Abstract
Omenn syndrome (OS) is a rare primary immunodeficiency characterized by the presence of activated/oligoclonal T cells, eosinophilia, and the absence of circulating B cells. OS patients carry leaky mutations of recombination activating genes (RAG1 or RAG2) resulting in partial V(D)J recombination activity, whereas null mutations cause severe combined immunodeficiency with absence of mature T and B cells (T-B- SCID). Here we describe somatic mosaicism due to multiple second-site mutations in a patient with RAG1 deficiency. We found that he is homozygous for a single base deletion in the RAG1 gene, which results in frameshift and likely abrogates the protein function. However, the patient showed typical OS features. Molecular analysis revealed that several second-site mutations, all of which restored the RAG1 reading frame and resulted in missense mutations, were demonstrated in his T cells. These findings suggest that his revertant T-cell mosaicism is responsible for OS phenotype switched from T-B- SCID. (Blood. 2005; 106:2099-2101)
Introduction
Omenn syndrome (OS) is an autosomal recessive primary immunodeficiency1,2 and caused by mutations of the recombination activating genes (RAG1 and RAG2).3 OS mutations maintain a residual recombination activity that allows limited T-cell receptor (TCR) gene rearrangements in the thymus, whereas null mutations cause a complete block of T- and B-cell development and lead to severe combined immunodeficiency (SCID) with absence of mature T and B lymphocytes (T-B- SCID).4 However, the occurrence of the same mutations in patients with T-B- SCID and OS suggests that “leaky” mutations in RAG genes may not be solely responsible for the development of OS.5
Somatic revertant mosaicism is a rare phenomenon that is increasingly being described in human genetic disorders.6,7 In all cases reported to date, revertant cells carried a single revertant sequence.6,7 It is also recognized that revertant mosaicism is an additional basis for milder phenotype in several primary immunodeficiencies such as adenosine deaminase deficiency,8 X-linked SCID,9 and Wiskott-Aldrich syndrome.10 Here we describe an unusual case of RAG1 deficiency presenting somatic T-cell mosaicism due to multiple second-site mutations and show that the patient's revertant T-cell mosaicism might have contributed to the modification of his clinical features.
Study design
Patient
The patient was the second child born to consanguineous, healthy Japanese parents. He developed generalized exudative erythroderma at age 1 month, followed by failure to thrive and persistent cough. At age 2 months, the patient was hospitalized for upper respiratory infections and otitis media. Two weeks later, he suffered from sepsis due to Pseudomonas aeruginosa. Laboratory evaluation at age 3 months showed moderate anemia, leukocytosis (104 × 109/L [104 000/μL]) with marked eosinophilia (21.8 × 109/L [21 800/μL]), and hypogammaglobulinemia (immunoglobulin G [IgG], 1.48 g/L [148 mg/dL]; IgA, less than 0.01 g/L [less than 1 mg/dL], IgM 0.02 g/L [2 mg/dL], and IgE less than 2 kIU/L). The level of soluble interleukin-2 receptor was markedly elevated at 19 400 kIU/L (normal, 220-530 kIU/L). Immunophenotypic analysis showed the absence of peripheral B cells and marked increase of both CD4+ and CD8+ T cells with activated/memory phenotypes. A skin biopsy revealed lymphocytic infiltration in the upper dermis with occasional eosinophils and destruction of epidermal-dermal junction. Based on these findings, a clinical diagnosis of OS was made.
Cell isolation, sequencing, and TCRVβ repertoire
CD4+ and CD8+ T cells were purified using magnetic beads as described.11 CD16+ natural killer (NK) cells and CD4+TCRVβ8+ and CD8+TCRVβ1+ T cells were separated from peripheral blood mononuclear cells (PBMCs) by an EPICS Elite flow cytometer (Beckman Coulter Fullerton, CA). Approval was obtained from the human research committee of Kanazawa University Graduate School of Medical Science for these studies, and informed consent was provided according to the Declaration of Helsinki. Mutation analysis of RAG genes, fluorescence-activated cell sorter (FACS) analysis of TCRVβ repertoire, and complementarity-determining region 3 (CDR3) spectratyping were performed as described.12,13
Results and discussion
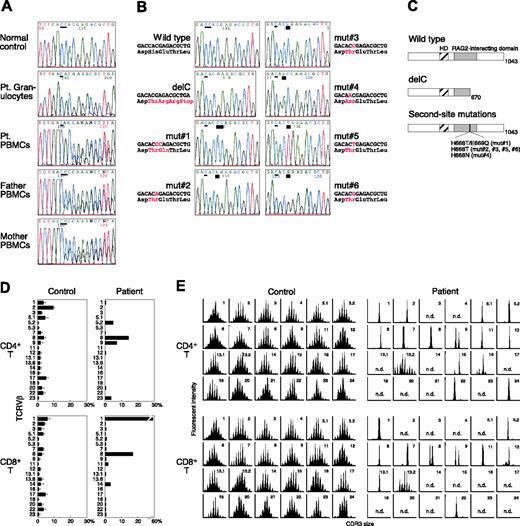
Inherited mutations in either the RAG1 or the RAG2 gene resulting in partial V(D)J recombination activity have been detected in most OS patients.14 We found that our patient is homozygous for a single base C deletion after nucleotide 2113 of the RAG1 gene (delC) in DNA from his granulocytes (Figure 1A). His parents were both heterozygous for this novel mutation. In contrast, DNA from the patient's PBMCs showed coexistence of the delC and other unexpected sequences (Figure 1A). When we analyzed such sequences in subcloned polymerase chain reaction (PCR) products obtained from his T cells, 6 different second-site mutations (mut no. 1-mut no. 6) were detected in addition to the delC mutation (Figure 1B). All of them restored the RAG1 reading frame and resulted in missense mutations, which were located in the RAG2-interacting domain (Figure 1C). Sequencing analysis in the general population excluded the possibility that they could be functional polymorphisms. The possibility that his T cells were derived from the maternal T-cell engraftment was ruled out by fluorescence in situ hybridization analysis for the detection of the X/Y chromosome and by standard molecular study of HLA typing (data not shown). In addition, the second-site mutations were not detectable in the mother's PBMCs. We therefore concluded that T cells carrying the second-site mutations originated from the patient's own hematopoietic cells in vivo.
Characterization of RAG1 gene mutations and T-cell receptor (TCR) Vβ repertoire. (A) The RAG1 gene was amplified from DNA extracted from normal PBMCs, the patient's granulocytes and PBMCs, and the parents' PBMCs. Direct sequencing was performed using an automated sequencer. A thin bar shows the position of the delC mutation. Pt indicates patient. (B) Sequence analysis of the same genomic region in subcloned PCR products obtained from the patient's T cells. A thick bar highlights the position of the second-site mutations. (C) Predicted structures of mutated RAG1 molecules. HD indicates homeodomain. (D) Expression profile of TCRVβ subfamilies. Peripheral blood samples were stained with monoclonal antibodies (mAbs) for individual TCRVβ together with anti-CD4 and anti-CD8 mAbs. The percentage of each TCRVβ expression within CD4+ or CD8+ T cells was analyzed by a flow cytometry. (E) CDR3 spectratyping. Each TCRVβ fragment was amplified from cDNA with one of the Vβ-specific primers. The size distribution of PCR products was determined by an automated sequencer and GeneScan software.
Characterization of RAG1 gene mutations and T-cell receptor (TCR) Vβ repertoire. (A) The RAG1 gene was amplified from DNA extracted from normal PBMCs, the patient's granulocytes and PBMCs, and the parents' PBMCs. Direct sequencing was performed using an automated sequencer. A thin bar shows the position of the delC mutation. Pt indicates patient. (B) Sequence analysis of the same genomic region in subcloned PCR products obtained from the patient's T cells. A thick bar highlights the position of the second-site mutations. (C) Predicted structures of mutated RAG1 molecules. HD indicates homeodomain. (D) Expression profile of TCRVβ subfamilies. Peripheral blood samples were stained with monoclonal antibodies (mAbs) for individual TCRVβ together with anti-CD4 and anti-CD8 mAbs. The percentage of each TCRVβ expression within CD4+ or CD8+ T cells was analyzed by a flow cytometry. (E) CDR3 spectratyping. Each TCRVβ fragment was amplified from cDNA with one of the Vβ-specific primers. The size distribution of PCR products was determined by an automated sequencer and GeneScan software.
The incidence of revertant mosaicism is considered rare, and revertant cells have been shown to carry a single revertant sequence in reported cases.6,7 Our studies, however, provide evidence for the presence of multiple and different second-site mutations in a single patient with nonmalignant diseases, an occurrence previously unreported. The mechanisms underlying these findings are presently unclear. Although mutational hotspots such as repeat sequences or CpG dinucleotides or increased genomic instability could be responsible for an increased rate of reversion events,6,7 this is unlikely the case in our patient.
The second-site mutations were only detectable among T lymphocytes including both CD4+ and CD8+ T cells and not among granulocytes, monocytes, and NK cells (Table 1). B lymphocytes were absent from his peripheral blood. These results suggest that the reversion events occurred in committed T-cell progenitors on one allele in the patient. Alternatively, some of the second-site mutations may have happened in more primitive hematopoietic progenitors such as common lymphoid progenitors, and the lack of circulating B cells, which is usually seen in typical OS patients, could be interpreted as the simple result of partial correction of V(D)J recombination activity. In OS, however, leaky differentiation of a very limited number of B cells is functional and results in the augmented IgE production. In contrast, serum IgE is usually undetectable in T-B- SCID with complete RAG deficiency, reflecting the impaired B-cell differentiation.14 Therefore, it is suggested that no revertant event occurred within B-cell lineages in this patient. On the other hand, some mutants such as the mut no. 2, mut no. 5, and mut no. 6 were detected only in CD4+ or CD8+ T cells (Table 1), indicating that these second-site mutations may have occurred in T-cell progenitors at a stage after CD4/CD8 lineage commitment. Studies of lymphocyte development from RAG1 and RAG2 knock-out mice, however, have demonstrated that RAG-deficient thymocytes accumulate as quiescent cells with a heat-stable antigen (HSA)-positive, CD25+, CD4-, c-kitlo phenotype resembling normal cells just prior to functional TCRβ chain expression.15-17 These findings suggest that the mut no. 2, mut no. 5, and mut no. 6 might be derived from the other precedent mutants by second somatic events after CD4/CD8 lineage commitment.
Genotypic analysis of lymphocyte subsets
. | . | . | Second-site mutations . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. . | delC . | No. 1 . | No. 2 . | No. 3 . | No. 4 . | No. 5 . | No. 6 . | |||||
| PBMCs | 81 | 52 | 25 | 1 | 0 | 3 | 0 | 0 | |||||
| CD4+ T cells | 168 | 91 | 30 | 0 | 19 | 12 | 7 | 9 | |||||
| CD8+ T cells | 130 | 63 | 43 | 13 | 7 | 4 | 0 | 0 | |||||
| CD4+ Vβ8+ T cells | 45 | 29 | 0 | 0 | 16 | 0 | 0 | 0 | |||||
| CD8+ Vβ1+ T cells | 43 | 21 | 21 | 0 | 1 | 0 | 0 | 0 | |||||
| CD16+ NK cells | 13 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Monocytes | 23 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Granulocytes | 24 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
. | . | . | Second-site mutations . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No. . | delC . | No. 1 . | No. 2 . | No. 3 . | No. 4 . | No. 5 . | No. 6 . | |||||
| PBMCs | 81 | 52 | 25 | 1 | 0 | 3 | 0 | 0 | |||||
| CD4+ T cells | 168 | 91 | 30 | 0 | 19 | 12 | 7 | 9 | |||||
| CD8+ T cells | 130 | 63 | 43 | 13 | 7 | 4 | 0 | 0 | |||||
| CD4+ Vβ8+ T cells | 45 | 29 | 0 | 0 | 16 | 0 | 0 | 0 | |||||
| CD8+ Vβ1+ T cells | 43 | 21 | 21 | 0 | 1 | 0 | 0 | 0 | |||||
| CD16+ NK cells | 13 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Monocytes | 23 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Granulocytes | 24 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
Sequence occurrence/total number of sequences.
RAG mutations lead to heterogeneous immune and clinical manifestations ranging from T-B- SCID to OS probably due to residual recombination activity.14 The frameshift mutation delC is expected to abrogate RAG1 function and should have resulted in a T-B- SCID phenotype when present on both alleles. However, our patient is classified as OS based on the clinical findings. Although we need to perform V(D)J recombination assay to determine the restored activity, all of the patient's second-site mutations are likely compatible with partial correction of the RAG1 activity. Indeed, a similar missense mutation resulting in an E669G substitution has been reported in a patient with typical OS.14 Accordingly, the revertant T cells of our patient showed mature and activated phenotype with a highly restricted TCR repertoire in the periphery (Figure 1D-E). It seems therefore reasonable that his clinical phenotype has changed from T-B- SCID to OS due to the revertant mosaicism.
Our studies provide significant implications of revertant mosaicism in the pathogenesis of OS. Recent advances in molecular genetics and cell enrichment techniques have allowed small levels of somatic mosaicism to be investigated.18 Thus, somatic revertant mosaicism may play a more important role in factors that influence phenotypic expression of diseases than previously thought and will help us to understand, at least in part, inconsistent genotype/phenotype correlation in genetic disorders.
Prepublished online as Blood First Edition Paper, April 21, 2005; DOI 10.1182/blood-2005-03-0936.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant from the Ministry of Health, Labour, and Welfare of Japan, Tokyo.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Harumi Matsukawa and Ms Mika Tamamura for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal