Abstract
Despite profound T-cell immunodeficiency, most patients treated with chemotherapy do not succumb to infection. The basis for residual protective immunity in lymphopenic patients is not known. We prospectively measured T-cell numbers, thymopoiesis, and T-cell memory in 73 children undergoing a 2-year chemotherapy regimen for acute lymphoblastic leukemia (ALL) and compared them to an age-matched cohort of 805 healthy children. Most patients had profound defects in CD4 and CD8 T-cell numbers at diagnosis that did not recover during the 2 years of therapy. Thymic output and the fraction of naive T cells were significantly lower than in healthy controls. However, the remaining T-cell compartment was enriched for antigen-experienced, memory T cells defined both by phenotype and by function. This relative sparing of T-cell memory may, in part, account for the maintenance of protective immunity in lymphopenic patients treated for ALL. Moreover, because the memory T-cell compartment is least affected by ALL and its treatment, strategies to induce immunity to pathogens or tumor antigens in cancer patients may be most successful if they seek to expand pre-existing memory T cells. (Blood. 2005; 106:1749-1754)
Introduction
Patients undergoing chemotherapy for cancer are susceptible to infection, particularly in hematologic malignancies such as acute lymphoblastic leukemia (ALL) where infections account for 80% of remission deaths.1-3 However, while infection represents a significant complication of chemotherapy, the number of patients who succumb to infection is low. For instance, fewer than 1% of children undergoing maintenance therapy for ALL die from infection.3 Although infection prophylaxis and good supportive care are likely to contribute to this low infectious mortality, the majority of patients with ALL appear to retain protective immunity against infection despite prolonged chemotherapy. The nature of this residual immune function in lymphopenic patients is not known.
In the normal host the peripheral T-cell compartment consists of naive T cells, which originate in the thymus, and memory T cells, which have differentiated from naive T cells in response to antigen exposure. Memory T cells can rapidly proliferate and acquire effector function on re-exposure to antigen. Together with antibody response, memory T cells are central to the maintenance of protective immunity. However, the fate of the memory T-cell compartment in patients treated with chemotherapy is poorly understood. Seminal studies by Mackall et al4-7 described the profound depletion seen in the T-cell compartment following chemotherapy. They showed that intensive chemotherapy is associated with a near-total loss of naive CD4 T cells, which do not reappear until several months after the end of treatment.4-7 Recent data using assays that quantify thymic output have also shown a marked decrease in thymic output in patients treated with chemotherapy and radiation, suggesting that direct thymic injury may account for this lack of naive T cells.8,9 Together, these studies have elegantly demonstrated that the production of naive, CD45RA+ T cells in chemotherapy-treated patients can be markedly reduced. However, it is not clear whether the remaining T cells are indeed true, antigen-experienced memory T cells. For instance, the studies by Mackall and colleagues report that residual T cells in chemotherapy-treated patients predominantly express CD45RO, a marker expressed on many memory T cells.5 However, recent data suggest that CD45RO can also be a marker of T-cell activation.10,11 Moreover in lymphopenic animal models, naive T cells undergoing homeostatic proliferation can “masquerade” as memory T cells by acquiring the phenotypic but not functional properties of memory T cells.12-14 Therefore it is not known whether the predominance of CD45RO on T cells in lymphopenic chemotherapy-treated patients reflects homeostatic expansion of naive T cells or the persistence of protective antigen-specific memory.
To characterize the fate of T-cell memory in chemotherapy-treated patients, we prospectively studied T-cell homeostasis and functional T-cell memory in a cohort of children with ALL undergoing a 24-month chemotherapy regimen. We showed that despite profound and sustained T-cell depletion, T-cell memory was enriched in the remaining T-cell pool, and may account for the low rate of infection seen in this population.
Patients, materials, and methods
Patient characteristics
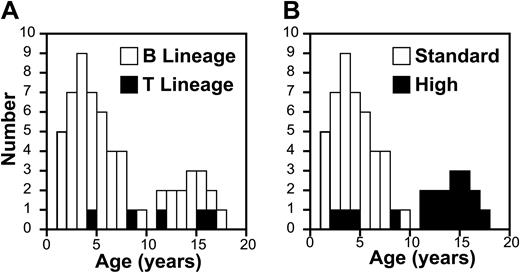
We studied T-cell homeostasis in 73 consecutive children with newly diagnosed ALL starting treatment on DFCI ALL protocol 00-001 at the Dana-Farber Cancer Institute between October 2000 and May 2003. The median age of the patients in this study was 4 years (range, 1-17 years) and followed a bimodal distribution, with a second age peak at 15 years (Figure 1). Most of the 23 patients (32%) with high-risk features or T-cell ALL (9%) were more than 10 years old (Figure 1). Only patients treated at the Dana-Farber Cancer Institute were included in this study in order to minimize sample variability caused by shipping from participating centers. Patients and guardians participating in this study gave informed consent according to the Declaration of Helsinki. All studies were approved by the Dana-Farber Cancer Institute institutional review board.
Treatment
Treatment for all patients consisted of an intensive, 5-drug remission induction phase, followed by a consolidation phase including weekly high-dose asparaginase, and then a continuation phase including every-3-week pulses of vincristine and prednisone. Full details of this protocol will be published elsewhere. Patients with high-risk ALL (presenting white cell count > 105/μL; age > 10 years; T-cell phenotype) also received doxorubicin during consolidation and a higher steroid dose during consolidation and maintenance; standard risk patients did not. Total duration of therapy was 24 months from complete remission. Sulfamethoxazole/trimethoprim was administered as Pneumocystis carinii pneumonia (PCP) prophylaxis to all patients.
Sample timing and preparation
Blood samples were obtained at diagnosis and on 5 occasions during therapy: at 5 months (during consolidation); 10 months (after consolidation); and at 15, 19, and 24 months (during maintenance). On-therapy blood samples were obtained at the start of 3-week cycles of treatment. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh, heparinized blood samples by density centrifugation and washed twice before use. Fresh, rather than cryopreserved, cells were used for all assays.
Healthy donors
Comparison of lymphocyte subset numbers and phenotype was made to a data set from 805 healthy children age 0 to 18 years provided by the Pediatric AIDS Clinical Trial group.15 Samples from additional healthy pediatric donors for functional studies were obtained from children presenting to their pediatricians for routine well-child visits, with approval of the institutional review board of Children's Hospital, Boston, MA.
Age distribution of subjects with ALL. Age distribution of patients with respect to disease type (A) and treatment arm (B).
Age distribution of subjects with ALL. Age distribution of patients with respect to disease type (A) and treatment arm (B).
PCR quantification of TREC content
CD3+ cells were immunomagnetically purified from fresh samples according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). Purity of more than 95% was routinely obtained. The quantification of signal-joint T-cell receptor excision circle DNA (sjTREC) in DNA purified from T cells was performed by real-time quantitative polymerase chain reaction (PCR), using the 5′ nuclease (TaqMan) assay with an ABI7700 system (Perkin-Elmer, Norwalk, CT) as described previously.16 sjTREC values in purified CD4 and CD8 T cells from healthy adult adults and children age birth to 18 years were used as controls. As the sjTREC values were not significantly different in T cells that had been separated based on CD4 or CD8 (P = .79), we considered that they represented a valid normal control set against which to compare sjTREC levels in total CD3 population.
Flow cytometry
PBMCs were stained with pretitrated quantities of antibodies to CD45, CD3, CD4, CD8, CD45RA, or CD62L conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or PE-Cyanine 5 (all from Beckmann-Coulter/Immunotech, Gainsville, FL). Flow cytometry data were acquired using an EPICS XL-MCL cytometer or a Cytomics FC500 (Beckman Coulter, Miami, FL), and analyzed using FlowJo software (Tree Star, San Carlos, CA). A lymphocyte gate was set based on orthogonal light scatter and staining with CD45. This allowed discrimination between large, CD45dim leukemic blasts, and small CD45bright normal lymphocytes in diagnostic blood samples. Within the lymphocyte gate, CD4+ cells were identified as CD3+/CD4+ cells and CD8+ as CD3+/CD8+. Subset analysis of CD4+ and CD8+ cells was achieved by staining with antibodies to CD45RA and CD62L, or CD45RA and CD45RO. T cells expressing with CD45RA and CD62L were considered phenotypically naive. CD45RO+/CD45RA- cells were considered phenotypically memory T cells.
Staining was done using whole blood in the case of control subjects and ficolled PBMCs in the case of patients with ALL. However, absolute counts for both sample types were calculated by multiplying the fraction of each subset within the lymphocyte gate by the absolute lymphocyte count determined on the same day by a clinical laboratory. We therefore considered that absolute T-cell numbers measured in control and ALL patient populations could be compared meaningfully.
Detection of antigen-specific responses
Antigen-specific responses were measured using a proliferation assay based on detection of dilution of the intracellular fluorescent dye 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) as previously described.17 Briefly, PBMCs were stained with CFSE (0.2 μM; Molecular Probes, Eugene, OR) for 5 minutes at 37°C per manufacturer's instructions and plated in 96-well round-bottomed plates at 2 × 105 cells/well in 200 μL RPMI (supplemented with 10% vol/vol, human AB serum, l-glutamine, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES]), along with the antigen of interest. Varicella Zoster Virus (VZV) cell lysates were obtained from Microbix (Toronto, ON, Canada) and used at 7 μg/mL and Tetanus Toxoid (25 μg/mL) from Calbiochem (San Diego, CA). Cells were incubated at 37°C for 7 days then harvested, washed, and stained with antibodies as described.17
Statistical analysis
Changes in T-cell subsets and sjTREC levels over time were assessed using mixed effects models; parameters were estimated by the method of maximum likelihood. The analysis included all available data points. Additional covariates considered were: age as a continuous variable; risk group (standard versus high); and immunophenotype (B- or T-lineage ALL). Interaction terms were included. Age-specific data from the healthy controls were used to calculate the fraction of patients falling below the 10th percentile of normal. Baseline comparisons between patients and healthy subjects were conducted using the Wilcoxon rank-sum test. All statistical tests were 2-sided with α equaling .05.
Results
Infectious morbidity and mortality is low in children treated for ALL
Previous reports have indicated that the rate of remission deaths caused by infection in children with ALL is low.3 We therefore determined if our cohort had a similarly low rate of life-threatening infection. We measured the frequency of serious infection, defined as invasive fungemia, bacteremia, or PCP infection and the rate of infectious deaths in the group of patients studied. There were 33 infections (31 episodes of fungemia or bacteremia, and 2 episodes of PCP pneumonia). Two patients each had more than one infection. Almost half of the infections (15 of 33) occurred within 30 days of diagnosis during induction chemotherapy. Thereafter, the rate of infection was low, and the adjusted rate of fungemia, bacteremia, or PCP pneumonia was 0.20 infections/patient-year in patients undergoing consolidation or maintenance. There were no deaths from infection among the 73 patients studied. Thus, the rates of infectious complications in this cohort were comparable to those reported by others.3
Thymopoiesis is impaired at diagnosis and during therapy
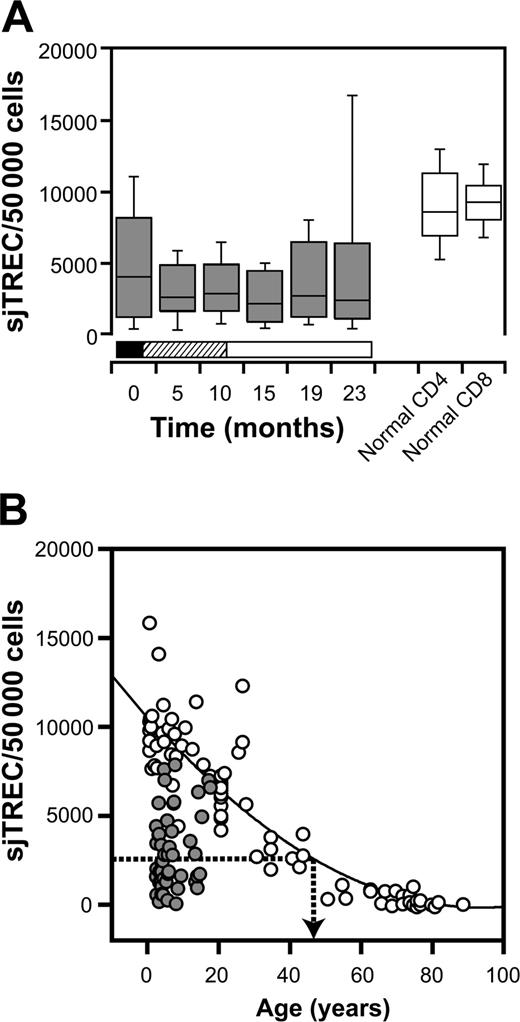
Adequate thymic function is essential for the generation of naive T cells capable of differentiation into memory cells. We therefore assessed the impact of ALL and its treatment on thymic function. sjTREC levels were measured in purified peripheral-blood CD3+ T cells and compared with age-matched healthy controls (Figure 2). At diagnosis, sjTREC levels in patients with ALL were significantly lower than in healthy children (P = .006). sjTREC levels remained low with respect to age-matched healthy controls during treatment. When measured at 5 timepoints during the 2-year treatment course, 86% of sjTREC values were less than the 10th percentile in healthy controls. Changes in sjTREC levels over time were assessed using mixed effects models as described in “Patients, materials, and methods.” There was no significant variation in sjTREC level with time, suggesting that sjTREC levels did not recover during treatment (Figure 2A).
sjTREC levels in patients compared with healthy controls. (A) Box-and-whisker plots for sjTREC levels in peripheral blood CD3+ T cells obtained at diagnosis and at 5 points during therapy (▦). Phases of treatment are shown by the bars below (▪ indicates induction; ▨, consolidation; and □, maintenance). sjTREC levels in CD4 and CD8 T cells from age-matched healthy donors are shown in the white box-and-whisker plots. Boxes indicate 2nd and 3rd quartiles; bar, median; and whiskers, range. (B) sjTREC values from individual patients averaged for all timepoints ( ) with respect to age overlaid on those from healthy donors (○). Regression line (solid line) is plotted from healthy donors, and dotted arrow indicates the median sjTREC level in patients with ALL.
) with respect to age overlaid on those from healthy donors (○). Regression line (solid line) is plotted from healthy donors, and dotted arrow indicates the median sjTREC level in patients with ALL.
sjTREC levels in patients compared with healthy controls. (A) Box-and-whisker plots for sjTREC levels in peripheral blood CD3+ T cells obtained at diagnosis and at 5 points during therapy (▦). Phases of treatment are shown by the bars below (▪ indicates induction; ▨, consolidation; and □, maintenance). sjTREC levels in CD4 and CD8 T cells from age-matched healthy donors are shown in the white box-and-whisker plots. Boxes indicate 2nd and 3rd quartiles; bar, median; and whiskers, range. (B) sjTREC values from individual patients averaged for all timepoints ( ) with respect to age overlaid on those from healthy donors (○). Regression line (solid line) is plotted from healthy donors, and dotted arrow indicates the median sjTREC level in patients with ALL.
) with respect to age overlaid on those from healthy donors (○). Regression line (solid line) is plotted from healthy donors, and dotted arrow indicates the median sjTREC level in patients with ALL.
In order to provide a context for this defect in thymic output, we compared the sjTREC levels in patients with ALL to the values in healthy controls with a wider range of ages (Figure 2B). Thymic function and sjTREC levels decline exponentially with age.16 The median sjTREC level in children treated for ALL was equivalent to that of 49-year-old controls (Figure 2B). These data suggest that thymopoiesis is markedly reduced both in newly diagnosed and in chemotherapy-treated patients with ALL, and fails to recover at any point during the 2-year treatment course.
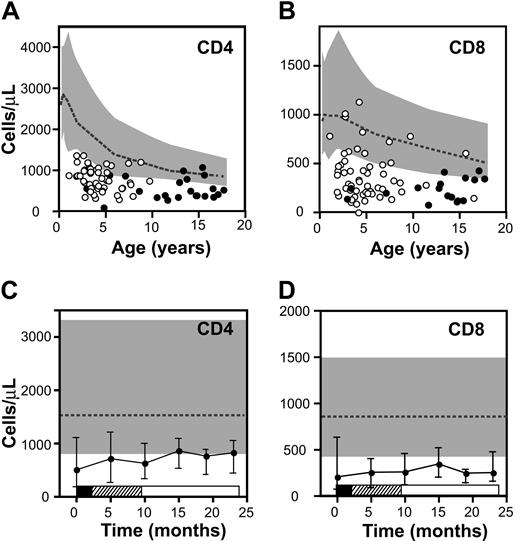
Absolute T-cell counts in patients with ALL at diagnosis and during therapy. (A-B) Mean CD4 (A) and CD8 (B) T-cell counts for individual patients with respect to age (circles represent average values for all timepoints for each patient; ○ indicates standard-risk patients; •, high-risk patients). Boundaries for 10th to 90th percentiles for healthy children are indicated by the gray bands, and the dotted line represents the median. (C-D) T-cell counts over time. CD4 counts (C) and CD8 counts (D) in the cohort of patients are shown at diagnosis and at 5 points during therapy. Points represent median values and error bars interquartile range. Phases of treatment are shown by the bars below (▪ indicates induction; ▨, consolidation; and □, maintenance). Gray band represents 10th to 90th percentiles, and the dotted line represents the median for healthy children.
Absolute T-cell counts in patients with ALL at diagnosis and during therapy. (A-B) Mean CD4 (A) and CD8 (B) T-cell counts for individual patients with respect to age (circles represent average values for all timepoints for each patient; ○ indicates standard-risk patients; •, high-risk patients). Boundaries for 10th to 90th percentiles for healthy children are indicated by the gray bands, and the dotted line represents the median. (C-D) T-cell counts over time. CD4 counts (C) and CD8 counts (D) in the cohort of patients are shown at diagnosis and at 5 points during therapy. Points represent median values and error bars interquartile range. Phases of treatment are shown by the bars below (▪ indicates induction; ▨, consolidation; and □, maintenance). Gray band represents 10th to 90th percentiles, and the dotted line represents the median for healthy children.
T-cell deficit at diagnosis and during therapy
Given the observed defect in thymic function, we measured the effect of ALL and its treatment on the peripheral T-cell compartment. Absolute T-cell counts were measured at diagnosis and during therapy. At diagnosis there was a marked defect in CD4 and CD8 T-cell numbers compared with healthy controls (P < .001; Figure 3). The median CD4 count was 508 cells/μL (range, 0-5035 cells/μL) and 55% of patients had CD4 counts less than the 10th percentile for age. Similarly, the median CD8 count at diagnosis was low at 204 cells/μL and was less than the 10th percentile for age in 77% patients. The T-cell counts were also measured at 5 timepoints during therapy (Figure 3C-D). CD4 and CD8 T-cell counts were below the 10th percentile for age in 45% and 88% of patients, respectively, and no significant recovery of the T-cell compartment occurred with time (P = .35 for CD4+ T cells and P = .56 for CD8+ T cells). As blood samples were collected at the start of 3-week cycles of chemotherapy, it is likely that samples obtained during the chemotherapy cycles would have shown even lower T-cell counts.
This defect in T-cell numbers was not due to persistence of marrow infiltration with leukemia, as patients rapidly cleared their leukemic burden (data not shown). Moreover, myelopoiesis recovered during therapy. At diagnosis, the median absolute neutrophil count (ANC) was low at 338 cells/μL (range, 0-11.6 × 103 cells/μL; data not shown). However, the median ANC increased over time (P = .001), and by 10 months after diagnosis, 70% of patients had an ANC within normal reference range for age, reflecting the less dose-intensive nature of maintenance therapy in the latter portion of the protocol. Thus, the T-cell defect seen at diagnosis and during therapy occurs in the absence of persistent leukemia and despite ongoing recovery of myelopoiesis.
Age and treatment intensity influence T-cell homeostasis
In order to investigate factors influencing T-cell homeostasis in this population, we performed statistical modeling using mixed effects models addressing age, treatment intensity, disease type (B-cell phenotype versus T-cell phenotype) and time on study. Age was strongly predictive of CD4 counts with older children having lower counts (P < .001), whereas the association between age and CD8 counts was weaker (P = .09). Treatment intensity also affected T-cell homeostasis, with patients treated on the high-risk arm having lower CD4 counts. There was a significant interaction between treatment group and age, such that being treated on the high-risk arm attenuated the benefit of young age on CD4 counts (P = .04). There was no association between disease type and T-cell numbers or phenotype.
Remaining T cells have a predominantly memory phenotype
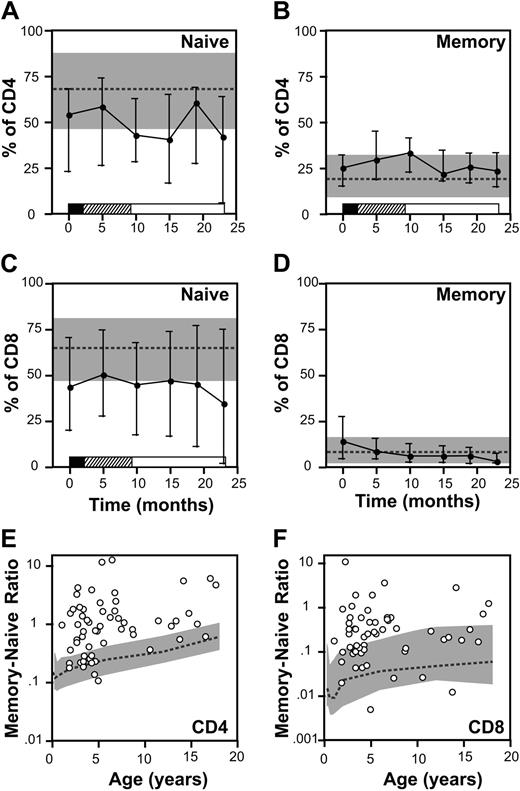
We next determined if the defect in T-cell number was predominantly found in the naive or in the memory T-cell compartment. At diagnosis the fraction of CD4+ and CD8+ T cells with a naive phenotype (CD45RA/CD62L) was significantly decreased with respect to age-matched controls (P < .001; Figure 4). The median CD4/CD45RA/CD62L fraction was 54% (range, 0%-88%) and 44% of patients had values less than the 10th percentile for age. Similarly, the CD8+ naive fraction was also reduced (P < .001) with a median value at diagnosis of 44% (range, 0%-76.8%) and 47% of patients had values below the 10th percentile values. Naive CD4+ and CD8+ T-cell fractions both remained below the 10th percentile for age in 53% of patients during therapy.
In contrast, the percentage of T cells with the phenotype of CD45RO was disproportionately elevated at diagnosis and throughout therapy (Figure 4). In newly diagnosed patients the fraction of CD4+ and CD8+ T cells with a CD45RO+ phenotype was increased (P < .001) and the percent CD4 CD45RO+ T cells remained significantly elevated in the entire cohort compared with healthy controls at all timepoints tested (P < .001). However, the absolute number of CD4+ or CD8+ T cells with a memory phenotype remained below the 10th percentile for age in 63% and 92% of patients, respectively.
To characterize the imbalance between T cells with a naive and memory phenotype for individual patients rather than across the cohort, we calculated ratios of memory-to-naive T cells in each sample. In healthy children, the ratio of memory-to-naive T cells gradually increases with age, presumably reflecting increasing exposure to infectious pathogens over time (Figure 4E-F). Compared with healthy children, patients with ALL had a significantly elevated ratio of memory-to-naive CD4 and CD8 T cells (P < .001). The ratio for CD4+ and CD8+ T cells was greater than the 90th percentile for age in 64% and 46% of patients, respectively. (Figure 4E-F). Thus, while the absolute number of T cells is reduced compared with healthy children, the T-cell compartment is proportionately enriched for CD45RO+ cells.
Memory and naive T cells. (A-D) Percentages of CD4 (A-B) and CD8 (C-D) cells staining with CD45RA and CD62L (A,C) or CD45RO (B,D) at diagnosis and at 5 points during therapy. Points represent median values; error bars, interquartile range. Phases of treatment are shown by the bars below (▪ indicates induction; ▨, consolidation; and □, maintenance). Gray band represents 10th to 90th percentiles for healthy children and the dotted line represents the median. (E-F) Ratio of CD45RO to CD45RA/CD62L cells in the CD4 (E) and CD8 (F) T-cell compartments with respect to age. Ratio is plotted on a log scale. Circles represent mean values for individual patients obtained at diagnosis and during therapy. Gray band represents 10th to 90th percentiles of age-specific ratios, and the dotted line represents the median for healthy children.
Memory and naive T cells. (A-D) Percentages of CD4 (A-B) and CD8 (C-D) cells staining with CD45RA and CD62L (A,C) or CD45RO (B,D) at diagnosis and at 5 points during therapy. Points represent median values; error bars, interquartile range. Phases of treatment are shown by the bars below (▪ indicates induction; ▨, consolidation; and □, maintenance). Gray band represents 10th to 90th percentiles for healthy children and the dotted line represents the median. (E-F) Ratio of CD45RO to CD45RA/CD62L cells in the CD4 (E) and CD8 (F) T-cell compartments with respect to age. Ratio is plotted on a log scale. Circles represent mean values for individual patients obtained at diagnosis and during therapy. Gray band represents 10th to 90th percentiles of age-specific ratios, and the dotted line represents the median for healthy children.
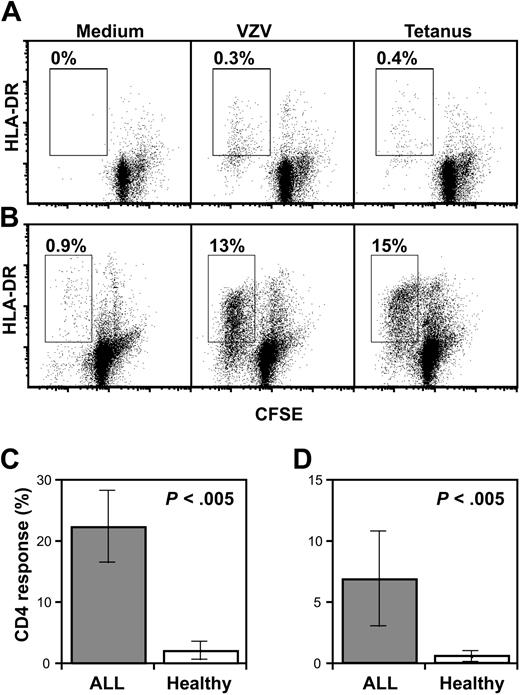
We used a functional assay to determine if the proportional increase in CD45RO+ T cells was associated with a relative increase in T-cell memory response to antigens encountered prior to the diagnosis of ALL. In order to minimize the contribution of T-cell responses to antigens that had been encountered after diagnosis, we studied T-cell response to antigens contained in routine childhood vaccines.18 Vaccination is not undertaken during therapy, therefore any response to these antigens must reflect pre-existing T-cell memory. T-cell proliferation to varicella zoster virus (VZV) lysate and tetanus toxoid (TT) was measured in 10 patients with ALL during treatment and compared with 10 control pediatric subjects using a sensitive, CFSE-based assay.17 As CD8+ T-cell proliferation to soluble antigens is limited, we focused our analysis on CD4+ T cells. Figure 5 shows that CD4+ T-cell proliferation both to VZV (mean 2.1%, range, 0%-15%) and to TT (mean 0.57%, range, 0%-4.5%) could be readily detected in most healthy controls. However, in patients treated for ALL, the fraction of T cells proliferating to both VZV (mean, 22%; range, 0.9%-54%) and TT (mean, 6.9%; range, 0.2%-39%) was significantly greater than in age-matched controls (P < .005). Thus, the increased frequency of T cells with a memory phenotype remaining in patients treated for ALL is associated with an increase in memory response to recall antigen in vitro.
Antigen-specific T-cell memory. (A-B) CD4 memory responses to VZV (middle) or TT (right) antigens or in control conditions (left) for a representative healthy donor (A) or patient with ALL (B). Gates represent proliferating/activated CD4 cells and percentages in gates are indicated on the dot plots. HLA-DR indicates human leukocyte antigen-DR. (C-D) CD4 response (percentage of CD4+ T cells proliferating) in patients with ALL (n = 10) or healthy children (n = 10) to VZV antigen (C) or TT (D). Error bars represent standard error of the mean.
Antigen-specific T-cell memory. (A-B) CD4 memory responses to VZV (middle) or TT (right) antigens or in control conditions (left) for a representative healthy donor (A) or patient with ALL (B). Gates represent proliferating/activated CD4 cells and percentages in gates are indicated on the dot plots. HLA-DR indicates human leukocyte antigen-DR. (C-D) CD4 response (percentage of CD4+ T cells proliferating) in patients with ALL (n = 10) or healthy children (n = 10) to VZV antigen (C) or TT (D). Error bars represent standard error of the mean.
Discussion
We show in this study that children undergoing a 2-year chemotherapy regimen for ALL have a persistent, marked deficit in T-cell numbers and thymopoiesis but retain a T-cell compartment that is enriched for T-cell memory.
This prospective study in a large cohort of children treated with a uniform chemotherapy regimen confirms the findings by Mackall et al4-7 and extends them. Our study confirms the profound loss of naive T cells and reduction in thymic output in children treated with chemotherapy. Moreover, we extend previous observations by defining the nature of the residual T-cell compartment. We show that the remaining T-cell compartment is enriched for T cells that not only have a memory phenotype but are also specific for previously encountered antigens—true memory T cells. Therefore, the relative excess of T cells with a memory phenotype in lymphopenic patients cannot simply be due to homeostatic proliferation of naive cells.
Why might the population of memory T cells be relatively spared in patients treated with chemotherapy? Our data show marked reduction in sjTREC levels and naive T cell fraction both at diagnosis and during therapy. Murine studies have shown that radiotherapy and chemotherapy lead to the death of thymic epithelial cells, resulting in reduced intrathymic interleukin 7 (IL-7) production and decreased T-cell neogenesis.19,20 Our study also demonstrates that reduced thymic output is evident not only during treatment, but also at diagnosis preceding chemotherapy. Therefore both the presence of ALL and its treatment impairs thymic function and is likely to decrease the production of naive T cells. Kinetic studies of T-cell turnover in mice and humans have shown that memory T cells in the peripheral blood proliferate faster than do naive cells.21-27 Prolonged suppression of T-cell neogenesis coupled with faster proliferation in the memory T-cell pool may therefore account for the observed disequilibrium between memory and naive T cells. Our findings also are compatible with alternative models. For instance the reduction in sjTREC levels may also represent an increased rate of death among recent thymic emigrants. However, we have found no evidence of an increase in sensitivity to chemotherapeutic agents in sorted naive compared with memory T cells in vitro (data not shown). Definitive understanding of the kinetics underlying the relative excess of memory T cells will require further study using techniques to quantify T-cell proliferation in intrathymic,28 naive, and memory compartments in the lymphopenic host.
The finding that the residual T-cell compartment is enriched for memory T cells has several implications for the therapeutic manipulation of immunity in patients with cancer. First, because the memory T-cell compartment is least affected by ALL and its treatment, strategies to expand antigen-specific T-cell responses in cancer patients to infectious pathogens or tumor antigens may be most successful if they target memory T cells. Our data emphasize the fact that the pool of naive T cells capable of participating in a response to previously unencountered antigens is severely contracted during chemotherapy and potentially for months or years thereafter. Vaccination against a neo-antigen—hepatitis B—during treatment for ALL has been reported to be unsuccessful in nearly 75% of children.29 We recently reported a phase 1 tumor vaccine trial in ALL,30 based on our previous observation that T cells specific for autologous tumor cells can be detected in many children with ALL.31-33 Several groups have also identified epitopes from candidate tumor antigens that could serve as targets for tumor vaccination in ALL.34-36 Our data suggest that the most successful approach for tumor vaccination in ALL will be the one that aims to expand antigen-experienced cells from within the relatively well-preserved memory T-cell pool, rather than priming T cells from the profoundly depleted naive compartment. Further studies of vaccination response in patients with ALL using both neo- and recall antigens will be necessary to determine if this hypothesis is correct.
Second, the low rate of infectious mortality that we and others have shown suggests that even a T-cell compartment that is greatly reduced in numbers may provide protective immunity if it is enriched for antigen-specific memory. The protective effect of memory T cells is supported by extensive clinical experience in patients treated with hematopoietic stem cell transplantation (HSCT). Analysis of immune reconstitution in recipients of unmanipulated grafts shows that for several months after transplantation, the T-cell compartment is composed principally of mature CD45RO+ T cells derived from the donor inoculum.37 The use of extensively T-cell-depleted grafts to decrease graft-versus-host disease is associated with lower T-cell counts following HSCT, reduces antigen-specific T-cell memory, and results in a higher rate of infectious complications.38-42 Conversely, enriching the peripheral T-cell pool with antigen-experienced T cells by the adoptive transfer of pathogen-specific T cells can effectively control Epstein-Barr virus-related disease in lymphopenic patients.43-46
Our observations indicate that although overall T-cell numbers are significantly reduced in children treated for ALL, the memory T-cell compartment is relatively well preserved. Given the low infectious mortality in these patients, our data suggest that a relatively small number of memory T cells may provide a clinically meaningful degree of protective immunity. Moreover, interventions that can expand T cells found in the memory compartment may be the most effective approach to improving antigen-specific T-cell immunity in patients with cancer.
Prepublished online as Blood First Edition Paper, May 26, 2005; DOI 10.1182/blood-2005-03-1082.
Supported by a Career Development Award from the American Society of Clinical Oncology (W.N.H.), by K08HL72 750 (W.N.H.) and by P01CA68 484 (W.N.H., L.B.S., E.C.G., L.M.N., S.E.S.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are indebted to the patients and families taking part in this study, and to the nurses and physicians who care for them. We greatly appreciate the support of Virginia Dalton and the 00-001 study team, and thank the National Institute of Allergy and Infectious Diseases and National Institute of Child Health and Human Development Pediatric AIDS Clinical Trials Group Protocol P1009 Team for use of their database of control lymphocyte subset values. We thank Angelo A. Cardoso and Ian Thornley for their critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal