Comment on Little et al, page 2050
Previous in vitro studies have shown that antineutrophil cytoplasmic autoantibodies (ANCAs) affect leukocyte-endothelial interactions, which are considered elementary in the development of necrotizing vasculitis. By intravital microscopy, Little and colleagues were able to study these interactions and their consequences in vivo.
Are antineutrophil cytoplasmic autoantibodies (ANCAs) pathogenic? Recent publications have provided compelling evidence that they are.1 But how do they contribute to the development of the necrotizing vasculitis lesions that are so characteristic of the ANCA-associated vasculitides? In this issue of Blood, Little and colleagues provide evidence that ANCAs enhance leukocyte–vessel wall interactions in a model of Wistar-Kyoto rats that developed experimental autoimmune vasculitis after immunization with human myeloperoxidase (MPO). In these rats, leukocyte–vessel wall interactions (adhesion and transmigration) were studied in mesenteric venules by intravital microscopy, a technique that brings us very close to actually watching the lesions of ANCA-associated vasculitis develop.
The choice of the mesenteric venules for the in vivo investigation of leukocyte–vessel wall interactions was most likely guided by practical purposes, as the venules were not affected by vasculitis. In fact, ANCA-associated mesenteric vasculitis is extremely rare; although it needs to be mentioned that it did occur in one of the first patients described with polyarteritis nodosa, in whom practically every organ was affected by the disease. In their famous case history from 1866, Kussmaul and Maier write that “in the mesentery... arterial branches are degenerated and thickened to the highest degree, in parts swollen to countless gray-yellow nodules from millet seed to peasized.”2(p11) In advanced ANCA-associated vasculitis, vessels from practically all organs may become involved, which underlines the statement by Little and colleagues that endothelial dysfunction in systemic vasculitis is global. However, it is remarkable that in limited or early disease, a predilection exists for vasculitis of the kidneys and lungs, which indeed is also the case in the Wistar-Kyoto rats that Little and colleagues used. This brings us to the point of organ specificity: it would be interesting to know which factor prevents the early development of vasculitis in the mesentery or otherwise enhances the development of vasculitis in the kidneys and lungs.
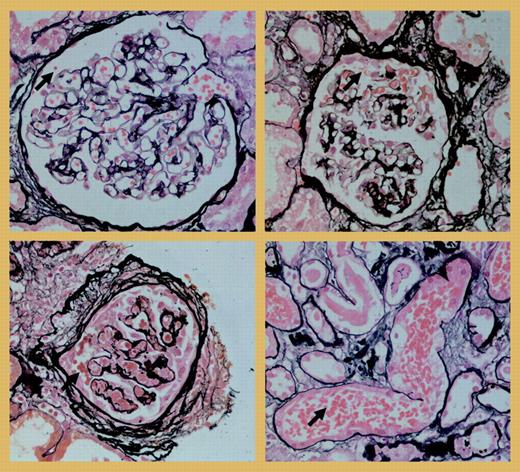
Another interesting point is whether the hemorrhage in the mesenteric vessels should be considered an early event in the cascade leading to necrotizing vasculitis. In the kidneys of the Wistar-Kyoto rats that were used in this experimental model, crescentic necrotizing lesions develop similar to those of human ANCA-associated glomerulonephritis, but it is unknown if hemorrhage precedes these lesions in the kidney. It could be argued that “glomerular hemorrhage” does occur in human ANCA-associated glomerulonephritis. The figureFIG1 shows examples of this phenomenon in a patient with anti-MPO antibodies in a glomerulus that is normal (top left), in one with a beginning crescent (top right), and in one with chronic changes of the Bowman capsule in the presence of extracapillary proliferation (bottom left). The leaking erythrocytes are transported by the tubules (bottom right), leading to erythrocyturia. However, this is a phenomenon occurring in many renal diseases, only some of which are known to be mediated by autoantibodies. In conclusion, Little and colleagues have elegantly demonstrated that ANCAs ameliorate leukocyte adhesion, transmigration, and hemorrhage in vivo. It is now time to further explore the link between these processes and the development of the necrotizing vasculitic lesion. ▪
“Glomerular hemorrhage” in a kidney biopsy of a patient with crescentic glomerulonephritis and anti-MPO antibodies, in an almost normal glomerulus (top left), in a glomerulus with beginning extracapillary proliferation (top right), and in a glomerulus with chronic lesions (bottom left). At the bottom right, erythrocytes from a glomerulus are present in a tubule.
“Glomerular hemorrhage” in a kidney biopsy of a patient with crescentic glomerulonephritis and anti-MPO antibodies, in an almost normal glomerulus (top left), in a glomerulus with beginning extracapillary proliferation (top right), and in a glomerulus with chronic lesions (bottom left). At the bottom right, erythrocytes from a glomerulus are present in a tubule.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal