Abstract
The erythroleukemia developed by spi-1/PU.1-transgenic mice is a model of multistage oncogenic process. Isolation of tumor cells representing discrete stages of leukemic progression enables the dissection of some of the critical events required for malignant transformation. To elucidate the molecular mechanisms of multistage leukemogenesis, we developed a microarray transcriptome analysis of nontumorigenic (HS1) and tumorigenic (HS2) proerythroblasts from spi-1-transgenic mice. The data show that transcriptional up-regulation of the sphingosine kinase gene (SPHK1) is a recurrent event associated with the tumorigenic phenotype of these transgenic proerythroblasts. SPHK1 is an enzyme of the metabolism of sphingolipids, which are essential in several biologic processes, including cell proliferation and apoptosis. HS1 erythroleukemic cells engineered to overexpress the SPHK1 protein exhibited growth proliferative advantage, increased clonogenicity, and resistance to apoptosis in reduced serum level by a mechanism involving activation of the extracellular signal-related kinases 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/AKT pathways. In addition, SPHK1-overexpressing HS1 cells acquired tumorigenicity when engrafted in vivo. Finally, enforced expression of a dominant-negative mutant of SPHK1 in HS2 tumorigenic cells or treatment with a pharmacologic inhibitor reduced both cell growth and apoptosis resistance. Altogether, these data suggest that overexpression of the sphingosine kinase may represent an oncogenic event during the multistep progression of an erythroleukemia. (Blood. 2005;106:1808-1816)
Introduction
The acute erythroleukemia caused by the Friend virus is a multistage neoplasm suitable to study the sequence of oncogenic events involved in the progression of erythroblastic cells toward malignancy.1 The early step of the Friend disease is characterized by a deregulation of growth and differentiation of erythropoietin (Epo)-independent erythroblasts. This phase is induced by the viral gp55 glycoprotein that constitutively activates the Epo receptor.2 At a later stage, a clonal population of proerythroblasts emerges that are arrested in their differentiation and tumorigenic in vivo.3,4 In these tumor cells, 2 recurrent genetic alterations have been identified: the transcriptional dysregulation of the spi-1 gene5 (also called PU.1) and the extinction of the p53 gene.6-8
Spi-1/PU.1 is a master gene in the development of B-lymphoid, monocyte, and neutrophilic lineages.9-11 Ectopic overexpression of spi-1/PU.1 in proerythroblasts leads to cell transformation as demonstrated in vitro in avian erythroid progenitors infected by spi-1-transducing retroviruses12 or in vivo in transgenic mice overexpressing spi-1 in hematopoietic cells.13 Spi-1-transgenic mice develop an erythroleukemia arising from the proliferation of proerythroblasts arrested in their differentiation (HS1 stage). At disease onset, survival and growth of erythroblasts are under the control of Epo. Thus, the major effect of spi-1 overexpression in transgenic mice is a blockade in the differentiation of the erythroid lineage. Later during disease progression, malignant proerythroblasts characterized by Epo-autonomous growth and tumorigenicity in vivo can be isolated (HS2 stage). This multistep process suggested that genetic lesions were required to confer full malignancy.13 Because of its recurrent alteration during Friend erythroleukemia,1,7,8 mutation of the tumor suppressor gene p53 was a prime candidate for an oncogenic event cooperating with spi-1 overexpression to promote malignant progression. Indeed, when spi-1-transgenic mice were produced in a p53-/- genetic background, mice developed erythroleukemia with an accelerated appearance compared with spi-1-transgenic mice carrying wild-type p53. However, p53-/--spi-1-transgenic proerythroblasts displayed a complex pattern of phenotypes with the ability to generate both Epo-dependent and Epo-independent cell lines and to produce tumors in vivo in only 70% of the case.14 These data indicated that deletion of p53 was not sufficient to render spi-1-transgenic proerythroblasts tumorigenic. Moreover, they showed that Epo independence and tumorigenicity were unrelated phenotypic characteristics, suggesting that multiple oncogenic events must occur to render fully malignant spi-1-transgenic erythroblasts.
To determine which genes contributed to the progression of the spi-1-transgenic proerythroblasts toward malignancy, we compared gene expression profilings from p53-/--spi-1-transgenic proerythroblasts, which were all Epo dependent but differed in their tumorigenic (DT+) or nontumorigenic (DT-) potential, and p53WT-spi-1-transgenic proerythroblasts, which were either Epo-dependent and nontumorigenic (HS1) or Epo independent and tumorigenic (HS2). Among genes differentially expressed, we focused our study on one gene coding for sphingosine kinase type 1 (SPHK1), which was up-regulated in all tumorigenic proerythroblasts. Sphingosine kinase phosphorylates sphingosine to form sphingosine-1-phosphate (S1P), a sphingolipid metabolite that can act either as an intracellular second messenger or as a ligand for G-protein-coupled cell-surface receptors.15,16 Sphingosine kinase is activated by various stimuli, and the resulting S1P behaves as a signaling molecule that regulates cell growth and suppresses apoptosis in diverse cell types.17-19 Conversely, the S1P precursors ceramide and sphingosine inhibit cell proliferation and promote apoptosis.20,21 These data led to the model of a dynamic regulation of apoptosis by the relative intracellular levels of ceramide and sphingosine versus S1P.18
In the present study, we show that overexpression of the sphingosine kinase gene was detected in a panel of tumors isolated from spi-1-transgenic mice. Furthermore, enforced expression of SPHK1 in nontumorigenic (HS1) spi-1-transgenic proerythroblasts resulted in significant resistance to apoptosis, cell growth advantage, and tumorigenesis by a mechanism involving activation of the extracellular signal-related kinases 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/AKT pathways. Altogether, these data demonstrate for the first time that the overexpression of SPHK1 may act as an oncogenic event that occurs during the multistep progression of an erythroleukemia.
Materials and methods
Cell lines, growth kinetics, and colony assays
Spi-1-transgenic cell lines, HS1 cells (633, 663, 806, and 812), HS2 cells (601, 606, 622, 921, and 931), DT- cells (124 and 378), and DT+ cells (108 and 126) were described previously.13,14 Cells were cultured in alpha minimum essential medium (αMEM; GibcoBRL, Grand Island, NY) supplemented with 10% or 1% fetal calf serum (FCS; GibcoBRL) and 1 U/mL recombinant human Epo (Cilag, Levallois-Perret, France) when indicated. Ba/F3, FDCP1, HeLa, HL-60, NRK, and NIH3T3 were from ATCC (American Type Culture Collection, Manassas, VA). 745-A22 and IW1-3223 were Friend erythroleukemic cell lines. N13 and N11 were mouse macrophage cell lines.24 All hematopoietic cell lines were cultured in αMEM containing 10% FCS. Rat NRK and murine NIH3T3 cells were maintained in Dulbecco modified Eagle medium (GibcoBRL) containing 10% FCS.
For growth kinetics, the cells were plated at a density of 2 × 105 cells/mL. The N,N-dimethylsphingosine (DMS; Biomol International, Ply-mouth Meeting, PA) was added at a concentration of 10 μM, the minimal dose that induced an arrest of the growth of HS1 cells cultured in the presence of 1% serum. Cell numbering and viability were monitored by staining with trypan blue and using Vi-cell cell analyzer (Beckman Coulter, Hialeah, FL).
Clonogenic assays were performed in methylcellulose medium (MethoCult M3134; Stem cell Technologies, Tebu, France) supplemented with 1% FCS and Epo (1 U/mL). Five-hundred growing cells were inoculated into 1.5 mL medium in 30-mm dishes, and colonies (50 cells at least) were counted on day 8 of incubation.
Sphingosine kinase cDNA cloning and mutagenesis
SPHK1 cDNA was amplified by reverse transcription-polymerase chain reaction (RT-PCR) from mRNA prepared from 921 cells. It was cloned with N-terminal c-myc epitope tags into pEF-BOS vector25 : pEF-BOS-MT-SPHK1 clone by standard cloning procedures. The SPHK1G81D mutant into the pEF-Bos expression vector was generated by mutagenesis of the wild-type SPHK1 cDNA using the quickchange site-directed mutagenesis system (Stratagene, La Jolla, CA) according to the manufacturer's recommendations.
Transfections
Fifty micrograms of the plasmids pEF-BOS or pEF-BOS-MT-SPHK1 and/or 5 μg of the selection vector pMSCV-Neo (Clontech, Palo Alto, CA) were transfected by electroporation (240 V, 960 μF) using a Biorad gene pulser (Biorad, Hercules, CA) in 2 × 107 663 HS1 cells or Ba/F3 cells. Stable transfectants were selected in growth medium containing 600 μg/mL G418 (Invitrogen, Frederick, MD). For siRNA transient expression, 2 × 106 663 and 812 HS1 cells were nucleofected with 5 μg siRNA (Dharmacon, Dallas, TX) using an Amaxa nucleofector (Biosystems) and G16 program. The sequence of RNAi for SPHK1 was 5′-aaGAGGCAGAGATAACCTTTAdtdt-3′.
Hybridization to oligonucleotide probe arrays
Affymetrix GeneChip MGU74A and U74B arrays were hybridized with cRNA probes synthesized with RNA from 124 and 378 DT- spleen, 108 and 126 DT+ spleen, 633 HS1 spleen, and 606 HS2 spleen. Hybridization data were analyzed using the MAS 5.0 (Affymetrix, Santa Clara, CA) NPGN (Novartis Pharmacogenetics Network) software.
Northern blotting analysis
Polyadenylated (poly A+) RNA (2 μg) was loaded onto 1.2% formaldehyde-agarose gels, then transferred to GeneScreen nylon membranes (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). After standard prehybridization, membranes were hybridized overnight at 65°C with a mouse SPHK1 cDNA probe labeled with [α32P] deoxycytidine triphosphate (dCTP) using random-primed labeling kit (Amersham Pharmacia Biotech) as described previously.13 RNA bands were detected by autoradiography and quantified using a phosphorImager (Molecular Dynamics, Sunnyvale, CA).
Western blotting analysis and antibodies
Cell extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 8% or 10% polyacrylamide gels as previously described.26 Proteins were blotted on Hybond nitrocellulose membrane (Amersham Pharmacia Biotech). Membranes were incubated in phosphate buffered saline (PBS) containing 5% nonfat dry milk and 0.1% Tween 20 with specific antibodies. After washing in PBS-Tween, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Proteins were visualized by the enhanced chemiluminescence Western blotting detection system (Amersham) and quantified with a fluoroimager. Anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-p44/p42 mitogen-activated protein (MAP) kinase, and anti-p44/42 MAP kinase were purchased from New England Biolabs (Beverly, MA); anti-αadaptin, from BD Biosciences Pharmingen (San Diego, CA); anti-β-actin, from Sigma-Aldrich (St Louis, MO); and anti-cleaved caspase-3 p17 (Asp175), from Cell Signaling Technology (Beverly, MA).
Determination of apoptotic cells by Hoechst staining
Cells were fixed by paraformaldehyde 4% in PBS for 10 minutes at room temperature, washed with PBS, and stained for 10 minutes with 10 μg/mL Hoechst 33342 (Molecular Probes, Eugene, OR) in PBS. Apoptotic cells were examined under an inverted fluorescence microscope. A minimum of 500 cells with condensed and fragmented nuclei were scored in double-blind manner by 2 investigators.
In vivo tumorigenicity of cells
Cells (107 cells/500 μL culture medium containing 2% FCS) were injected subcutaneously into 8- to 10-week-old nude mice. Tumor nodules were taken off when tumor mass reached at least 0.5 cm in diameter.
Sphingosine kinase activity
The sphingosine kinase activity was measured according to the procedure of Kohama et al27 from cells cultured in 1% serum. It was expressed as picomoles of S1P formed/min per milligram of protein.
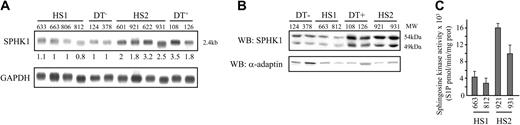
Transcriptional overexpression of the sphingosine kinase gene in tumorigenic HS2 cells. (A) Poly A+ RNA was isolated from the indicated spleen from diseased spi-1-transgenic mice. Northern blot was carried out using a SPHK1 cDNA probe. As a control of RNA loading, the same filter was stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe.28 The hybridized filter was exposed in a phosphorimager (Molecular Dynamics, Bondoufle, France) and the resulting signal quantified using the ImageQuant software package. Values are normalized against GAPDH expression. Basal message level is 1 in 663 HS1 cells. (B) Expression of SPHK1 in HS2 cells. Whole-cell extracts were subjected to Western blot analysis (WB) with antibodies against SPHK1 protein and α-adaptin as loading control. (C) Sphingosine kinase activity of SPHK1 in HS1 and HS2 cells was measured on cell lysates from 663 and 812 HS1 and 606 and 921 HS2 cells as described in “Materials and methods.” Data are means ± SD of duplicated samples in 3 independent experiments.
Transcriptional overexpression of the sphingosine kinase gene in tumorigenic HS2 cells. (A) Poly A+ RNA was isolated from the indicated spleen from diseased spi-1-transgenic mice. Northern blot was carried out using a SPHK1 cDNA probe. As a control of RNA loading, the same filter was stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe.28 The hybridized filter was exposed in a phosphorimager (Molecular Dynamics, Bondoufle, France) and the resulting signal quantified using the ImageQuant software package. Values are normalized against GAPDH expression. Basal message level is 1 in 663 HS1 cells. (B) Expression of SPHK1 in HS2 cells. Whole-cell extracts were subjected to Western blot analysis (WB) with antibodies against SPHK1 protein and α-adaptin as loading control. (C) Sphingosine kinase activity of SPHK1 in HS1 and HS2 cells was measured on cell lysates from 663 and 812 HS1 and 606 and 921 HS2 cells as described in “Materials and methods.” Data are means ± SD of duplicated samples in 3 independent experiments.
Results
SPHK1 expression is up-regulated in tumorigenic spi-1-transgenic proerythroblasts
To assess gene expression changes that could play a role in the leukemic progression of spi-1-transgenic proerythroblasts, we used oligonucleotide microarrays to profile transcripts from tumorigenic and nontumorigenic cells. RNA was prepared from 4 types of erythroleukemic spleens. Cells obtained from spi-1-transgenic donors with a wild-type p53 (p53WT) genotype were either nontumorigenic and Epo dependent (HS1) or tumorigenic and Epo independent (HS2), while cells isolated from p53-deficient spi-1-transgenic mice were all Epo dependent and either nontumorigenic (DT-) or tumorigenic (DT+). Genes differentially expressed between the tumorigenic and nontumorigenic samples derived either from p53WT-orp53-/--spi-1-transgenic mice were considered the best candidates to give insight into the mechanisms of tumorigenicity. The present study was focused on the sphingosine kinase (SPHK1) gene whose transcription was increased about 2-fold in all tumorigenic samples.
Then, the expression of the SPHK1 gene was analyzed by Northern blotting, in a panel of nontumorigenic (HS1 and DT-) and tumorigenic (HS2 and DT+) spi-1-transgenic cell lines. The SPHK1 gene was transcribed as a unique 2.4-kilobase (kb) messenger RNA detected in all samples (Figure 1A). Quantification of the SPHK1 transcripts revealed a 1.8- to 3.5-fold increase in all tumorigenic samples (HS2 and DT+) when compared with nontumorigenic samples (HS1 and DT-).
To extend this observation, we analyzed the SPHK1 protein amount in the various cell lines by Western blotting using whole-cell extracts. A polyclonal antibody directed against the 16 carboxy-terminal amino acids of the mouse SPHK1 protein29 revealed the presence of 2 bands with 49 kDa and 54 kDa apparent molecular weights (Figure 1B) that were 6- to 8-fold increased in tumorigenic compared with nontumorigenic cell extracts. Finally, the sphingosine kinase enzymatic activity was determined in cell lysates from 2 nontumorigenic and 2 tumorigenic cell lines (Figure 1C). It was increased about 4-fold in lysates from HS2 cells compared with HS1 cells. Altogether, these data show that overexpression of the SPHK1 is a representative feature found in tumorigenic proerythroblasts.
Due to the genetic instability of the p53-/- cells, all further studies were performed with the HS1 and HS2 cells derived from p53WT-spi-1-transgenic mice.
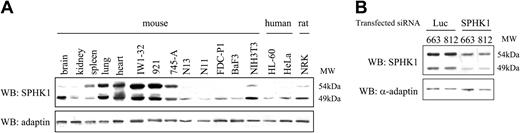
Expression of SPHK1 in murine, rat, and human tissues or cell lines. (A) Whole-cell lysates were subjected to Western blot analysis (WB) with antibodies directed against SPHK1 protein and α-adaptin as loading control. Among hematopoietic cell lines, IW1-32 and 745-A are erythroleukemic cell lines derived from Friend murine leukemia virus (F-MuLV)- or spleen focus-forming virus (SFFV)-induced erythroid tumor cells, 921 is an HS2 erythroleukemic cell line from spi-1-transgenic mice, N13 and N11 are macrophagic cell lines, FDC-P1 is a multipotent progenitor cell line, Ba/F3 is a pro-B-cell line, and HL-60 is a myeloblastic cell line. NIH3T3 and NRK (normal rat kidney) are fibroblastic cell lines. (B) Western blot with anti-SPHK1 antibodies of whole-cell lysates harvested 24 hours after transfection with Luc siRNA and SPHK1 siRNA. HeLa cells are epithelial cells.
Expression of SPHK1 in murine, rat, and human tissues or cell lines. (A) Whole-cell lysates were subjected to Western blot analysis (WB) with antibodies directed against SPHK1 protein and α-adaptin as loading control. Among hematopoietic cell lines, IW1-32 and 745-A are erythroleukemic cell lines derived from Friend murine leukemia virus (F-MuLV)- or spleen focus-forming virus (SFFV)-induced erythroid tumor cells, 921 is an HS2 erythroleukemic cell line from spi-1-transgenic mice, N13 and N11 are macrophagic cell lines, FDC-P1 is a multipotent progenitor cell line, Ba/F3 is a pro-B-cell line, and HL-60 is a myeloblastic cell line. NIH3T3 and NRK (normal rat kidney) are fibroblastic cell lines. (B) Western blot with anti-SPHK1 antibodies of whole-cell lysates harvested 24 hours after transfection with Luc siRNA and SPHK1 siRNA. HeLa cells are epithelial cells.
SPHK1 is expressed as 2 isoforms
It was somewhat surprising to detect 2 proteins in mouse erythroid cells, since SPHK1 is usually described as a single 49-kDa protein in humans and rats.29,30 We thus investigated the expression pattern of SPHK1 in various murine tissues and hematopoietic lineages by immunoblotting using the anti-SPHK1 antibody (Figure 2A). The 49-kDa and 54-kDa proteins were detected in different murine tissues including lung, spleen, and heart. In hematopoietic cells, the level of both proteins was high in erythroid cell lines, while only the 49-kDa protein was seen in macrophagic, multipotent, and pro-B cell lines at a very low level. In fibroblastic cells, both proteins were expressed, though the 54 kDa was revealed only after prolonged exposure of the membrane. In agreement with other works,29,30 only the 49 kDa was detected in human HL-60 and HeLa cells. These data revealed that SPHK1 is highly expressed in murine erythroblastic cell lines and suggested the existence of 2 isoforms of the protein.
Until now, 2 open reading frames of 381 and 388 amino acids differing by 10 amino acids at the N-terminus (named SPHK1a and SPHK1b) have been described.27 By RT-PCR, we observed that both SPHK1a and SPHK1b were transcribed in erythroleukemic cells (data not shown), suggesting that the faint band detected above the 49-kDa SPHK1a protein (Figure 2A) most likely corresponds to SPHK1b. To ascertain that the 49-kDa and 54-kDa proteins were encoded by the SPHK1 gene, we designed a siRNA duplex directed against the SPHK1 gene. The SPHK1 siRNA and an irrelevant control siRNA (Luciferase) were transiently transfected into 2 independent HS1 cells (663 and 812). A significant reduction of both the 49-kDa and 54-kDa proteins was observed in cells transfected with SPHK1 siRNA, demonstrating that the 2 bands corresponded to the SPHK1 protein (Figure 2B). The function of the 54-kDa isoform remains to be determined.
Overexpression of SPHK1 does not confer Epo independence to HS1 spi-1-transgenic proerythroblasts
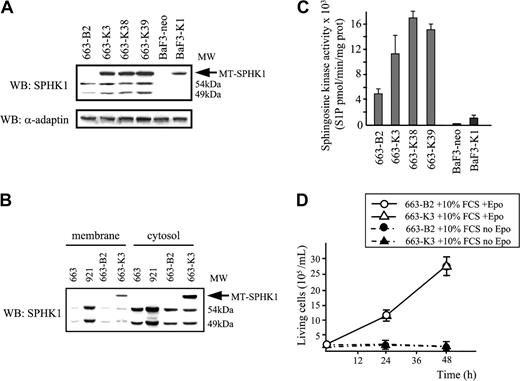
To determine the phenotypic consequences of an increased level of SPHK1 in leukemic cells, a c-myc-tagged SPHK1 vector (MT-SPHK1) encoding the 49-kDa SPHK1 species was introduced in nontumorigenic HS1 cells (line 663). Stable G418-resistant transfected pools and clones were generated. In pools (K2 and K3) or clones (K38 and K39) transfected with MT-SPHK1, Western blotting shows a protein of 62 kDa (Figure 3A), which is absent in control cells transfected with the empty vector (663-B2). Quantification of the 62-kDa protein indicated an expression level approximately 10-fold over the endogenous proteins. With regard to the subcellular localization of exogenous (62 kDa) and endogenous (49 and 54 kDa) sphingosine kinases (Figure 3B), both partitioned similarly into cytosolic and particulate fractions with a predominant cytosolic fraction (approximately 80%).
Characterization of HS1 cells overexpressing SPHK1. (A) The pEF-BOS empty vector and expression construct for MT-SPHK1 were transfected into 663 cells or Ba/F3 cells. Expression of MT-SPHK1 in whole-cell lysates from pools of cells transfected with pEF-BOS and pMSCV-Neo (663-B2 and Ba/F3-neo), from pools of cells transfected with pEF-BOS-MT-SPHK1 and pMSCV-Neo (663-K3 and Ba/F3-SK1), and from cell clones transfected with pEF-BOS-MT-SPHK1 and pMSCV-Neo (663-K38 and 663-K39) was analyzed by Western blotting using anti-SPHK1 antibodies. Reprobing the same membrane with anti-α adaptin was used as loading control. The 49 and 54 kDa indicate the apparent molecular weights (MW) of endogenous SPHK1. (B) Western blot analysis of the expression of endogenous and/or exogenous SPHK1 in membrane and cytosolic compartments from 663 HS1, 921 HS2, 663-B2, and 663-K3 cells using anti-SPHK1 antibodies. (C) Sphingosine kinase activity in 663-B2 and Ba/F3-neo control cells and in 663-K3, 663-K38, 663-K39, and Ba/F3-SK1 cells. Data are means ± SD of duplicated samples in 3 independent experiments. (D) Overexpression of SPHK1 does not confer Epo independence to HS1 cells. Proliferation of 663 cells stably expressing MT-SPHK1 (663-K3) in culture medium containing 10% serum in the presence or absence of Epo (1 U/mL). Cells transfected with pEF-BOS vector (663-B2) were used as control. ○ indicates 663-B2 + 10% FCS + Epo; ▵, 663-K3 + 10% FCS + Epo; •, 663-B2 + 10% FCS, no Epo; and ▴, 663-K3 + 10% FCS, no Epo. Data are means ± SD of 4 experiments in duplicate.
Characterization of HS1 cells overexpressing SPHK1. (A) The pEF-BOS empty vector and expression construct for MT-SPHK1 were transfected into 663 cells or Ba/F3 cells. Expression of MT-SPHK1 in whole-cell lysates from pools of cells transfected with pEF-BOS and pMSCV-Neo (663-B2 and Ba/F3-neo), from pools of cells transfected with pEF-BOS-MT-SPHK1 and pMSCV-Neo (663-K3 and Ba/F3-SK1), and from cell clones transfected with pEF-BOS-MT-SPHK1 and pMSCV-Neo (663-K38 and 663-K39) was analyzed by Western blotting using anti-SPHK1 antibodies. Reprobing the same membrane with anti-α adaptin was used as loading control. The 49 and 54 kDa indicate the apparent molecular weights (MW) of endogenous SPHK1. (B) Western blot analysis of the expression of endogenous and/or exogenous SPHK1 in membrane and cytosolic compartments from 663 HS1, 921 HS2, 663-B2, and 663-K3 cells using anti-SPHK1 antibodies. (C) Sphingosine kinase activity in 663-B2 and Ba/F3-neo control cells and in 663-K3, 663-K38, 663-K39, and Ba/F3-SK1 cells. Data are means ± SD of duplicated samples in 3 independent experiments. (D) Overexpression of SPHK1 does not confer Epo independence to HS1 cells. Proliferation of 663 cells stably expressing MT-SPHK1 (663-K3) in culture medium containing 10% serum in the presence or absence of Epo (1 U/mL). Cells transfected with pEF-BOS vector (663-B2) were used as control. ○ indicates 663-B2 + 10% FCS + Epo; ▵, 663-K3 + 10% FCS + Epo; •, 663-B2 + 10% FCS, no Epo; and ▴, 663-K3 + 10% FCS, no Epo. Data are means ± SD of 4 experiments in duplicate.
The MT-SPHK1 expression vector was also transfected in Ba/F3 cells. In the MT-SPHK1-transfected Ba/F3 cells, the endogenous SPHK1 expression appears insignificant in comparison with the ectopic MT-SPHK1 (Figure 3A). Thus, extracts from these cells were used to control the enzymatic activity of the MT-SPHK1. Figure 3C shows that expression of MT-SPHK1 produces a substantial increase (around 4-fold) in the sphingosine kinase activity in Ba/F3 cells. This indicated that MT-SPHK1 was functional and active in the stable transfectants.
In the various pool (663-K3) and clones (663-K38 or K39 cells) of MT-SPHK1-transfected cells, cytosolic SPHK1 activity was increased 3- to 4-fold over control vector-transfected 663-B2 cells (Figure 3C). Thus, in agreement with its subcellular localization and its enzymatic activity, exogenous MT-SPHK1 appears functional in HS1-transfected cells. Moreover, the SPHK1 enzymatic activity in cytosolic extracts from MT-SPHK1-transfected cells is very similar to that measured in cytosolic extracts from HS2 cells (Figure 1C), thus making analysis of the biologic consequences of SPHK1 overexpression in HS1 cells relevant.
We first compared the growth curves of 663-K3 cells overexpressing SPHK1 cells and 663-B2 control cells. As determined by viable cell number and saturation density, growth characteristics of 663-K3 cells were indistinguishable from those of 663-B2 cells when cultures were performed in the presence of 10% serum and 1 U/mL Epo (Figure 3D). When Epo was omitted, both 663-K3 and 663-B2 cells died within 48 hours, although culture medium contained 10% of serum (Figure 3D). This indicated that SPHK1 overexpression was not sufficient to abolish the Epo dependency of HS1 cells for growth and survival. We also determined that SPHK1 overexpression did not induce Epo hypersensitivity in HS1 cells when cultured in the presence of limiting concentrations (0.01 and 0.05 U/mL) of Epo (data not shown).
Overexpression of SPHK1 enhances growth and survival of HS1 spi-1-transgenic proerythroblasts
When the 663-K3 and 663-B2 cells were cultured in the presence of low serum concentration (1%) and with Epo (1 U/mL), the 2 cell types behaved differently. A marked expansion in living 663-K3 cells number was observed with an approximately 2-fold increase over 48 hours compared with the 663-B2 control cells (Figure 4A). Addition to the cell culture medium of a specific inhibitor of SPHK1, DMS (10 μM), significantly reduced the proliferative advantage of 663-K3 cells (Figure 4A), whereas it impeded the proliferation of 663-B2 cells. Similar data were observed with other 663-K and 663-B cells (not shown). Together, these results suggest that enforced expression of SPHK1 in HS1 cells reduces serum dependency without changing the Epo requirement. The fact that DMS is capable of impeding the growth of HS1 cells and of reverting the growth advantage due to SPHK1 overexpression suggests that the enzymatic activity of SPHK1 must be important for the growth of the leukemic proerythroblasts.
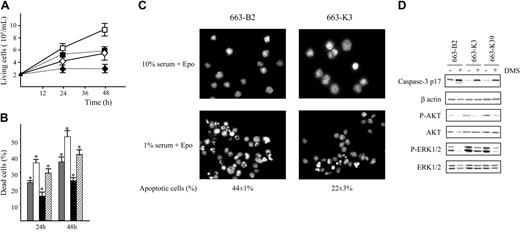
Overexpression of SPHK1 protects HS1 cells from apoptosis. (A) Proliferation of pEF-BOS-transfected cells (663-B2) and MT-SPHK1-transfected cells (663-K3 cells) in a culture medium containing 1% serum and Epo (1 U/mL) in the presence or absence of DMS 10 μM. The graphs are means ± SD of 5 independent experiments performed in duplicate. ⋄ indicates 663-B2 + 1% FCS + Epo; ♦, 663-B2 + 1% FCS + Epo + DMS; □, 663-K3 + 1% FCS + Epo; and ▪, 663-K3 +1% FCS + Epo + DMS. (B) Percentages of dead cells were determined by trypan blue exclusion assay on 663-B2 and 663-K3 cells cultured for 24 hours and 48 hours in a medium containing 1% serum and Epo (1 U/mL) in the presence or absence of 10 μM DMS. Data are means ± SD of 5 experiments in duplicate. * indicates statistical significance by Student t test: P < .05 compared with the control. ▦ indicates 663-B2; □, 663-B2 + DMS; ▪, 663-K3; and ▨, 663-K3 + DMS. (C) Detection of fragmented and condensed nuclei in apoptotic cells by fluorescence microscopy. Representative images after Hoechst staining of 663-B2 and 663-K3 cells cultured in a medium containing either 10% or 1% serum and Epo (1 U/mL) for 48 hours. Magnification, × 63. Cells were observed using a Nikon Eclipse TE300 microscope (Nikon, Champigny sur Marne, France) with 40 × objective magnification. Images were acquired with Nikon Coolpix 950 and processed using Adobe Photoshop (Adobe Systems, San Jose, CA). Three different fields (500 cells per field) were scored. (D) Processing of caspase-3 and activation of AKT and ERK1/2 in 663-B2, 663-K3, and 663-K39 cells cultured in a medium containing 1% serum and Epo (1 U/mL) in the presence (+) or absence (-) of DMS (10 μM) for 24 hours. Cell lysates were subjected to immunoblotting with antibodies indicated on the left of the blots. Blots were reprobed with a β-actin antibody to control loading of the gels. Western blots are from a representative experiment. Similar results were obtained in 3 independent experiments.
Overexpression of SPHK1 protects HS1 cells from apoptosis. (A) Proliferation of pEF-BOS-transfected cells (663-B2) and MT-SPHK1-transfected cells (663-K3 cells) in a culture medium containing 1% serum and Epo (1 U/mL) in the presence or absence of DMS 10 μM. The graphs are means ± SD of 5 independent experiments performed in duplicate. ⋄ indicates 663-B2 + 1% FCS + Epo; ♦, 663-B2 + 1% FCS + Epo + DMS; □, 663-K3 + 1% FCS + Epo; and ▪, 663-K3 +1% FCS + Epo + DMS. (B) Percentages of dead cells were determined by trypan blue exclusion assay on 663-B2 and 663-K3 cells cultured for 24 hours and 48 hours in a medium containing 1% serum and Epo (1 U/mL) in the presence or absence of 10 μM DMS. Data are means ± SD of 5 experiments in duplicate. * indicates statistical significance by Student t test: P < .05 compared with the control. ▦ indicates 663-B2; □, 663-B2 + DMS; ▪, 663-K3; and ▨, 663-K3 + DMS. (C) Detection of fragmented and condensed nuclei in apoptotic cells by fluorescence microscopy. Representative images after Hoechst staining of 663-B2 and 663-K3 cells cultured in a medium containing either 10% or 1% serum and Epo (1 U/mL) for 48 hours. Magnification, × 63. Cells were observed using a Nikon Eclipse TE300 microscope (Nikon, Champigny sur Marne, France) with 40 × objective magnification. Images were acquired with Nikon Coolpix 950 and processed using Adobe Photoshop (Adobe Systems, San Jose, CA). Three different fields (500 cells per field) were scored. (D) Processing of caspase-3 and activation of AKT and ERK1/2 in 663-B2, 663-K3, and 663-K39 cells cultured in a medium containing 1% serum and Epo (1 U/mL) in the presence (+) or absence (-) of DMS (10 μM) for 24 hours. Cell lysates were subjected to immunoblotting with antibodies indicated on the left of the blots. Blots were reprobed with a β-actin antibody to control loading of the gels. Western blots are from a representative experiment. Similar results were obtained in 3 independent experiments.
Then, we analyzed the viability of 663-K3 and 663-B2 cells cultured in medium containing 1% serum and Epo by the trypan blue exclusion assay. Figure 4B shows that 24% and 36% of the 663-B2 cells were dead at 24 hours and 48 hours, respectively. In contrast, the 663-K3 cells exhibited a reduced mortality with 15% and 25% of dead cells at 24 and 48 hours, showing that overexpression of SPHK1 confers resistance to cell death induced by low serum concentration. Addition of DMS (10 μM) abrogated the survival advantage of 663-K3 cells.
To gain further insight into the protective effect of SPHK1 overexpression, we checked for apoptotic features by using Hoechst staining. While no fragmentated nuclei could be detected in 663-B2 cells and 663-K3 cells cultured in the presence of 10% serum and Epo, numerous fragmentated nuclei indicative of apoptosis were seen in both types of cells grown in 1% serum and Epo (Figure 4C). However, when fragmented nuclei were numbered, the percentage of apoptotic cells was 2-fold reduced in 663-K3 cells (22 ± 3%) compared with 663-B2 cells (44 ± 1%). This indicates that increased SPHK1 expression led to a significant reduction of apoptosis induced by low serum level.
Then, the processing of caspase-3 that functions as an effector of apoptosis was used as a molecular read-out of apoptosis.31 Pro-caspase-3 is activated by a proteolytic cleavage generating a mature active form (17 kDa). By Western blotting using an antibody specific for the p17 subunit, the active form of caspase-3 was clearly detected in extracts from 663-B2 cells cultured for 24 hours in 1% serum and Epo (1 U/mL) (Figure 4D). In 663-K3 and 663-K39 cells, the level of p17 caspase-3 was strongly reduced. Addition of DMS to the cell culture medium increased the amount of active caspase-3 in 663-B2 cells as well as in MT-SPHK1-transfected cells. These results show that DMS counteracts the antiapoptotic effects related to SPHK1 overexpression and is effective on the endogenous and ectopically expressed proteins.
It is postulated that the PI3K/AKT and ERK1/ERK2 pathways are downstream targets of SPHK1 activation30,32-34 ; we therefore examined the activations of AKT and ERK1/2 in SPHK1-transfected cells cultured in medium containing 1% serum and Epo (1 U/mL) by measuring the total and activated levels of AKT and ERK1/2 with appropriate antibodies. 663-B2 cells exhibited a significant amount of phosphorylated AKT and ERK1/2 (Figure 4D), albeit at a noticeably lower level than 663-K3 and 663-K39 cells. DMS treatment abrogated AKT and ERK1/2 phosphorylations in 663-B2 cells and strongly reduced them in 663-K3 and 663-K39 cells. These results indicate that activations of the ERK and PI3K/AKT pathways are downstream signaling events for SPHK1 in proerythroblasts. They also demonstrate that SPHK1 overexpression leads to an increase in the activation of these signaling pathways in HS1 cells cultured in suboptimal conditions.
Overexpression of SPHK1 increases clonogenicity and promotes tumorigenesis of HS1 spi-1-transgenic proerythroblasts
To study the oncogenic potential resulting from SPHK1 overexpression in spi-1-transgenic proerythroblasts, we first compared the clonogenic capacity of control cells and SPHK1-tranfected cells in semisolid medium containing 1% serum and Epo (1 U/mL). A poor cloning efficiency (around 1.6%) was seen for control cells, in contrast to a 3.5-fold increment in colony formation for SPHK1-transfected cells (Figure 5A). In addition, colonies produced by SPHK1-transfected cells were much larger than controls (Figure 5B). Thus, SPHK1 overexpression in HS1 cells confers a significant clonogenic and proliferative advantage in vitro.
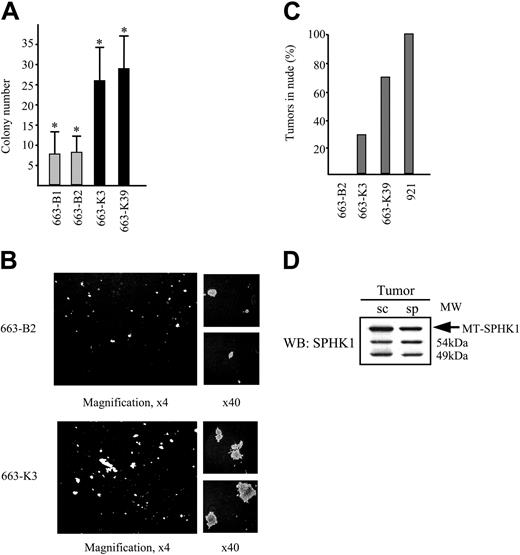
Overexpression of SPHK1 in HS1 cells enhances their proliferation and cloning efficiency and confers tumorigenicity. (A) Number of colonies formed by control 663-B1 and 663-B2 cells and SPHK1-transfected 663-K3 and 663-K39 cells. Five hundred cells were inoculated in semisolid medium containing 1% serum and Epo (1 U/mL) and the colonies were numbered after 8 days. The representative plotted data are shown as the average colony number (±SD) from four experiments in duplicate, determined in a doubled-blinded manner. * indicates statistical significance by Student t test: P < .005 compared with the control. (B) Colony-formation assay with control 663-B2 cells and MT-SPHK1-transfected 663-K3 cells. Images were captured as in Figure 4C, except for given magnifications. (C) Percentage of recipient mice bearing tumors after subcutaneous injection of control 663-B2 cells, SPHK1-transfected 663-K3 and 663-K39 cells, and 921 HS2 cells (7 mice/cell line). (D) Expression of the MT-SPHK1 and endogenous SPHK1 in the subcutaneous (sc) and spleen tumor (sp) isolated in the same nude mice grafted with 663-K39 cells was analyzed by Western blotting using anti-SPHK1 antibodies.
Overexpression of SPHK1 in HS1 cells enhances their proliferation and cloning efficiency and confers tumorigenicity. (A) Number of colonies formed by control 663-B1 and 663-B2 cells and SPHK1-transfected 663-K3 and 663-K39 cells. Five hundred cells were inoculated in semisolid medium containing 1% serum and Epo (1 U/mL) and the colonies were numbered after 8 days. The representative plotted data are shown as the average colony number (±SD) from four experiments in duplicate, determined in a doubled-blinded manner. * indicates statistical significance by Student t test: P < .005 compared with the control. (B) Colony-formation assay with control 663-B2 cells and MT-SPHK1-transfected 663-K3 cells. Images were captured as in Figure 4C, except for given magnifications. (C) Percentage of recipient mice bearing tumors after subcutaneous injection of control 663-B2 cells, SPHK1-transfected 663-K3 and 663-K39 cells, and 921 HS2 cells (7 mice/cell line). (D) Expression of the MT-SPHK1 and endogenous SPHK1 in the subcutaneous (sc) and spleen tumor (sp) isolated in the same nude mice grafted with 663-K39 cells was analyzed by Western blotting using anti-SPHK1 antibodies.
DMS inhibits the growth and increases apoptosis of HS1 and HS2 cells cultured in low serum level. (A) 663 HS1 cells and 921 HS2 cells were cultured for the indicated times in a medium containing 10% serum in the presence or absence of 10 μM DMS and in the presence of Epo (1 U/mL) for 663 HS1 cells. The means and standard deviations were determined from 5 experiments. ○ indicates 663; •, 663 + DMS; ▵, 921; and ▴, 921 + DMS. (B) Percentages of dead cells were determined by trypan blue exclusion assay on 663 and 921 cells cultured in a medium containing 1% serum in the presence or absence of 10 μM DMS for 24 hours or 48 hours. Data are means ± SD of 5 experiments performed in duplicate. * indicates statistical significance by Student t test: P < .01 compared with the control. ▦ indicates 663; □, 663 + DMS; ▪, 921; and ▨, 921 + DMS. (C) The pEF-BOS empty vector and expression construct for MT-SPHK1G81D were transfected into 921 cells. Expression of MT-SPHK1G81D in whole-cell lysates from cell clones transfected with pEF-BOS and pMSCV-Neo (921-B) and with pEF-BOS-MT-SPHK1G81D and pMSCV-Neo (921-G3 and 921-G5) was analyzed by Western blotting using anti-SPHK1 antibodies. Reprobing the same membrane with anti-α adaptin was used as loading control. (D) Proliferation of pEF-BOS-transfected cells (921-B1) and MT-SPHK1G81D-transfected cells (921-G3 and 921-G5) in a culture medium containing 1% serum. The graphs depict means ± SD of 4 independent experiments performed in duplicate. □ indicates 921-B; ▵, 921-G3; and •, 921-G5. (E) Percentages of dead cells were determined by trypan blue exclusion assay on 921-B1, 921-G3, and 921-G5 cells cultured for 24 hours and 48 hours in a medium containing 1% serum. Data are means (±SD) of 4 experiments in duplicate. * indicates statistical significance by Student t test: P < .05 compared with the control. ▪ indicates 921-B1; ▦, 921-G3; and ▨, 921-G5.
DMS inhibits the growth and increases apoptosis of HS1 and HS2 cells cultured in low serum level. (A) 663 HS1 cells and 921 HS2 cells were cultured for the indicated times in a medium containing 10% serum in the presence or absence of 10 μM DMS and in the presence of Epo (1 U/mL) for 663 HS1 cells. The means and standard deviations were determined from 5 experiments. ○ indicates 663; •, 663 + DMS; ▵, 921; and ▴, 921 + DMS. (B) Percentages of dead cells were determined by trypan blue exclusion assay on 663 and 921 cells cultured in a medium containing 1% serum in the presence or absence of 10 μM DMS for 24 hours or 48 hours. Data are means ± SD of 5 experiments performed in duplicate. * indicates statistical significance by Student t test: P < .01 compared with the control. ▦ indicates 663; □, 663 + DMS; ▪, 921; and ▨, 921 + DMS. (C) The pEF-BOS empty vector and expression construct for MT-SPHK1G81D were transfected into 921 cells. Expression of MT-SPHK1G81D in whole-cell lysates from cell clones transfected with pEF-BOS and pMSCV-Neo (921-B) and with pEF-BOS-MT-SPHK1G81D and pMSCV-Neo (921-G3 and 921-G5) was analyzed by Western blotting using anti-SPHK1 antibodies. Reprobing the same membrane with anti-α adaptin was used as loading control. (D) Proliferation of pEF-BOS-transfected cells (921-B1) and MT-SPHK1G81D-transfected cells (921-G3 and 921-G5) in a culture medium containing 1% serum. The graphs depict means ± SD of 4 independent experiments performed in duplicate. □ indicates 921-B; ▵, 921-G3; and •, 921-G5. (E) Percentages of dead cells were determined by trypan blue exclusion assay on 921-B1, 921-G3, and 921-G5 cells cultured for 24 hours and 48 hours in a medium containing 1% serum. Data are means (±SD) of 4 experiments in duplicate. * indicates statistical significance by Student t test: P < .05 compared with the control. ▪ indicates 921-B1; ▦, 921-G3; and ▨, 921-G5.
Then, 663-K3, 663-K39, or 663-B2 cells were injected subcutaneously in nude mice. As control for the tumorigenesis assay in vivo, 921 HS2 cells were grafted in parallel. They induced tumors in 100% of recipients 5 weeks after graft. Between 2 and 5 months after grafting, 2 recipients injected with 633-K3 cells and 5 recipients injected with 663-K39 cells developed subcutaneous tumors (0.5 to 1.5 cm across) (Figure 5C). Three of these recipients also presented a massively invaded spleen. In contrast, no tumors developed in recipients engrafted with 663-B2 cells even after up to 7 months. Protein analysis by Western blotting of cells taken from either subcutaneous or spleen tumors revealed the presence of ectopic MT-SPHK1, demonstrating that tumor cells indeed derived from the injected cells (Figure 5D). Thus, SPHK1 overexpression confers tumorigenicity to HS1 cells.
Role of SPHK1 in survival and growth of malignant HS2 spi-1-transgenic proerythroblasts
To investigate further the role of SPHK1 up-regulation in tumorigenic cells, we compared the proliferation of 663 HS1 and 921 HS2 cells when cultured in medium containing 1% serum and Epo (1 U/mL). Figure 6A shows that the growth of 663 HS1 cells was more sensitive to suboptimal culture conditions compared with 921 HS2 cells, whereas HS1 and HS2 cells grow in a similar way in a culture medium containing 10% serum.26 In the presence of DMS (10 μM), cell proliferation was reduced by about 20% at 48 hours for 921 HS2 cells and arrested for 663 HS1 cells. Then, we analyzed the viability of HS1 and HS2 cells when cultured in medium containing 1% serum and Epo (1 U/mL). The number of dead cells was higher for 663 HS1 cells than for 921 HS2 cells (37 ± 7% versus 20 ± 2%, respectively, at 48 hours). Addition of 10 μM DMS significantly increased the number of dead cells in both cell lines (Figure 6B). Similar data (not shown) were observed with 2 other HS1 and HS2 cell lines (812 and 606, respectively). These results are reminiscent of the effects of DMS reported in Figure 4A-B on the SPHK1-transfected HS1 cells. Thus, HS2 cells are more resistant than HS1 cells to cell death induced by culture at a low serum level, and this resistance involves a SPHK1 activity.
Alternatively, we investigated the involvement of SPHK1 in proliferation and survival of HS2 cells by expressing a dominant-negative mutant of SPHK1. The mutation G82D in the adenosine triphosphate (ATP)-binding region is known to inactivate the human SPHK1.34,35 We introduced the homologous mutation (G81D) in the myc-tagged murine SPHK1 and generated stable clones of 921 HS2 cells with expression constructs for MT-SPHK1G81D. HS2 cells were also transfected with empty vector to serve as controls (921-B cells). Whole-cell extracts were analyzed for MT-SPHK1G81D expression through Western blotting using an anti-SPHK1 antibody. Two cell clones (921-G3 and 921-G5) that express MT-SPHK1G81D at a level approximately 6- to 8-fold over the endogenous SPHK1 (Figure 6C) were retained for further study. The growth curves of 921-G3 and 921-G5 cells showed that expression of SPHK1G81D reduced their growth by about 50% at 48 hours compared with the control 921-B cells. This decrease in proliferation was related to an increase in number of dead cells (25% ± 5% for 921-G3 and 27% ± 2% for 921-G5 cells versus 16% ± 2% for 921-B1 cells at 48 hours) (Figure 6E). Taken together, the expression of the dominant-negative SPHK1 mutant induced similar inhibitory effects to DMS treatment, demonstrating that SPHK1 plays a critical role in the growth advantage and the protection of erythroleukemic cells against apoptosis.
Discussion
Leukemia progression in spi-1-transgenic mice involves proerythroblasts displaying different phenotypic characteristics. As a consequence of Spi-1/PU.1 ectopic expression, these cells have in common a blockage at the basophilic stage of erythroid differentiation. However, spi-1-transformed proerythroblasts differ by at least 2 main properties: (1) an autonomy or a strict Epo requirement for survival and growth and (2) an ability to grow as solid tumors in vivo or a nontumorigenic potential. In the present report, we used a comparative analysis of the transcriptional profiles of proerythroblasts with different phenotypes to identify signatures distinctive of the tumorigenic state. Our data show that a transcriptional up-regulation of the sphingosine kinase gene correlated to an increase in protein level, and SPHK1 enzymatic activity was a recurrent event detected in malignant erythroblasts (HS2 cells). The phenotypic changes originating from overexpression of SPHK1 in HS1 cells were a proliferative advantage, an increased clonogenicity, a resistance to apoptosis induced by culture with low serum level, and a tumorigenic potential in vivo. Thus, overexpression of SPHK1 in spi-1-transgenic proerythroblasts induces features that are considered to be characteristic of oncogenic factors.
SPHK1 mediates the intracellular conversion of sphingosine to S1P. Mammalian cells have a low basal level of SPHK1 activity. When cells are stimulated with growth factors or other agonists (reviewed in Pyne and Pyne15 ), SPHK1 is rapidly activated resulting in increased intracellular levels of S1P. Thus, in response to various stimuli such as phorbol esters, ceramide,18 tumor necrosis factor α (TNF-α),36 or serum deprivation,16,37,38 S1P promotes cell growth and acts as a protective factor against apoptosis. The observation that SPHK1 was overexpressed in tumorigenic HS2 cells raised the question of its function in the leukemic proerythroblast. It is conceivable that overexpression of SPHK1 activity can simultaneously clear the cell of proapoptotic mediators (ie, sphingosine and ceramide) and maintain a high level of antiapoptotic cellular S1P. HS1 cells are strictly dependent upon Epo for their survival and proliferation. Nevertheless, despite the presence of supraoptimal concentrations of Epo, reduction in serum level induced a massive apoptosis in HS1 cells. Overexpression of SPHK1 conferred neither Epo independency nor Epo hypersensitivity to HS1 cells, indicating that the SPHK1/S1P pathway was not a mediator for the mitogenic action of Epo in the proerythroblast. Moreover, SPHK1 when overexpressed reduces death of HS1 cells cultured in low serum level. In addition, the growth and survival of HS2 cells were less sensitive to low serum level than those of HS1 cells, and this resistance was dependent on the SPHK1 activity. Altogether, these data show that the function of SPHK1 in the HS1 proerythroblasts may be to protect cells from death induced by stress conditions. This implies that SPHK1 overexpression itself may maintain survival signals in HS2 cells, ultimately leading to tumorigenesis.
In NIH3T3 fibroblasts, overexpression of SPHK1 reduces cell death caused by serum deprivation and promotes cell proliferation. The growth advantage was related to an increased proportion of cells in S phase.37 We do not observe modifications in cell cycle in HS1 cells that overexpress SPHK1 (data not shown). Although not formally demonstrated, these data suggest that protection from apoptosis may account for the growth advantage of the HS1 cells overexpressing SPHK1.
S1P can act either as an extracellular ligand for receptors of the endothelial differentiation gene (EDG) family coupled to heterotrimeric G proteins or as an intracellular second messager.19 The spi-1-transgenic proerythroblasts express at least 3 EDG receptor subtypes (E.L.S., personal data, May 2004). Thus, SPHK1 overexpression may generate S1P that is able to activate the EDG receptors in an autocrine and paracrine manner. However, inhibition of EDG signaling by blocking Gi-protein-coupled cell surface receptors with pertussis toxin had no appreciable effects on the growth and survival of either HS1 or HS2 cells (data not shown). In contrast, the cytoprotective effects mediated by SPHK1 overexpression in HS1 cells are abolished by DMS. Hence, S1P receptors are dispensable for the effects of SPHK1 in spi-1-transgenic proerythroblasts, and we thus postulate that S1P behaves as an intracellular second messenger. Several studies support the notion that S1P has an intracellular function in regulating cell growth and suppressing apoptosis.34,39-41 Notably, overexpression of SPHK1 in fibroblasts from embryonic mice that do not express S1P receptors promotes their growth and survival.42 In view of our observations, it is likely that SPHK1 modulates intracellular pathways, leading to the regulation of the growth and survival of HS1 cells.
Depending on the cellular context, the AKT and ERK1/ERK2 pathways are downstream targets for SPHK1 activity.30,32-34,36,43 In the spi-1-transgenic proerythroblasts cultured under stress conditions, the activations of the PI3K/AKT and ERK pathways were abrogated by the SPHK1 inhibitor. Hence, in tumorigenic proerythroblasts, the increase in SPHK1 activity may amplify the activation of these signaling pathways participating in apoptosis resistance and cell proliferation.
An implication of SPHK1 in oncogenesis has been described in classical models of transformation with SPHK1-transfected cells in culture.38,44 Recently, elevated levels of SPHK1 mRNA have been reported in various human solid tumors.21,45 Our study shows for the first time that an up-regulation of the expression of the sphingosine kinase gene is associated with a leukemic process in vivo. Indeed, an enforced expression of SPHK1 in HS1 cells conferred in vivo tumorigenicity. Nevertheless, SPHK1-overexpressing HS1 cells were less aggressive than HS2 cells, which promote tumor formation with 100% efficiency in 3 to 6 weeks.13 In addition, SPHK1 overexpression did not release the HS1 cells from their growth-factor dependency. This indicates that overexpression of SPHK1 is an oncogenic event participating in leukemic progression, but additional somatic alterations cooperating with SPHK1 overexpression are required to induce a fully malignant phenotype, The search for such oncogenic alterations is currently under way.
The cause for SPHK1 up-regulation in tumorigenic proerythroblasts remains to be elucidated. The increase in SPHK1 mRNA could result from an enhanced transcription or an increased mRNA stability. It will be of interest to determine whether somatic mutations occur in cis- and trans-regulatory elements controlling sphingosine kinase gene transcription to settle whether SPHK1 overexpression is a causal event in leukemic progression.
Development of cancer is a stepwise process in which multiple somatic mutations give rise to expansion of malignant cell clones. Classically, mutations blocking cell differentiation and inducing survival and proliferation cooperate for transformation. Because Spi-1 contributes to leukemogenesis by blocking differentiation of the proerythroblast, the expected roles of cooperating oncogenes may be to favor cell proliferation and increase resistance to apoptosis. In that context, transcriptional up-regulation of the sphingosine kinase gene appears as one oncogenic event cooperating with Spi-1 during the progression of erythroleukemia. Such an observation should provide a starting point for checking for genetic deregulation of sphingolipid metabolism associated with various leukemic disorders in humans.
Prepublished online as Blood First Edition Paper, May 12, 2005; DOI 10.1182/blood-2004-12-4832.
E.L.S. was a recipient of successive fellowships from the Ministère de la Recherche et de la Technologie, the Ligue Nationale Contre Le Cancer, and the Société Française d'Hématologie. This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Consortium National de Recherche en Génomique, the Association pour la Recherche sur la Cancer, the Fondation de France, the Ligue Nationale de Recherche contre le Cancer, Centpoursanglavie, and the Institut Curie (Paris, France).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank N. Brandon (Inserm U528) for molecular biology procedures, S. Carpentier (Inserm U466) for technical assistance in sphingosine kinase assays, P. Arduin and A. Rouches (Institut Gustave Roussy, Villejuif, France) for helpful assistance with the animals, and Cilag (Levallois-Perret, France) for the gift of recombinant human Epo. We are grateful to C. Guillouf, O. Kosmider, R. Monni, and L. Delva for many helpful discussions and F. Wendling and J. de Gunzburg for their comments on this manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal