Abstract
The production of high-affinity antibodies to T-dependent antigens requires the interaction of B cells and T helper cells expressing receptors specific for the same antigen. Although several mechanisms have been elucidated that regulate B-cell trafficking within lymphoid organs, less is known about molecular cues that guide the small subpopulation of CD4+ follicular T helper cells to B-cell follicles. Using adoptive transfer of transgenic T cells in mice, we demonstrate that antigen-induced activation leads to a finely tuned positioning of T cells either to the T-cell area or the B-cell follicle. We show that expression of CXCR5 is indispensable for T cells to enter B-cell follicles, whereas expression of CCR7 provides a counteracting signal to retain activated T cells in the T-cell area. Although only few T cells transiently migrate from the T-cell area to the B-cell follicle of peripheral lymph nodes following antigenic challenge, this step is essential to provide the help B cells require to produce antibodies efficiently. Thus, we demonstrate that the balanced expression of CCR7 and CXCR5 determines the positioning and proper function of follicular T helper cells.
Introduction
To effectively fight invading pathogens, mammals have developed a repertoire of adaptive immune mechanisms that include the production of high-affinity antibodies. A critical event in the initiation of the humoral immune response reflects the timely interaction of B and T cells specific for the same antigen. The border between T-cell zone and B cell-rich follicles in lymph nodes (LNs) and the white pulp of the spleen are commonly considered as the place where the initial interaction between antigen-specific T and B cells occurs.1 After receiving signals from activated CD4 T cells via the interaction of CD40/CD40L and further costimulatory molecules, some B cells differentiate into plasmablasts, which migrate to the red pulp of the spleen to become short-lived plasma cells secreting low-affinity antibodies. Following the interaction with T cells, other B cells migrate into the B follicle, start to proliferate, and form germinal centers (GCs).2 It is widely accepted that isotype switching and affinity maturation of B cells depend on CD4 T helper cells. Some of these might also migrate into the follicle and GC. Thus, the coordinated migration of B and T cells appears to represent a decisive event that allows lymphocytes to make contact with each other.
Ample evidence indicates that the migration of lymphocytes into secondary lymphoid organs is controlled by a chemokine-driven process. During the last decade several chemokines have been identified that are expressed in lymphoid organs, which guide resting or activated cells to their point of destination. Naive T cells express the chemokine receptor CCR7, which allows recruitment of these cells into T-cell areas of LNs or to the splenic white pulp through interaction with its ligands CCL19 and CCL21. These chemokines are expressed at specialized high endothelial venules (HEVs) in LNs and by lymphoid and nonlymphoid cells in the T-cell area of LNs and spleen.3,4 In addition to CXCR4, CCR6, and CXCR5, B cells also express CCR7 to be able to enter lymphoid organs efficiently. Once entered these cells require CXCR5 to follow a chemokine gradient build up by CXL13 that is constitutively expressed on stromal cells in the follicles.5 The differential localization of B cells during the different phases of an antigen-specific immune response is facilitated by the balanced responsiveness of these cells toward the chemokines CXCL13 and CCL19/CCL21.6
In contrast to the situation described for B cells, less is known about molecular mechanisms that guide the small subpopulation of CD4+ helper T cells into the B-cell follicle. These cells lack a distinct TH1 or TH2 cytokine expression profile but express costimulatory molecules such as inducible costimulator (ICOS) and CD40L and induce B cells to produce IgG and IgA in vitro.7,8 Based on this ability and due to the fact that these cells express CXCR5, a chemokine receptor known to be essential for the localization of B cells to the follicle, we and others termed these T cells “follicular B helper T cells” (TFH).7,8 Following stimulation in vitro and in vivo a subpopulation of naive CCR7+ T cells starts to express CXCR5 and becomes less responsive to CCR7 ligands in vitro.5 Although these data suggest that CXCR5 guiding of TFH into B-cell follicles experimental, evidence supporting this hypothesis is still missing.
Using various adoptive transfer experiments with T cells carrying a major histocompatability complex class II (MHCII)-restricted T-cell receptor (TCR) specific for ovalbumin (OVA), we now demonstrate that the migration of CD4+ T cells into B-cell follicles entirely depends on the expression of CXCR5. We show that the majority of CXCR5+ helper cells isolated from murine Peyer patches (PPs) lack CCR7, whereas this receptor is always expressed on these TFH cells generated during an adoptive immune response in peripheral LNs. Furthermore, we demonstrate that the balanced expression of CCR7 and CXCR5 determines their localization into the B or T area within LNs. Our data reveal that this migration contributes essentially to an effective T cell-dependent immunoglobulin secretion.
Materials and methods
Mice
A breeder pair of DO11.10 mice that carry a I-Ad-restricted transgenic T-cell receptor specific for OVA was kindly provided by Thomas Blankenstein (Max Delbrück Center, Berlin, Germany). OT-II mice were kindly provided by Arne von Bonin (Bernhard-Nocht Institute, Hamburg, Germany). All mice were kept under specific pathogen-free (SPF) conditions at Hannover Medical School's central animal facility and were used at the age of 8 to 12 weeks. All animal experiments have been approved by the local government and were conducted according to the guidelines of Hannover Medical School.
CXCR5-/- (mixed CD-1 × 129Sv/Ev genetic background crossed for 6 generations to 129SV/En), CXCR5-/- (crossed for 8 generations to C57BL6), and CCR7-/- mice (mixed Balb/c × 129Sv/Ev genetic background) were described earlier.3,9 CXCR5- and CCR7-deficient mice were bred to DO11.10 mice to obtain chemokine receptor-deficient, T-cell receptor-transgenic (TCR-tg) mice. Wild-type C57BL6, Balb/c, and 129SV mice were purchased from Charles River (Sulzfeld, Germany) and intercrossed. The F1 offspring of Balb/c × 129SV intercrosses served as recipients in some adoptive transfer experiments.
Flow cytometry
To obtain single-cell suspensions, LNs were minced through a 45-μm nylon mesh and washed with phosphate-buffered saline (PBS) supplemented with 3% fetal calf serum (FCS) and then stained as previously described.10 TCR-tg cells of DO11.10 mice were detected using the biotinylated or allophycocyanin (APC)-labeled clonotypic monoclonal antibody (mAb) KJ1-26 (Caltag, Hamburg, Germany). CXCR5 was stained with the rat anti-mouse CXCR5 mAb (clone 2G8)9 and mouse anti-rat cyanin 5 (Cy5; Dianova, Hamburg, Germany). CCR7 expression was detected by staining cells with a CCL19-hIgG1 fusion protein followed by goat anti-human phycoerythrin (PE; Dianova) as described previously.11 P-selectin ligand (P-Sel-Lig) was stained with the P-Sel-hIgG1 fusion protein (R&D Systems, Minneapolis, MN) followed by goat anti-human PE (Dianova). ICOS was revealed with biotinylated mAb (eBioscience, San Diego, CA) followed by streptavidin-peridinin chlorophyll protein (PerCP; Becton Dickinson, Heidelberg, Germany). Antihuman EBNA1 mAb (E1-BS 1H4, IgG2a) or anti-human CXCR5 (RF8B2, IgG2a) served as control for anti-mouse CXCR5. A CCL25-hIgG1 fusion protein that does not bind to wild-type nor CCR9-deficient lymphocytes (R.F., unpublished data, May 2003) served as a control for CCR7 and P-Sel-Lig staining.
Adoptive transfer
Adoptive transfer of T and B cells was performed as previously described.3 In brief, single-cell suspensions from LNs and spleen from DO11.10 or CXCR5-/-DO11.10 mice were used either untreated or labeled with fluorescein diacetate succinimidyl ester (CFSE) as described elsewhere.12 Single-cell suspension from LNs and spleen of CCR7-/-DO11.10, CXCR5-/-DO11.10, or DO11.10 mice were transferred by intravenous injection into Balb/c × 129SV F1 recipients. In some experiments recipients of CCR7-/-DO11.10 cells were treated with FTY720 (kindly provided by Volker Brinkmann, Novartis, Basel, Switzerland) by adding 50 μg/mL in drinking water for 4 days.
Cytokine production
Single-cell suspensions from LNs were incubated in RPMI with 10% FCS and stimulated with 50 ng/mL phorbol myristate acetate (PMA; Sigma, St Louis, MO) and 500 ng/mL ionomycin at 37°C for 2 hours. Subsequently 10 μg/mL brefeldin A (Sigma) was added and cells were incubated for a further 2 hours. TCR-tg T cells were identified using the KJ1-26 mAb. Cells were then fixed with 2% paraformaldehyde (PFA) and treated with 0.1% saponin (Sigma) for 30 minutes. Intracellular cytokine detection was done using mAbs against interferon γ (INF-γ; PE) and interleukin 4 (IL-4; APC; Becton Dickinson).
B-cell help
B-cell priming. B cells were purified by magnetic-activated cell sorting (MACS; B220 microbeads, Miltenyi Biotec, Bergisch Gladbach, Germany) from spleens of Balb/c × 129SV F1 mice that had been immunized 14 days earlier with 100 μg trinitrophenol (TNP)/bovine serum albumin in 200 μL alum (HCI Biosector, Frederikssund, Denmark).
In vivo activation of T cells. Cells from CXCR5-/-DO11.10 or CXCR5+/+DO11.10 mice were transferred intravenously into Balb/c × 129SV F1 recipients as described under “Adoptive transfer.” To generate activated T cells, recipient mice were immunized subcutaneously with 250 μg OVA (Sigma) and 1 μg cholera toxin (CT; Sigma) in 200 μL PBS (100 μL/flank). After 4 days mice were killed, cells from the draining inguinal LNs were isolated, and the percentage of KJ1-26+ cells was determined by flow cytometry.
For in vivo assays, sublethally (500 rad) irradiated recipients (Balb/c × 129SV) received either 107 purified B cells (as described at the beginning of this section) alone or in combination with either 105 naive DO11.10 T cells or with 105 DO11.10 T cells that had been activated for 4 days in vivo. One day after adoptive transfer, recipients were immunized intraperitoneally with 100 μg TNP-OVA (Biosearch Technologies, Novato, CA) in 200 μL alum. Serum was collected after 9 days and analyzed for TNP-specific IgG by enzyme-linked immunosorbent assay (ELISA). For in vitro assays, 106 purified primed B cells were either cultured alone or in combination with 105 B cell-depleted (MACS, B220 microbeads) KJ1-26+ T cells that had been activated in vivo. These cells were then cultured for 10 days before supernatants were analyzed for the presence of TNP-specific IgG.
Migration assay
One day before immunization, DO11.10 transgenic lymphocytes were adoptively transferred to Balb/c recipients. Recipient mice were immunized subcutaneously with 250 μg OVA plus 1 μg CT in 200 μL PBS (100 μL/flank) 1 day after transfer. Three days later, mice were killed and cells from the draining LNs were isolated. Naïve cells were taken from peripheral LNs of DO11.10 mice. The transwell membranes (5-μm pores; Costar, Cambridge, MA) were coated overnight with 20 μg/mL murine collagen type IV (Becton Dickinson) and then washed with PBS. The chemokine CCL21 was diluted with RPMI medium to final concentrations ranging from 100 to 1000 ng/mL. For migration experiments, 1 × 106 cells were applied to the upper part of each transwell. Cells had been stained before with anti-CD4-PerCP (Becton Dickinson), anti-CD19-PE (Southern Biotechnologies, Birmingham, AL), and KJ1-26 (Caltag). After 5 hours cells were collected from the lower chamber, counted, and analyzed by flow cytometry. Migration of naive and activated KJ1-26+ cells was calculated as the migration index, which is the ratio of number of migrated cells in the presence of CCL21 to the number of migrated cells in the absence of CCL21.
ELISA
ELISAs were performed by coating 96-well plates (Nunc, Wiesbaden, Germany) with 5 μg/mL TNP-human serum albumin, followed by blocking with PBS/10% FCS. Mouse serum was added and IgG was detected by using biotinylated anti-mouse IgG1 or anti-mouse IgG2a (Becton Dickinson) followed by streptavidin-peroxidase (Dianova). For the detection of peroxidase activity, tetramethylbenzidine (TMB) substrate (Sigma) was used and optical density (OD) was determined at 450 nm. The inflection points of the titer curves were determined and used as units of immunoglobulin.
Immunohistochemistry
Immunohistologic analysis of LNs was performed as previously described9,11 using a motorized Axiovert M200 microscope (Carl Zeiss MicroImaging, Oberkochen, Germany), a × 10/NA 0.45 objective, and a Hamamatsu/Orca ER camera. Cryosections (8 μm) of LNs were blocked with rat serum and stained with the following antibodies: B220-Cy5 (clone TIB146), CD3-Cy3 (clone 17A2), and TCR clonotype antibody KJ1-26-biotin followed by streptavidin-Cy3 (Dianova). Color channels were assembled automatically with the AxioVision3.0 software (Carl Zeiss Imaging).
Immunization of CXCR5-/- mice
Wild-type and CXCR5-/- mice on a 129SV as well as on a C57BL6 background were either immunized intraperitoneally with 200 μg TNP-BSA plus 5 μg CT in PBS or with 200 μg TNP-BSA plus 200 μg polyinosinic-polycytidylic acid (poly I:C; Sigma). Serum was taken after 6, 9, and 14 days and analyzed for TNP-specific IgG by ELISA.
Statistical analysis
The Student paired t test was used to determine the significance of the differences between 2 sets of data. P less than or equal to .05 was considered significant.
Results
Up-regulation of CXCR5 on activated antigen-specific T cells
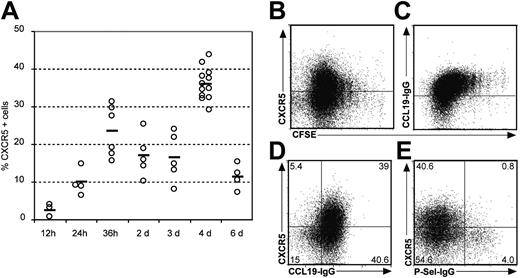
Recently, we and others have shown that a CXCR5-expressing subpopulation of CD4+ T cells is able to provide B-cell help in vitro and localizes to the B-cell follicles in human tonsils.7,8 Furthermore, in vitro activation of peripheral blood T cells indicates that CXCR5 up-regulation is an early event during T-cell activation,13 possibly reflecting early differentiation of the T cell toward defined effector cell populations. To further study the function of CXCR5 on T helper cells in vivo we used an adoptive T-cell transfer model that enabled us to study the antigen-specific induction of CXCR5 and its correlation to T-cell activation, positioning, and function. To address this experimentally, we subcutaneously immunized Balb/c mice with OVA plus CT that had received OVA-specific T cells from DO11.10 mice and followed the immune response of the antigen-specific cells in situ. Twelve hours to 6 days after immunization draining inguinal lymph nodes (dLNs), nondraining facial LNs as well as peripheral blood and spleen were isolated and analyzed by flow cytometry and immunohistology. Transferred cells were identified with the clonotypic KJ1-26 mAb and stained for the expression of specific homing markers including CXCR5 (TFH), CCR7 (naive and central memory T cells), and P-Sel-Lig (effector T cells). Studying the expression pattern during this time course, we observed a rapid induction of CXCR5 expression in the dLNs. Even before cells started to proliferate, approximately 25% of all tg T cells expressed CXCR5 within 36 hours after immunization (Figure 1A and data not shown). The highest percentage of cells expressing CXCR5 was reached at day 4. At later time points, the number of CXCR5+DO11.10 cells dropped sharply (Figure 1A). To analyze whether expression of the investigated homing receptor correlates with cell proliferation, we transferred CFSE-labeled DO11.10 T cells into wild-type recipients followed by the same immunization protocol as described above. Analyzing the dLNs 4 days later we observed that most CXCR5+ cells expressed intermediate levels of this receptor during the first 5 divisions, whereas some degree of polarization into a CXCR5+ and a CXCR5- population could be observed later (Figure 1B), indicating that after 4 days some degree of polarization occurred regarding CXCR5 expression. Interestingly, looking at CCR7, a different pattern of CFSE labeling and chemokine receptor expression could be observed. Increasing numbers of cell divisions were paralleled by a weak but continuous down-regulation of CCR7 (Figure 1C) generating a population of CXCR5+CCR7+ cells (Figure 1D). To further characterize these CXCR5+ cells, we investigated the expression of P-Sel-Lig and found that expression of CXCR5 and P-Sel-Lig is mutually exclusive (Figure 1E). These data suggest that profound differentiation processes of CD4+ cells specific for the same antigen had already occurred within the first few days after exposure to antigen.
Expression of CXCR5, CCR7, and P-Sel-Lig on adoptively transferred DO11.10 T cells. After transferring CFSE-labeled lymphocytes from DO11.10 mice into Balb/c mice recipients were immunized with OVA plus CT. Cells from the dLNs were isolated at the time points indicated. (A) The percentage of KJ1-26+ cells expressing CXCR5 is shown for individual mice (○); bars represent mean values (data shown derive from 4 independent experiments). The expression of CXCR5 (B) and CCR7 (C) on KJ1-26+ T cells 4 days after antigen application is shown in relation to CSFE dilution, which indicates cell proliferation. (C) Expression of CXCR5 and CCR7 (D) and CXCR5 and P-Sel-Lig (E) on KJ1-26+ cells 4 days after immunization. Similar data were obtained in 3 to 6 additional experiments.
Expression of CXCR5, CCR7, and P-Sel-Lig on adoptively transferred DO11.10 T cells. After transferring CFSE-labeled lymphocytes from DO11.10 mice into Balb/c mice recipients were immunized with OVA plus CT. Cells from the dLNs were isolated at the time points indicated. (A) The percentage of KJ1-26+ cells expressing CXCR5 is shown for individual mice (○); bars represent mean values (data shown derive from 4 independent experiments). The expression of CXCR5 (B) and CCR7 (C) on KJ1-26+ T cells 4 days after antigen application is shown in relation to CSFE dilution, which indicates cell proliferation. (C) Expression of CXCR5 and CCR7 (D) and CXCR5 and P-Sel-Lig (E) on KJ1-26+ cells 4 days after immunization. Similar data were obtained in 3 to 6 additional experiments.
Expression of CXCR5 and positioning in situ
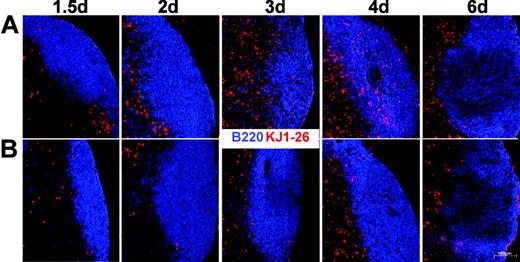
Migration of activated T cells into B-cell follicles has been reported previously14,15 and it has been suggested that this might depend on the expression of CXCR5. To test this hypothesis we transferred DO11.10 cells from a wild-type (Balb/c × 129SV) or CXCR5-/- genetic background (Balb/c × 129SV) into Balb/c × 129SV F1 recipients and immunized the animals with OVA plus CT as described earlier, under “Adoptive transfers.” Immunohistology of the dLNs revealed that expression of CXCR5 on CD4 cells does not inevitably result in the migration of these cells into follicles (Figure 2A). DO11.10 T cells were identified within the follicles only at day 3 or day 4 after immunization but not at earlier time points, where up to 25% of all DO11.10 cells already expressed CXCR5 (Figure 1A). In the ongoing immune response we observed few tg T cells that stayed in or relocalized to the B-cell follicles of LNs (analyzed until day 14; data not shown). Interestingly, our data also demonstrate that the migration of T cells to B-cell follicles critically depends on the presence of CXCR5 because DO11.10 cells deficient for this chemokine receptor are excluded from B-cell follicles (Figure 2B). These data clearly demonstrate that CXCR5 is instrumental in guiding activated T cells to B-cell follicles at any time point of an ongoing immune response.
CXCR5-/- T cells fail to migrate to B-cell follicles. Immunohistology of cryostat sections from dLNs of mice (F1; Balb/c × 129SV) immunized with OVA plus CT that had received lymphocytes from CXCR5+/+DO11.10 (mixed Balb/c × 129SV background; A) or CXCR5-/-DO11.10 (mixed Balb/c × 129SV background; B) mice. At the time points indicated animals were killed and LN sections were stained with KJ1-26 (red) and anti-B220 (blue) to identify transferred cells and the B-cell follicle, respectively. A representative set of data from 2 to 4 independent experiments using 2 mice at each time point is shown.
CXCR5-/- T cells fail to migrate to B-cell follicles. Immunohistology of cryostat sections from dLNs of mice (F1; Balb/c × 129SV) immunized with OVA plus CT that had received lymphocytes from CXCR5+/+DO11.10 (mixed Balb/c × 129SV background; A) or CXCR5-/-DO11.10 (mixed Balb/c × 129SV background; B) mice. At the time points indicated animals were killed and LN sections were stained with KJ1-26 (red) and anti-B220 (blue) to identify transferred cells and the B-cell follicle, respectively. A representative set of data from 2 to 4 independent experiments using 2 mice at each time point is shown.
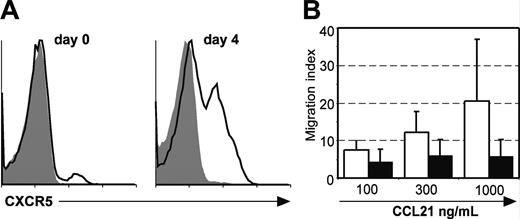
To identify the mechanisms responsible for the difference in the localization of CXCR5+ cells 36 hours or 4 days after immunization (T-cell area versus B follicle), we compared the levels of CXCR5 surface expression on DO11.10 cells. We observed a constant up-regulation of CXCR5 expression up to day 4. Whereas at day 0 only few naive T cells expressed CXCR5, this chemokine receptor was present on 30% to 40% of all DO11.10 cells (Figure 3A and data not shown). These results suggest that, to migrate to the B-cell follicles, T cells have to accumulate a certain amount of surface CXCR5 to combat signals such as those mediated through CCR7 that might retain these cells in the T area. In addition it also seems possible that activated T cells are less responsive toward CCR7 ligands compared to naive cells. To address this experimentally, we compared the chemotactic response of naive and activated KJ-1-26+ cells toward CCL21 in transwell migration assays. Following adoptive transfer of DO11.10 cells Balb/c recipients were immunized as described. At day 4 after immunization the dLN was removed and cells were isolated. These cells and naive cells obtained from DO11.10 mice were assayed for their migratory response toward CCL21. Compared to naive KJ1-26+ cells we found that activated KJ1-26+ cells had a strongly diminished responsiveness toward CCL21 (Figure 3B), demonstrating that T cells change their chemotactic responsiveness during the immune response, which might allow the relocalization of T cells within lymphoid organs.
Increased surface expression of CXCR5 and reduced migration toward CCL21 on T cells following activation. (A) Expression of CXCR5 on naive DO11.10 cells and adoptively transferred DO11.10 cells isolated from the dLNs of Balb/c recipients at day 4 after immunization with OVA/CT. CXCR5 expression on KJ1-26+ cells is shown. The shaded area represents the isotype staining. Representative data are shown from 2 to 4 mice analyzed at each time point. Similar results were obtained in 1 to 2 additional experiments. (B) DO11.10 cells isolated from the dLNs of Balb/c recipients at day 4 after adoptive transfer and immunization with OVA/CT (▪) or from the spleen of naive DO11.10 donors (□) were used in transwell assay to assess their responsiveness toward different concentrations of CCL21. (Mean + SD; n = 3 independent experiments).
Increased surface expression of CXCR5 and reduced migration toward CCL21 on T cells following activation. (A) Expression of CXCR5 on naive DO11.10 cells and adoptively transferred DO11.10 cells isolated from the dLNs of Balb/c recipients at day 4 after immunization with OVA/CT. CXCR5 expression on KJ1-26+ cells is shown. The shaded area represents the isotype staining. Representative data are shown from 2 to 4 mice analyzed at each time point. Similar results were obtained in 1 to 2 additional experiments. (B) DO11.10 cells isolated from the dLNs of Balb/c recipients at day 4 after adoptive transfer and immunization with OVA/CT (▪) or from the spleen of naive DO11.10 donors (□) were used in transwell assay to assess their responsiveness toward different concentrations of CCL21. (Mean + SD; n = 3 independent experiments).
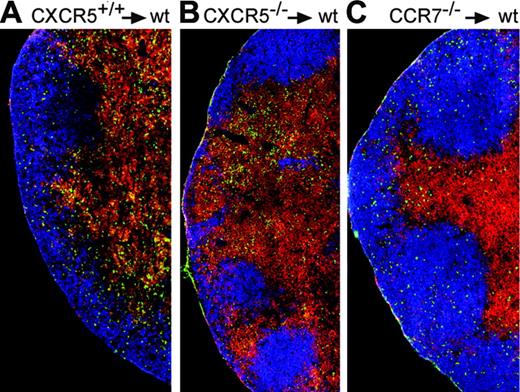
To test the hypothesis that a balanced responsiveness toward CXCR5 and CCR7 ligands might determine the positioning of CXCR5+ T cells within LNs, we applied adoptive transfers. To this end we intravenously injected CSFE-labeled OVA-specific tg T cells from DO11.10 mice (mixed Balb/c × 129SV) of a CXCR5+/+ background, a CXCR5-/- background, and a CCR7-/- background to Balb/c × 129SV F1 recipients. Because CCR7-deficient T cells are defective for homing into LNs,3 all recipients were treated with FTY720 because we showed previously that this drug rescues the homing defect of lymphocytes to LNs in CCR7-deficient mice.16 Thus, recipients were treated with this drug to allow T-cell entry into lymphoid organs irrespective of their genetic background to investigate the positioning of adoptively transferred DO11.10 cells in LNs. After adoptive transfer, recipients were immunized with OVA/CT. Immunohistology on LNs isolated 4 days later confirmed that only a small fraction of DO11.10 CXCR5+/+ cells could be identified in B-cell follicles (Figure 4A), whereas CXCR5-deficient DO11.10 cells were excluded from the follicle (Figure 4B). In contrast, under these experimental in vivo conditions the vast majority of CCR7-deficient DO11.10 cells did not stay in the T-cell area but migrated into the B-cell follicle (Figure 4C). These results are in accordance with those reported by Ansel et al,5 demonstrating reduced responsiveness of activated T cells toward CCL21/CCL19 while gaining responsiveness toward CXCL13. To check whether FTY720 treatment influences the positioning of activated T cells in the lymph node in this experimental setup, we determined the percentage of KJ1-26+ cells within the B-cell follicle in the absence or presence of FTY720 4 days after immunization. Independent of FTY720 treatment we found the same percentage of KJ1-26+ T cells residing within the follicles (21.9% and 22.3% in the absence or presence of FTY720, respectively; data derived from 3 animals each; data not shown; compare also Figure 4A and Figure 2A day 4).
Balanced expression of CXCR5 and CCR7 determines positioning of T cells to the T-cell area or B-cell follicles. Immunohistology of cryostat sections from dLNs of mice immunized 4 days earlier with OVA plus CT that had also received lymphocytes from CXCR5+/+DO11.10 (A), CXCR5-/-DO11.10 (B), or CCR7-/-DO11.10 (C) mice. LN sections were stained with KJ1-26 (green), anti-B220 (blue), and anti-CD3 (red). Data are representative for 3 mice analyzed per group. Further details are given under “Expression of CXCR5 and positioning in situ.”
Balanced expression of CXCR5 and CCR7 determines positioning of T cells to the T-cell area or B-cell follicles. Immunohistology of cryostat sections from dLNs of mice immunized 4 days earlier with OVA plus CT that had also received lymphocytes from CXCR5+/+DO11.10 (A), CXCR5-/-DO11.10 (B), or CCR7-/-DO11.10 (C) mice. LN sections were stained with KJ1-26 (green), anti-B220 (blue), and anti-CD3 (red). Data are representative for 3 mice analyzed per group. Further details are given under “Expression of CXCR5 and positioning in situ.”
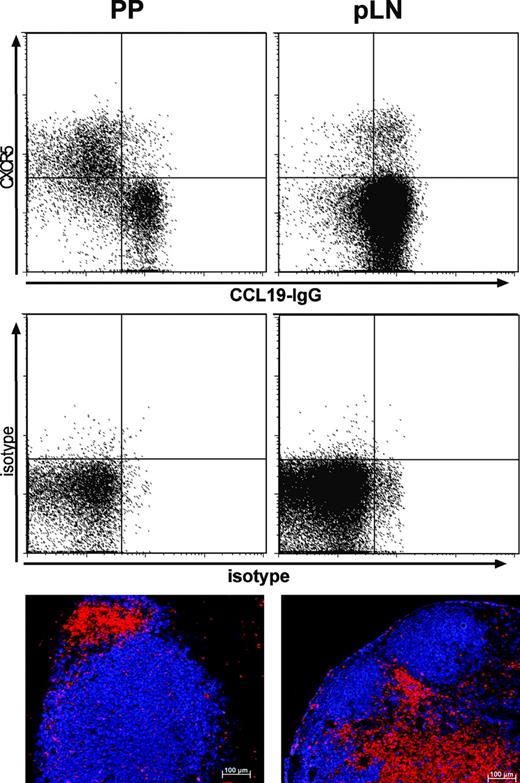
CXCR5 and CCR7 are expressed differently on LN and PP T helper cells
Because our data suggest that the balanced expression of CXCR5 and CCR7 determines the positioning of follicular T helper cells to the T-cell area or the B-cell follicle, we analyzed the expression of both receptors on TFH derived from different lymphoid organs of untreated mice. Analysis of peripheral LNs revealed that the vast majority of CXCR5+ TFH coexpressed CCR7 and that hardly any of these cells localized to the B-cell follicles (Figure 5 right column). In contrast 35% to 45% of all CD4 cells isolated from PPs express CXCR5 and most of these cells lack CCR7 (Figure 5 left column). Interestingly, lack of CCR7 expression is paralleled by a high frequency of T cells locating to B-cell follicles in PPs as revealed by immunohistology. These data further confirm our hypothesis that expression of CXCR5 and CCR7 is tightly regulated to determine the positioning of T cells within lymphoid organs.
CXCR5-/- T cells provide poor B-cell help in vivo
To study functional consequences caused by lack of CXCR5 on T helper cells and subsequent exclusion of these cells from B-cell follicles, we analyzed the capacity of follicular homing T cells to provide B-cell help in vivo. We used an adoptive carrier-hapten system in which sublethally irradiated recipients received B cells from TNP-immunized mice alone or together with naive or activated OVA-specific T cells.17,18 These mice were then immunized with TNP-OVA in alum and the TNP-specific IgG response was analyzed in the serum collected 9 days later (Figure 6A). Whereas both naive wild-type and naive CXCR5-deficient T cells failed to support IgG production, activated wild-type cells very potently supported B cells to produce specific antibodies. In contrast, activated T cells that failed to migrate into the follicle due to the lack of CXCR5 only weakly supported B cells in producing immunoglobulin (Figure 6A). These data demonstrate that TFH cells require CXCR5 to efficiently support B cells in vivo. We subsequently investigated whether this reduced capacity is due to an impairment of these cells to migrate to B-cell follicles or whether these cells have an “inborn” defect in supporting B cells. Therefore, we analyzed their B-cell supporting capacity in an in vitro assay that allows intensive physical contact of antigen-specific T and B cells. TNP-primed B cells were cultured for 10 days alone or in the presence of in vivo activated T cells, either derived from DO11.10 donors of wild-type or CXCR5-deficient genetic background. Analyzing the supernatant for the presence of TNP-specific IgG1, we failed to observe any significant difference between both groups (Figure 6B). These data suggest that the priming of both T cells and B cells would work properly in CXCR5-deficient animals but that the lack of this chemokine receptor prevents forwarding of T helper cells into the follicle where they efficiently provide B-cell help.
Expression of CXCR5 and CCR7 and positioning of T cells to B-cell follicles in LNs and PPs. Peripheral LNs (right column) and PPs (left column) from C57BL6 mice were stained with anti-CD4-PerCP, anti-CXCR5 followed by mouse anti-rat-Cy5 and a CCL19-IgG fusion protein detected with goat anti-human-IgG-PE to reveal CCR7 expression (upper panel). The expression of CXCR5 and CCR7 is shown for CD4+ cells. The central panel shows corresponding isotype controls (1H4; and a nonbinding CCL25-IgG fusion). Immunohistology of cryostat sections from pLNs and PPs using anti-B220 (blue) and anti-CD3 (red). Data are representative for 6 mice. Comparable results were obtained analyzing 4 Balb/c mice.
Expression of CXCR5 and CCR7 and positioning of T cells to B-cell follicles in LNs and PPs. Peripheral LNs (right column) and PPs (left column) from C57BL6 mice were stained with anti-CD4-PerCP, anti-CXCR5 followed by mouse anti-rat-Cy5 and a CCL19-IgG fusion protein detected with goat anti-human-IgG-PE to reveal CCR7 expression (upper panel). The expression of CXCR5 and CCR7 is shown for CD4+ cells. The central panel shows corresponding isotype controls (1H4; and a nonbinding CCL25-IgG fusion). Immunohistology of cryostat sections from pLNs and PPs using anti-B220 (blue) and anti-CD3 (red). Data are representative for 6 mice. Comparable results were obtained analyzing 4 Balb/c mice.
Antigen-specific B-cell help from CXCR5-/- T helper cells is impaired in vivo. (A) Serum TNP-specific IgG1 was determined by ELISA from sublethally irradiated mice that received TNP-activated B cells isolated from third donors alone or together with either naive or activated CXCR5-/-DO11.10 or CXCR5+/+DO11.10 cells. Data represents mean + SD derived from 3 mice of each group. Similar results were obtained in 2 to 3 additional experiments; ***P < .001. (B) TNP-specific IgG1 in supernatants of in vitro cultures of in vivo activated B cells in the absence or presence of activated CXCR5-/-DO11.10 or CXCR5+/+DO11.10 cells. Supernatants were collected after 10 days and pooled from 6 wells. Similar data were obtained in 2 additional experiments. For further details see “Materials and methods” and “Results.”
Antigen-specific B-cell help from CXCR5-/- T helper cells is impaired in vivo. (A) Serum TNP-specific IgG1 was determined by ELISA from sublethally irradiated mice that received TNP-activated B cells isolated from third donors alone or together with either naive or activated CXCR5-/-DO11.10 or CXCR5+/+DO11.10 cells. Data represents mean + SD derived from 3 mice of each group. Similar results were obtained in 2 to 3 additional experiments; ***P < .001. (B) TNP-specific IgG1 in supernatants of in vitro cultures of in vivo activated B cells in the absence or presence of activated CXCR5-/-DO11.10 or CXCR5+/+DO11.10 cells. Supernatants were collected after 10 days and pooled from 6 wells. Similar data were obtained in 2 additional experiments. For further details see “Materials and methods” and “Results.”
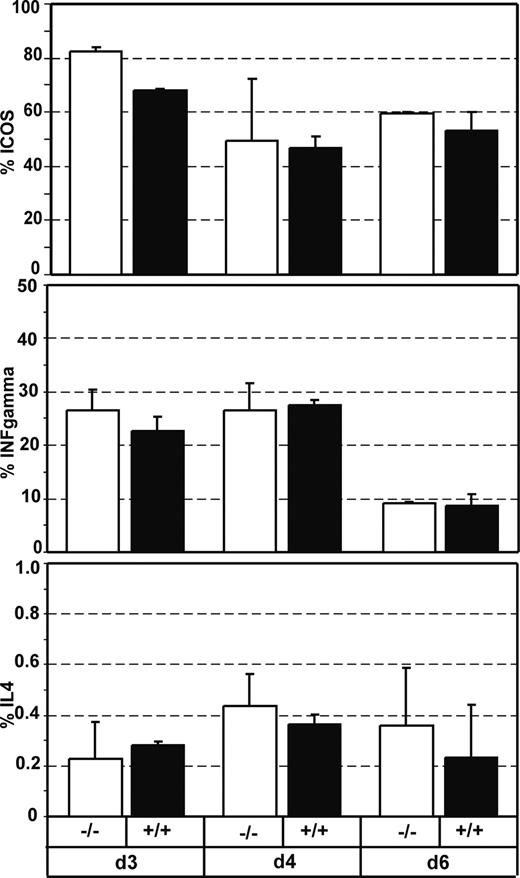
To confirm the idea that CXCR5-deficient T cells can be as efficiently primed as wild-type T cells, we adoptively transferred DO11.10 cells from wild-type CXCR5+/+ or CXCR5-/- genetic background to wild-type recipients and analyzed the expression of costimulatory molecules and cytokines following immunization with antigen plus CT. At 3, 4, and 6 days after immunization we analyzed DO11.10 CD4+ cell for the expression of ICOS, CD40L, IFN-γ, and IL-4. We failed to observe any significant difference between wild-type and CXCR5-/-DO11.10 cells (Figure 7; data not shown for CD40L). Accordingly, immunohistology of cryosections from dLNs using anti-ICOS or anti-CD40L mAb showed no differences between CXCR5-deficient and wild-type DO11.10 cells regarding the expression of both molecules (data not shown).
CXCR5-deficient and wild-type T cells produce similar levels of T helper cytokines and costimulatory molecules. The percentage of CD4+KJ1-26+ T cells expressing ICOS (A), IFN-γ (B), or IL-4 (C) was determined by flow cytometry on cells isolated from the dLNs at the time points indicated after immunization with OVA/CT. Recipient mice (Balb/c × 129SV) had received lymphocytes from either CXCR5-/-DO11.10 mice (□) or CXCR5+/+DO11.10 mice (▪) 1 day before immunization. (Mean + SD, 2 mice per group; similar results were obtained in 2 additional experiments).
CXCR5-deficient and wild-type T cells produce similar levels of T helper cytokines and costimulatory molecules. The percentage of CD4+KJ1-26+ T cells expressing ICOS (A), IFN-γ (B), or IL-4 (C) was determined by flow cytometry on cells isolated from the dLNs at the time points indicated after immunization with OVA/CT. Recipient mice (Balb/c × 129SV) had received lymphocytes from either CXCR5-/-DO11.10 mice (□) or CXCR5+/+DO11.10 mice (▪) 1 day before immunization. (Mean + SD, 2 mice per group; similar results were obtained in 2 additional experiments).
Impaired B-cell response in CXCR5-deficient mice
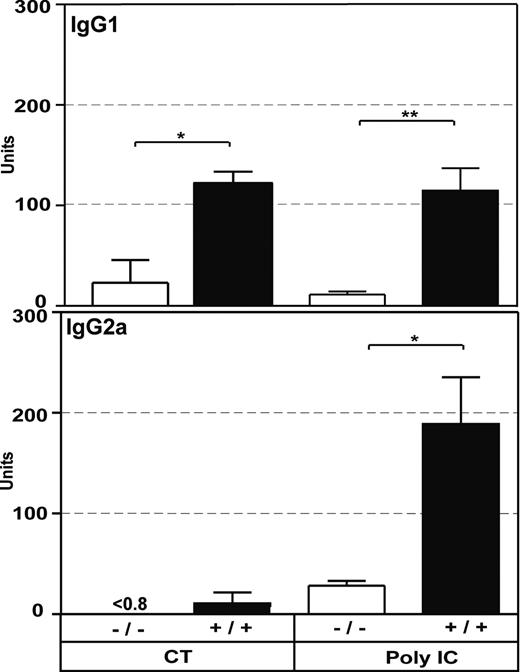
To investigate whether CXCR5 deficiency results in impaired B-cell responses in vivo, we immunized CXCR5-/- on a 129SV or a C57BL6 background intraperitoneally with TNP-BSA plus adjuvant (CT or poly I:C). Poly I:C, a synthetic dsRNA analog and potent inducer of IFN-αβ,19 is known to support class switching toward IgG2a via the induction of type 1 IFN,20 whereas CT has been reported to induce IgG1.21 Serum was collected at day 9 after immunization and TNP-specific IgM, IgG1, and IgG2a was determined by ELISA. CXCR5-/- and wild-type mice produced the same levels of IgM (data not shown), whereas CXCR5-deficient mice had reduced levels of IgG1 and also of IgG2a (Figure 8).
Discussion
The differentiation- and maturation-related expression of chemokine receptors contributed substantially to our understanding regarding the functional differentiation and distribution of T cells within the body. For example, expression of CXCR3 on T cells is restricted to armed effectors to allow entry of these cells into inflamed tissue,22,23 whereas CCR7 is expressed on those cells that continuously recirculate through secondary lymphoid organs, that is, naive and central memory cells.24 Furthermore, CCR9 and CCR10 have been implicated in homing processes of T cells into mucosal surfaces,25-27 whereas CCR4, CCR8, and CCR10 seem to mediate homing of T cells into the skin.28,29 The observations reported here add complexity to the network of chemokine-controlled T-cell migration by providing the first genetic evidence that CXCR5 represents the chemokine receptor that guides a subset of T helper cells into the B-cell follicles to provide help to B cells. CXCR5 is ubiquitously expressed on B cells. Either CXCR5-deficient mice, or gene-targeted mice deficient for the chemokine CXCL13, the sole ligand for this receptor, show severely impaired homing of B cells to the B-cell follicle.5,9 In addition to B cells, a small subpopulation of CD4+ cells expresses this receptor in the blood and peripheral lymphoid organs.3,30 Functional analysis of CXCR5+ T cells isolated from human peripheral blood or tonsils revealed their capacity to provide B-cell help in vitro. Furthermore, CXCR5+ T cells localize to B-cell follicles in human tonsils. Therefore, we and others termed these cells “follicular B helper T cells (TFH)”.7,8 Other have shown that the CXCR5+ population can be divided into CD57+ and CD57- cells and that only the CXCR5+CD57+ cells efficiently provide B help.31 So far no markers have been described that allow discriminating CXCR5+ T cells in the mouse accordingly. Campbell et al17 characterized the TFH cell further in mice by showing that T cells, which are responsive toward CXCL13, are negative for P-Sel-Lig and CD62L and fail to induce a delayed-type hypersensitivity (DTH) response, but support antibody production more efficiently than the P-Sel-Lig+ effector population.
The characteristic expression pattern of CXCR5 on T cells that locate to the B-cell follicles suggested that this receptor might be implicated in the migration of T cells to the B-cell follicles. Based on this assumption, in the present study we investigated the functional aspects of CXCR5 expression during the initial activation of T cells. Applying an adoptive transfer model, we aimed to clarify the implication of this receptor for the migration of activated T cells into the B-cell follicle and the capacity of providing B-cell help.
The adoptive transfer model used in this study allowed the identification of TCR-tg T cells that migrate into the follicles following TCR-specific stimulation with antigen (Figure 2). Several lines of evidence suggest that this migration is the result of the altered responsiveness of these follicular T cells to the chemokines expressed in the T-cell area (CCL19, CCL21) and B-cell follicle (CXCL13), respectively. (1) During the course of an immune response the up-regulation of CXCR5 at day 4 correlates with the localization of a fraction of transferred T cells to the follicle (Figures 1, 2). (2) The inability of CXCR5-deficient TCR-tg T cells to enter the follicle at any time point demonstrates that this migration process depends on functional CXCR5 (Figure 2B). (3) Our data show that expression of CCR7 retains the majority of T cells in the T-cell area. Once this receptor is deleted by gene targeting, the vast majority of all adoptively transferred and activated T cells steer for the B-cell follicle (Figure 4). Complementary to the scenario discussed here, it has been shown for spleen cells that the positioning of activated B cells to the follicle or to the T-cell zone depends on the up-regulation of CCR7.6 The concomitant increased migration of B cells toward CCL19 and CCL21 shifts their balanced responsiveness toward T zone chemokines and allows B cells to migrate toward the B/T boundary to facilitate the initial interaction of B and T cells specific for the same antigen.6
Impaired IgG production in CXCR5-/- mice. Serum TNP-specific IgG1 or IgG2a levels were determined 9 days after intraperitoneal immunization of CXCR5-/- (□; 129SV background) and 129SV wild-type (▪) with TNP-BSA/poly I:C or 9 days after intraperitoneal immunization of CXCR5-/- (C57BL6 background) and C57BL6 mice with TNP-BSA plus CT. (Mean + SEM; n = 3 for CT; n = 6-8 for poly I:C; **P < .01, *P < .05; unpaired 2-tailed Student t test).
Impaired IgG production in CXCR5-/- mice. Serum TNP-specific IgG1 or IgG2a levels were determined 9 days after intraperitoneal immunization of CXCR5-/- (□; 129SV background) and 129SV wild-type (▪) with TNP-BSA/poly I:C or 9 days after intraperitoneal immunization of CXCR5-/- (C57BL6 background) and C57BL6 mice with TNP-BSA plus CT. (Mean + SEM; n = 3 for CT; n = 6-8 for poly I:C; **P < .01, *P < .05; unpaired 2-tailed Student t test).
Interestingly, the early up-regulation of CXCR5 observed within 36 hours, a phenomenon also described for in vitro activated human peripheral blood lymphocytes,13 does not suffice to trigger T-cell migration into B-cell follicles. It appears that this initial event precedes the onset of T-cell proliferation and differentiation and that distinct thresholds of CXCR5 and CCR7 expression are required to enable some T cells to temporarily migrate to the B-cell compartment (Figure 2). Early up-regulation of CXCR5 might be the result of the interaction between T cells and antigen-presenting dendritic cells (DCs) because OX40L/OX40 signaling has been shown to promote CXCR5 up-regulation.32 In addition, it is plausible that the T-cell activation process needs to be completed before T cell can migrate to their next destination. This might be true for CXCR5+ cells as well as P-Sel-Lig+ cells, which are prone to migrate to the follicle or to become peripheral effector cells, respectively. Both are still present in the T area at early time points of the immune response (Figure 1).
Although we have shown here that the expression of CXCR5 is indispensable for T-cell migration into the B-cell follicle, it is currently unknown why a considerable proportion of T cells remain in the T-cell area despite expressing CXCR5 at high levels. It is well known that chemokine-driven migration not only depends on the expression of its cognate receptor, but also requires efficient intracellular signaling pathways. Chemokine receptor signaling is known to be regulated at several levels including G protein exchange factors (GEFs), G protein dissociation inhibitors, and arrestins. Arrestins have been reported to bind to activated/phosphorylated chemokine receptors, thereby regulating further interactions of G proteins.33,34 However, the processes investigated here are dynamic. In addition, activation of individual T cells may occur both at different times during antigenic stimulus and with varying intensities. Considering that the T cells reside only transiently in the B-cell follicle, a substantial proportion of them is expected to be found in the T area at any time point investigated.
In CXCR5-deficient mice, GCs dislocate to the T-cell area where they develop around the central artery of the splenic white pulp. Of interest, studies by Berek and colleagues demonstrated that these dislocated GCs perform regular functions because affinity maturation is unimpaired in CXCR5-deficient mice,35 suggesting that the initial T/B interaction is also not affected by lack of CXCR5. However, data presented here indicate that an additional T/B interaction, which occurs within the B-cell follicle to provide efficient help to B cells, depends on the expression of CXCR5 on a subpopulation of CD4+ T cells. Therefore, a regular onset of an adaptive immune response but a compromised progress at later stages should be observed in mice lacking CXCR5. Indeed, immunization of CXCR5-deficient mice with TNP-BSA together with the adjuvants CT or poly I:C also resulted in impaired production of IgG1 and IgG2a, whereas IgM production was not affected. In the initial description of the CXCR5-deficient mouse,9 we failed to observe significant differences in serum dinitrophenyl (DNP) IgG titers between wild-type and CXCR5-deficient mice after immunization with DNP-keyhole-limpet hemocyanin (KLH) in complete Freund adjuvant (CFA). The reasons for these different results are currently unclear, but they might be due to the fact that different antigens and adjuvants have been used in both studies. Because CXCR5-deficient mice on a 129SV as well as on a C57BL6 background gave similar results in the present study, we consider genetic variations between the mouse strains used in both studies as less likely responsible for the observed differences. In addition, it should be mentioned that B-cell migration is severely impaired in CXCR5-deficient mice and it seems likely that this defect also contributes to impaired antibody production in CXCR5-deficient mice.
Regarding T-dependent B-cell responses in peripheral LNs we would like to summarize our current knowledge in the context of the following model. T cells, expressing an antigen-matching TCR, are activated in the T area by antigen-presenting cells (APCs), stay there for 1 to 2 days,1 and start to up-regulate CXCR5 (Figure 1). At day 2 some of them move to the edges of follicle where the first contact with B cells specific for the same antigen occurs.1 Starting at day 3 some of the activated T cells migrate into the follicle, a process regulated by the balanced expression of CXCR5 and CCR7. At later time points, most of the T cells disappear from the follicle and only a minor population of antigen-specific T cells remains inside this compartment at least until day 14 (not shown). Data from our adoptive transfer experiment would suggest that the ongoing presence of T cells in the follicle is required to support antibody production.
Another interesting finding of the present study is the observation that, in contrast to B-cell follicles of LNs and spleen of untreated or immunized mice, PPs contain considerable numbers of T cells (Figure 5) resembling to the situation described for human tonsils.30 Human tonsils, as well as mouse PPs, represent lymphoid environments that differ considerably from LNs or spleen because a high proportion of lymphocytes are permanently activated in these structures due to sustained antigenic load coming along with food. Therefore, it is conceivable to assume that the mucosal environment favors the development of T-cell subsets with distinct chemokine receptor expression profiles. Whereas the CXCR5+ CD4 T cells in mouse LNs are predominantly CCR7+, the CXCR5+ CD4 T cells in PPs are primarily CCR7- (Figure 5). It is currently unclear which factor determines this differential CCR7 and CXCR5 expression in mucosa-associated versus non-mucosa-associated lymphoid tissue. It has been recently suggested that dendritic cells have an imprinting capacity on the homing potential of T-cell subsets generated in the mucosa-associated lymphoid tissue,36 thereby substantiating the environmental input on T-subset diversification.
In summary, our findings show that activated T cells up-regulate CXCR5 and that the balanced expression of CCR7 and CXCR5 determines their localization into the B- or T-cell area within the LN. Furthermore, the entry of T cells into the B-cell follicle entirely depends on CXCR5 and T cells that lack this receptor only weakly support effective antibody production by B cells in vivo.
Prepublished online as Blood First Edition Paper, May 17, 2005; DOI 10.1182/blood-2004-11-4494.
Supported by Deutsche Forschungsgemeinschaft grant Fo334/1-1 (R.F.).
S.H. performed the experiments and together with R.F. wrote the manuscript. R.F. and L.O. designed the study and provided scientific support.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank S. Krautwald for providing the CCL19-IgG fusion protein, E. Stüwe for expert technical assistance, and G. Bernhardt for valuable suggestions on the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal