamide, vincristine, and prednisone) followed by involved field irradiation to sites of original nodal involvement. Patients were deemed eligible for enrollment if they were aged 16 to 61 years with newly diagnosed, treatment-naive, advanced-stage indolent non-Hodgkin lymphoma. Advanced stage disease was defined as Ann Arbor stage III or IV, or stage II with B symptoms, nonradioencompassable disease, or bulk of 10 cm or greater in maximum diameter at any individual tumor site. Patients were otherwise unselected other than to exclude those with independent organ dysfunction that would have made administration of the chemotherapy unacceptably toxic. Approval to review, analyze, and publish the data in this study was given by the University of British Columbia-British Columbia Cancer Agency Research Ethics Board. Informed consent was provided according to the Declaration of Helsinki. The source of pathology specimens for diagnosis included only lymph node biopsies. All patients had indolent lymphoma confirmed by standard histologic, immunohistochemical, and immunophenotypic and cytogenetic methods. Pathology was reviewed after enrollment but was not an inclusion criterion. A single pathologist (R.D.G.) classified all cases as indolent according to the Working Formulation for Clinical Usage and later updated them according to the World Health Organization (WHO) classification.34 For this study of tumor-associated macrophages, 2 pathologists (R.D.G. and B.F.S.) re-reviewed all of the FL cases, graded them according to the WHO criteria, and recorded those with evidence of marginal zone differentiation.34 The histologic diagnoses of the other cases, not included in this study, were small lymphocytic lymphoma, lymphoplasmacytic lymphoma, diffuse follicle-center lymphoma, mantle-cell lymphoma, and marginal-zone lymphoma.
Tissue microarray construction
A tissue microarray (TMA) was constructed using a tissue arrayer device (Beecher Instruments, Silver Spring, MD). Only those FL cases with adequate biopsy material and available blocks could be included. Of the 126 cases of FL, paraffin blocks were available for 110 patients. Of these, 105 had adequate material remaining in the block to be used for the TMA. Duplicate 1.0-mm cores were used to construct the TMA. Slides from the TMA block were cut at 4 microns.
Histology and immunohistochemistry
A hematoxylin and eosin (H&E) stain of the TMA was prepared using routine methods. All of the cores contained neoplastic follicles, and no discrepant results were seen between duplicate cores. A battery of immunohistochemical stains were performed, including CD20 (L26), CD3, CD4, CD7, CD8, CD10, CD21, CD57, MIB-1, T-cell intracellular antigen-1 (TIA-1), Bcl-2, Bcl-6, Bcl-XL, and CD68 (Table 1). Immunostaining was performed using a Dako autostainer and the EnVision polymer detection system, except for CD10 detection in formalin fixation, which employs the PowerVision system (all from DakoCytomation, Glostrup, Denmark). Antigen retrieval was used for all antibodies and included enzyme predigestion for CD21 and pressure-cooking (5 minutes) for the remainder. A variety of buffers were used depending on the specific antibody to allow optimal detection of the antigens. The chromogen in all cases was diaminobenzidine.
Antibodies used in the study
Antibody . | Clone . | Source . | Dilution . |
|---|---|---|---|
| Bcl-2 | 124 | DAKO | 1:20 |
| Bcl-XL | 2H12 | ZYMED | 1:50 |
| Bcl-6 | PG-B6p | DAKO | 1:10 |
| CD3 | — | Cell Marque/Novocastra | 1:100 |
| CD4 | 4B12 | Novocastra | 1:50 |
| CD7 | CD7-272 | Novocastra | 1:30 |
| CD8 | C8/144B | DAKO | 1:50 |
| CD10 | 56C6 | Novocastra | 1:50 |
| CD20 | L26 | DAKO | 1:500 |
| Ki67 | MIB1 | DAKO | 1:100 |
| CD21 | 1F8 | DAKO | 1:30 |
| CD57 | NK-1 | Novocastra | 1:30 |
| CD68 | KP1 | DAKO | 1:2000 |
| TIA-1 | 2G9A10F5 | Beckman | 1:250 |
Antibody . | Clone . | Source . | Dilution . |
|---|---|---|---|
| Bcl-2 | 124 | DAKO | 1:20 |
| Bcl-XL | 2H12 | ZYMED | 1:50 |
| Bcl-6 | PG-B6p | DAKO | 1:10 |
| CD3 | — | Cell Marque/Novocastra | 1:100 |
| CD4 | 4B12 | Novocastra | 1:50 |
| CD7 | CD7-272 | Novocastra | 1:30 |
| CD8 | C8/144B | DAKO | 1:50 |
| CD10 | 56C6 | Novocastra | 1:50 |
| CD20 | L26 | DAKO | 1:500 |
| Ki67 | MIB1 | DAKO | 1:100 |
| CD21 | 1F8 | DAKO | 1:30 |
| CD57 | NK-1 | Novocastra | 1:30 |
| CD68 | KP1 | DAKO | 1:2000 |
| TIA-1 | 2G9A10F5 | Beckman | 1:250 |
ZYMED (Invitrogen, Carlsbad, CA); Cell Marque (Hot Springs, AR); Novocastra (Newcastle upon Tyne, United Kingdom).
— indicates CD3 clone PS1 (Ultra Marque CD3).
The immunostained TMA slides were scored both for architectural pattern and the number of positively stained cells. In total, 14 biomarkers were analyzed in this study. For CD10, CD20, Bcl-2, Bcl-6 and Bcl-XL, the cases were scored qualitatively, as duplicate cores were either completely positive or negative. In addition to the qualitative CD10 scoring, the presence or absence of interfollicular CD10+ neoplastic cells was noted. The proliferation rate of the neoplastic cells was scored based on the percentage of the MIB-1+ cells within neoplastic follicles (MIB-1 ≤ 10% = 1; MIB-1 > 10 and < 50% = 2; MIB-1 > 50% = 3). T-cell markers (CD3, CD4, CD7, CD8, and CD57) were evaluated both for their distribution in relation to the neoplastic follicles (interfollicular or perifollicular compartments), the predominance of CD4 versus CD8 cells, and counting the mean number of the CD57+ cells. Cytotoxic granule TIA-1+ cells were scored qualitatively, with cases called positive in the presence of more than 10% of positive T cells. Follicular dendritic-cell (FDC) meshworks (CD21+) were morphologically classified either as follicular pattern (tight concentric FDC cytoplasmic processes) or expanded when there was dissolution of the normal pattern and coalescence of FDC processes in a loose arrangement between follicles.
Representative tissue microarray cores of patients with follicular lymphoma. The image on the left shows the uncommon finding of large numbers of macrophages both within and surrounding neoplastic follicles (n = 12). The image on the right shows the more common finding of very few macrophages (n = 87). The insert shows the typical strong cytoplasmic staining of reactive macrophages with anti-CD68 antibody. Images were acquired using a Nikon Eclipse E600 clinical microscope and Dxm1200 digital camera and software (Nikon, Tokyo, Japan). Original magnification × 100 (objective, 10 ×/0.3 NA) for the larger panels and × 1000 (objective, 100 ×/1.3 NA) for the insert.
Representative tissue microarray cores of patients with follicular lymphoma. The image on the left shows the uncommon finding of large numbers of macrophages both within and surrounding neoplastic follicles (n = 12). The image on the right shows the more common finding of very few macrophages (n = 87). The insert shows the typical strong cytoplasmic staining of reactive macrophages with anti-CD68 antibody. Images were acquired using a Nikon Eclipse E600 clinical microscope and Dxm1200 digital camera and software (Nikon, Tokyo, Japan). Original magnification × 100 (objective, 10 ×/0.3 NA) for the larger panels and × 1000 (objective, 100 ×/1.3 NA) for the insert.
CD68+ macrophages were first evaluated for their consistency between duplicate cores and then qualitatively. The initial analysis of CD68 staining revealed that macrophage content could be divided into 2 groups, cases with either none or few cells positive versus many positive cells. To make the determination of macrophage numbers more objective, we counted cells using high-power magnification (× 1000 oil lens). This high magnification was used to avoid counting neutrophils and intercellular debris occasionally stained with this antibody. Five representative fields per case were counted. We chose to count in areas where the staining was the strongest and most uniform. In virtually all of the cases scoring was made easier by the fact that cases were either clearly positive or negative (Figure 1). Neither FDCs nor neoplastic B cells stained with anti-CD68 in any case.
Statistics and survival analysis
Progression-free survival (PFS) was defined as the interval between diagnosis and death or lymphoma progression, whichever came first; disease-specific survival (DSS) was defined as the interval from diagnosis until death when the underlying cause of death was lymphoma or treatment toxicity, with all other causes of death censored; and overall survival (OS) was defined as the interval from date of diagnosis until death from any cause. International Prognostic Index (IPI) score originally developed for large-cell lymphomas, histologic grade, the presence of marginal zone differentiation, age, sex, and each of the 14 biomarkers (Table 2) including LAM were evaluated for prognostic significance. The relationships between LAM and other potential prognostic factors were assessed by the Pearson χ2 test. Survival estimates were calculated using the Kaplan-Meier method.35 Significance levels, estimates of hazard ratios (HR), and their 95% confidence intervals (CIs) were calculated using the proportional-hazards regression model.36
Biomarkers in follicular lymphoma
Biomarker . | No. failed . | No. positive (%) . | Morphologic pattern . | OS P . | Comments . |
|---|---|---|---|---|---|
| Bcl-2 | 2 | 92 (89) | — | NS | |
| Bcl-XL | 1 | 15 (14) | — | NS | >10% lymphoma cells |
| Bcl-6 | 1 | 81 (78) | — | NS | |
| CD10 | 2 | 94 (91) | — | NS | |
| Interfollicular CD10 | 2 | 78 (76) | — | NS | Presence of interfollicular CD10+ neoplastic cells |
| Ki-67 (MIB-1) | 5 | 62 (62) | — | NS | > 50% neoplastic nuclei |
| CD4 | 2 | 78 (76) | — | NS | Majority of T cells |
| CD7 pattern | 1 | 73 (70) | Interfollicular | NS | Interfollicular CD7 predominance |
| CD4/CD8 pattern | 5 | 71 (71) | Follicular | NS | Intrafollicular CD4 and perifollicular CD8 |
| FDC (CD21) pattern | 19 | 27 (31) | Expanded follicles | NS | Coalescence of FDC meshwork |
| CD57 | 1 | 67 (64) | — | NS | >10% of T cells |
| CD57 pattern | 13 | 68 (63) | Follicular | NS | Intrafollicular CD57 predominance |
| TIA-1 | 1 | 47 (45) | — | NS | >10% of T cells |
| CD68+ macrophages | 6 | 12 (12) | — | <.001 | >15 macrophages/hpf (X 1 000) |
Biomarker . | No. failed . | No. positive (%) . | Morphologic pattern . | OS P . | Comments . |
|---|---|---|---|---|---|
| Bcl-2 | 2 | 92 (89) | — | NS | |
| Bcl-XL | 1 | 15 (14) | — | NS | >10% lymphoma cells |
| Bcl-6 | 1 | 81 (78) | — | NS | |
| CD10 | 2 | 94 (91) | — | NS | |
| Interfollicular CD10 | 2 | 78 (76) | — | NS | Presence of interfollicular CD10+ neoplastic cells |
| Ki-67 (MIB-1) | 5 | 62 (62) | — | NS | > 50% neoplastic nuclei |
| CD4 | 2 | 78 (76) | — | NS | Majority of T cells |
| CD7 pattern | 1 | 73 (70) | Interfollicular | NS | Interfollicular CD7 predominance |
| CD4/CD8 pattern | 5 | 71 (71) | Follicular | NS | Intrafollicular CD4 and perifollicular CD8 |
| FDC (CD21) pattern | 19 | 27 (31) | Expanded follicles | NS | Coalescence of FDC meshwork |
| CD57 | 1 | 67 (64) | — | NS | >10% of T cells |
| CD57 pattern | 13 | 68 (63) | Follicular | NS | Intrafollicular CD57 predominance |
| TIA-1 | 1 | 47 (45) | — | NS | >10% of T cells |
| CD68+ macrophages | 6 | 12 (12) | — | <.001 | >15 macrophages/hpf (X 1 000) |
—indicates not evaluated; NS, not significant.
Results
Clinical characteristics and outcome
A total of 99 patients were included in this study, representing the patients with FL from the original clinical cohort (n = 126) with available blocks, sufficient tissue remaining in the blocks, and successful TMA construction and interpretation. The median age of the evaluable patients was 44 years (range, 19-61 years). There were 49 women and 50 men. The distribution of IPI score included 58 patients with IPI scores of 0/1 (group 1), 40 with IPI scores of 2/3 (group 2), and only a single patient with an IPI score of 4/5 (group 3). For analysis purposes, the single patient in IPI group 3 was included with IPI group 2 in order to eliminate a clinical group with only 1 patient. The median follow-up of living patients (n = 55) was 12.5 years. The median OS was 16.5 years. The IPI was highly significant as a predictor of OS, disease-specific survival (DSS) and progression-free survival (PFS) (P = .001, .003, and .007, respectively).
Pathology variables
Histologic grading of FL subtypes according to the WHO classification included 77 with grade 1, 15 with grade 2, and 7 with grade 3a. A total of 15 patients were determined to have marginal zone differentiation, as defined by a zone of pale cells at least 3 cells in thickness, expressing CD20 and surrounding neoplastic follicles. Neither histologic grade nor the presence of marginal zone differentiation had an effect on OS, DSS, or PFS (data not shown).
Some cores were lost during the preparation of the sections or were uninterruptible due to poor fixation and/or inadequate staining (varies with biomarker). The percentage of informative individual cores was 96%, which varied with the different biomarkers (1%-18%; Table 2). Results of the biomarkers are summarized in Table 2. The percentage of the positive cases for Bcl-2, Bcl-6, and CD10 were in line with previous reports. In total, 16% of the CD10+ FL had no or minimal interfollicular involvement by CD10+ neoplastic cells. Only a small number of cases (14%) had more than 10% Bcl-XL+ cells. There was a correlation between the proliferative rate (MIB-1) and the histologic grade, at least for the small number of grade 3a cases. The majority (83%) of grade 3a FL cases had a proliferative rate greater than 50%. Grade 2 FL cases showed more heterogeneity, with 62% having a proliferative rate between 10% and 50%, while the bulk of the remaining cases had more than 50% MIB-1+ cells. Only 50% of the grade 1 FL cases revealed less than 10% MIB-1+ cells, with the majority of the remaining cases demonstrating proliferative rates between 10% and 50%. These data indicate the imperfect relationship between histologic grade and proliferative rate. Importantly, neither grade nor MIB-1 score were predictive of outcome (data not shown).
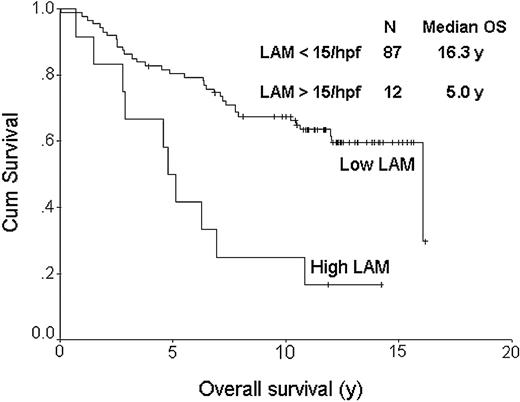
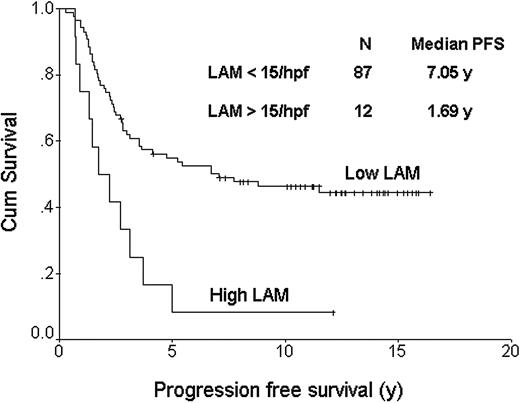
The FDC pattern (CD21) was disrupted with expansion of the meshwork and fusion of the follicles in 27% of the cases. The reactive T cells showed a predominantly perifollicular distribution in most cases (70%), as well as a CD4 phenotype (76%). In 71% of the cases there was a unique follicular pattern, with CD4 cells inside the follicles surrounded by CD8 positive cells outside the follicles, whereas in other cases the distribution was diffuse for both subsets. CD57+ cells represented more than 10% of the T cells in most cases (64%) and they had a clear follicular pattern in 68%, with most of the positive cells localized inside the follicles. In 82% of these cases the CD57 follicular pattern correlated with the CD4/CD8 follicular pattern and both CD57 and CD4 cells had an equal distribution within the follicles. Cases with more than 10% cytotoxic TIA-1 granule+ cells were found in 45% of the cases. The numbers of CD68+ macrophages (LAMs) were initially determined using low-power magnification. None of the cases showed staining of neoplastic B cells with anti-CD68 antibody. It was clear that most cases had few or no macrophages, while a small number had many (Figure 1). The distribution of these CD68+ cells was uneven throughout cores with both intrafollicular and perifollicular macrophages observed. We then used high-power magnification (× 1000 oil lens) to count the CD68+ macrophages, which ranged between 1 and 25 cells per high-power field (hpf). Because many of the cases had no or few macrophages, we established a cut-off of 15 cells/hpf for analysis purposes. Importantly, most of the negative cases had less than 10 cells (82%), and the positive cases had more than 20 cells (58%) per hpf, respectively. Table 3 shows the distribution of clinical and pathologic variables for the FL cases with high LAM (n = 12) versus those with low LAM content (n = 87). Only histologic grade was unevenly distributed between these 2 groups and thus was included in a multivariate model along with the IPI. All biomarker determinations were measured without knowledge of the clinical outcome. Except for the LAM score, none of the other markers showed a significant impact on outcome. Cases with less and more than 15 CD68+ macrophages/hpf had median OSs of 16.3 and 5.0 years, respectively (P < .001; Figure 2). Figure 3 shows the progression-free survival curve. Cases with less and more than 15 CD68+ macrophages/hpf had a median PFS of 7.05 and 1.69 years, respectively (P = .001). A multivariate proportional hazards model that included the LAM content, the IPI score, and histologic grade showed that LAM is an independent predictor of OS (HR = 2.4, 95%CI = 1.3-4.5, P = .004). The inferior PFS and OS of the patients with a high LAM score cannot be explained by a lower response rate to treatment (Table 3), nor an increased risk of histologic transformation (0 of 12 developed transformed disease) nor lack of appropriate treatment for relapse (all patients with relapse were treated with systemic chemotherapy, 3 with high-dose chemotherapy and hematopoietic stem cell transplantation). All but 1 of the 10 deaths among the patients with high LAM scores resulted from progressive lymphoma.
Distribution of clinical and pathologic variables between high LAM and low LAM cases
Feature . | No. patients with high LAM (%) . | No. patients with low LAM (%) . | Total (%) . | P . |
|---|---|---|---|---|
| No. | 12 (12) | 87 (88) | 99 (100) | — |
| Clinical features | 058* | |||
| IPI Group 1 (0/1) | 4 (33) | 54 (62) | 58 (59) | |
| Group 2 (2/3) | 8 (66) | 32 (37) | 40 (40) | |
| Group 3 (4/5) | 0 (0) | 1 (1) | 1 (1) | |
| BM involvement | 6 (50) | 44 (51) | 50 (51) | |
| Treatment response | NS† | |||
| CR | 6 (50) | 48 (55) | 54 (55) | |
| PR | 6 (50) | 36 (41) | 42 (42) | |
| NR | 0 (0) | 3 (3) | 3 (3) | |
| Pathology features | 046‡ | |||
| FL grade | ||||
| Grade 1 | 6 (50) | 67 (77) | 73 (74) | |
| Grade 2 | 4 (33) | 16 (18) | 20 (20) | |
| Grade 3 | 2 (17) | 4 (5) | 6 (61) | |
| MZ differentiation | 3 (25) | 22 (25) | 25 (25) | NS |
| Transformation | 0 (0) | 8 (9) | 8 (8) | NS |
Feature . | No. patients with high LAM (%) . | No. patients with low LAM (%) . | Total (%) . | P . |
|---|---|---|---|---|
| No. | 12 (12) | 87 (88) | 99 (100) | — |
| Clinical features | 058* | |||
| IPI Group 1 (0/1) | 4 (33) | 54 (62) | 58 (59) | |
| Group 2 (2/3) | 8 (66) | 32 (37) | 40 (40) | |
| Group 3 (4/5) | 0 (0) | 1 (1) | 1 (1) | |
| BM involvement | 6 (50) | 44 (51) | 50 (51) | |
| Treatment response | NS† | |||
| CR | 6 (50) | 48 (55) | 54 (55) | |
| PR | 6 (50) | 36 (41) | 42 (42) | |
| NR | 0 (0) | 3 (3) | 3 (3) | |
| Pathology features | 046‡ | |||
| FL grade | ||||
| Grade 1 | 6 (50) | 67 (77) | 73 (74) | |
| Grade 2 | 4 (33) | 16 (18) | 20 (20) | |
| Grade 3 | 2 (17) | 4 (5) | 6 (61) | |
| MZ differentiation | 3 (25) | 22 (25) | 25 (25) | NS |
| Transformation | 0 (0) | 8 (9) | 8 (8) | NS |
IPI indicates International Prognostic Index score; BM, bone marrow; CR, complete remission; PR, partial remission; NR, no response; NS, not significant; —, ____; and MZ, presence of marginal zone differentiation.
IPI group 1 versus 2/3.
CR versus PR/NR.
Grade 1 versus 2/3.
Overall survival curve based on lymphoma associated macrophage (LAM) content. The top curve represents cases with less than 15 CD68+ macrophages/hpf; the bottom curve, those cases with more than 15 CD68+ macrophages/hpf. The median OSs were 16.3 and 5.0 years, respectively (P < .001).
Overall survival curve based on lymphoma associated macrophage (LAM) content. The top curve represents cases with less than 15 CD68+ macrophages/hpf; the bottom curve, those cases with more than 15 CD68+ macrophages/hpf. The median OSs were 16.3 and 5.0 years, respectively (P < .001).
Discussion
FL represents a lymphoma subtype characterized by a significant degree of clinical heterogeneity.2 The recent development of a clinical prognostic model (FLIPI) may help to define those patients at risk of failing current therapies, but the degree to which this index will be helpful in planning initial therapy for newly diagnosed patients remains undefined.3 Importantly, this model includes clinical surrogates for the underlying molecular genetic alterations and thus will not help to identify novel targets for therapy. An improved understanding of the molecular abnormalities that underlie the survival differences among patients with FL may lead to improvements in risk stratification, but more importantly would offer the possibility of identifying new targets for rationale design of future treatments. To a large extent, the future of tailored or personalized therapies in cancer will depend on this very hypothesis.
Progression-free survival curve based on lymphoma-associated macrophage (LAM) content. The top curve represents cases with less than 15 CD68+ macrophages/hpf; the bottom curve, those cases with more than 15 CD68+ macrophages/hpf. The median PFSs were 7.05 and 1.69 years, respectively (P = .001).
Progression-free survival curve based on lymphoma-associated macrophage (LAM) content. The top curve represents cases with less than 15 CD68+ macrophages/hpf; the bottom curve, those cases with more than 15 CD68+ macrophages/hpf. The median PFSs were 7.05 and 1.69 years, respectively (P = .001).
Most published data for FL suggest that the molecular alterations that characterize this lymphoma result from a combination of abnormalities present at the time of diagnosis and ongoing stochastic events that occur in association with clonal evolution.5,8,11,13,33 Because FL is a germinal center derived neoplasm and frequently reveals intraclonal heterogeneity at the level of the immunoglobulin heavy chain, there is a belief that additional molecular alterations are occurring over time and contribute to disease progression. However, a number of other possibilities exist to explain disease progression in FL, including the outgrowth of subclones that may have been present as minor populations at diagnosis.24,37 Whatever the relative contribution of these 2 processes, there is little doubt that the molecular signature present in initial diagnostic biopsies plays a prominent role in predicting outcome in FL. The recent gene expression profiling studies of the LLMPP have clearly shown that the signatures from coordinately expressed genes play a dominant role in affecting survival, as evidenced by widely divergent survivals.33 The most important gene expression signatures in the recently developed molecular predictor were not derived from neoplastic B cells but instead were expressed by nonneoplastic immune response cells. One of these signatures, called IR-2, included a number of genes that appeared to correspond to the repertoire expressed by macrophages and was associated with inferior survival. These results suggest that benign macrophages play a role in the biology of FL. Data supporting such a role for reactive macrophages as biomarkers in cancer have been described in a number of different tumors including breast, prostate, ovarian, and cervical cancer.31 A suspected role in lymphoma was untested until recently conducted studies by the LLMPP consortium.
The current study clearly shows that LAM content is an independent predictor of survival in uniformly treated FL. Not unexpectedly, the IPI strongly predicted survival in this study (P = .003). The content of LAM was, however, also predictive of survival, including OS (P = .003), disease-specific (P < .001), and progression-free (P = .001) survivals, and remained an independent variable distinct from the IPI in a Cox multivariate model (P = .004). These data agree with similar observations made in epithelial cancers that increased numbers of macrophages portend a worse outcome.31,32 Moreover, these results also agree with the recent gene expression studies and provide indirect validation at the protein level for the role of IR-2. Ongoing investigations of the biologic functions of macrophages in FL continue, as do studies to validate gene expression in FL by studying the expression of other proteins using paraffin-embedded material.
The precise biologic function of tissue-based macrophages in this setting remains undefined, but they may be providing important growth signals to the FL cells in the form of cytokine stimulation or specific chemokine production. Macrophage heterogeneity is well studied in the mouse, but less is known about human macrophages.31,38,39 The plasticity of macrophage function is evident in the diverse range of functions of these cells, including inflammatory responses, immune reactions, tissue remodeling, and morphogenesis. Depending on the microenvironment, macrophages appear to be either “classic” M1 (inflammatory responses induced by interleukin-12 [IL-12] and tumor necrosis factor-α [TNF-α]) or “alternative” M2 (tissue remodeling and morphogenesis induced by IL-4, IL-13, and IL-10) subtypes, referred to as polarized macrophages. These 2 types differ in their receptor expression and cytokine/chemokine profiles.39
In advance of this study we studied macrophage content and distribution in a series of reactive lymph nodes (n = 10; data not shown). Virtually all cases showed large numbers of tissue-based macrophages both within and between reactive lymphoid follicles. Thus, we were surprised to find that most cases of FL in this study had no or very few macrophages. We hypothesize that in the minority of cases of FL with large numbers of macrophages the neoplastic B cells display signals that attract and/or retain predominantly M2-type macrophages. Alternatively, an inflammatory microenvironment might be created by the antitumor-responsive T cells that attract or retain the macrophages. Once in the lymph node, these cells might provide the neoplastic B cells with trophic/survival signals, facilitate tumoral invasion by disrupting basement membranes, promote angiogenesis by secreting angiogenic factors, alter the pheno-type and/or function of antigen-presenting dendritic cells, or amplify regulatory T cells, leading to an immunosuppressive intratumoral environment.40 At present, these remain untested hypotheses. Nonetheless, an improved understanding of the interactions between nonneoplastic macrophages and neoplastic B cells in FL should provide important insights into the biology of this common tumor. Ultimately it may be possible to manipulate the microenvironment of the lymph node in favor of the host and turn off any trophic survival signals derived from nonneoplastic tissue-based macrophages. Finally, this study provides compelling data that support a role for benign macrophages in predicting outcome in aggressively treated patients with FL and further substantiate the recent gene expression profiling data highlighting the importance of nonneoplastic cells in determining prognosis in FL.
Prepublished online as Blood First Edition Paper, June 2, 2005; DOI 10.1182/blood-2005-04-1565.
Supported by an unrestricted educational grant and a molecular pathology fellowship from Berlex Canada, Berlex US, and AG Schering; and Canadian Institute of Health Research (CIHR no. STP-53912) and the Fundacao para a Ciencia e a Tecnologia (BD 13230/2003) from Portugal (P.F.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank all of the treating physicians of the Lymphoma Tumor Group for allowing us to include their patients. In addition we greatly appreciated the cooperation from all of the pathologists throughout British Columbia for their support of the provincial lymphoma pathology service.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal