Abstract
Growth and survival of chronic lymphocytic leukemia (CLL) B cells are favored by interactions between CLL and nontumoral accessory cells. CLL cells express CXCR4 chemokine receptors that direct leukemia cell chemotaxis. Marrow stromal cells or nurselike cells constitutively secrete CXCL12, the ligand for CXCR4, thereby attracting and rescuing CLL B cells from apoptosis in a contact-dependent fashion. Therefore, the CXCR4-CXCL12 axis represents a potential therapeutic target in CLL. We evaluated the most active CXCR4-specific antagonists (T140, TC14012, TN14003) for their capacity to inhibit CXCL12 responses in CLL cells. T140, or its analogs, inhibited actin polymerization, chemotaxis, and migration of CLL cells beneath stromal cells. CXCL12-induced phosphorylation of p44/42 mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 3 (STAT3) was abolished by CXCR4 antagonists. TC14012 and TN14003 antagonized the antiapoptotic effect of synthetic CXCL12 and stromal cell-mediated protection of CLL cells from spontaneous apoptosis. Furthermore, we found that stromal cells protected CLL cells from chemotherapy-induced apoptosis. Treatment with CXCR4 antagonists resensitized CLL cells cultured with stromal cells to fludarabine-induced apoptosis. These findings demonstrate that CXCR4 blocking agents effectively antagonize CXCL12-induced migratory and signaling responses and stromal protection of CLL cells from spontaneous or fludarabine-induced apoptosis. As such, small molecular CXCR4 antagonists may have activity in the treatment of patients with this disease. (Blood. 2005;106:1824-1830)
Introduction
B-cell chronic lymphocytic leukemia (CLL) is characterized by the accumulation of a monoclonal population of CD5+ neoplastic B cells in secondary lymphoid organs, marrow, and blood. Because most of the circulating leukemia cells are arrested in the G0/G1 phase of the cell cycle, the primary defect may be one of resistance to programmed cell death rather than accelerated cell division.1,2 However, CLL cells can rapidly undergo spontaneous apoptosis under culture conditions that support the growth of human B-cell lines. This implies that such ex vivo conditions lack factors necessary for leukemia cell survival or that the resistance to apoptosis is not intrinsic to the leukemia B cell.
The leukemia cell microenvironment in the marrow or in secondary lymphoid tissues may contribute to the noted resistance of CLL cells to apoptosis in vivo.3,4 Normal B-cell development depends on complex interactions with accessory cells that define the so-called specialized microenvironments. T cells and a variety of different types of adherent cells, generally defined as stromal cells, are the main elements of the microenvironment.3 In patients with CLL, the marrow invariably is infiltrated with CLL B cells, and the pattern and extent of marrow involvement correlates with clinical stage and prognosis.5,6 As such, interactions with stromal cells in the marrow microenvironment appear to play a role in disease progression and resistance to therapy.7-9
In addition, we found that a small proportion of the mononuclear cells from the blood of patients with CLL can differentiate into large, round, adherent cells that attract CLL cells and protect them from undergoing spontaneous or drug-induced cell death.10,11 Because these cells share features with thymic nurse cells that nurture developing thymocytes, we designated them nurselike cells (NLCs). Although NLCs differentiate from blood mononuclear cells after several days in vitro, fully differentiated NLCs can be found in the spleen and secondary lymphoid tissue of patients with CLL,11 where they might play a role in protecting CLL cells from apoptosis in vivo. This model implies that CLL cells depend on specific extrinsic factors from NLCs and other stromal elements for their survival. Conceivably, CLL cells recirculate from the blood through secondary lymphoid tissues and back into the systemic circulation in response to certain chemokines.
One such chemokine is stromal cell-derived factor-1/pre-B cell growth-stimulating factor (SDF-1/PBSF), which recently has been designated CXCL12. CXCL12 is a member of a family of chemotactic cytokines (chemokines) that initially were characterized as growth-stimulating factors for B-cell precursors.12 CXCR4 is a primary physiologic receptor for CXCL12 and functions as a coreceptor for entry of T-tropic strains of HIV-1. Mutant mice with targeted gene disruption of CXCL12 or CXCR4 have defects in the production of new B cells, implicating an important role for CXCL12 in the regulation of early B-cell development.
We previously demonstrated that stromal cells can attract CLL cells through the production of CXCL12.13 In addition, NLCs express CXCL12 and can attract CLL cells by elaboration of this chemokine.10 Because CXCL12 may attract leukemia cells to stromal cells and NLCs in vivo, the inhibition of CXCL12-CXCR4 interactions may disrupt the interactions of leukemia cells with protective microenvironments, possibly rendering the CLL cells or other CXCL12-dependent leukemia cells14 more susceptible to spontaneous or drug-induced apoptosis. Because of this, we examined whether the CXCR4-specific chemokine receptor antagonist T140, a 14-residue polypeptide downsized from a naturally occurring horseshoe crab self-defense peptide, and its analogs15 could modulate CXCL12-induced activation and survival of CLL B cells and related signaling cascades. In addition, we examined whether CXCR4 receptor antagonists could inhibit the protective effect of stromal cells or NLCs on leukemia cell survival and drug-induced apoptosis.
Patients, materials, and methods
Cell purification, cell culture, chemokines, antibodies, and flow cytometry
After informed consent, blood samples were collected from patients at Freiburg University Hospital who fulfilled diagnostic and immunophenotypic criteria for common B-cell CLL. The patients had not been previously treated with fludarabine and had not received recombinant growth factors or exogenous cytokines. CLL B cells were isolated by density-gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Cells were used fresh or viably frozen in fetal calf serum containing 5% dimethyl sulfoxide for storage in liquid nitrogen. The viability of CLL cells after recovery was always greater than 85%. All CLL samples contained greater than 90% CLL B cells, as determined by fluorescence-activated cell sorter (FACS) analysis with anti-CD19, anti-CD5, and anti-CD23 monoclonal antibody (mAb). The murine stromal cell line M2-10B4 was obtained from the American Type Culture Collection (ATCC; Manassas, VA). Cells were maintained in RPMI 1640 medium containing 10% fetal calf serum (FCS) and penicillin-streptomycin-glutamine (Gibco BRL, Grand Island, NY) and were plated into 24-well plates at a concentration of 1.5 × 105 cells/well.
For experiments with NLCs, CLL cells were plated into 12-well plates at a concentration of 2 × 107 cells/well and were left for 14 days in culture to allow the adherent NLCs to spread out. After 14 days, CLL cells were removed from the NLCs, washed, and added back to the NLCs or, as controls, were put into fresh plates without NLCs, as described previously.7
Synthetic human CXCL12 was purchased from Upstate Biotechnology (Lake Placid, NY). The CXCR4 chemokine receptor antagonist T140, a 14-amino acid residue peptide,16 and its analogs TC14012 and TN14003, which have higher stability in serum because they are C-terminally amidated,15,16 were developed by us. Molecular weights of these compounds are approximately 2500. The highest concentration used in the experiments, 100 μg/mL, corresponds to 40 μM. The CXCR4 receptor antagonist AMD3100 was purchased from Sigma-Aldrich (Schnelldorf, Germany). Fludarabine was purchased from Medac Schering Onkologie (Munich, Germany) and was dephosphorylated with alkaline phosphatase (Gibco BRL) to the dephosphorylated nucleoside arabinosyl-2-fluoroadenine (F-ara-A) according to the protocol described by Huang and Plunkett.17 Antibodies against phospho-p42/44, p42/44, phospho-signal transducer and activator of transcription 3 (phospho-STAT3), and STAT3 were purchased from Cell Signaling Technology (Beverly, MA).
For flow cytometry, the cells were analyzed on a FACScalibur (Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed using the FlowJo 3.3 software (Tree Star, San Carlos, CA).
Actin polymerization assay
Actin polymerization was tested as described.18,19 Briefly, cells (1.25 × 106/mL) were suspended in RPMI 1640 medium with 0.5% bovine serum albumin (BSA) at 37°C and incubated with 100 ng/mL CXCL12. At the indicated time points, 400 μL cell suspension was added to 100 μL of a solution containing 4 × 10-7 M fluorescein isothiocyanate (FITC)-labeled phalloidin (Sigma-Aldrich), 0.5 mg/mL 1-α-lysophosphatidylcholine (Sigma-Aldrich, Germany), and 18% formaldehyde in phosphate-buffered saline (PBS). Fixed cells were analyzed by flow cytometry on a FACScalibur, and all time points were plotted relative to the mean fluorescence of the sample before chemokine was added.
Chemotaxis assay
CLL cells were suspended in RPMI 1640 with 0.5% BSA. For CXCR4 inhibition experiments, CLL cells were preincubated for 30 minutes with different concentrations of T140 before they were added to the inserts. A total of 100 μL, containing 5 × 105 cells, was added to the top chamber of a 6.5-mm diameter Transwell culture insert (Costar, Cambridge, MA) with a pore size of 5 μm. Filters then were transferred to wells containing medium with or without CXCL12. Chambers were incubated for 2 hours at 37°C in 5% CO2. After this incubation, the cells in the lower chamber were suspended and divided into aliquots for counting with a FACScalibur for 20 seconds as described earlier.13 Chemotaxis was expressed as the migration index, which was calculated according to the following formula: number of cells in the lower well/([number of cells in the lower well + number of cells in the upper chamber] × 100).
In vitro migration of CLL cells beneath stromal cells
Migration beneath stromal cells was performed as described previously.13 Briefly, M2-10B4 stromal cells (ATCC) were seeded onto collagen-coated 24-well plates at a concentration of 1.5 × 105 cells/well in medium. After overnight culture, CLL cells were incubated with inhibitors for 30 minutes at room temperature and were added onto the confluent stromal cell layer to a final concentration of 5 × 106 cells/well. Plates were incubated at 37°C in 5% CO2. After incubation for 4 to 6 hours, the cells that had not migrated to the stromal cell layer were removed by vigorous washing of the wells 3 times with RPMI 1640. The complete removal of nonmigrated cells and the integrity of the stromal cell layer containing transmigrated cells were assessed by phase-contrast microscopy and were documented photographically. Pseudoemperipolesis, the spontaneous migration of CLL cells beneath stromal cells, is characterized by the dark appearance of cells that have migrated to the same focal plane as the stromal cells, whereas the more superficial nonmigrated cells remain refractile. The stromal cell layer containing the migrated cells then was detached by incubation with trypsin/EDTA (ethylenediaminetetraacetic acid) solution (Gibco BRL) resuspended in 0.5 mL medium for counting by flow cytometry. A lymphocyte gate was set using the different relative size and granularity (forward scatter and side scatter) characteristics to exclude stromal cells. Duplicate samples were counted at high flow rates for 20 seconds to determine the relative number of migrated cells. A 1:20 diluted sample of supernatant cells resuspended to 0.5 mL was counted under the same conditions to determine the relative proportion of migrated cells.
Western blotting
For immunoblot analyses, 1 × 107 CLL cells were serum starved for 2 hours, treated with CXCL12 at 200 ng/mL for the indicated time points, and used to make lysates. Equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to polyvinylidene difluoride (PDVF) membranes. Immunoblot analysis was performed using p44/42, STAT3, phopho-p44/42, and phospho-STAT3 antibodies that specifically recognize the phosphorylated Thr202/Tyr204 of p44/42 MAPK protein and the phosphorylated serine (Ser727) of STAT3, respectively (Cell Signaling Technology). Immunoreactive bands were visualized using horseradish peroxidase-conjugated goat antirabbit secondary antibody and the enhanced chemiluminescence system (Amersham Biosciences, Freiburg, Germany).
Measurement of cell viability
Determination of CLL cell viability was based on the analysis of mitochondrial transmembrane potential by 3,3-dihexyloxocarbocyanine iodine (DiOC6) and cell membrane permeability to propidium iodide (PI), as described.20,21 For viability assays, 200 μL cell suspension was collected at the indicated time points and transferred to FACS tubes containing 200 μL of 60 nM DiOC6 (Molecular Probes, Eugene, OR) and 10 μg/mL PI (Molecular Probes) in RPMI with 0.5% BSA. Cells were then incubated at 37°C for 15 minutes and analyzed within 30 minutes by flow cytometry using a FACScalibur (Becton Dickinson). Fluorescence was recorded at 525 nm (FL-1) for DiOC6 and at 600 nm (FL-3) for PI.
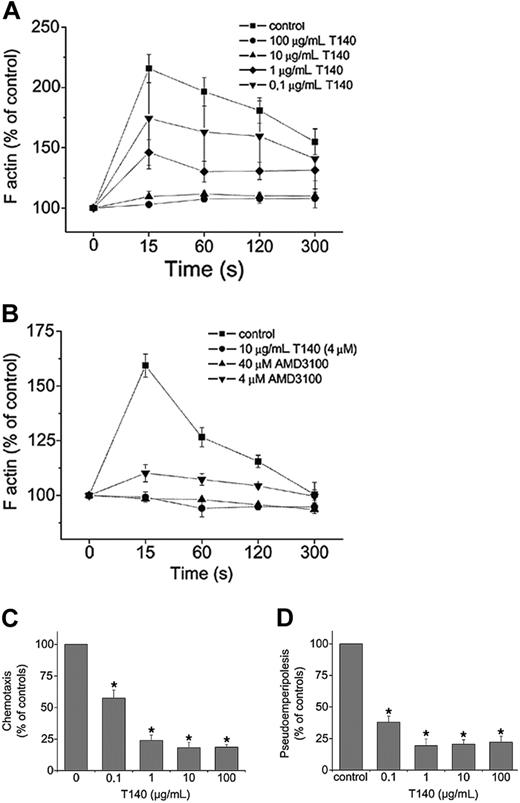
Inhibition of CXCL12-induced responses in CLL cells by T140 and AMD3100. (A) Actin polymerization of CLL cells in response to CXCL12 preincubated with different concentrations of T140. Intracellular F-actin in cells was measured using FITC-labeled phalloidin 15, 60, 120, and 300 seconds after the addition of 200 mg/mL CXCL12. Results are shown as percentages of intracellular F-actin relative to the values before the addition of CXCL12 and are mean ± SEM of 9 experiments. (B) Inhibition of actin polymerization of CLL cells in response to CXCL12 by the CXCR4 antagonists T140 and AMD3100. Cells were preincubated with 4 μM or 40 μM AMD3100 before stimulation with CXCL12. Inhibition of the response by 10 μg/mL T140 (4 μM) is shown for comparison. Results are displayed as percentages of intracellular F-actin relative to the values before the addition of CXCL12 and are the mean ± SEM of 4 CLL samples from different patients. (C) CXCL12-induced chemotaxis is inhibited by T140. Results indicate relative migration compared with control samples migrating to 200 ng/mL CXCL12 (100%) and samples preincubated with different concentrations of T140, representing the mean ± SEM values of 12 experiments with CLL cells from different patients (*P < .05). (D) In vitro migration of CLL cells beneath stromal cells (pseudoemperipolesis) is inhibited to T140. Cells were seeded onto M2-10B4 cells and allowed to migrate to the stromal cell layer. After vigorous washings, the remaining CLL cells were quantified using flow cytometry. Results are represented relative to untreated controls (100%). T140 was used at different concentrations, as indicated in the figure. Data shown are mean ± SEM values of 10 experiments with CLL cells from different patients (*P < .05).
Inhibition of CXCL12-induced responses in CLL cells by T140 and AMD3100. (A) Actin polymerization of CLL cells in response to CXCL12 preincubated with different concentrations of T140. Intracellular F-actin in cells was measured using FITC-labeled phalloidin 15, 60, 120, and 300 seconds after the addition of 200 mg/mL CXCL12. Results are shown as percentages of intracellular F-actin relative to the values before the addition of CXCL12 and are mean ± SEM of 9 experiments. (B) Inhibition of actin polymerization of CLL cells in response to CXCL12 by the CXCR4 antagonists T140 and AMD3100. Cells were preincubated with 4 μM or 40 μM AMD3100 before stimulation with CXCL12. Inhibition of the response by 10 μg/mL T140 (4 μM) is shown for comparison. Results are displayed as percentages of intracellular F-actin relative to the values before the addition of CXCL12 and are the mean ± SEM of 4 CLL samples from different patients. (C) CXCL12-induced chemotaxis is inhibited by T140. Results indicate relative migration compared with control samples migrating to 200 ng/mL CXCL12 (100%) and samples preincubated with different concentrations of T140, representing the mean ± SEM values of 12 experiments with CLL cells from different patients (*P < .05). (D) In vitro migration of CLL cells beneath stromal cells (pseudoemperipolesis) is inhibited to T140. Cells were seeded onto M2-10B4 cells and allowed to migrate to the stromal cell layer. After vigorous washings, the remaining CLL cells were quantified using flow cytometry. Results are represented relative to untreated controls (100%). T140 was used at different concentrations, as indicated in the figure. Data shown are mean ± SEM values of 10 experiments with CLL cells from different patients (*P < .05).
Statistical analysis
Results are shown as mean plus or minus SD, or standard error of the mean (SEM), of at least 3 experiments each. For statistical comparison between groups, the Student paired t test or Bonferroni t test was used. Analyses were performed using the biostatistics software developed by Stanton A. Glantz (University of California at San Francisco). Flow cytometry data were analyzed using the FlowJo 3.3 software.
Results
T140 inhibits CXCL12-induced actin polymerization, chemotaxis, and pseudoemperipolesis in CLL B cells
Reorganization of the actin cytoskeleton is an early event in the migratory response to chemokines.22 CXCL12 induces changes in the actin cytoskeleton of CLL cells.13 Typically, a significant, transient increase in F-actin occurs within 15 seconds of exposure to CXCL12, followed by subsequent depolymerization. We examined whether this response to CXCL12 could be blocked by T140. As shown in Figure 1A, CXCL12-induced actin polymerization is inhibited in a dose-dependent manner. At higher concentrations (10 and 100 μg/mL), actin polymerization is completely abolished. Data shown are mean ± SEM of 9 experiments with CLL B cells from different patients.
Figure 1B depicts the inhibition of actin polymerization in response to CXCL12 by another CXCR4 antagonist, AMD3100, in comparison with T140. At a concentration of 4 μM AMD3100, actin polymerization was approximately 80% abolished, and at a concentration of 40 μM, the reaction was completely abolished. For comparison, we used 4 μM T140 (10 μg/mL), which completely abolished the reaction.
We also examined whether T140 could inhibit CXCL12-induced chemotaxis of CLL cells. As shown in Figure 1C, T140 inhibited chemotaxis relative to that of control cultures in a dose-dependent fashion, resulting in a 42.5% inhibition of chemotaxis at 100 ng/mL, 76% inhibition at 1 μg/mL, and greater than 80% inhibition at 10 μg/mL (4 μM; Figure 1C).
Coculture of CLL cells with marrow cells resulted in the spontaneous migration of CLL cells to the stromal cell layer (pseudoemperipolesis). We previously demonstrated that the CXCR4-CXCL12 interaction plays a key role for this migration phenomenon. To assess the efficacy of CXCR4 antagonists to inhibit this migration, pseudoemperipolesis of CLL cells was quantified by flow cytometry with or without preincubation of CLL cells with T140. We found that T140 inhibited pseudoemperipolesis in a dose-dependent manner (Figure 1D). Maximum inhibition of pseudoemperipolesis was achieved at concentrations of 1 μg/mL (0.4 μM). This inhibition of pseudoemperipolesis was confirmed by phase-contrast microscopy before detachment of the stromal layer for flow cytometry.
TC14012 and TN14003 inhibit antiapoptotic effects of CXCL12 and marrow stromal cells
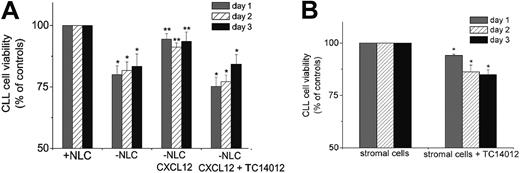
We examined whether T140 could inhibit the protective effect(s) of NLCs on CLL cells in vitro. As shown in Figure 2A, CLL cells that were grown on NLCs for 2 weeks and then reseeded into plates without NLCs lost approximately 20% viability within 48 hours compared with control cells reseeded on NLCs. Adding 500 ng/mL CXCL12 to the medium of CLL cells plated without NLCs increased CLL cell viability to almost the same viability as CLL cells seeded onto NLCs (94.4% ± 7.3% on day 1, 91.2% ± 5.1% on day 2, and 93.5% ± 10.7% on day 3 compared with a relative viability of 100% for CLL cells cultured on NLCs). On the other hand, adding TC14012 to the CLL cells cultured in the presence of CXCL12 reduced the viability of CLL cells to 75.2% ± 3.7%, 77.1% ± 2.7%, and 84.2% ± 3.9% of the control cultures on days 1, 2, and 3, respectively. Similar results were obtained with TN14003 (64.6% ± 3.7%, 70.6% ± 3.0%, and 68.8% ± 5.8% viability on days 1, 2, and 3, respectively), which is comparable to the viability of CLL cells cultured without NLCs. Adding TC14012 to CLL cells cultured in the presence of NLCs induced only a minor decrease in viability of CLL cells (less than 5% decrease in viability compared with controls); however, this difference was not significant, possibly because of other NLC-associated antiapoptotic factors, as outlined under “Discussion.”
TC14012 inhibits protective effects of CXCL12 and accessory cells. (A) CLL cells were grown on NLCs for 2 weeks and then reseeded on the NLCs (controls; 100% viability) or seeded into wells without NLCs and treated with or without CXCL12 and with CXCL12 and TC14012 (100 μ/mL), as indicated. Presented are the mean ± SEM relative viabilities related to the viabilities of CLL cells remaining on NLCs of 10 experiments with cells from different CLL patients. CLL cells cultured without NLCs or without NLCs in the presence of CXCL12 and TC14012 had significantly reduced viability at each of the indicated time points (*P < .05). Synthetic CXCL12 partially restores the antiapoptotic effect of NLCs, resulting in a significantly higher viability of CLL cells without NLCs in the presence of synthetic CXCL12 (third block; **P < .05) when compared with CLL cells without NLCs (second block). (B) CLL cells were seeded onto M1-10B4 stromal cells with or without TC14012, and cell viability was measured after 24, 48, and 72 hours. Cell viability was related to the viability of M1-10B4 stromal cells (100%). Presented are the mean ± SEM relative viabilities related to the viabilities of CLL cells remaining on M2-10B4 cells of 8 experiments with cells from different patients (*P < .05).
TC14012 inhibits protective effects of CXCL12 and accessory cells. (A) CLL cells were grown on NLCs for 2 weeks and then reseeded on the NLCs (controls; 100% viability) or seeded into wells without NLCs and treated with or without CXCL12 and with CXCL12 and TC14012 (100 μ/mL), as indicated. Presented are the mean ± SEM relative viabilities related to the viabilities of CLL cells remaining on NLCs of 10 experiments with cells from different CLL patients. CLL cells cultured without NLCs or without NLCs in the presence of CXCL12 and TC14012 had significantly reduced viability at each of the indicated time points (*P < .05). Synthetic CXCL12 partially restores the antiapoptotic effect of NLCs, resulting in a significantly higher viability of CLL cells without NLCs in the presence of synthetic CXCL12 (third block; **P < .05) when compared with CLL cells without NLCs (second block). (B) CLL cells were seeded onto M1-10B4 stromal cells with or without TC14012, and cell viability was measured after 24, 48, and 72 hours. Cell viability was related to the viability of M1-10B4 stromal cells (100%). Presented are the mean ± SEM relative viabilities related to the viabilities of CLL cells remaining on M2-10B4 cells of 8 experiments with cells from different patients (*P < .05).
In parallel, the inhibitors were added to CLL cells from 3 patients and to lymphocytes from 3 healthy volunteers to rule out toxic effects of the inhibitors. Treatment of CLL cells and lymphocytes with the same concentrations (0.1-100 μg/mL) of the inhibitors showed no toxic effect in comparison with untreated control cells on either of the cells on days 1, 2, and 3 (data not shown). The declining viability that occurs over time when CLL cells are cultured alone, as stated, was not affected by the addition of the inhibitors. Because mononuclear cells from patients showed differences in the presence and in the number of outgrowing NLCs, we subsequently focused on experiments with the stromal cell line M2-10B4. These cells constitutively secrete high amounts of CXCL12 and protect CLL cells from spontaneous apoptosis in a fashion similar to that for NLCs. CLL cells cultivated on M2-10B4 cells remained viable for a long period of time in culture. In addition, as shown in Figure 2B, adding the inhibitor TC14012 reduced the relative viability of CLL cells to 93.9% ± 0.8% on day 1, 86.2% ± 3.2% on day 2, and 84.9% ± 2.6% on day 3 compared with untreated controls.
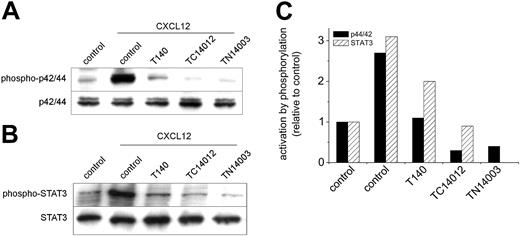
T140, TC14012, and TN14003 inhibit CXCL12-induced p42/44 MAPK activation and STAT3 serine phosphorylation. Cells were preincubated with 100 μg/mL T140, TC14012, and TN14003 for 30 minutes before stimulation with 200 ng/mL CXCL12 for 10 minutes. (A) Western blot analysis was performed on cell lysates with anti-phospho-p42/44 (top blot) and anti-p42/44 (bottom blot) antibodies. The blot shows representative results from 1 of 5 experiments with CLL B cells from different patients. (B) Western blot analysis was performed on cytosolic fractions with an antibody specific for serine phosphorylation of STAT3 (top blot). The bottom blot shows the membrane reprobed with an anti-STAT3 antibody. (C) Densitometry of the Western blots confirmed the reduction in p42/44 MAPK and STAT3 activation by preincubation with CXCR4 antagonists. Bars represent the phosphorylation of p42/44 MAPK and STAT3, as displayed in panels A and B after analysis by densitometry relative to the unstimulated controls (left-hand block).
T140, TC14012, and TN14003 inhibit CXCL12-induced p42/44 MAPK activation and STAT3 serine phosphorylation. Cells were preincubated with 100 μg/mL T140, TC14012, and TN14003 for 30 minutes before stimulation with 200 ng/mL CXCL12 for 10 minutes. (A) Western blot analysis was performed on cell lysates with anti-phospho-p42/44 (top blot) and anti-p42/44 (bottom blot) antibodies. The blot shows representative results from 1 of 5 experiments with CLL B cells from different patients. (B) Western blot analysis was performed on cytosolic fractions with an antibody specific for serine phosphorylation of STAT3 (top blot). The bottom blot shows the membrane reprobed with an anti-STAT3 antibody. (C) Densitometry of the Western blots confirmed the reduction in p42/44 MAPK and STAT3 activation by preincubation with CXCR4 antagonists. Bars represent the phosphorylation of p42/44 MAPK and STAT3, as displayed in panels A and B after analysis by densitometry relative to the unstimulated controls (left-hand block).
T140, TC14012, and TN14003 inhibit CXCL12-induced p44/42 MAPK activation
We have previously shown that engagement of CXCR4 by CXCL12 in CLL cells induces robust, prolonged p42/44 MAPK signaling. Preincubation of CLL cells with the CXCR4 inhibitors T140, TC14012, and TN14003 before stimulation with CXCL12 completely abolished the phosphorylation of p42/44. Figure 3A shows a representative Western blot of 5 experiments with cells from different patients.
T140, TC14012, and TN14003 inhibit CXCL12-induced serine phosphorylation of STAT3 in cytosolic fractions
As described earlier, Ser727 of STAT3 is constitutively phosphorylated in CLL B cells.23 We were able to show significant additional STAT3 phosphorylation on stimulation with 200 ng/mL CXCL12 for 10 minutes. As shown in Figure 3B, this CXCL12-induced phosphorylation could also be inhibited by all 3 inhibitors tested. Densitometry was performed to quantify the relative changes in phosphorylation of p42/44 MAPK and STAT3, and the data corresponding to the Western blots (Figure 3A-B) are displayed in Figure 3C.
Inhibition of protective effects of marrow stroma on CLL cells against drug-induced apoptosis
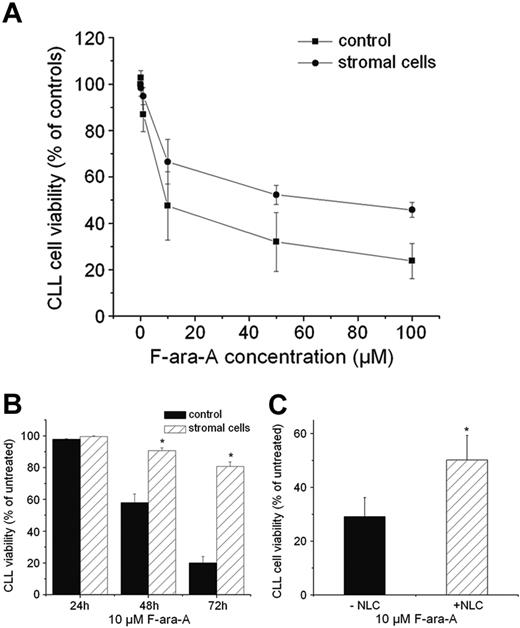
We treated CLL cells with different concentrations of F-ara-A (0.1, 1, 10, 50, and 100 μM), the dephosphorylated form of fludarabine, which is the most common chemotherapeutic treatment for CLL patients. CLL cells were plated with or without M2-10B4 cells, and cell viability was measured after 24, 48, and 72 hours of treatment. We found that M2-10B4 cells protected CLL cells from apoptosis at all drug concentrations tested. Data of one representative experiment with different concentrations of F-ara-A for 48 hours are displayed in Figure 4A. Figure 4B shows the protective effect of M2-10B4 cells on the survival of CLL cells treated with 10 μM F-ara-A for 24, 48, and 72 hours with or without stromal cells (99.6% ± 4% vs 97.8% ± 0.5%, 90.7% ± 1.8% vs 57.8% ± 5.5%, and 80.8 % ± 2.8% vs 20% ± 4.1% viability of the CLL cells; mean ±SEM; n = 10).
As shown in Figure 4C, a comparable protective effect was seen when CLL cells were treated with F-ara-A in the presence or absence of NLCs. After 48 hours of treatment with F-ara-A, the survival of CLL cells was significantly higher when cultured in the presence of NLCs.
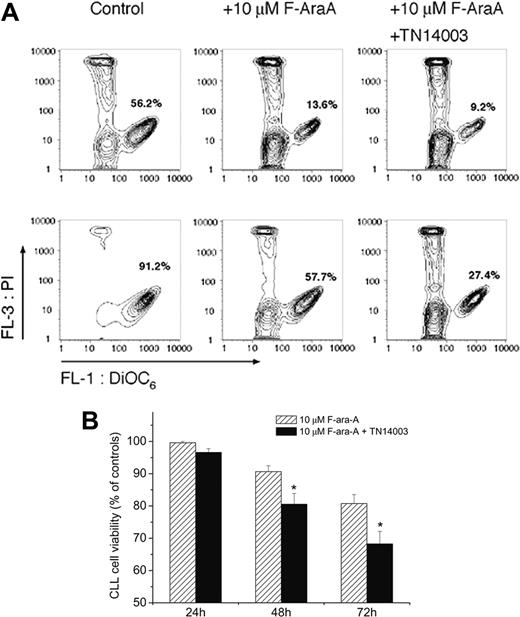
To test whether the protective effect of M2-10B4 cells on chemotherapy-induced apoptosis can be abolished by the inhibitors, we compared the apoptosis of CLL cells treated with F-ara-A or with a combination of F-ara-A (10 μM) and TC14012 (100 μg/mL). Figure 5A displays FACS analysis of CLL cell viability of cells taken from a patient with CLL and cultivated with and without M2-10B4 cells and treated with 10 μM F-ara-A or a combination of F-ara-A and TN14003 72 hours after treatment. Whereas the addition of TN14003 to CLL cells cultured without stromal cells did not lead to a significant additional decrease in viability, the addition of TN14003 decreased the viability of cells cultured on stromal cells from 58% to 27% in this case. Figure 5B demonstrates the inhibitory effect of TN14003 on stromal cell-mediated protection of CLL cells from F-ara-A-induced apoptosis. CLL cells cultured on M2-10B4 cells treated with F-ara-A and TN14003 displayed a significant decrease in viability compared with CLL cells treated without TN14003 (80.6 % ± 3.3% vs 90.7% ± 1.8% and 68.3% ± 3.9% vs 80.8% ± 2.8% on days 2 and 3 respectively; mean ± SEM; n = 10; P < .05).
Marrow stromal cells and NLCs protect CLL cells against F-ara-A-induced apoptosis. (A-B) Cultivation on stromal cells protected CLL cells from F-ara-A-induced apoptosis. (A) CLL cell viability after 48-hour treatment with the indicated F-ara-A concentrations. Cells were cultivated with or without M2-10B4 stromal cells and treated with the indicated F-ara-A concentrations. CLL cell viability was determined after 48-hour treatment. Results are represented relative to untreated controls (100%) and are the mean ± SEM values of 4 different patients. (B) CLL cell viability with or without M2-10B4 stromal cells after 24-, 48-, and 72-hour treatment with 10 μM F-ara-A. Results are represented relative to untreated controls (100%) and are the mean ± SEM values of 10 patients. *Significant differences of CLL cell viability with stromal cells compared with CLL cell viability without stromal cells (P < .05). (C) NLCs protect CLL B cells against F-ara-A-induced apoptosis. CLL cells were grown in the presence of NLCs for 14 days and reseeded with or without NLCs before treatment with 10 μM F-ara-A. CLL cell viability was determined after 48 hours. Data are the mean ± SEM values of 6 patients (*P < .05).
Marrow stromal cells and NLCs protect CLL cells against F-ara-A-induced apoptosis. (A-B) Cultivation on stromal cells protected CLL cells from F-ara-A-induced apoptosis. (A) CLL cell viability after 48-hour treatment with the indicated F-ara-A concentrations. Cells were cultivated with or without M2-10B4 stromal cells and treated with the indicated F-ara-A concentrations. CLL cell viability was determined after 48-hour treatment. Results are represented relative to untreated controls (100%) and are the mean ± SEM values of 4 different patients. (B) CLL cell viability with or without M2-10B4 stromal cells after 24-, 48-, and 72-hour treatment with 10 μM F-ara-A. Results are represented relative to untreated controls (100%) and are the mean ± SEM values of 10 patients. *Significant differences of CLL cell viability with stromal cells compared with CLL cell viability without stromal cells (P < .05). (C) NLCs protect CLL B cells against F-ara-A-induced apoptosis. CLL cells were grown in the presence of NLCs for 14 days and reseeded with or without NLCs before treatment with 10 μM F-ara-A. CLL cell viability was determined after 48 hours. Data are the mean ± SEM values of 6 patients (*P < .05).
TN14003 antagonizes protective effects of marrow stromal cells. (A) Determination of cell viability by staining with DiOC6 and PI. Presented are contour maps of CLL B cells from one patient defining the relative green (DiOC6) and red (PI) fluorescence intensities of CLL cells on the horizontal and vertical axes, respectively. The vital cell population (DiOC6bright, PIexclusion) was determined for CLL cells cultured in the presence (top row) or absence (bottom row) of M2-10B4 cells and 72 hours after treatment with 10 μM F-ara-A and TN14003. The percentage of vital cells is displayed in each contour map. (B) CLL cells were cultured on M2-10B4 stromal cells and treated with 10 μM F-ara-A or the combination of F-ara-A and 10 μg/mL TN14003. Cell viability was measured 24, 48, and 72 hours after treatment, as indicated on the horizontal axis. Data represent the mean relative viability of CLL cells treated with 10 μM F-ara-A (▨) or 10 μM F-ara-A and TN14003 (▪) compared with the respective viability of CLL cells cultured on M2-10B4 stromal cells without F-ara-A or TN14003 (controls). Displayed are the mean ± SEM values of 10 patient samples (*P < .05).
TN14003 antagonizes protective effects of marrow stromal cells. (A) Determination of cell viability by staining with DiOC6 and PI. Presented are contour maps of CLL B cells from one patient defining the relative green (DiOC6) and red (PI) fluorescence intensities of CLL cells on the horizontal and vertical axes, respectively. The vital cell population (DiOC6bright, PIexclusion) was determined for CLL cells cultured in the presence (top row) or absence (bottom row) of M2-10B4 cells and 72 hours after treatment with 10 μM F-ara-A and TN14003. The percentage of vital cells is displayed in each contour map. (B) CLL cells were cultured on M2-10B4 stromal cells and treated with 10 μM F-ara-A or the combination of F-ara-A and 10 μg/mL TN14003. Cell viability was measured 24, 48, and 72 hours after treatment, as indicated on the horizontal axis. Data represent the mean relative viability of CLL cells treated with 10 μM F-ara-A (▨) or 10 μM F-ara-A and TN14003 (▪) compared with the respective viability of CLL cells cultured on M2-10B4 stromal cells without F-ara-A or TN14003 (controls). Displayed are the mean ± SEM values of 10 patient samples (*P < .05).
Discussion
Cell adhesion has been considered an important mechanism of primary drug resistance in malignant diseases such as acute and chronic leukemia, lymphoma, lung cancer, and breast cancer. Evidence is accumulating that interactions between CLL B cells and accessory cells confer a growth advantage, an extended cell survival, and drug resistance to chronic lymphoid tumors of B-cell type (for reviews, see Caligaris-Cappio3 and Chiorazzi et al4 ). This mechanism of primary drug resistance has been termed cell adhesion-mediated drug resistance (CAM-DR).24 Therefore, the identification of molecular targets that link malignant B cells to the microenvironment may lead to new therapeutic avenues for CLL patients.
In this study we demonstrate that small peptide inhibitors that specifically block the CXCR4 chemokine receptor inhibit the activation of CLL cells in response to stimulation with CXCL12 (SDF-1). Furthermore, CXCR4 receptor antagonists inhibit chemotaxis of CLL cells and their migration beneath marrow stromal cells (pseudoemperipolesis). To further investigate the effects of CXCR4 antagonists on CXCR4 downstream signaling events related to prosurvival or antiapoptotic effects, we tested for p42/44 MAPK activation and STAT3 phosphorylation. By pretreatment of CLL cells with T140 or its analogs, we were able to block almost completely the robust CXCL12-induced p42/44 phosphorylation, as displayed in Figure 3A. Another new finding in this study is that activation of the CXCR4 receptor results in the serine phosphorylation of STAT3 protein. STAT proteins are critical in mediating the response of hematopoietic cells to a diverse spectrum of cytokines. Frank et al23 described constitutive serine phosphorylation in CLL samples, whereas Jurlander et al25 described STAT3 activation in CLL B cells on interleukin-10 (IL-10) stimulation. As shown in Figure 3B, we noticed little constitutive STAT3 phosphorylation, but CXCL12 induced a strong serine phosphorylation of STAT3 protein. STAT proteins usually become activated on tyrosine and serine phosphorylation and are subsequently translocated from the cytosol to the nucleus, where they exert DNA-binding activity elements26 and modulate the expression of target genes. As demonstrated in Figure 3B, the serine phosphorylation of CLL cells is completely inhibited by T140 and its analogs.
Although conventional tissue culture conditions result in a rapid decline in CLL cell viability (as outlined in the introduction), culture with stromal or nurselike cells protects CLL cells from apoptosis in vitro and thus appears to reflect interactions with the protective microenvironment in vivo. We found that this protective effect of stromal cells and partially of NLCs on the spontaneous apoptosis of CLL cells was antagonized by CXCR4 inhibitors. The antiapoptotic effect of marrow stromal cells was significantly reduced by CXCR4 antagonists (Figure 2B), whereas the effect of CXCR4 antagonists was lower in cocultures with NLCs and did not reach statistical significance. This difference may be attributed to several factors. First, the outgrowth and dependency of CLL cells on NLC support varies among patient samples, which makes the results heterogeneous (J.A.B., unpublished observations, January 1999). Moreover, NLCs express B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) belonging to the tumor necrosis factor (TNF) family that can protect CLL B cells from spontaneous apoptosis in a paracrine manner, as reported by Nishio et al.27 Deaglio et al28 recently reported that NCLs from patients with B-CLL also express CD31 and plexin-B1 (high-affinity CD100 ligand), which deliver additional growth or survival signals to CLL B cells.28 These molecules protect CLL B cells by activating molecular pathways that are distinct from those activated by NLC-derived CXCL12.27 Our initial observation that CXCL12 is a central molecule in NLC-mediated protection of CLL cells from apoptosis has been confirmed recently,27 but the data discussed here indicate that NLC and CLL B cells are engaged in a complex molecular interaction that makes it more difficult to effectively block the antiapoptotic NLC effect with a single agent. Therefore, we decided to perform subsequent viability experiments with a supportive marrow stromal cell line with the advantages of consistent culture conditions along with a high constitutive CXCL12 secretion that protects CLL and other leukemia cells, as noted earlier by us and others.10,14,29
Comparable results with CXCR4 inhibitors have recently been described by Juarez et al30 in acute lymphoblastic leukemia (ALL) cells. In a nonobese diabetes/severe combined immunodeficiency (NOD/SCID) mouse model of human high-grade NHL, CXCR4 neutralization by anti-CXCR4 mAbs had impressive efficacy without toxicity.31
Interestingly, we furthermore demonstrated that stromal cells protected CLL cells from fludarabine (F-ara-A)-induced apoptosis as an example of cell adhesion-mediated drug resistance. Treatment of CLL cells with CXCR4 receptor antagonists partially resensitized the CLL cells to F-ara-A, suggesting that treatment of CLL patients with such antagonists could mobilize CLL cells from protective microenvironments and make them more accessible to conventional therapy. The general feasibility and safety of such an approach has been demonstrated by recent studies in which CXCR4 antagonists were administered to patients or volunteers. Mobilization of hematopoietic progenitors in 26 healthy volunteers by the CXCR4 antagonist AMD3100 has recently been reported by Liles et al.32 Single subcutaneous injection at doses up to 240 μg/kg per day induced rapid, generalized leukocytosis associated with a transient increase (up to 10-fold) in peripheral blood CD34+ cells. Administration was well tolerated and produced only mild, transient toxicity. In addition, Devine et al33 recently reported AMD3100 as a safe and effective agent for the mobilization of CD34+ cells in patients with multiple myeloma and non-Hodgkin lymphoma who previously underwent chemotherapy. Premature ventricular contractions were observed in 2 of 40 HIV-infected patients in another clinical study with AMD3100, which resulted in the discontinuation of this trial.34
Several CXCR4 antagonists have been described, of which 2 (AMD3100 and ALX40-4C) have been administered to human subjects, as outlined here. Recent studies that compared the structural basis for the interaction of T140 and AMD3100 with CXCR4 demonstrated that the mechanisms used by these agents are different.35,36 AMD3100 and ALX40-4C display weak, partial agonist (CXCL12-like) activity, whereas T140 has been characterized as an inverse agonist. Because agonist activity could be a disadvantage in treatment of diseases in which CXCR4 activation provides a survival signal (such as in CLL cells), T140-derived CXCR4 antagonists might have some advantage in clinical development compared with AMD3100 and ALX40-4C. Meanwhile, several animal studies have demonstrated the efficacy and safety of T140-derived CXCR4 antagonists that provide the basis for a clinical trial with these agents.15,30,37-39
However, the adhesive interactions between CLL cells and accessory cells and their protective effect on CLL cell survival was not completely abolished by the CXCR4 antagonists. The multistep paradigm of leukocyte activation by chemokines, initially described by Springer,40 requires cooperation between integrin binding to their respective ligands along with chemokine receptor activation to sustain cell-cell adhesion. De la Fuente et al41 demonstrated that adhesion of CLL B cells to the fibronectin fragment H89, a ligand for CD49d (α4β1 integrins/VLA4), prevents their spontaneous apoptosis in vitro and that CLL B cells cultured on H89 during treatment with fludarabine showed significantly higher mean viability than cells cultured on control polylysine. We previously demonstrated that CLL B cells that migrate beneath marrow stromal cells displayed higher levels of CD49d than nonmigrated cells,13 suggesting that α4β1 integrin molecules indeed cooperate with CXCR4 receptors during CLL cell migration beneath marrow stromal cells. It is, therefore, likely that integrin-mediated survival signals may partially protect and compensate for CXCL12 survival signals in the direct culture system used here. Therefore, further studies will have to prove whether treatment with T140 analogs, along with integrin antagonists, synergize in inducing apoptosis in CLL cells.
In summary, this study demonstrates that the CXCR4 inhibitor T140 and its analogs effectively block all previously described functions of CXCL12 on CLL cells. Thereby, CXCR4 antagonists modulate biologically important functions of the leukemia cells that are essential for their localization and survival in a protective microenvironment. As such, a treatment concept that would first mobilize CLL cells from their protective microenvironment by using CXCR4 antagonists and then attack CLL B cells with cytotoxic agents such as fludarabine might increase the efficacy of current treatments. However, the mobilization of normal hematopoietic progenitor cells by CXCR4 antagonists is an important concern because the coadministration of a cytotoxic agent might increase the toxicity to normal hematopoiesis. B-cell targeted therapy, in combination with CXCR4 antagonists, could avert this potential hazard. Overall, our data suggest a potential role for CXCR4 antagonists in the treatment of B-cell CLL and also suggest that further in vivo investigation is warranted.
Prepublished online as Blood First Edition Paper, May 19, 2005; DOI 10.1182/blood-2004-12-4918.
Supported by Deutsche José Carreras Leukämiestiftung grant DJCLS R02/08 (J.A.B.), Deutsche Forschungsgemeinschaft (DFG) grant BU/1159/3-1 (M.B), and Deutsche Krebshilfe grant 10-1688-Bu (J.A.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Barbara Rogalsky for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal