Abstract
Severe acquired aplastic anaemia (SAA) is a life-threatening disease characterized by pancytopenia and hypoplastic bone marrow. Autologous T lymphocytes are thought to cause bone marrow failure by immune-mediated excessive apoptosis of stem and progenitor cells. The disease is subclassified into a severe (neutrophil count, > 0.2 × 109/L [> 200/μL]) and a very severe (< 0.2 × 109/L [< 200/μL]) (vSAA) form. We report the results of a prospective multicenter trial with a combined immunosuppressive regimen of cyclosporin A (CSA), anti-thymocyte globulin (ATG) and, in cases with neutrophil counts fewer than 0.5 × 109/L (< 500/μL), granulocyte colony-stimulating factor (G-CSF) for treatment of SAA in children. Children with vSAA showed a higher rate of complete response than did children with SAA (68% versus 45%; P = .009), as well as better survival (93% versus 81%; P < .001). Thus, in children with SAA a more severe disease stage at diagnosis indicates a favorable outcome with immunosuppressive therapy. (Blood. 2005;106:2102-2104)
Introduction
A more severe form of a given disease negatively affects outcome in almost all human diseases. This seemed likely to apply to severe acquired aplastic anaemia (SAA), the pathophysiology of which is characterized by immune-mediated bone marrow failure.1 A predominant oligoclonal immune response2 by autologous T lymphocytes was shown to cause excessive apoptosis in stem and progenitor cells.3,4 The molecular target of this T-cell response is still unknown, although in vitro a T-cell-mediated inhibition of aneuploid hematopoietic cells was demonstrated in patients with myelodysplastic syndrome.5
Immunosuppressive therapy (IST)6 seems to be the appropriate treatment for an autoimmune disease, but tissue replacement by bone marrow transplantation (BMT) is still the treatment of first choice7,8 because there have been 20% to 30% IST nonresponders, a significant proportion of patients with subnormal blood counts9 and a high risk of relapse10,11 and clonal disease.12,13 Because of the long latency of response to IST, identification of predictors of response and survival would be helpful to assign patients to the appropriate regimen.
In children very SAA (vSAA) is predominant (> 60%). Patients with vSAA are especially prone to develop life-threatening infections. As most of those high-risk patients lack an HLA-identical sibling donor (matched sibling donor; MSD), searching for a matched unrelated donor (MUD) was recommended, despite that BMT from a MUD is still complicated by a high risk of graft rejection and graft-versus-host disease. This recommendation was well founded on the basis of a previous retrospective analysis14 in which the outcome for children with vSAA was the worst with IST consisting of antithymocyte-globulin (ATG) plus androgens and/or steroids (probability of survival: vSAA, 37%; SAA, 56%).
Combined IST with ATG and cyclosporin A (CSA) has been shown to be superior to ATG alone or in combination with androgens in adults,15,16 and the addition of granulocyte colony-stimulating factor (G-CSF) to the treatment regimen was able to improve granulocyte recovery.17,18
We conducted a prospective multicenter trial comparing combined IST plus G-CSF and BMT19 to identify prognostic factors for response to therapy and survival.
Study design
Two hundred and thirteen patients newly diagnosed with SAA younger than the age of 17 years in 53 centers in Germany and Austria were included in the study between November 1993 and December 2001 (Table 1). The treatment protocol was approved by the local Ethics Committees. Informed consent was given by parents. For this study approval was obtained from the institutional review board of the Medical Department of the Ludwig-Maximilians University of Munich. Fanconi anemia was excluded by chromosomal fragility test. The diagnosis of SAA was based on morphology and blood counts.20 Zytogenetics and/or fluorescence in situ hybridization (FISH) analysis for monosomy 7 and trisomy 8 were performed in 158 of 213 patients at diagnosis. Clonal disease was detected during follow-up in 15 patients who received IST (8 cases with monosomy 7); in 3 of them the aberration was already present in the initial bone marrow (BM).
Patient characteristics in children treated with IST or BMT for severe aplastic anemia
. | All patients . | Patients with SAA . | Patients with vSAA . | P . |
|---|---|---|---|---|
| No. patients | 213 | 76 | 137 | |
| Age, median, y (range) | 8.9 (0.91-16.95) | 10.43 (1.42-16.93) | 8.06 (0.91-16.95) | .010* |
| Sex, no. (%) | .146† | |||
| Male | 125 (59) | 50 (66) | 75 (55) | |
| Female | 88 (41) | 26 (34) | 62 (45) | |
| Etiology, no. (%) | .874‡ | |||
| Idiopathic | 1.71 (80) | 63 (83) | 108 (79) | |
| Toxic | 9 (4) | 3 (4) | 6 (4) | |
| After hepatitis | 18 (9) | 6 (8) | 12 (9) | |
| Postviral infection | 15 (7) | 4 (5) | 11 (8) | |
| Interval between diagnosis and treatment, median, mo (range) | 0.90 (0.03-8.94) | 1.26 (0.20-8.94) | 0.92 (0.03-8.21) | .002§ |
. | All patients . | Patients with SAA . | Patients with vSAA . | P . |
|---|---|---|---|---|
| No. patients | 213 | 76 | 137 | |
| Age, median, y (range) | 8.9 (0.91-16.95) | 10.43 (1.42-16.93) | 8.06 (0.91-16.95) | .010* |
| Sex, no. (%) | .146† | |||
| Male | 125 (59) | 50 (66) | 75 (55) | |
| Female | 88 (41) | 26 (34) | 62 (45) | |
| Etiology, no. (%) | .874‡ | |||
| Idiopathic | 1.71 (80) | 63 (83) | 108 (79) | |
| Toxic | 9 (4) | 3 (4) | 6 (4) | |
| After hepatitis | 18 (9) | 6 (8) | 12 (9) | |
| Postviral infection | 15 (7) | 4 (5) | 11 (8) | |
| Interval between diagnosis and treatment, median, mo (range) | 0.90 (0.03-8.94) | 1.26 (0.20-8.94) | 0.92 (0.03-8.21) | .002§ |
Unpaired t test.
Fisher exact test.
χ2 test.
Mann-Whitney U test.
By biologic selection depending on the availability of an MSD, patients were assigned to either the BMT (n = 67) or the IST group (n = 146). Sixty-two patients received BMT after a conditioning treatment with ATG (horse; Sangstatt/Genzyme, Neu Isenburg, Germany) (0.75 mL/kg body weight [BW] for 4 days) and cyclophosphamide (50 mg/kg BW for 4 days). In the remaining 5 patients, 3 with SAA and 2 with vSAA, parents refused BMT. One hundred fifty-one children (151; 5 with an MSD) were treated with combined IST, including ATG (horse; 0.75 mL/kg BW for 8 days; Sangstatt/Genzyme), CSA (5 mg/kg BW, adjusted to blood levels), prednisolone (1-2 mg/kg tapered until day 28), and, in cases with neutrophil count (polymorphonuclear leukocytes; PMNs) fewer than 0.5 × 109/L [500/μL], with G-CSF (5 μg/kg BW) in addition (8 SAA patients did not receive G-CSF). After 28 days the G-CSF dose was increased to 10 μg/kg when PMNs were fewer than 1.5 × 109/L (1500/μL), whereas, when PMNs were more than 1.5 × 109/L (1500/μL), G-CSF was slowly tapered.
Response to IST and survival were evaluated on days 28, 42, and 112, at 6 months, and every 6 months afterward. Complete response (CR) was registered when hemoglobin reached age-adjusted normal values, platelet count was more than 100.0 × 109/L (>100 000/μL), and PMNs were more than 1.5 × 109/L (> 1500/μL). All criteria had to be fulfilled. Partial response (PR) was diagnosed in patients with transfusion independency, when reticulocyte count was more than 30.0 × 109/L (> 30 000/μL), platelet count was greater than 30.0 × 109/L (> 30 000/μL), and PMNs were more than 0.5 × 109/L (> 500/μL) above baseline. Minimum projected follow-up was 2 years; median follow-up at time of analysis was 50 months (range, 1-116 months). The analysis was based on intention to treat.
Results and discussion
In 137 (64%) of 213 patients vSAA was diagnosed. Within the BMT group, patients with vSAA (n = 40) and with SAA (n = 27) reached comparable survival rates (5-year survival rate: vSAA, 89%; 95% confidence interval [CI], 80%-99%; SAA, 96%; 95% CI, 89%-100%).
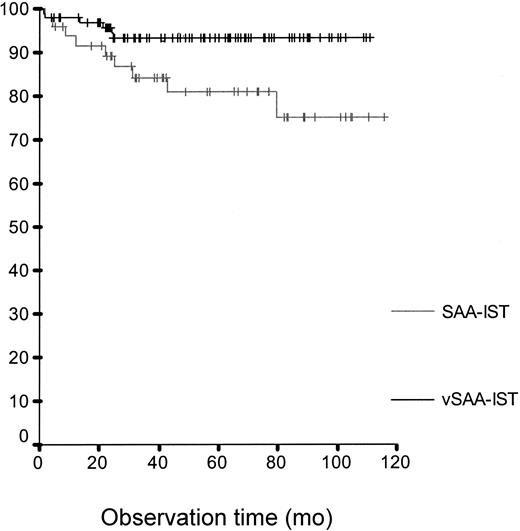
Survival rate after IST for children with vSAA (93% after 5 years; 95% CI, 88%-98%) and SAA (81% after 5 years; 95% CI, 69%-93%; P <.001).
Survival rate after IST for children with vSAA (93% after 5 years; 95% CI, 88%-98%) and SAA (81% after 5 years; 95% CI, 69%-93%; P <.001).
Ninety-seven vSAA patients without MSD were selected to receive IST. In this high-risk group the 5-year survival rate was 93% (95% CI, 88%-98%), a clear improvement compared with previously published data. Surprisingly, patients with SAA (n = 49) in the IST group showed a lower 5-year survival rate, namely 81% (95% CI, 69%-93%; P of log-rank test < .001) (Figure 1). This was due to a better response to IST by patients with vSAA, who reached CR in 69% (95% CI, 60%-78%), compared with 44% of patients with SAA (95% CI, 30%-58%; P = .004, Fisher exact test) (best response 1 year after start of treatment). There was a tendency for better relapse-free survival for patients with vSAA compared with patients with SAA (80% versus 67% at 5 years; 95% CI, 70%-91% versus 51%-83%) (P < .065, log-rank [LR] test). Age and time to therapy were different between SAA and vSAA (Table 1). In a multivariate Cox regression model adjusted for age the interaction between severity and intended therapy proved to be relevant. Time to therapy had no prognostic relevance there.
After 6 months, response to IST strongly predicted further survival in all patients then alive, with a 5-year survival of 100% in CR, 97% in PR (95% CI, 92%-100%), and 67% in patients with no response (NR) (95% CI, 48%-86%; P value of LR test: CR and PR versus NR < .001, CR versus PR = .271).
We conclude that in children with AA, disease severity no longer predicts inferior survival, when no MSD is available. In vSAA, treatment with combined IST and G-CSF converts the former bad prognosis factor severe granulocytopenia (< 0.2 × 109/L [< 200/μL]) into an indicator of excellent response and survival. Part of this progress may also be due to improved supportive care. Thus, early BMT from MUD is no longer recommended in this good-prognosis group.
After 6 months, response to IST predicted further survival.21 Nonresponders at that time should proceed to MUD BMT. However, the long latency period of recovery after IST disqualifies response to therapy as a tool for early therapeutic decisions.
A different treatment approach was chosen by a Japanese group. They administered androgens in addition to ATG, CSA, and G-CSF, with comparable results.22 In their study no difference in outcome between SAA and vSAA was observed, which was likely due to a number of cases with androgen-responsive congenital bone marrow failure hidden in the SAA group. Because of the excellent outcome in our study and the severe side effects of androgens, we strongly recommend that these drugs should be omitted from SAA treatment in children.
Our findings clearly suggest that especially in children with vSAA the immune system plays the key role in the pathomechanism of bone marrow failure, and the per se healthy stem cell compartment is able to compensate for the loss of stem cells as soon as the T-cell-mediated destruction is interrupted.23 Severe granulocytopenia at diagnosis may therefore serve as a surrogate marker for an immune-mediated disease instead of a primary stem cell disorder.
Appendix
Centers in Germany: Aachen, Universitäts-Kinderklinik; Augsburg, Kinderklinik im Klinikum; Berlin, Kinderklinik Charité Campus Virchow; Berlin, Kinderklinik Klinikum Berlin-Buch; Bielefeld, Kinderklinik Bethel-Krankenan-stalten; Bonn, Universitäts-Kinderklinik; Braunschweig, Kinderklinik im Klinikum; Chemnitz, Klinik für Kinderund Jugendmedizin; Cottbus, Kinderklinik Carl-Thiem Klinikum; Datteln, Vestische Kinderklinik; Detmold, Kinderklinik Klinikum Lippe; Dortmund, Städtische Kliniken; Dresden, Universitäts-Kinderklinik; Düsseldorf, Universitäts-Kinderklinik; Erfurt, Kinderklinik Helios Klinikum GmbH; Erlangen, Universitäts-Kinderklinik; Essen, Universitäts-Kinderklinik; Freiburg, Universitäts-Kinderklinik; Frankfurt, Universitäts-Kinderklinik; Gießen, Universitäts-Kinderklinik; Greifswald, Universitäts-Kinderklinik; Halle, Universitäts-Kinderklinik; Hamburg, Universitäts-Kinderklinik; Hannover, Kinderklinik der Medizinischen Hochschule; Heidelberg, Universitäts-Kinderklinik; Homburg, Universitäts-Kinderklinik; Jena, Universitäts-Kinderklinik; Kassel, Kinderklinik im Klinikum; Kiel, Universitäts-Kinderklinik; Koblenz, Kinderklinik Städt. Klinikum Kemperhof; Köln, Universitäts-Kinderklinik; Krefeld, Kinderklinik im Klinikum; Leipzig, Universitäts-Kinderklinik; Lübeck, Universitäts-Kinderklinik; Magdeburg, Universitäts-Kinderklinik; Mainz, Universitäts-Kinderklinik; München, Kinderklinik Klinikum Harlaching; München, Kinderklinik der Technischen Universität; München, Kinderpoliklinik der Universität; München, Dr. von Haunersches Kinderspital; Münster, Universitäts-Kinderklinik; Nürnberg, Cnopf'sche Kinderklinik; Oldenburg, Elisabeth-Kinderkrankenhaus; Regensburg, Kinderklinik St. Hedwig; Rostock, Universitäts-Kinderklinik; Siegen, DRK-Kinderklinik; Stuttgart, Olgaspital-Pädiatrisches Zentrum; Tübingen, Universitäts-Kinderklinik; Ulm, Universitäts-Kinderklinik; Würzburg, Universitäts-Kinderklinik
Centers in Austria: Graz, Universitätskinderklinik; Salzburg, Kinderspital; Wien, St. Anna Kinderspital
Prepublished online as Blood First Edition Paper, June 2, 2005; DOI 10.1182/blood-2005-03-0874.
A complete list of the members of the German/Austrian Aplastic Anemia Working Group appears in the “Appendix.”
Supported by the Friedrich-Baur-Stiftung (43/98), the Wilhelm-Sander-Stiftung (97.080.1), the Dr Sepp und Hanne Sturm Gedächtnisstiftung, and AMGEN Germany.
M.F., U.R., C.N., G.J.-S., W.F., A.B., and C.B.-G. conceived the study, designed the approach, and interpreted the data. M.F. wrote the paper. A.F. performed and interpreted the statistical analysis. I.B. reviewed the bone marrow biopsies to establish the correct diagnosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mrs Heike Preissler, Mrs Marianne Gerusel-Bleck, and Mrs Sonja Bruss for their excellent work in the laboratory and the study organization.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal