Abstract
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disease that occurs mainly in young adults. Acquired cases are usually a result of antibodies directed against ADAMTS13 (a disintegrin-like and metalloprotease [reprolysin type] with thrombospondin type 1 motif 13), a protease that cleaves the von Willebrand factor multimers. Prognosis has been improved by plasma therapy, but some acute severe forms are refractory to this treatment and achieving a sustained remission is still a challenge in chronic relapsing forms. We therefore conducted a multicentric open-label prospective trial to test the efficacy of rituximab, an anti–B-cell monoclonal antibody, as a curative and prophylactic treatment in patients with TTP as a result of anti-ADAMTS13 antibodies. Six patients were included during an acute refractory TTP episode. Five patients with severe relapsing TTP and persistent anti-ADAMTS13 antibodies were prophylactically treated during remission. All patients received 4 weekly infusions of rituximab. The target of treatment was to restore a significant ADAMTS13 plasma activity (> 10%). Treatment with rituximab led to clinical remission in all cases of acute refractory TTP. In all patients, anti-ADAMTS13 antibodies disappeared, and a significant (18%-75%) plasma ADAMTS13 activity was detected following treatment. Tolerance of rituximab was good. Rituximab is a promising first-line immunosuppressive treatment in patients with acute refractory and severe relapsing TTP related to anti-ADAMTS13 antibodies.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disease, characterized by microangiopathic hemolytic anemia, thrombocytopenia, neurologic disturbances, and renal failure.1 These symptoms are related to the presence of von Willebrand factor (VWF)–rich platelet thrombi in the arterioles and capillaries. VWF is a multimeric plasma glycoprotein crucial for both platelet adhesion and aggregation, especially at the high shear rates in the microvasculature. The size of VWF multimers is physiologically regulated in vivo by a specific metalloprotease, ADAMTS13 (a disintegrin-like and metalloprotease [reprolysin type] with thrombospondin type 1 motif 13), that cleaves the largest multimers to prevent the spontaneous formation of platelet thrombi in the microcirculation. Depending on inclusion criteria, 33% to greater than 90% of idiopathic TTP cases are related to a severe functional deficiency of ADAMTS13 in plasma. ADAMTS13 deficiency may be due either to mutations in the ADAMTS13 gene in the very rare inherited forms of TTP (Upshaw-Schulman syndrome) or to circulating auto-antibodies (Abs) to ADAMTS13 in the more frequently acquired forms of TTP.2 Circulating auto-Abs to ADAMTS13 are inhibitory in most cases, but some nonneutralizing Abs have also been reported.3-5
Plasma therapy, including both plasma infusion (PI) and plasma exchange (PE), has been empirically initiated since the 1970s, and it has dramatically improved TTP prognosis, decreasing the mortality rate from 90% to less than 20%.6 However, a subset of patients with acquired TTP requires very long-term plasma therapy to prevent fatal outcome and to achieve a sustained remission. In these patients, complementary treatments, including immunosuppressive agents (corticosteroids, vincristine, cyclophosphamide, azathioprine, cyclosporine A), high-dose intravenous immunoglobulin (Ig), staphylococcal protein A immunoadsorption, and splenectomy, have been proposed.7-11 Despite all these therapeutic options, about one third of all patients with acquired TTP develop multiple relapses or persistent disease.1
The prediction of which patients are at high risk of either prolonged course or short- or long-term relapses remains a challenging issue. These “refractory” forms of TTP have been suggested to be associated with high titers of inhibitory anti-ADAMTS13 Abs in one prospective study involving 37 patients with ADAMTS13-undetectable acute TTP.12 However, the follow-up of 18 patients from the Oklahoma Registry on thrombotic microangiopathies reports that the occurrence of relapses was not related to the strength of the inhibitor activity detected during the acute phase.13
From 2002, rituximab, an anti-CD20 monoclonal Ab, has proved efficiency in the treatment of CD20+ lymphoproliferative disorders14 and some autoimmune diseases.15-18 It has been also used as a curative treatment in small retrospective cohorts of patients with acute refractory TTP associated with a severe ADAMTS13 deficiency related to inhibitory anti-ADAMTS13 Abs.19-29 Twenty-five of 26 patients achieved complete remission, and their follow-up revealed long-term remission in most cases. Rituximab has never been used as a prophylactic treatment in patients with highly recurrent TTP out of an acute episode.
The current study had a double aim: first, to test the efficiency of rituximab in prospectively enrolled patients with an acute refractory TTP; second to evaluate the use of rituximab as a prophylactic treatment in selected patients with recurrent TTP out of an acute episode.
Patients, materials, and methods
The trial was conducted between February 2004 and January 2005 in 4 French centers (Hôpital Necker-Enfants Malades, Hôpital de la Pitié-Salpêtrière [Paris], Hôpital de la Conception [Marseille], and Hôpital de Valenciennes [Valenciennes]) and 1 Italian center (Mario Negri Institute, Bergamo). Approval was obtained from the Hôpital Necker-Enfants Malades institutional review board for this study. Appropriate consent was obtained from all patients.
Inclusion criteria
Patients included during an acute phase of TTP.
All the following criteria were required for inclusion: (1) age of at least 18 years; (2) a current acute TTP episode defined by a microangiopathic hemolytic anemia (hemoglobin level <100 g/L [<10 g/dL], lactate dehydrogenase [LDH] > 460 IU/L, undetectable serum haptoglobin and the presence of schizocytes on blood smear) and a thrombocytopenia (platelet count < 50 × 109/L), eventually associated with visceral ischemic signs; (3) a current undetectable ADAMTS13 plasma activity (< 5%; normal, 50%-150%) related to a circulating anti-ADAMTS13 inhibitory Ab; and (4) the absence of remission after at least 3 weeks of plasma exchange.
Patients included during a remission phase of TTP.
All the following criteria were required for inclusion: (1) age of at least 18 years, (2) at least 2 previous acute episodes of TTP, (3) at least one extra-hematologic symptom during one or several previous relapses, (4) failure of at least one treatment other than plasma (vincristine, intravenous Ig, splenectomy, etc) before the inclusion in the current study, (5) current clinical remission, (6) undetectable ADAMTS13 activity in plasma related to a circulating anti-ADAMTS13 inhibitory Ab, and (7) the use of contraception (excluding estrogen-progesterone pills) in sexually active women.
Exclusion criteria
One of the following criteria was enough for exclusion in both previous groups of patients: (1) ADAMTS13 deficiency (plasma activity < 5%) in the absence of detectable anti-ADAMTS13 inhibitory antibodies, (2) acquired or constitutional immune deficiencies, and (3) active viral infections (hepatitis C and B, HIV).
Treatment with rituximab
All patients included in the study received 4 weekly infusions of rituximab (375 mg/m2; MabThera; Roche, Meylan, France) with a premedication using steroids (30 mg intravenously), dexchlorpheniramine (10 mg intravenously), and paracetamol (1 g intravenously).
In patients prospectively included during a refractory acute TTP, PEs were discontinued before treatment with rituximab and were not resumed afterward. Plasma infusions (15-25 mL/kg/d) were continued for at least 3 weeks and progressively tapered after the induction of clinical remission. In contrast, in selected patients with a relapsing TTP, rituximab was the only treatment.
Criteria for clinical and biologic remission
TTP clinical remission was defined by both the regression of visceral ischemic signs (if initially present) and the normalization of standard blood parameters (platelet count > 150 × 109/L and hemoglobin level > 120 g/L [> 12 g/dL]). Biologic remission was defined by the recovery of an ADAMTS13 activity in plasma greater than 10% with no detectable circulating inhibitor. The threshold of 10% was chosen based on observations made in patients with congenital TTP because of ADAMTS13 gene mutations and treated with prophylactic infusions of fresh-frozen plasma (FFP).30 A minimal ADAMTS13 activity of 10%, achieved using repeated FFP infusions, is usually sufficient to avoid a TTP relapse in these patients.
ADAMTS13 assays
In all patients, venous blood was first collected at inclusion, before any treatment. The other blood collections were performed during follow-up (see “Follow-up”). Functional assays for ADAMTS13 plasma activity and testing for circulating inhibitory anti-ADAMTS13 antibodies were performed as previously described.31,32 Briefly, measurement of ADAMTS13 activity was performed by immunoradiometric assay. Platelet-poor plasma from 25 healthy volunteers was arbitrarily defined as containing 100% ADAMTS13 and was used as an internal control. ADAMTS13 inhibitor was assayed by measuring the residual ADAMTS13 activity in mixtures of patient plasma and plasma from healthy volunteers at 3 different volume-to-volume ratios (1:1, 2:1, and 3:1) after a 30-minute preincubation at room temperature.
Follow-up
After clinical remission, patients were subsequently seen in the participating centers every 3 months for a clinical evaluation; standard laboratory tests; measurement of CD19+ and CD20+ cell counts, total Ig level, ADAMTS13 plasma activity, and testing for anti-ADAMTS13 inhibitory Abs.
Results
Patient characteristics at inclusion
Eleven patients were enrolled in the study, including 6 patients during an acute TTP episode and 5 patients during a clinical remission of a severe relapsing TTP. These 11 patients represent all patients seen in the 5 participating institutions over a period of 1 year and fulfilling the inclusion criteria. Patient features at the time of inclusion are summarized in Table 1. All patients were white.
Baseline features of patients with TTP
Patient no. . | Sex . | Age at inclusion, y . | Duration of TTP, y . | Total no. of TTP episodes (TTP episodes/y) . | Kidney/brain involvement . | Previous treatments . | Time since last immunosuppressive treatment, mo . |
|---|---|---|---|---|---|---|---|
| 1 | F | 21 | First episode | 1 | –/altered cse | Plasma, vincristine | 1 |
| 2 | F | 58 | First episode | 1 | –/TCA seizures | Plasma, vincristine | 1 |
| 3 | F | 42 | 2 | 3 (1.5) | –/TCA | Plasma, vincristine | 2 |
| 4 | F | 21 | 2 | 3 (1.5) | –/– | Plasma, splenectomy | – |
| 5 | M | 36 | 5 | 12 (2.4) | SCr 150/TCA | Plasma, vincristine, IV Ig, splenectomy | 24 |
| 6 | M | 40 | 1 | 2 (2) | SCr 330/– | Plasma, rituximab | 10 |
| 7 | M | 31 | 4 | 4 (1) | SCr 710/– | Plasma, vincristine | 1 |
| 8 | M | 40 | 11 | 4 (0.4) | SCr 200/altered cse | Plasma, splenectomy | – |
| 9 | F | 40 | 7 | 4 (0.6) | –/altered cse | Plasma, vincristine, splenectomy | 48 |
| 10 | M | 60 | 12 | 11 (0.9) | SCr 880/TCA seizures | Plasma, vincristine, cyclosporine, intravenous Ig, splenectomy, rituximab | 17 |
| 11 | M | 40 | 28 | 15 (0.6) | SCr 180/TCA seizures | Plasma, vincristine, intravenous Ig, splenectomy, rituximab | 20 |
Patient no. . | Sex . | Age at inclusion, y . | Duration of TTP, y . | Total no. of TTP episodes (TTP episodes/y) . | Kidney/brain involvement . | Previous treatments . | Time since last immunosuppressive treatment, mo . |
|---|---|---|---|---|---|---|---|
| 1 | F | 21 | First episode | 1 | –/altered cse | Plasma, vincristine | 1 |
| 2 | F | 58 | First episode | 1 | –/TCA seizures | Plasma, vincristine | 1 |
| 3 | F | 42 | 2 | 3 (1.5) | –/TCA | Plasma, vincristine | 2 |
| 4 | F | 21 | 2 | 3 (1.5) | –/– | Plasma, splenectomy | – |
| 5 | M | 36 | 5 | 12 (2.4) | SCr 150/TCA | Plasma, vincristine, IV Ig, splenectomy | 24 |
| 6 | M | 40 | 1 | 2 (2) | SCr 330/– | Plasma, rituximab | 10 |
| 7 | M | 31 | 4 | 4 (1) | SCr 710/– | Plasma, vincristine | 1 |
| 8 | M | 40 | 11 | 4 (0.4) | SCr 200/altered cse | Plasma, splenectomy | – |
| 9 | F | 40 | 7 | 4 (0.6) | –/altered cse | Plasma, vincristine, splenectomy | 48 |
| 10 | M | 60 | 12 | 11 (0.9) | SCr 880/TCA seizures | Plasma, vincristine, cyclosporine, intravenous Ig, splenectomy, rituximab | 17 |
| 11 | M | 40 | 28 | 15 (0.6) | SCr 180/TCA seizures | Plasma, vincristine, intravenous Ig, splenectomy, rituximab | 20 |
Patients 1 to 6 were included during an acute episode of refractory TTP. Patients 7 to 11 were included during clinical remission. cse indicates consciousness; TCA, transient cerebrovascular attacks; SCr, maximal serum creatinine (μM); and –, absence.
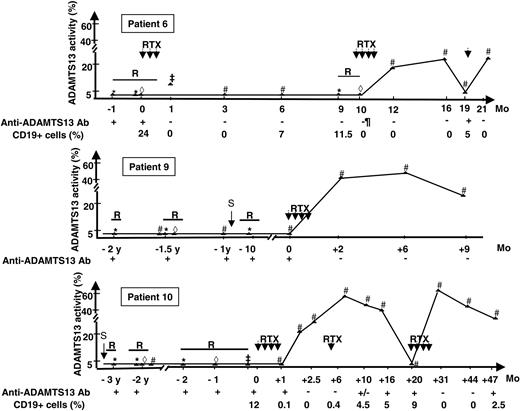
Among patients enrolled during an acute TTP episode (patients 1 to 6), 2 patients had a first TTP episode, whereas 4 patients previously experienced several TTP relapses before their inclusion (Table 1). All patients but one exhibited kidney (n = 2) or brain (n = 4) ischemic manifestations, including one patient with both. All patients had previously received plasma therapy and various additional treatments, including vincristine (n = 3), intravenous Ig (n = 1), or splenectomy (n = 2). At inclusion in the study, immunosuppressive treatments had been stopped for at least one month (range, 1-24 months). Plasma therapy was the only treatment performed for 3 to 12 weeks. All patients received concomitant steroid treatment. Patient 6 had been treated with rituximab for a first severe refractory episode of TTP. Subsequently, remission was obtained and anti-ADAMTS13 antibodies were undetectable using plasma-phase assays. However, plasma ADAMTS13 activity remained undetectable (< 5%) even during a relapse of TTP 9 months later. At that time, a previously described enzyme-linked immunosorbent assay (ELISA) test using recombinant ADAMTS134 showed the persistence of high-titer (1/200) anti-ADAMTS13 antibodies (IgG and IgM) with the absence of ADAMTS13 antigen in the plasma. Protracted PE failed to control the disease; mesenteric ischemia as well as severe systemic reaction to FFP occurred, and PEs were discontinued. The patient was then included in the study during this second TTP episode.
Patients included during a remission phase of the disease (patients 7 to 11) had previously experienced 4 to 15 severe TTP episodes (Table 1). The duration of remission before inclusion was 1, 12, 15, 17, and 19 months for patients 7, 8, 9, 10, and 11, respectively. Kidney involvement was present in 4 cases and brain ischemia in 4 cases, including 3 patients with both. All patients had previously received plasma therapy and various additional treatments, including vincristine (n = 4), splenectomy (n = 4), intravenous Ig (n = 2), or cyclosporine (n = 1). Patient 8 had never received any immunosuppressive treatment before. In patients 7 and 9, vincristine was the only immunosuppressive treatment administered 1 and 48 months, respectively, before. Patients 10 and 11 had already been treated once with rituximab 17 and 20 months, respectively, before their inclusion in the study.
After the first treatment with rituximab, patient 10 recovered a detectable ADAMTS13 activity in plasma (21%) associated with the disappearance of the inhibitor. Eleven months later, although B-cell count remained lower than normal, anti-ADAMTS13 inhibitor reappeared and ADAMTS13 activity became undetectable (< 5%).
The case of patient 11 has already been reported in part.33 Following the first rituximab treatment, patient 11 recovered a significant ADAMTS13 activity in plasma (45%) together with the disappearance of the anti-ADAMTS13 inhibitor. Nine months after rituximab treatment, the recovery of a normal B-cell count was followed 2 months later by the reappearance of ADAMTS13 severe deficiency (plasma activity < 5%) related to the presence of an inhibitor and rituximab was resumed.
Initial response to treatment
In all patients enrolled during an acute TTP episode, rituximab induced a clinical remission in 5 to 14 days after the 4th rituximab infusion. A recovery of a significant ADAMTS13 plasma activity was obtained as early as 8 weeks after rituximab treatment and discontinuation of plasma therapy.
In patients included during a remission phase of TTP, no clinical relapse occurred as expected. Biologic remission, defined as the disappearance of anti-ADAMTS13 inhibitory antibodies together with the recovery of a detectable ADAMTS13 activity in plasma (range, 29%-75%), was achieved in all patients 7 to 24 weeks after the last rituximab infusion.
Follow-up
Follow-up ranged from 6 to 11 months. In all patients of both groups, clinical remission persisted during follow-up. Table 2 summarizes 3-, 6-, and 9-month follow-up in the curative and in the prophylactic treatments groups.
Follow-up of 11 patients with TTP treated with rituximab
. | 3-mo follow-up . | . | 6-mo follow-up . | . | 9-mo follow-up . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | ADAMTS13 activity in plasma, % . | Inhibitory activity to ADAMTS13 in plasma . | ADAMTS13 activity in plasma, % . | Inhibitory activity to ADAMTS13 in plasma . | ADAMTS13 activity in plasma, % . | Inhibitory activity to ADAMTS13 in plasma . | |||
| 1 | < 5 | Positive | 18 | Negative | 26 | Negative | |||
| 2 | < 5 | Negative | 27 | Negative | 47 | Negative | |||
| 3 | < 5 | Positive | 33 | Negative | 38 | Negative | |||
| 4 | 35 | Negative | 35 | Negative | NA | NA | |||
| 5 | 56 | Negative | 54 | Negative | 32 | Negative | |||
| 6 | 18 | Negative | 21 | Negative | 10* | Negative* | |||
| 7 | 33 | Negative | 56 | Negative | 46 | Positive (weak) | |||
| 8 | < 5 | Positive | 75 | Negative | NA | NA | |||
| 9 | 46 | Negative | 29 | Negative | 18* | Negative* | |||
| 10 | 24 | Negative | 32 | Negative | 18 | Negative | |||
| 11 | 67 | Negative | 65 | Negative | 29 | Negative | |||
. | 3-mo follow-up . | . | 6-mo follow-up . | . | 9-mo follow-up . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | ADAMTS13 activity in plasma, % . | Inhibitory activity to ADAMTS13 in plasma . | ADAMTS13 activity in plasma, % . | Inhibitory activity to ADAMTS13 in plasma . | ADAMTS13 activity in plasma, % . | Inhibitory activity to ADAMTS13 in plasma . | |||
| 1 | < 5 | Positive | 18 | Negative | 26 | Negative | |||
| 2 | < 5 | Negative | 27 | Negative | 47 | Negative | |||
| 3 | < 5 | Positive | 33 | Negative | 38 | Negative | |||
| 4 | 35 | Negative | 35 | Negative | NA | NA | |||
| 5 | 56 | Negative | 54 | Negative | 32 | Negative | |||
| 6 | 18 | Negative | 21 | Negative | 10* | Negative* | |||
| 7 | 33 | Negative | 56 | Negative | 46 | Positive (weak) | |||
| 8 | < 5 | Positive | 75 | Negative | NA | NA | |||
| 9 | 46 | Negative | 29 | Negative | 18* | Negative* | |||
| 10 | 24 | Negative | 32 | Negative | 18 | Negative | |||
| 11 | 67 | Negative | 65 | Negative | 29 | Negative | |||
Patients 1 to 6 received curative rituximab treatment during an acute refractory episode of TTP, and patients 7 to 11 were included during clinical remission for prophylactic rituximab treatment.
NA indicates not available.
Data at 11 months of follow-up
At the 3-month follow-up, undetectable ADAMTS13 activity in plasma related to an inhibitor was still noted in 2 patients who underwent a curative rituximab treatment and in only 1 patient who received a prophylactic rituximab treatment. In contrast, biologic remission consisting in ADAMTS13 activity in plasma ranging from 18% to 75% together with no detectable inhibitor was achieved in all patients at the 6-month follow-up (Table 2). Biologic remission was still observed in all patients, except patient 6, after 6 to 9 months of follow-up.
The evolution of ADAMTS13 plasma therapy before and after rituximab in patients 6, 9, and 11 for whom sequential assays of ADAMTS13 plasma activity are available over several years is shown in Figure 1.
B-cell depletion (< 1% and < 5/mm3) was achieved in all patients except in patient 9 (lowest CD19+ cell value, 17/mm3).At last follow-up all patients had recovered a significant B-cell count (CD19+ > 100/mm3) (data not available for patients 4, 7, 8, and 10).
Tolerance
Mild hypotension occurred during the first rituximab infusion in patient 1 and resolved after the infusion rate was decreased. No side effects were noted during follow-up. Total immunoglobulin level remained unchanged in all patients (data not available in patients 4 and 7).
Discussion
Rituximab is a monoclonal chimeric anti-CD20 antibody mainly used in the treatment of B-cell malignancies.14 It induces a B-cell depletion that lasts 6 to 9 months with the recovery of a normal B-cell count usually occurring 12 months after treatment. The tolerance of rituximab is good; the main side effects are cytokine-related systemic reactions usually occurring during the first infusion and, rarely, infections, mainly viral (eg, cytomegalovirus [CMV], hepatitis B reactivation). The efficacy of rituximab also has been reported in various autoimmune disorders such as autoimmune hemolytic anemia, rheumatoid arthritis, systemic lupus erythematous, systemic vasculitis, especially in severe forms resistant to conventional immunosuppressive treatment.15-18
Time course of ADAMTS13 plasma activity in 3 patients for whom sequential measurements of ADAMTS13 plasma activity over several years are available. ADAMTS13 activity was measured during (1) a disease relapse before (*) and during plasma exchange (⋄) or infusions of fresh-frozen plasma (‡) and (2) remission (#). R indicates relapse; RTX, rituximab; S, splenectomy; and Ab, antibody. Anti-ADAMTS13 antibodies were detected using the ELISA test. Sequential measurements of CD19+ cells are not available for patient 9. The length of the solid bars indicates the duration of TTP relapse.
Time course of ADAMTS13 plasma activity in 3 patients for whom sequential measurements of ADAMTS13 plasma activity over several years are available. ADAMTS13 activity was measured during (1) a disease relapse before (*) and during plasma exchange (⋄) or infusions of fresh-frozen plasma (‡) and (2) remission (#). R indicates relapse; RTX, rituximab; S, splenectomy; and Ab, antibody. Anti-ADAMTS13 antibodies were detected using the ELISA test. Sequential measurements of CD19+ cells are not available for patient 9. The length of the solid bars indicates the duration of TTP relapse.
Most cases of acquired TTP are to be considered and treated as an autoimmune disease because anti-ADAMTS13 Abs are detected in 33% to greater than 90% of idiopathic TTP of patients.2,3 In the presence of high-titer anti-ADAMTS13 antibodies, FFP infusions and PE rarely lead to the disappearance of these antibodies, and ADAMTS13 plasma activity remains undetectable12 as clearly illustrated in patients 6, 9, and 10 (Figure 1). Even though remission may occur in the presence of undetectable ADAMTS13 activity, relapse rate is high (43%).13 Several treatments (eg, cyclophosphamide, vincristine, intravenous Ig) have been previously used in patients with TTP in single cases or in small series published before the role of ADAMTS13 was identified. Results were highly variable, and the treatment of severe relapsing TTP remains challenging.
Rituximab has been previously used successfully in TTP resulting from anti-ADAMTS13 antibodies. These reports consisted of small retrospective series or single case reports.19-29 Moreover, patients usually received concomitant additional immunosuppressive treatments, including vincristine and cyclophosphamide.
We report the first prospective open-label trial of rituximab in TTP due to anti-ADAMTS13 antibodies. First, our results confirm previous preliminary reports concerning the efficacy of rituximab in patients with acute refractory TTP. In the absence of any clear evidence supporting the efficacy of other therapies in this setting, our data suggest that rituximab is a promising first-line immunosuppressive therapy in patients with acute refractory TTP. However, the efficiency of rituximab in inducing sustained TTP remission remains to be evaluated in prospective controlled studies. These studies must also address the issue of the optimal timing because the use of rituximab remains to be determined: after at least 3 weeks of plasma therapy, or earlier in patients with high-titer anti-ADAMTS13 antibodies?
Second, our results show for the first time that rituximab is a promising prophylactic first-line treatment in selected patients with severe relapsing TTP and persistent anti-ADAMTS13 antibodies. The rationale underlying the use of prophylactic rituximab therapy in these patients is based on (1) the high frequency of TTP relapses (4-15 episodes) despite at least one previous prophylactic treatment, (2) the severity of previous TTP episodes with a increased risk of mortality and morbidity in these young adults, and (3) the high risk of relapse in the presence of anti-ADAMTS13 antibodies (43%).13 Thus, the aim of prophylactic treatment is to restore a significant (> 10%) ADAMTS13 activity. In our patients (cases 7-11), FFP and PE as well as previous immunosuppressive treatments and/or splenectomy have failed to clear anti-ADAMTS13 antibodies and to restore a significant ADAMTS13 plasma activity. This was achieved following treatment with rituximab, as the sole treatment in our patients.
In addition, the tolerance of rituximab treatment was excellent in both groups, especially when compared with the side effects reported with immunosuppressive agents previously used in TTP.
These data clearly support the use of rituximab as a first-line prophylactic treatment in patients with TTP with persistent anti-ADAMTS13 antibodies.
Data obtained in patient 2 confirm a previous report by Scheiflinger et al4 concerning the presence of non-neutralizing anti-ADAMTS13 antibodies in patients with TTP and suggest that several types of anti-ADAMTS13 antibodies may coexist. Nonneutralizing antibodies are not detected by functional plasma-phase assays because they are probably not directed to the catalytic site of ADAMTS13. These antibodies are probably pathogenic because they seem to interfere with the ADAMTS13 binding to endothelial cells or increase its clearance from the plasma. However, one cannot exclude that plasma-phase tests fail to detect some neutralizing antibodies by lack of sensitivity and that other types of autoantibodies (such as anti-CD36) may contribute to the pathogenesis of TTP.
Several issues regarding the maintenance treatment with rituximab in TTP remain unsolved. Replenishment of the B-cell pool 6 to 12 months after rituximab therapy is frequently (but not invariably) associated with the reappearance of autoantibodies. In 6 of 9 patients assessed at 9 months, the ADAMTS13 level decreased from the 6-month measurement, even though it remained > 10%, the threshold required to prevent TTP relapse. This may be due to minimal residual autoimmune disease leading to the persistence or reappearance of low-titer antibodies not detected by functional tests.
Clearly, a maintenance treatment with rituximab is necessary in some patients with severe TTP as in patients 6, 10, and 11. The modalities of this treatment are still open to debate. What are the optimal time (before or after the recovery of B cells? Before or after the reappearance of autoantibodies?), frequency (every 6 months?), and dose (1 perfusion?) of maintenance therapy? What will be the effect of such treatment on the incidence of anti–rituximab antibodies and on the subsequent efficacy of the drug? Finally, it remains to be determined why, in some patients with autoimmune disorders, autoantibodies do not reappear despite the recovery of a normal B-cell count.
Despite these promising data, the effect of rituximab on long-term evolution of TTP remains to be ascertained. Especially, extended follow-up is necessary to evaluate whether rituximab significantly decreases the frequency of TTP relapses.
In summary, our data indicate that rituximab is (1) an effective curative therapy in patients with acute refractory TTP related to anti-ADAMTS13 antibodies and (2) a promising prophylactic treatment in selected patients with severe relapsing TTP and persistent anti-ADAMTS13 antibodies.
Prepublished online as Blood First Edition Paper, June 2, 2005; DOI 10.1182/blood-2005-03-0848.
Supported by the Délégation Régionale à la Recherche Clinique (Assistance Publique-Hôpitaux de Paris) (RITHA13 study, P030605), which sponsored the research, and by the Laboratoire Roche France, who provided rituximab (MabThera). Roche France had no role in the design of the study, interpretation of the data, the writing of the report, or in the decision to submit the paper for publication.
F.F. designed the study, analyzed the results, and wrote the article. The study protocol was discussed with J.-P.G., P.L., G.R., J.-P.V., A.V., M.W., and O.H., A.V. and M.W. were responsible for the ADAMTS13 assays. G.K., R.B., M.R., F.S., P.P., B.D., R.D., and P.V. were in charge of the patients.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank François Lefrère, Francoise Audat (Service d'Hémaphérèse, Hôpital Necker, Paris, France), Patrick Lefèvre (Service d'Hémaphérèse et Autotransfusion, Hôpital de La Conception, Marseille, France), Marina Noris, Miriam Galbusera (Mario Negri Institute for Pharmacological Research, Bergamo, Italy), Enrico Pogliani (Hematology Unit, Ospedale San Gerardo Monza, Italy), Pr Jean-Christophe Thalabard (Unité de Recherche Clinique, Hôpital Necker-Enfants Malades, Paris, France), and Sophia Hilaly (Délégation Régionale à la Recherche Clinique, Paris, France) for their valuable help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal