Abstract
To elucidate interpatient variability in thioguanine nucleotide (TGN) concentrations in acute lymphoblastic leukemia (ALL) cells, we determined the TGN concentrations in leukemic blasts from 82 children with newly diagnosed ALL after intravenous administration of mercaptopurine (MP). Patients treated with MP alone achieved higher TGN concentrations than those treated with the combination of methotrexate plus mercaptopurine (MTX + MP). Analysis of the expression of approximately 9600 genes in ALL cells obtained at diagnosis identified 60 gene probes significantly associated with TGN accumulation in patients treated with MP alone and 75 gene probes in patients treated with MTX + MP, with no overlap between the 2 sets of genes. Genes significantly associated with intracellular TGN accumulation after MP alone included those encoding MP metabolic enzymes and transporters (eg, SLC29A1). Inhibition of SLC29A1 by nitrobenzylmercaptopurine ribonucleoside (NBMPR) caused a 33% to 45% reduction of TGN in ALL cells in vitro (P < .006), consistent with the gene expression findings. Genes associated with TGN concentration after combination therapy included those involved in protein and adenosine triphosphate (ATP)-biosynthesis. Together, these in vivo and in vitro data provide new insight into the genomic basis of interpatient differences in intracellular TGN accumulation and reveal significant differences between treatment with MP alone and treatment with MP and MTX. (Blood. 2005;106:1778-1785)
Introduction
Mercaptopurine (MP) is a purine antimetabolite widely used in the treatment of acute lymphoblastic leukemia (ALL).1 MP is an inactive prodrug that requires intracellular activation catalyzed by multiple enzymes to exert cytotoxicity (Figure A in Supplemental Document S1, available on the Blood website; see the Supplemental Document link at the top of the online article).2-5 MP is converted by hypoxanthine guanine phosphoribosyltransferase (HGPRT) to thioinosine monophosphate (TIMP) and subsequently to thioguanosine monophosphate (TGMP) by a 2-step process involving inosine monophosphate dehydrogenase (IMPDH) and guanosine monophosphate synthetase (GMPS).2 Subsequently, TGMP can be converted to the active thioguanine nucleotides (TGNs) by adenosine kinase,2,6,7 Cytotoxic effects of MP are achieved primarily through the incorporation of TGN into RNA and DNA.8,9 The incorporation of TGN, as deoxy-TGN triphosphate, inhibits the function of several enzymes involved in DNA replication and repair10,11 and induces DNA damage, such as single-strand breaks, DNA protein cross-links, interstrand cross-links, and sister chromatid exchanges.12-15 The pathway leading to TGN synthesis is in competition with inactivation pathways catalyzed by xanthine oxidase (XDH) and thiopurine methyltransferase (TPMT).16
Numerous clinical studies have reported substantial interpatient variability in intracellular TGN concentrations during continuation therapy for ALL.5,17-19 Determinants of this variability include differences in MP absorption, genetic polymorphism of TPMT, and patient compliance with treatment.20-23 Interpatient differences in intracellular TGN concentrations have been associated with treatment outcome in children with ALL, whereas variability in MP plasma concentrations has not. Patients with TGN accumulation greater than the population average had better 5-year relapse-free survival compared with patients with TGN concentrations below the population average when they were treated with antimetabolite-based chemotherapy regimens.5,24
In most treatment protocols for childhood ALL, MP is administered in combination with methotrexate (MTX). The pharmacologic rationale for this combination is based in part on a direct effect of MTX on enzymes involved in TGN metabolism25 or on an indirect effect on plasma purines.17,26,27 Recently, it has been shown that patients treated with this combination in the presence of circulating ALL cells accumulate lower intracellular TGN concentrations in bone marrow leukemic lymphoblasts, peripheral blasts, and erythrocytes than patients treated with MP alone.28 For reasons that are largely unknown, however, there were substantial interindividual differences in TGN accumulation in ALL cells after each treatment (MP alone, 0.21-16.6; MTX + MP, 0.08-1.74; range, 10th-90th percentiles [pmol/5 × 106 cells]).
The current investigation was undertaken to identify genes significantly associated with the accumulation of TGN in ALL cells in vivo and to identify gene expression patterns that differed in leukemia cells that accumulated high compared with low intracellular concentrations of TGN after the administration of MP alone or in combination with MTX.
Patients, materials, and methods
Patients and treatment
Eighty-two patients aged 18 years or younger with newly diagnosed ALL, enrolled on the St Jude Children's Research Hospital Total Therapy XIIIB protocol,29 were included in the study. The institutional review board approved the study, and informed consent was obtained from patients, parents, or legal guardians, as appropriate. The diagnosis of ALL was based on morphology, cytochemical staining, immunophenotyping, cytogenetic analysis, and genetic characterization using methods previously described.1,30 Patients were randomly assigned to receive 1 of 3 initial therapies (Figure B in Document S1). Patients were administered intravenous MP alone (200 mg/m2 over a period of 20 minutes plus 800 mg/m2 over a period of 5 hours and 40 minutes); low-dose oral MTX (30 mg/m2 every 6 hours for 6 doses) followed by intravenous MP as described (LDMTX + MP); or high-dose intravenous MTX (200 mg/m2 push plus 800 mg/m2 over a 24-hour period) followed by intravenous MP as described (HDMTX + MP). Patients with contraindications to urate oxidase or rasburicase31,32 were administered allopurinol orally. As previously described,28 the use of allopurinol at least 72 hours before bone marrow aspiration did not alter thiopurine metabolite levels among treatment groups. For TGN analysis, we divided patients into 3 groups according to the randomized treatment arms: MP (n = 33), HDMTX + MP (n = 29), and LDMTX + MP (n = 20).
Isolation of ALL blasts from bone marrow aspirate
For all 82 patients, ALL cells at the time of diagnosis and 20 hours after initiation of the MP infusion (after treatment) were obtained and processed as previously described.33 The percentages of leukemia blasts in bone marrow aspirates at diagnosis and after treatment were similar (median, 97% and 95%, respectively). The final cell yield was determined by hemocytometer, and viability was determined by trypan blue exclusion.
Determination of intracellular thiopurine nucleotide concentrations in bone marrow ALL cells
We used a reverse-phase high-performance liquid chromatography (HPLC) method reported previously34 to determine the amount of TGNs in bone marrow cells 20 hours after in vivo MP treatment of patients. Briefly, 5 × 106 ALL cells were sonicated for 10 seconds, and proteins were removed by ultrafiltration. Thiopurine nucleotides were calculated by subtracting the thiopurine ribonucleotides from thiopurine ribonucleosides after conversion with acid phosphatase. The concentration of TGN is expressed as picomoles per 5 × 106 cells.
Mercaptopurine pharmacokinetic analysis
To assess whether plasma MP and MP metabolite concentrations were associated with intracellular TGN concentrations, we measured the plasma concentrations of mercaptopurine, mercaptopurine riboside, thioguanine, thioguanine riboside, and thiouric acid at 3 and 6 hours after the start of MP in 63 evaluable patients (MP, n = 23; HDMTX + MP, n = 17; LDMTX + MP, n = 23) using the HPLC method we have previously reported.34
TPMT phenotype and genotype
RNA extraction, gene expression profiling, and quantitative real-time-polymerase chain reaction
Patients for whom gene expression was determined (all 82) had sufficient blasts in their bone marrow aspirates to permit RNA isolation from 5 to 10 × 106 leukemia cells. We extracted high-quality total RNA with TriReagent (MRC, Cincinnati, OH) from mononuclear cells of bone marrow aspirates at diagnosis. As previously described,37,38 high-quality total RNA was processed and hybridized to the HG-U95Av2 oligonucleotide microarray (Affymetrix; Santa Clara, CA; see manufacturer's manual for details).
To further establish the validity of gene expression determined by microarray analysis, we performed quantitative real-time-polymerase chain reaction (RT-PCR) in 4 patient samples for 3 genes (GAB1, GRB2-associated binding protein 1; HNRPF, heterogeneous nuclear ribonucleoprotein F; DPM2, dolichol-phosphate mannosyltransferase polypeptide 2, regulatory subunit).
Determination of TGN accumulation in leukemia cells in the presence or absence of a specific inhibitor of the SLC29A1 transporter
We used the reverse-phase HPLC method reported previously34 to determine intracellular TGN concentration after treatment with 10 μM MP (Sigma, St Louis, MO) in the presence or absence of a specific SLC29A1 inhibitor, nitrobenzylmercaptopurine ribonucleoside (NBMPR) (Sigma). CEM, Nalm-6, and MOLT-4 human leukemia cells (American Type Culture Collection [ATCC], Manassas, VA) (50 × 106 cells in 50 mL media) were incubated for 24 hours at 37°C, and the effect of SLC29A1 transport inhibition was assessed by adding 100 nM NBMPR 20 minutes before MP exposure. As a negative control, we treated CEM cells with 1 nM adenine (Sigma), which is not a substrate for SLC29A1,39,40 and measured the intracellular adenine concentration after 24-hour exposure in the presence or absence of 100 nM NBMPR. These experiments were performed 3 times with 3 replicates.
Statistical analysis
Pairwise comparisons using the Wilcoxon rank sum test were used to assess differences in posttreatment TGN concentrations among patients in the 3 treatment arms. The Holm method was used to adjust for multiple testing.41 The Wilcoxon rank sum test was used to compare plasma MP and MP-metabolite concentrations between patients randomly assigned to HDMTX + MP and LDMTX + MP. Multiple regression analysis was used to assess the association between intracellular TGN concentrations and plasma MP and MP-metabolite concentrations separately in the group of patients treated with MP alone and in that treated with the combination of MTX + MP. Spearman rank correlation was used to assess the association between TPMT activity and TGN concentration. Default settings of Affymetrix Microarray Suite software version 5 (MAS 5.0; Affymetrix) were used to calculate gene expression values. Expression values were scaled to the target intensity of 2500 and were log-transformed. From the total of 12 599 probe sets, those expressed in less than 5% of the 82 patients were omitted, leaving 5013 probe sets for subsequent analyses. Analyses were performed separately in the 2 groups of patients treated either with MP alone or with the combination of MTX plus MP. Spearman rank correlation and false discovery rate (FDR) using the Storey q-value42 were computed to identify genes associated with TGN concentration. Leave-one-out cross-validation using the support vector machine (SVM) based on Spearman rank correlation was used to assess the validity of association between gene expression profiles and TGN concentrations.
To assess whether the expression of gene probe sets associated with intracellular TGN concentration in patients treated with MP alone was also associated with MP sensitivity in the 60 human tumor cell line panel (NCI60), we correlated the expression levels of those genes with the growth inhibition values (GI50) obtained after treatment with MP in these 60 cell lines. The GI50 values and the HG-U95Av2 gene expression data are publicly available through the National Cancer Institute's Developmental Therapeutics Program (http://dtp.nci.nih.gov/mtargets/download.html). Spearman rank correlation was used to assess the association between gene expression and GI50 values.
R 1.8.0 statistical software (http://www.r-project.org) was used to perform these analyses. Principal component analysis (PCA) and 2-dimensional hierarchical clustering were performed using GeneMaths 2.1 software (AppliedMaths, St Martens-Latem, Belgium).
Results
Differences in TGN accumulation in ALL cells of patients randomly assigned to 3 initial treatments
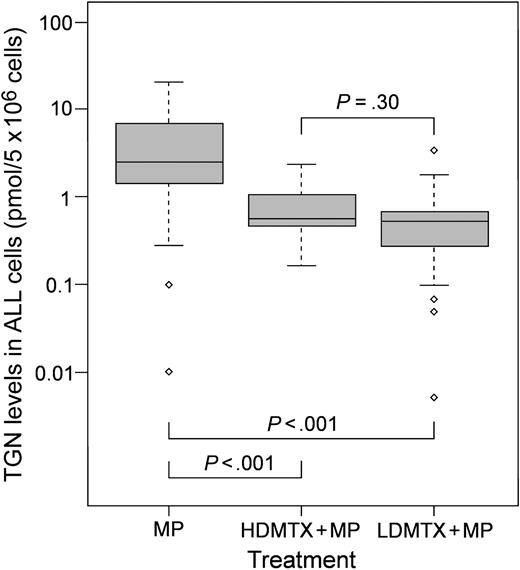
As shown in Figure 1, 33 patients who received MP alone (2.46 ± 5.36 pmol/5 × 106 cells; median ± SD) accumulated significantly higher concentrations of TGN in ALL cells than 29 patients treated with either LDMTX + MP (0.51 ± 0.64 pmol/5 × 106 cells; P < .001) or 20 patients treated with HDMTX + MP (0.57 ± 0.54 pmol/5 × 106 cells; P < .001). There were no differences in TGN concentrations between patients randomly assigned to receive LDMTX + MP and those randomly assigned to receive HDMTX + MP (P = .30). Patient characteristics (age, sex, race, white blood cell [WBC] count, ALL subtype) were similar among the 3 groups of patients (Figure B and Table A in Document S1).
Therefore, because of the results of our current analysis and our previous findings that showed no difference in TGN accumulation when MP was given after low-dose and high-dose MTX,28 in subsequent analyses we combined patients treated with either combination of HDMTX + MP and LDMTX + MP (MTX + MP; n = 49) into one group. Multiple regression analyses did not show any association between TGN concentration and patient age, sex, initial WBC count, or ALL subtype (Table B in Document S1) in either group of patients.
Intracellular concentrations of TGNs in patients after treatment with MP alone or in combination with MTX. Box plot of TGN concentrations in ALL cells for patients randomly assigned to treatment with MP alone (n = 33), HDMTX + MP (n = 20), or LDMTX + MP (n = 29). Horizontal line indicates the median for each group; box depicts the 25th and 75th percentile ranges. ⋄ indicates outlier values. P was determined by pairwise comparison using the Wilcoxon rank sum test adjusted for multiple testing.
Intracellular concentrations of TGNs in patients after treatment with MP alone or in combination with MTX. Box plot of TGN concentrations in ALL cells for patients randomly assigned to treatment with MP alone (n = 33), HDMTX + MP (n = 20), or LDMTX + MP (n = 29). Horizontal line indicates the median for each group; box depicts the 25th and 75th percentile ranges. ⋄ indicates outlier values. P was determined by pairwise comparison using the Wilcoxon rank sum test adjusted for multiple testing.
Correlation between MP pharmacokinetic parameters and intracellular TGN accumulation
Plasma concentrations of MP and MP metabolites at 3 and 6 hours after MP treatment were similar between patients randomly assigned to treatment with HDMTX + MP or LDMTX + MP.28 Plasma concentrations of thioguanine and thioguanine riboside were negligible (data not shown). Multiple regression analyses did not show any significant association between plasma concentrations of mercaptopurine, mercaptopurine riboside, or thiouric acid and intracellular TGN accumulation in ALL cells after treatment with MP alone (P > .29; n = 23) or with the combination of MTX + MP (P > .20; n = 40) (Table C in Document S1). The power to detect an association of R2 = 0.20 at the α= 0.10 level for 23 or 40 persons was 68.7% or 90%, respectively.
Association between TPMT phenotype/genotype and TGN accumulation in bone marrow ALL cells
In patients treated with MP alone (n = 24) or in patients treated with the combination of MTX + MP (n = 36), intracellular TGN concentrations were not associated with TPMT activity measured at the time of diagnosis (MP, R2 = 0.01; MTX + MP, R2 = 0.02). However, among the 60 patients who underwent genotyping, only 2 had TPMT heterozygosity and none were deficient in TPMT.
Relation between gene expression and intracellular TGN accumulation
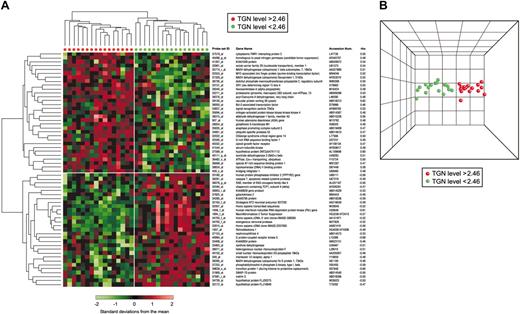
Unsupervised hierarchical clustering, which groups patients based on predominant similarities in overall gene expression, did not cluster patients according to ALL TGN concentrations (Figure C in Document S1). However, Spearman rank correlation and leave-one-out cross-validation using SVM identified 60 gene probe sets (ρ= 0.60; P < .001; FDR = 45%) (Table D in Document S1) that were associated with TGN concentrations in ALL cells after treatment with MP alone (n = 33). Hierarchical clustering using these selected gene probe sets distinctly separated the 33ALL patients treated with MP alone into 2 major groups with either a low (less than 2.46 pmol/5 × 106 cells) or a high (more than 2.46 pmol/5 × 106 cells) TGN concentration (Figure 2A). PCA illustrates the degree of discrimination between the 2 groups of patients (Figure 2B).
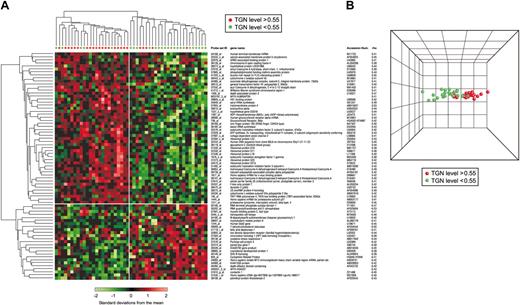
In a separate analysis, 75 gene probe sets (ρ= 0.65; P < .001; FDR = 25%) (Table E in Document S1) were significantly associated with TGN concentrations in ALL cells after treatment MTX + MP (n = 49). Two major branches were also found for patients treated with MTX + MP (less than 0.55 pmol/5 × 106 cells and more than 0.55 pmol/5 × 106 cells; Figure 3A), and the degree of discrimination between these 2 groups of patients is evident by PCA (Figure 3B).
Interestingly, there was no overlap between the 2 sets of genes. Among genes associated with TGN accumulation in ALL cells after treatment with MP alone, 31 were positively and 29 were negatively correlated with TGN concentration. In the group of patients treated with MTX + MP, 50 genes were positively correlated and 25 were negatively correlated with TGN accumulation. Genes significantly associated with TGN concentration in patients treated with MP alone included xanthine oxidase (XDH)(ρ= -0.51; P = .002), solute carrier family 29 member 1 (SLC29A1) (ρ= 0.54; P = .001), aldehyde dehydrogenase 1 family member A2 (ALDH1A2)(ρ= 0.56; P < .001), adenosine deaminase (ADA)(ρ= 0.48; P = .004), and genes related to cell proliferation and apoptosis (caspase 7 [CASP7], DNA topoisomerase II-binding protein [TOPBP1], anaphase-promoting complex subunit 5 [ANAPC5], and chaperonin-containing TCP1 subunit 4 [CCT4]). In the group of patients treated with MTX + MP, we found genes involved in adenosine triphosphate (ATP) synthesis (solute carrier family 25, mitochondrial carrier member 3 [SLC25A3], ATP synthetase, H+ transporting, mitochondrial complex, O subunit [ATP50], cytochrome c oxidase subunit Vb [COX5B], and VIIa polypeptide 2-like [COX7A2L]) and several genes implicated in protein synthesis, such as ribosomal proteins (RPS19, RPL18, RPS25, RPL23) and translation factors (EEF1G, EIF3S5, eIF3k).
Clustering of genes associated with intracellular concentrations of TGN in leukemia cells of patients treated with MP alone. (A) Hierarchical clustering of 33 ALL patients using 60 gene probe sets significantly associated with TGN accumulation in ALL cells after treatment with MP alone. Each row represents a probe set and each column a patient. Standardized expression values are shown according to the scale. Red and green symbols indicate patients with TGN concentrations higher and lower than 2.46 pmol/5 × 106 cells, respectively. Listed are the Affymetrix probe set IDs, gene names, GenBank accession numbers, and coefficients of correlation (rho). A positive rho indicates a positive correlation, and a negative rho indicates negative correlation between gene expression and intracellular TGN concentration. (B) PCA of ALL cells with high (red) and low (green) TGN concentrations based on the selected probe sets. Similarities are visualized in 3-dimensional space.
Clustering of genes associated with intracellular concentrations of TGN in leukemia cells of patients treated with MP alone. (A) Hierarchical clustering of 33 ALL patients using 60 gene probe sets significantly associated with TGN accumulation in ALL cells after treatment with MP alone. Each row represents a probe set and each column a patient. Standardized expression values are shown according to the scale. Red and green symbols indicate patients with TGN concentrations higher and lower than 2.46 pmol/5 × 106 cells, respectively. Listed are the Affymetrix probe set IDs, gene names, GenBank accession numbers, and coefficients of correlation (rho). A positive rho indicates a positive correlation, and a negative rho indicates negative correlation between gene expression and intracellular TGN concentration. (B) PCA of ALL cells with high (red) and low (green) TGN concentrations based on the selected probe sets. Similarities are visualized in 3-dimensional space.
Correlation between gene expression and GI50 using NCI60 cancer cell lines
Using Spearman rank correlation, we found that among the 60 gene probe sets associated with TGN accumulation in ALL cells after in vivo treatment with MP alone, 10 genes were significantly associated with the 50% growth inhibitory concentration (GI50) of MP in vitro sensitivity (P < .05) measured in a panel of 60 human tumor cell lines. Nine genes were negatively and one was positively correlated with GI50. Six of the negatively correlated genes were positively correlated with TGN accumulation in ALL cells in our patients. These genes encode for transporters (SLC29A1, ATP2A3), proteins involved in oxidative phosphorylation (NADH dehydrogenase flavoprotein 1 [NDUFV1]), and carbohydrate metabolism (isocitrate dehydrogenase 3 β [IDH3B] and DNA replication ([TOPBP1]) (Table 1; Figure D in Document S1).
Genes significantly associated with GI50for MP in NCI60 human tumor cell lines
Probe set ID . | Gene name . | P GI50 . | ρ GI50 . | P TGN . | ρ TGN . |
|---|---|---|---|---|---|
| 35835_at | Anaphase promoting complex subunit 5* | < .001 | −.45 | .007 | .47 |
| 41544_at | Serum-inducible kinase | .001 | .41 | .004 | .49 |
| 37329_at | NADH dehydrogenase flavoprotein 1* | .003 | −.39 | .001 | .55 |
| 33901_at | Solute carrier family 29, member 1* | .003 | −.38 | .003 | .54 |
| 32007_at | Homo sapiens-transcribed sequences | .004 | −.37 | .002 | −.52 |
| 38834_at | Topoisomerase (DNA) II binding protein* | .012 | −.32 | .001 | .58 |
| 37825_at | Galactokinase 2 | .016 | −.31 | .007 | −.46 |
| 36482_s_at | ATPase, Ca++ transporting, ubiquitous* | .022 | −.30 | .004 | .50 |
| 40111_g_at | Isocitrate dehydrogenase 3 (NAD+) β* | .031 | −.28 | .003 | .51 |
| 1937_at | Retinoblastoma 1 | .046 | −.26 | .004 | −.49 |
Probe set ID . | Gene name . | P GI50 . | ρ GI50 . | P TGN . | ρ TGN . |
|---|---|---|---|---|---|
| 35835_at | Anaphase promoting complex subunit 5* | < .001 | −.45 | .007 | .47 |
| 41544_at | Serum-inducible kinase | .001 | .41 | .004 | .49 |
| 37329_at | NADH dehydrogenase flavoprotein 1* | .003 | −.39 | .001 | .55 |
| 33901_at | Solute carrier family 29, member 1* | .003 | −.38 | .003 | .54 |
| 32007_at | Homo sapiens-transcribed sequences | .004 | −.37 | .002 | −.52 |
| 38834_at | Topoisomerase (DNA) II binding protein* | .012 | −.32 | .001 | .58 |
| 37825_at | Galactokinase 2 | .016 | −.31 | .007 | −.46 |
| 36482_s_at | ATPase, Ca++ transporting, ubiquitous* | .022 | −.30 | .004 | .50 |
| 40111_g_at | Isocitrate dehydrogenase 3 (NAD+) β* | .031 | −.28 | .003 | .51 |
| 1937_at | Retinoblastoma 1 | .046 | −.26 | .004 | −.49 |
Genes with negative correlation with NCI60 GI50 and positive correlation with TGN concentration.
TGN accumulation in leukemia cells differed after in vitro treatment with MP alone or in the presence of NBMPR
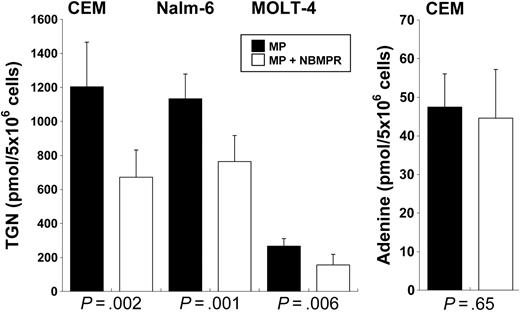
NBMPR is an inhibitor of the SLC29A1 nucleoside transporter.43 As depicted in Figure 4, in CEM human leukemia cells treated for 24 hours with MP + NBMPR, concentrations of TGN were 45% lower in cells treated with MP alone (672 ± 160 vs 1204 ± 262 pmol/5 × 106 cells; P < .002), 33% lower in Nalm-6 cells (764 ± 153 vs 1133 ± 146 pmol/5 × 106 cells; P = .001), and 42% lower in MOLT-4 cells (156 ± 62 vs 267 ± 44 pmol/5 × 106 cells; P = .006), comparing use of inhibitor with no inhibitor, respectively, for each cell line. The intracellular accumulation of adenine, which is not a substrate of SLC29A1,39 was not different in CEM cells treated with adenine in the presence or the absence of NBMPR (47.44 ± 8.6 vs 44.6 ± 12.6 pmol/5 × 106 cells; P = .65).
Validation of gene expression
Gene expression levels determined by microarray analysis were validated using quantitative real-time-polymerase chain reaction (RT-PCR) in 4 patient samples for 3 genes (GAB1, HNRPF, DPM2). Correlation (R2) between mRNA levels determined by RT-PCR and microarray was 0.97 for GAB1, 0.77 for HNRPF, and 0.83 for DPM2, confirming expression levels determined by the gene expression array (Figure E in Document S1).
Discussion
Intracellular accumulation of TGNs is a critical event for the cytotoxic effects of mercaptopurine.8,9 Previous studies have shown that several factors, including the activity of enzymes involved in MP metabolism, MP dose intensity, drug-drug interactions, and patient compliance, significantly influence interpatient differences in TGN concentrations.18,44,45 Furthermore, these interpatient differences have been associated with treatment efficacy3,5,24 and MP toxicity.23,46
Well-defined sources of variability include inherited differences in TPMT36,46-48 and inhibition of xanthine oxidase by allopurinol.49 Consistent with previous reports from our laboratory and others,26,28 the current study revealed a significant in vivo effect of MTX on intracellular accumulation of TGN compared with treatment with MP given alone (P < .001). The current study also documented substantial interpatient variability in TGN accumulation not explained by these drug interactions or by inherited differences in TPMT activity.
Hierarchical clustering using genes associated with intracellular concentrations of TGN in ALL cells of patients treated with MP + MTX. (A) Hierarchical clustering of 49 ALL patients using 75 probe sets significantly associated with TGN accumulation in ALL cells after treatment with MTX + MP. Each row represents a probe set and each column a patient. Standardized expression values are shown according to the scale. Red symbols depict patients with TGN concentrations greater than 0.55 pmol/5 × 106 cells, and green symbols depict those with concentrations less than 0.55 pmol/5 × 106 cells. Listed are the Affymetrix probe set IDs, gene names, GenBank accession numbers, and coefficients of correlation (rho). A positive rho indicates a positive correlation, and a negative rho indicates a negative correlation between gene expression and intracellular TGN concentration. (B) PCA of ALL cells with high (red) and low (green) TGN concentration based on the selected probe sets. Similarities are visualized in 3-dimensional space.
Hierarchical clustering using genes associated with intracellular concentrations of TGN in ALL cells of patients treated with MP + MTX. (A) Hierarchical clustering of 49 ALL patients using 75 probe sets significantly associated with TGN accumulation in ALL cells after treatment with MTX + MP. Each row represents a probe set and each column a patient. Standardized expression values are shown according to the scale. Red symbols depict patients with TGN concentrations greater than 0.55 pmol/5 × 106 cells, and green symbols depict those with concentrations less than 0.55 pmol/5 × 106 cells. Listed are the Affymetrix probe set IDs, gene names, GenBank accession numbers, and coefficients of correlation (rho). A positive rho indicates a positive correlation, and a negative rho indicates a negative correlation between gene expression and intracellular TGN concentration. (B) PCA of ALL cells with high (red) and low (green) TGN concentration based on the selected probe sets. Similarities are visualized in 3-dimensional space.
We evaluated MP with or without MTX as initial therapy because that is the only time it is possible to assess MP dispositions and effects in primary cells after in vivo treatment (by week 4, most patients have no ALL cells in their peripheral blood or bone marrow). In our study, we were focused on treatment-naive ALL and its ability to accumulate active MP metabolites in vivo. This necessitated that we assess gene expression at diagnosis and TGN accumulation after in vivo MP treatment during the first 4 days of treatment.
To better understand the biologic basis underlying interpatient variability in TGN accumulation in ALL cells after MP treatment, we used oligonucleotide microarrays to perform a genomewide determination of pretreatment gene expression in ALL cells to identify genes and gene expression patterns significantly associated with high or low TGN accumulation in leukemia cells. This revealed 60 gene probe sets (49 genes, 11 cDNA clones) highly related to the extent of TGN accumulation in leukemia cells of patients treated with MP alone and 75 gene probe sets (68 genes, 7 cDNA clones) of patients treated with MTX + MP. Notably, there was no overlap between the 2 sets of genes, indicating that different genes influence the accumulation of TGN when MP is given alone and when it is given in combination with MTX. Previous studies have shown that in vivo gene expression changes are treatment specific in ALL cells and that the nature of these cellular drug responses is different when medications are given alone and when they are given in combination with a second agent.37,50 Of note, gene expression was not biased by low-dose or high-dose MTX + MP shown by “unsupervised” clustering analysis using all genes and not by using genes related to TGN accumulation (Figure C in Document S1; P = .5).
Effect of concomitant treatment with nucleoside transporter inhibitor NBMPR on TGN and adenine concentrations in ALL cells. (Left) Shown are intracellular TGN concentrations in CEM, Nalm-6, and MOLT-4 cells after treatment with MP in the presence or absence of nitrobenzylmercaptopurine ribonucleoside (NBMPR), a nucleoside transport (SLC29A1) inhibitor. (Right) Shown is the intracellular adenine concentration in CEM cells after incubation with adenine, in the presence or absence of NBMPR. Columns represent the averages of 3 independent experiments, each with 3 replicates; error bars represent range.
Effect of concomitant treatment with nucleoside transporter inhibitor NBMPR on TGN and adenine concentrations in ALL cells. (Left) Shown are intracellular TGN concentrations in CEM, Nalm-6, and MOLT-4 cells after treatment with MP in the presence or absence of nitrobenzylmercaptopurine ribonucleoside (NBMPR), a nucleoside transport (SLC29A1) inhibitor. (Right) Shown is the intracellular adenine concentration in CEM cells after incubation with adenine, in the presence or absence of NBMPR. Columns represent the averages of 3 independent experiments, each with 3 replicates; error bars represent range.
After treatment with MP alone, genes significantly related to TGN accumulation in ALL cells included those encoding transporters (SLC29A1, ATP2A3, NDUFS1), enzymes involved in purine metabolism (ADA, XDH), regulation of cell cycle (ANAPC5, CCT4), and apoptosis (CASP7, PRKR). Interestingly, only 2 (XDH, ADA) of the genes identified in the current study have been previously associated with MP metabolism and disposition. Expression of SLC29A1 (or ENT1), a member of the family of the equilibrative nucleoside transporters that mediates the uptake of nucleosides and chemotherapeutic agents,39,51 has been shown to be positively associated with cytotoxicity of nucleoside analogs (MP, azacytidine, cytarabine, inosine-glycodialdehyde) in human cancer cell lines52 and clinical response to cytarabine in patients with acute myeloid leukemia.53 Consistent with this, in our patients, SLC29A1 expression was high in ALL cells that accumulated high posttreatment TGN concentrations (ρ= 0.54; P = .0015).
We measured TGN accumulations in CEM, Nalm-6, and MOLT-4 human leukemia cells after exposure to MP in the presence or absence of NBMPR, a specific inhibitor of the SLC29A1 transporter. Inhibition of SLC29A1 by NBMPR reduced the concentrations of TGN at 24 hours by 45%, 33%, and 42%, respectively (P < .006). Together, these in vivo and in vitro data provide the first evidence that the inhibition of SLC29A1 reduced steady state TGN accumulation by 45% to 33%, and it also indicates the existence of other MP and TGN transport and metabolic mechanisms in ALL cells.
XDH encodes xanthine oxidase, an important enzyme involved in the first-pass metabolism of MP.16 This enzyme, predominantly expressed in the intestinal mucosa and liver, converts MP to inactive thiouric acid,54,55 a principal pathway for MP catabolism. In ALL, XDH was expressed at a relatively low level (median, 1575, Affymetrix scaled signal). The significant negative correlation between XDH expression and TGN concentration in ALL cells (ρ= -0.52; P = .003) is consistent with the role of this enzyme in the catabolism of MP, but it may be related to concordant variability of XDH in other tissues (eg, liver). The nonsignificant correlation of XDH expression and TGN (ρ= 0.08; P = .54) in patients treated with MTX + MP may be attributed to the inhibitory effects of MTX on xanthine oxidase activity.25
Another gene that showed a positive relation between gene expression and TGN accumulation (ρ= 0.48; P = .005) was human adenosine deaminase (ADA). ADA encodes a purine-metabolizing enzyme that indirectly influences the metabolism of MP through perturbation of the intracellular pool of enzymes involved in nucleotide synthesis. ADA has been extensively studied in ALL patients, and low ADA activity has been associated with poor outcome after in vivo treatment with the structurally similar 6-thioguanine.56 It is postulated that high ADA expression results in the accumulation of inosine and subsequently inosine monophosphate (IMP),57 which inhibits phosphoribosyl pyrophosphate aminotransferase (PPAT), thereby increasing intracellular phosphoribosyl pyrophosphate (PRPP), a rate-limiting precursor for the formation of TGN.58
Interestingly, among the 60 gene probe sets significantly associated with TGN accumulation in patients treated with MP alone, 10 were significantly correlated with MP sensitivity (GI50) in NCI60 human tissue cell lines. Notably, 6 of these genes were negatively correlated with GI50 in cell lines and positively correlated with TGN accumulation in our patients. These findings indicate that increased expression of genes associated with TGN accumulation is associated with increased sensitivity to MP.
Among genes related to TGN accumulation after MTX + MP treatment were several involved in mitochondrial activities (eg, ATP biosynthesis and fatty acid metabolism) and protein synthesis (eg, ribosomal proteins and translation factors). To our knowledge, none of those 68 genes has been previously implicated in MP metabolism, TGN accumulation, or thiopurine resistance. Four of these genes, SLC25A3, ATP50, COX5B, and COX7A2L, are involved in the synthesis of the structurally similar nucleotide, ATP. SLC25A3 encodes the phosphate carrier PCH, an integral protein of the mitochondrial inner membrane involved in the terminal process of oxidative phosphorylation.59 ATP50 encodes for the oligomycin sensitivity conferring protein (OSCP) subunit of the stalk of the mitochondrial respiratory chain F1F0-ATP synthase.60 Finally, COX5B and COX7A2L encode for subunits of the cytochrome c oxidase, the terminal enzyme of the mitochondrial electron transport chain. ATP is a cofactor for GMPS,61 an important enzyme involved in the conversion of thioxanthosine monophosphate (TXMP) to TGMP, an essential step in TGN formation. Low ATP levels have been associated with significantly lower intracellular TGN accumulation in leukemia cells treated in vitro with MP.62 MTX, through the inhibition of de novo purine synthesis, decreases intracellular levels of ATP.44,63 Therefore, it is plausible that patients with increased expression of SLC25A3, ATP50, COX5B, and COX7A2L have higher ATP levels and are, therefore, less susceptible to the inhibition of ATP synthesis by MTX and, as a consequence, achieve relatively higher intracellular TGN concentrations than other patients treated with MTX + MP. Expression of these genes was not significantly associated with TGN concentrations in the group of patients treated with MP alone. Together, these in vivo and in vitro data provide new insight into the genomic basis of interpatient differences in TGN accumulation in ALL cells and reveal significant differences when MP is given alone or in combination with MTX.
Prepublished online as Blood First Edition Paper, May 19, 2005; DOI 10.1182/blood-2005-01-0143.
Supported in part by National Institutes of Health grants R37 CA36401 (W.E.E., M.V.R., C.H.P.), R01 CA78224 (W.E.E., M.V.R., C.H.P.), RO1 CA51001 (M.V.R., C.H.P.), and U01 GM61393 (M.V.R., W.E.E.) and Cancer Center Support Grant CA21765; by an F.M. Kirby Clinical Research Professorship from the American Cancer Society (C.H.P.); and by the American Lebanese Syrian Associated Charities.
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the following people for their outstanding support: Dr Natalia Krynetskaia, Dr Evgeny Krynetsky, Yaqin Chu, Margaret Needham, Nancy Kornegay, and Dr Clayton Naeve and his staff in the Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children's Research Hospital. We also thank Paxton Baker, Cong Ding, Nancy Duran, Emily Melton, and Siamac Salehy for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal