Abstract
Primary mediastinal large B-cell lymphoma (MLBCL) shares important clinical and molecular features with classic Hodgkin lymphoma, including nuclear localization of the nuclear factor κB (NFκB) subunit c-REL (reticuloendotheliosis viral oncogene homolog) in a pilot series. Herein, we analyzed c-REL subcellular localization in additional primary MLBCLs and characterized NFκB activity and function in a MLBCL cell line. The new primary MLBCLs had prominent c-REL nuclear staining, and the MLBCL cell line exhibited high levels of NFκB binding activity. MLBCL cells expressing a superrepressor form of inhibitor of kappa B alpha signaling (IκBα) had a markedly higher rate of apoptosis, implicating constitutive NFκB activity in MLBCL cell survival. The transcriptional profiles of newly diagnosed primary MLBCLs and diffuse large B-cell lymphomas (DLBCLs) were then used to characterize the NFκB target gene signatures of MLBCL and specific DLBCL subtypes. MLBCLs expressed increased levels of NFκB targets that promote cell survival and favor antiapoptotic tumor necrosis factor α (TNFα) signaling. In contrast, activated B cell (ABC)-like DLBCLs had a more restricted, potentially developmentally regulated, NFκB target gene signature. Of interest, the newly characterized host response DLBCL subtype had a robust NFκB target gene signature that partially overlapped that of primary MLBCL. In this large series of primary MLBCLs and DLBCLs, NFκB activation was not associated with amplification of the cREL locus, suggesting alternative pathogenetic mechanisms. (Blood. 2005;106:1392-1399)
Introduction
The large B-cell lymphomas (LBCLs) include several distinct subtypes characterized by specific clinical features and/or transcriptional profiles. In addition to recognized entities such as primary mediastinal large B-cell lymphoma (MLBCL),1-3 there are recently described subtypes of diffuse large B-cell lymphoma (DLBCL) with likely differences in normal cell(s) of origin (COO), genetic bases for transformation, and comprehensive transcriptional signatures.4-6 With the newly available molecular signatures, it is now possible to evaluate the role of specific survival pathways in discrete LBCL subtypes.
The nuclear factor κB (NFκB) signaling pathway regulates the survival of normal and malignant B cells by controlling the expression of cell death regulatory genes.7,8 The extrinsic apoptotic pathway is triggered by engagement of tumor necrosis factor (TNF) family death receptors (TNF receptor 1 and TNF receptor superfamily member 6 [FAS, CD95]), and the intrinsic apoptotic pathway is activated by the translocation of proapoptotic B-CLL/lymphoma 2 (BCL2) family members to the mitochondria and subsequent release of cytochrome c.7 Depending on the cellular context, TNFα signaling and other stimuli (including B-cell receptor, CD40, and Toll-receptor engagement) also activate the NFκB pathway and augment the transcription of NFκB target genes.7 These NFκB target genes enhance cell survival by modulating TNFα signaling, inhibiting FAS-mediated apoptosis, and limiting the activity of proapoptotic BCL2 family members, in addition to multiple other effects.7
Inactive NFκB heterodimers (primarily reticuloendotheliosis viral oncogene homolog [c-REL] or REL homolog A [RELA] and NFκB1 [protein 50 {p50}]) reside in the cytoplasm where they are complexed with an inhibitor of kappa B signaling (IκB).7 In response to a variety of signals, Ikappa kinase (IκK) phosphorylates IκB, resulting in the inhibitor's dissociation from the cytoplasmic NFκB heterodimer. Thereafter, phosphorylated IκB is degraded via the proteosome, and the freed (active) NFκB heterodimer translocates to the nucleus where it induces the transcription of NFκB target genes.7 For this reason, the activity of the NFκB pathway in a B-cell tumor can be preliminarily assessed by determining the subcellular localization of NFκB subunits (nuclear vs cytoplasmic). This type of analysis was previously used to identify the likely role of c-REL-containing heterodimers and NFκB activation in classic Hodgkin lymphomas (cHLs).9
We recently characterized the transcriptional profile of primary MLBCL and identified important shared features with cHL.2 Like Hodgkin Reed-Sternberg (HRS) cells, MLBCLs had low levels of expression of multiple B-cell signaling components and coreceptors.2 MLBCLs also had high levels of expression of cytokine pathway components, TNF family members, and extracellular matrix elements previously identified in cHL.2 These observations were of particular interest because MLBCL and the most common subtype of cHL (nodular sclerosis) have similar clinical presentations (ie, in younger patients with local/mediastinal tumors characterized by reactive fibrosis).
Given the known role of the NFκB survival pathway in cHL,9-11 and the striking similarities between the cHL and MLBCL transcriptional profiles, including up-regulation of specific NFκB target genes, we assessed the subcellular localization of c-REL in a small pilot series of primary MLBCLs.2 In almost all cases, the c-REL NFκB subunit was localized to the nucleus, underscoring the potential role of constitutive NFκB activation in primary MLBCLs.2
Previous studies also implicate NFκB signaling in the survival of a subset of DLBCLs. DLBCLs are thought to arise from normal antigen-exposed B cells that have migrated to or through germinal centers (GCs) in secondary lymphoid organs.12 A series of recent profiling studies highlighted the similarities between subsets of DLBCL and their putative normal B-cell counterparts. These DLBCL subsets shared certain features with normal GC B cells (GC-type) or in vitro-activated peripheral blood B cells (activated B cell [ABC]-type); an additional group of tumors (type 3 or Other) could not be classified by COO.4,5 In associated functional analyses, DLBCL cell lines with ABC-type signatures had high levels of NFκB activity and increased sensitivity to NFκB inhibition, specifically implicating the NFκB survival pathway in this DLBCL subset.13
More recently, our group used a large series of newly diagnosed DLBCLs, whole genome arrays, and multiple clustering methods to identify 3 robust DLBCL subtypes with unique comprehensive transcriptional signatures—oxidative phosphorylation, B-cell receptor/proliferation, and host response (HR).6 HR tumors have increased expression of T/natural killer (NK)-cell receptor and activation pathway components, complement cascade members, macrophage/dendritic cell markers, and inflammatory mediators; these tumors also contain significantly higher numbers of CD2+/CD3+ tumor-infiltrating lymphocytes and interdigitating S100+/gamma-interferon-inducible lysosomal thiol reductase [GILT+]/CD1a-/CD123- dendritic cells.6 HR DLBCLs share features of histologically defined T-cell/histiocyte-rich LBCL, including fewer genetic abnormalities and presentation in younger patients with frequent splenic and bone marrow involvement.6 To date, the potential role of NFκB activation in HR tumors and the additional comprehensive clusters (CCs) has not been defined.
In LBCLs with likely NFκB activation, the genetic bases for constitutive NFκB signaling are not yet known. In cHL, gains of chromosome 2p12-16 (the cREL locus) have been associated with the accumulation of nuclear c-REL, suggesting that cREL amplification leads to increased NFκB activity.9 A small number of DLBCLs and MLBCLs also have gains of chromosome 2p12-16, prompting speculation regarding a similar mechanism of NFκB activation in LBCLs.14,15 However, recent studies suggest that cREL amplification is (1) more common in GC-type DLBCLs than ABC-type DLBCLs4 ; and (2) infrequently associated with nuclear c-REL expression.16
Given the likely role of NFκB in promoting normal and malignant B-cell survival, the NFκB pathway includes promising rational therapeutic targets.17 For these reasons, we have analyzed the role of NFκB activation in MLBCL and DLBCL subtypes using a combination of c-REL immunolocalization, molecular inhibition of NFκB in informative cell lines, and analyses of NFκB target gene signatures and cREL amplification in well-defined primary LBCLs.
Materials and methods
Cell lines
The Karpas 1106 MLBCL cell line (gift of A. Karpas, Cambridge, United Kingdom18 ), 2 DLBCL cell lines (DHL6 and OCI-LY1019 ), and a Hodgkin lymphoma cell line (KM-H2; German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were cultured at 37°C in 5% CO2 in RPMI-1640 medium (Mediatech Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (Tissue Culture Biologicals, Tulare, CA).
Immunohistochemistry
Immunohistochemistry (IHC) was performed on an additional series of primary MLBCLs obtained from the archives of Brigham & Women's Hospital using 5-μ thick formalin-paraffin-embedded tissue sections. Slides were deparaffinized and pretreated with 10 mM citrate (pH 6.0; Zymed, South San Francisco, CA) in a steam pressure cooker (Decloaking Chamber; BioCare Medical, Walnut Creek, CA) and subsequently washed in distilled water. All further steps were performed at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (DAKO USA, Carpinteria, CA) for 5 minutes to quench endogenous peroxidase activity. Primary rabbit anti-c-Rel antibody (AB-1; 1:1000 dilution; Oncogene Research Products, San Diego, CA) was applied in DAKO diluent (DAKO USA) for 1 hour. The specificity of the c-REL antibody was previously confirmed by (1) immunoblotting of a c-REL-positive control cell line and demonstrating reactivity with a single band of appropriate molecular weight (manufacturer's information [Oncogene, San Diego, CA]); and (2) detecting nuclear translocation of c-REL in a c-REL-positive control cell line stimulated with CD40 ligand.20 Slides were washed in 50 mM Tris (tris(hydroxymethyl)aminomethane)-Cl (pH 7.4), and anti-rabbit horseradish peroxidase (HRP)-conjugated antibody solution (Envision+ detection kit; DAKO USA) was applied for 30 minutes. After further washing, immunoperoxidase staining was developed with diaminobenzidine (DAB) chromogen (DAKO USA), and slides were counterstained with Harris hematoxylin (Polyscientific, Bay Shore, NY). c-REL IHC staining was evaluated using 3 distinct categories: absent (-), weak-to-intermediate (+), and intense (++) staining of tumor cell nuclei using an Olympus BX41 microscope equipped with an Olympus UPlanFL 40 ×/0.75 objective lens (Olympus, Melville, NY). Cytoplasmic staining was used as an internal control and reference to score the intensity of nuclear staining. Tumors were considered to have significant nuclear c-REL if 40% or more tumor cell nuclei exhibited + or ++ nuclear c-REL staining. The pictures were taken using Olympus QColor3 and analyzed with acquisition software QCapture v2.60 (QImaging, Burnaby, BC) and Adobe Photoshop 6.0 (Adobe, San Jose, CA).
NFκB activation assay
NFκB activity was assessed using a colorimetric assay21 that detects binding of cellular p50 (NFκB1) to immobilized NFκB target consensus oligonucleotide sequences (Active Motif, Carlsbad, CA). In brief, 5 μg of each whole-cell lysate was added to microwells containing immobilized NFκB-specific target probes. Following incubation and washing, cellular p50 bound to the immobilized NFκB target sequences was detected using a p50-specific polyclonal antibody, an HRP-conjugated secondary antibody, and colorimetric quantification. NFκB binding activity in study samples was compared with that of standardized negative and positive controls provided by the manufacturer (2.5 μg nuclear extract from phorbol 12-O-tetradecanoylphorbol-13-acetate [TPA]-stimulated Jurkat cells preincubated with NFκB target sequences [wild-type sequences, negative control; mutated sequences, positive control]).
Molecular cloning and retroviral transduction
An IκBα superrepressor construct in which serines 32 and 36 were replaced with alanines (SR-IκBα; gift of A. Rabson and C. Gelinas22 ) was used to inhibit NFκB activation in Karpas 1106 cells. SR-IκBα, which cannot be phosphorylated by IκK, remains complexed to the NFκB heterodimer, inhibiting NFκB translocation and activation of NFκB targets. SR-IκBα was cloned into a modified murine stem cell virus (MSCV) vector (Clontech, Palo Alto, CA) that expresses enhanced green fluorescent protein (eGFP) in a bicistronic manner with the gene of interest.23 MSCV-eGFP-SR-IκBα or MSCV-eGFP alone was cotransfected into 293T cells with pKAT (an amphotropic packaging plasmid) and pCMV-VSV-G (a vector encoding the vesicular stomatitis virus G-glycoprotein) using lipofectamine 2000 (Invitrogen, Carlsbad, CA). Supernatants containing retrovirus were harvested at 48 and 72 hours.
Karpas 1106 cells were washed in phosphate-buffered saline (PBS; Mediatech/Cellgro) and resuspended at 1 × 106 cells/mL in fresh media containing Polybrene (final concentration 8 mg/mL; Sigma, St Louis, MO). Karpas 1106 (500 μL) was added to individual wells of a 24-well plate, and 500 μL retroviral supernatant (MSCV-eGFP-SR-IκBα or vector only) was added thereafter. Cells were then spinoculated at 670 g for 90 minutes, incubated at 37°C in 5% CO2 overnight, and subsequently washed and resuspended in fresh medium. GFP expression was analyzed at 24 hours, and GFP-positive cells were isolated by fluorescence-activated cell sorting (FACS Vantage; Becton Dickinson, San Jose, CA). Statistical comparisons of NFκB activity in SR-IκBα- and vector-only-transduced cells were performed using a one-sided Student t test.
Cell viability and apoptosis
Proliferation of MSCV-eGFP-SR-IκBα- or vector-only-transduced Karpas 1106 cells was assessed by measuring MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium; Promega, Madison, WI) dye absorbance at 0, 24, 48, and 72 hours after cell sorting.19 Statistical comparisons of proliferation in SR-IκBα- and vector-only-transduced cells were performed using an analysis of variance (ANOVA) model on the natural logarithm of the data including the cell type (SR-IκB, empty vector, or parental cells) and time after sorting as variables. Apoptosis was assessed using annexin V conjugated to the red-fluorescent dye, Alexa 568, according to the manufacturer's recommendations (Roche Applied Science, Penzberg, Germany). The percentage of apoptotic cells was defined as the number of cells that coexpressed annexin V (Alexa 568) and GFP within the entire GFP+ cell population.
Microarray analysis: description of data set
The expression of NFκB target genes in primary large B-cell lymphomas was evaluated using a recently described data set of 176 DLBCL and 34 MLBCL transcriptional profiles2,6 (http://www.broad.mit.edu/mpr/publications/projects/Lymphoma/Mediastinal.res.gz). All primary DLBCL and MLBCL tumor specimens were nodal or mediastinal biopsies from newly diagnosed, previously untreated patients. The data set included the top 15 000 genes from the Affymetrix U133A/U133B arrays, ranked using a median absolute deviation (MAD) variation filter across all samples.2 The DLBCL tumor samples were previously assigned to the developmentally related COO categories: GCB, ABC, and Other using linear predictive scores.5,6 The DLBCL tumor samples were also assigned to the more recently described comprehensive consensus clusters: oxidative phosphorylation (OXP), B-cell receptor signaling/proliferation (BCR), and HR.6
Curation of microarray-based NFκB target gene sets
Three NFκB target gene sets were selected for detailed analysis based on the gene sets' relevance to the biology of normal and malignant B cells: (gene set 1) NFκB target genes that were down-regulated in Hodgkin lymphoma cell lines following introduction of NFκB superrepressor IκBΔN11 ; (gene set 2) previously described NFκB target genes that were differentially expressed at specific stages of normal B-cell development and/or in DLBCLs with ABC features13,24 ; (gene set 3) NFκB target genes that were down-regulated after siRNA silencing of REL-A (p65) in TNFα-stimulated HeLa cells.25 The complete gene sets and their associated microarray probe sets are included as Supplemental Material, available on the Blood website; see the Supplemental Document link at the top of the online article.
Enrichment test for NFκB genes in MLBCL
Gene set enrichment analysis (GSEA)2,6,26 was performed as previously described using the NFκB target gene sets and the MLBCL and DLBCL array data. Enrichment was assessed by (1) ranking the 15 000 genes with respect to the phenotype MLBCL versus DLBCL; (2) locating the represented members of a given NFκB target gene set within the ranked gene list; (3) measuring the proximity of the gene set to the overexpressed end of the ranked list with a Kolmogorov-Smirnoff (KS) score (with a higher score corresponding to a higher proximity); and (4) comparing the observed KS score to the distribution of 100 permuted KS scores for all gene sets. The P values were obtained by pooling the permuted KS scores for all the gene sets tested, and by locating the observed KS scores within the resulting permutation distribution (Supplemental Document S1).
Supervised analysis in the space of NFκB target genes
The 3 sets of NFκB target genes were combined for additional supervised analyses in specific lymphoma subsets (MLBCL vs DLBCL, ABC vs non-ABC DLBCL, HR vs non-HR DLBCL). For inclusion in the supervised analysis, NFκB target genes had to be represented in the 15 000-gene data set. Sixty-four of the 68 NFκB target genes met these criteria (Supplemental Document S1). NFκB target genes correlating with the class distinction of interest (ie, MLBCL vs DLBCL, ABC DLBCL vs non-ABC DLBCL, HR or non-HR DLBCL) were identified by ranking the target genes according to their signal-noise ratio (SNR) based on medians. Thereafter, the observed values in the data were compared with the 99th percentile of the permutation distribution (1000 permutations; Supplemental Document S1). In addition, the fold difference in expression of a given NFκB target was calculated by dividing the median expression value for the class of interest by the median expression value of the comparison group (ie, MLBCL/DLBCL). NFκB target genes that met the 99th percentile of the permutation distribution and exhibited a 30% or more difference in median expression values were considered to be differentially expressed (Supplemental Document S1).
The same method was used to assess differential expression of NFκB target genes in an independent set of 38 MLBCLs and 26 DLBCLs with available cDNA microarray (lymphochip) profiles.27 Ninety-two percent (59/64) of the combined set of NFκB target genes included in the 15 000 gene data set were also represented on the lymphochip platform (Supplemental Document S1 and Rosenwald et al3 ).
Quantitative PCR analysis of cREL amplification
Quantitative polymerase chain reaction (PCR) was used to measure cREL copy numbers in genomic DNA isolated using a published method.28 In brief, real-time PCR was performed using the ABI Prism 7700 sequence detector system (Applied Biosystems, Foster City, CA). cREL copy numbers were compared with those of 2 control genes (beta-2-microglobulin, albumin), which map to loci that are rarely involved in chromosomal gains or losses (primer sequences and reaction conditions available upon request). On each 96-well reaction plate, a dilution series of normal human genomic spleen DNA was used as reference. Further, each reaction plate included a negative control (normal human genomic DNA; Applied Biosystems) and 2 positive controls (genomic DNA isolated from 2 Hodgkin lymphoma cell lines with known cREL amplification, KM-H2 and L-42829 ; German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). All measurements were performed in triplicate. Cases with a ratio of cREL/beta-2-microglobulin and cREL/albumin copy number higher than 2 were considered positive for cREL amplification.
Results
Confirmation of nuclear subcellular localization of c-REL in MLBCL
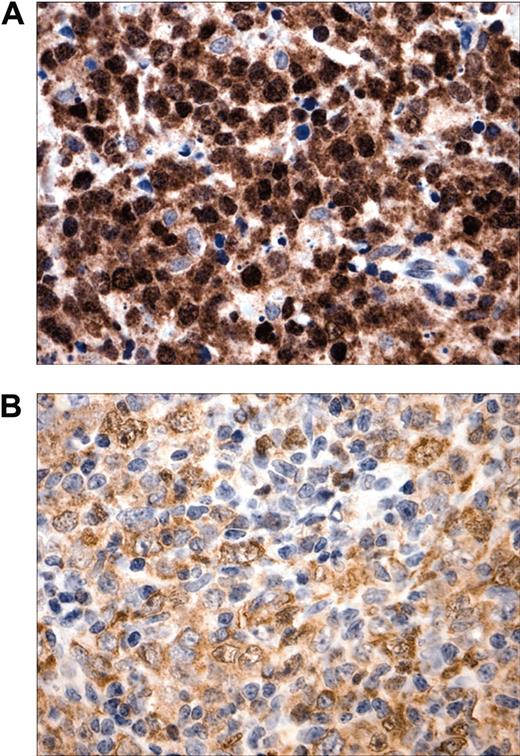
To confirm and extend our original observations regarding c-REL nuclear localization in MLBCL, we analyzed a new series of primary tumors with a robust c-REL immunohistochemical assay. As before, there was prominent c-REL nuclear staining in 7 of 7 new primary MLBCLs (Figure 1). c-REL staining intensity and subcellular localization were more variable in DLBCLs, consistent with recent observations.16
Subcellular localization of c-REL in MLBCL. (A) Prominent intense c-REL nuclear staining in a representative primary MLBCL. (B) Variable c-REL staining intensity and subcellular localization in a DLBCL. Original magnification, × 400.
Subcellular localization of c-REL in MLBCL. (A) Prominent intense c-REL nuclear staining in a representative primary MLBCL. (B) Variable c-REL staining intensity and subcellular localization in a DLBCL. Original magnification, × 400.
NFκB activity is required for cell survival of an MLBCL cell line
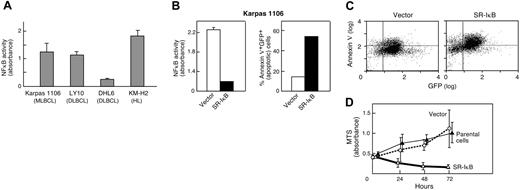
After demonstrating c-REL nuclear staining in the additional primary MLBCLs, we sought functional evidence of NFκB activation in an MLBCL cell line (Karpas 1106). Initially, NFκB activity in Karpas 1106 was measured and compared with that in lymphoma cell lines with previously reported high or low levels of NFκB activity (Hodgkin lymphoma line [KM-H2] and DLBCL line [OCI-Ly10], high; and DLBCL line [DHL6], low). Like KMH2 and OCI-Ly10, the Karpas 1106 MLBCL cell line had high levels of constitutive NFκB activity, over 4-fold greater than that of DHL6 cells (Figure 2A).
NFκB activity and inhibition in an MLBCL cell line. (A) NFκB DNA-binding activity of the MLBCL cell line, Karpas 1106; 2 DLBCL cell lines, OCI LY10 and DHL6; and a Hodgkin lymphoma cell line, KM-H2. NFκB DNA-binding activity was measured using a colorimetric assay. Measurements were performed in triplicates. (B) NFκB activity and apoptosis in MLBCLs expressing an IκBα superrepressor. (Left) NFκB DNA-binding activity in MLBCL cells transduced with MSCV-eGFP (vector) alone or MSCV-eGFP-SR-IκBα. Measurements were performed in triplicate. NFκB activity was significantly lower in SR-IκBα cells than in vector-only cells (P < .001, one-sided Student t test). (Right) The apoptotic fraction (percentage annexin V+ GFP+) of cells transduced with MSCV-eGFP-SR-IκBα is much higher than that of cells transduced with vector alone. (C) Apoptosis in MLBCL cells expressing an IκB superrepressor. GFP-positive cells expressing vector alone or MSCV-eGFP-SR-IκBα were analyzed for expression of annexin V (Alexa 568). GFP, x-axis; annexin V, y-axis. (D) Proliferation of MLBCL cells expressing an IκBα superrepressor. The proliferation (MTS absorbance) of parental and GFP+ vector-only- and MSCV-eGFP-SR-IκBα-transduced MLBCL cells was measured at 0, 24, 48, and 72 hours and assessed with an ANOVA model that included the type of treatment and time of measurement. The difference between SR-IκBα-transduced MLBCL cells and either vector-only or parental cells was significant (P < .001 in each case), whereas there was no difference between empty vector and parental cells (P = NS). The measurements were performed in triplicate. All experiments (A-D) were performed 3 to 4 times with comparable results; representative experiments are shown. Error bars indicate the standard deviation within triplicate experiments.
NFκB activity and inhibition in an MLBCL cell line. (A) NFκB DNA-binding activity of the MLBCL cell line, Karpas 1106; 2 DLBCL cell lines, OCI LY10 and DHL6; and a Hodgkin lymphoma cell line, KM-H2. NFκB DNA-binding activity was measured using a colorimetric assay. Measurements were performed in triplicates. (B) NFκB activity and apoptosis in MLBCLs expressing an IκBα superrepressor. (Left) NFκB DNA-binding activity in MLBCL cells transduced with MSCV-eGFP (vector) alone or MSCV-eGFP-SR-IκBα. Measurements were performed in triplicate. NFκB activity was significantly lower in SR-IκBα cells than in vector-only cells (P < .001, one-sided Student t test). (Right) The apoptotic fraction (percentage annexin V+ GFP+) of cells transduced with MSCV-eGFP-SR-IκBα is much higher than that of cells transduced with vector alone. (C) Apoptosis in MLBCL cells expressing an IκB superrepressor. GFP-positive cells expressing vector alone or MSCV-eGFP-SR-IκBα were analyzed for expression of annexin V (Alexa 568). GFP, x-axis; annexin V, y-axis. (D) Proliferation of MLBCL cells expressing an IκBα superrepressor. The proliferation (MTS absorbance) of parental and GFP+ vector-only- and MSCV-eGFP-SR-IκBα-transduced MLBCL cells was measured at 0, 24, 48, and 72 hours and assessed with an ANOVA model that included the type of treatment and time of measurement. The difference between SR-IκBα-transduced MLBCL cells and either vector-only or parental cells was significant (P < .001 in each case), whereas there was no difference between empty vector and parental cells (P = NS). The measurements were performed in triplicate. All experiments (A-D) were performed 3 to 4 times with comparable results; representative experiments are shown. Error bars indicate the standard deviation within triplicate experiments.
We next assessed the consequences of NFκB inhibition on the survival and proliferation of Karpas 1106 MLBCL cells. The MLBCL cells were transduced with a retrovirus encoding GFP and the superrepressor form of IκBα (SR-IκBα) or GFP alone. Thereafter, GFP-expressing SR-IκBα and vector-only MLBCL cells were isolated by FACS and analyzed for NFκB activity. As expected, SR-IκBα MLBCL cells had approximately 6-fold less NFκB activity than cells expressing the vector alone (P < .001, one-sided Student t test; Figure 2B). SR-IκBα MLBCL cells had a 4-fold higher rate of apoptosis (annexin V expression) than cells expressing the vector alone (SR-IκBα [54% apoptosis] vs vector-only [14% apoptosis]; Figure 2B [right panel],C). SR-IκBα MLBCL cells also failed to proliferate in culture, whereas vector-only cells grew at the same rate as parental Karpas 1106 cells (Figure 2D). The difference between SR-IκB-transduced MLBCL cells and either empty vector-expressing or parental cells was significant (P < .001 in each case), whereas there was no difference between empty vector and parental cells (P = not significant [NS]). Taken together, these data indicate that constitutive NFκB activity is essential for the survival of this MLBCL cell line.
The primary MLBCL signature is enriched for NFκB target genes
After demonstrating the importance of NFκB activity for the proliferation and viability of a MLBCL cell line, we assessed the consequences of NFκB activation in our series of primary MLBCLs with available transcriptional profiles. Specifically, we asked whether previously defined, coregulated sets of NFκB target genes were up-regulated in primary MLBCLs (compared with DLBCLs) using GSEA. Three independently defined sets of NFκB target genes with likely biologic relevance in MLBCL were used: a series of target genes responsive to NFκB inhibition in Hodgkin lymphoma cell lines; a series of target genes responsive to NFκB inhibition in TNF-stimulated adherent cells11,25 ; and an additional series of previously described NFκB targets that were more abundant in in vitro-activated normal peripheral blood B cells and ABC-like DLBCLs.13,24 The primary MLBCL signature was significantly enriched for the credentialed NFκB targets from Hodgkin lymphoma cell lines (gene set 1, P = .05) and the likely NFκB targets in normal ABCs and ABC-like DLBCLs (gene set 2, P = .02). In addition, there was a trend toward more abundant TNF-induced NFκB target transcripts in primary MLBCLs (gene set 3, P = .08).
MLBCLs are characterized by a broad NFκB activation signature
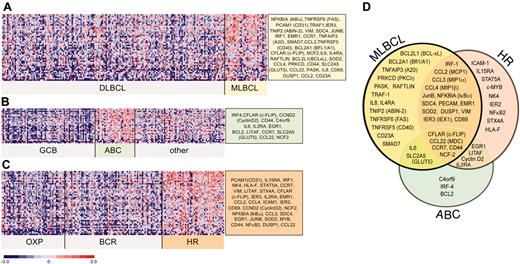
Given the partial overlap between the 3 sets of NFκB target genes used for GSEA, we then combined these gene sets for more detailed analyses of NFκB activation signatures in primary MLBCLs and previously defined subsets of DLBCLs. A large series of NFκB targets were expressed at significantly higher levels in MLBCL than DLBCL (Figure 3A), including genes regulating cell viability. To compare the NFκB target gene signature of MLBCL with that of ABC-like DLBCLs, we also identified the differentially expressed NFκB target genes in ABC-like versus non-ABC-like DLBCLs (Figure 3B). Not surprisingly, the ABC-like DLBCLs in our series had more abundant expression of previously described ABC NFκB targets including BCL-2, interferon regulatory factor 4 (IRF4), cyclin D2, and CD44. However, there was a much smaller series of abundant NFκB targets in ABC-like DLBCLs and only limited overlap with the NFκB activation signature in primary MLBCLs (compare Figure 3A, B, and D).
Confirmation of NFκB activation signature in MLBCL in an independent data set
We next assessed the reproducibility of the identified NFκB target gene signatures in MLBCL and DLBCL subsets using an independent series of primary tumors (38 MLBCLs and 13 ABC-like and 13 GC-like DLBCLs) with publicly available transcriptional profiles.3 This independent series of primary MLBCLs also had significantly higher expression of 79% of the NFκB target genes that were identified in our series and represented on both platforms (compare Figures 3A and 4A; and Supplemental Document S1). Within the independent series of DLBCLs, tumors with ABC features also had significantly higher expression of many of the identified NFκB targets in our series (compare Figures 3B and 4B; and Supplemental Document S1).
Differential expression of NFκB target genes in large B-cell lymphoma subtypes. NFκB target genes that met the 99th percentile of the permutation distribution and exhibited a 30% or more difference in median expression values were considered to be differentially expressed. Differentially expressed NFκB target genes in (A) primary MLBCL versus DLBCL; (B) ABC-like DLBCLs versus GCB-like and other DLBCLs; and (C) HR DLBCLs versus OxPhos and BCR/proliferation DLBCLs. (D) Comparison of the NFκB target gene signatures from primary MLBCL, HR DLBCLs, and ABC-like DLBCLs.
Differential expression of NFκB target genes in large B-cell lymphoma subtypes. NFκB target genes that met the 99th percentile of the permutation distribution and exhibited a 30% or more difference in median expression values were considered to be differentially expressed. Differentially expressed NFκB target genes in (A) primary MLBCL versus DLBCL; (B) ABC-like DLBCLs versus GCB-like and other DLBCLs; and (C) HR DLBCLs versus OxPhos and BCR/proliferation DLBCLs. (D) Comparison of the NFκB target gene signatures from primary MLBCL, HR DLBCLs, and ABC-like DLBCLs.
Amplification of the cREL locus is associated with high c-REL transcript abundance but not with an NFκB activation signature
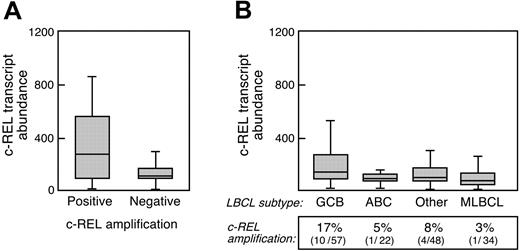
A small number of DLBCLs and primary MLBCLs have amplification of the cREL locus, raising the possibility that higher cREL copy numbers increase c-REL transcript abundance and augment NFκB activity. To address this issue in our lymphoma series, we analyzed cREL copy numbers in the primary tumors and compared cREL amplification and transcript abundance with the identified NFκB activation signature. As expected, primary DLBCLs with an amplified cREL locus (15/127 examined tumors) had significantly more abundant c-REL transcripts (2.5 × higher, P < .001; Figure 5A). cREL amplification was somewhat more common in DLBCLs with GC features, as previously described (% cREL amplification in GC vs non-GC DLBCLs, P = .06; Figure 5B).4 Consistent with this observation, GC-like DLBCLs also had more abundant c-REL transcripts than non-GC DLBCLs (1.7 × higher c-REL transcripts, P < .005; Figure 5B). Although c-REL transcripts were more abundant in GC-like DLBCLs, these tumors did not exhibit up-regulation of the identified NFκB target genes (Figure 3B), and primary GC-like DLBCLs had largely cytoplasmic c-REL expression (4/6 tumors cytoplasmic only, 2/6 tumors cytoplasmic with < 5% nuclear staining; data not shown). In addition, GC-like DLBCL cell lines had low levels of NFκB activity (Figure 2A and Davis et al13 ). Conversely, our series of MLBCLs had evidence of NFκB activation, although cREL amplification was rare (1/34 tumors) and c-REL transcripts were not increased (Figures 3A,5B). Taken together, the data indicate that in LBCLs, increased abundance of cREL per se does not translate into increased NFκB activity, and NFκB activation is not primarily dependent on c-REL transcript abundance.
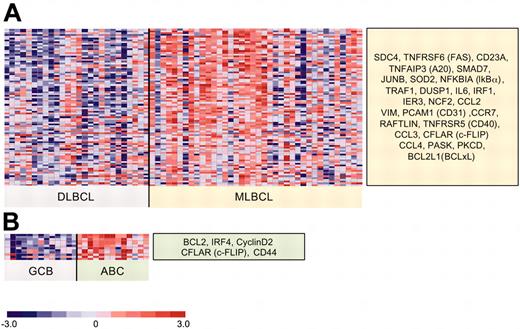
Differential expression of NFκB target genes in an independent series of primary MLBCLs and DLBCLs. The independent data set includes all primary MLBCLs (38 tumors) and DLBCLs (26 tumors: 13 GC-type and 13 ABC-like) that were made available at the NIH Lymphoma/Leukemia Molecular Profiling Project website.27 Differentially expressed NFκB target genes were defined as in Figure 3. (A) MLBCLs versus DLBCLs. This independent series of primary MLBCLs had significantly higher expression of 79% (26/33) of the NFκB target genes that were identified in our series and represented on both platforms; and (B) GC-type versus ABC-like DLBCLs.
Differential expression of NFκB target genes in an independent series of primary MLBCLs and DLBCLs. The independent data set includes all primary MLBCLs (38 tumors) and DLBCLs (26 tumors: 13 GC-type and 13 ABC-like) that were made available at the NIH Lymphoma/Leukemia Molecular Profiling Project website.27 Differentially expressed NFκB target genes were defined as in Figure 3. (A) MLBCLs versus DLBCLs. This independent series of primary MLBCLs had significantly higher expression of 79% (26/33) of the NFκB target genes that were identified in our series and represented on both platforms; and (B) GC-type versus ABC-like DLBCLs.
NFκB-activation signature in HRS-type lymphomas
In addition to recognized LBCL subtypes (such as MLBCL) and DLBCLs with shared developmental features (ABC- and GC-like tumors), DLBCL subsets with highly reproducible, comprehensive transcriptional profiles have been identified (OxPhos, BCR/proliferation, host response [HR]6 ). To evaluate the potential role of the NFκB pathway in these newly described DLBCL subtypes, we compared NFκB target gene expression in each group of tumors. Of interest, HR tumors had increased expression of a broad series of NFκB targets that partially overlapped those expressed by primary MLBCLs (compare Figure 3A, C, and D). To more specifically define the NFκB target gene signature in HR DLBCLs, we performed GSEA with the individual NFκB target lists in HR versus non-HR tumors. The HR signature was significantly enriched for TNF-induced NFκB targets (P = .01) but not the other NFκB target gene sets. These data suggest that the consequences of NFκB activation may vary in tumors with different developmental features and specific microenvironmental signals (Figure 3D).
Discussion
Herein, we confirmed the nuclear accumulation of c-REL-containing NFκB heterodimers in an additional series of primary MLBCLs and directly implicated NFκB in the survival of an MLBCL cell line. As in Hodgkin lymphoma, the nuclear localization of c-REL may prove to be a useful diagnostic feature in primary MLBCL.30 The direct evidence of constitutive NFκB activation and NFκB-dependent survival of an MLBCL cell line also indicates that this pathway may be a promising rational therapeutic target.
In addition, we characterized the NFκB target gene signature of primary MLBCLs and compared their signature with that of additional LBCL subtypes (ABC-like DLBCLs and HR tumors). Primary MLBCLs expressed increased levels of NFκB targets that promote TNFα-induced cell survival (TNF-receptor-associated factor 1 [TRAF1],7 BCL2-related protein A1 [BFL1/A1],31,32 protein kinase C delta [PKCdelta],33 superoxide dismutase 2 [SOD2]34 ) and regulate TNFα signaling (TNFα-induced protein 3 [TNFAIP3, A20]35 and TNFA1P3 interacting protein 2 [TNIP2, ABIN2]36 ) (Figure 3A,D). In addition to BFL1/A1, MLBCL NFκB target genes include another antiapoptotic BCL2 family member, BCLxL.7 Primary MLBCLs also had increased expression of the critical NFκB target and key inhibitor of FAS-mediated apoptosis and caspase-8, caspase-8 and FADD-like apoptosis regulator (CFLAR, c-FLIP) (Figure 3A,D).37,38 These results are of particular interest given the documented role of c-FLIP-mediated resistance to death receptor-induced apoptosis in Hodgkin Reed-Sternberg cells.39,40 Like Hodgkin lymphoma, primary MLBCLs also express increased levels of the NFκB target and activator protein 1 (AP-1) transcription factor, JunB protooncogene (JUNB).41
Although primary ABC-like DLBCLs had a more restricted NFκB target gene signature than primary MLBCLs, ABC-like tumors also had more abundant c-FLIP transcripts (Figure 3B,D). Whereas primary MLBCLs expressed increased levels of BFL1/A1 and BCLxL, ABC-like DLBCLs had more abundant expression of a different NFκB target and antiapoptotic BCL2 family member, BCL2. Modulators of TNFα-induced cell survival, inflammatory cytokines, and adhesion molecules that were part of the MLBCL NFκB target signature were not seen in ABC-like DLBCL (Figure 3B,D). As expected, several of the NFκB targets that were more abundant in ABC-type DLBCLs (IRF-4, BCL2, cyclin D2) were also expressed at high levels in normal in vitro activated B cells,24 suggesting that these targets may be developmentally regulated in this LBCL subset.
cREL amplification and transcript abundance in large B-cell lymphoma subtypes. (A) c-REL transcript abundance in primary DLBCLs with an amplified cREL locus (positive, 15/127 examined tumors) or no cREL amplification (negative, 112/127 examined tumors). (B) c-REL transcript abundance and cREL amplification in DLBCLs arranged by cell of origin (GCB, ABC, and other) and primary MLBCL. Error bars indicate the standard deviation within triplicate experiments.
cREL amplification and transcript abundance in large B-cell lymphoma subtypes. (A) c-REL transcript abundance in primary DLBCLs with an amplified cREL locus (positive, 15/127 examined tumors) or no cREL amplification (negative, 112/127 examined tumors). (B) c-REL transcript abundance and cREL amplification in DLBCLs arranged by cell of origin (GCB, ABC, and other) and primary MLBCL. Error bars indicate the standard deviation within triplicate experiments.
In contrast to primary MLBCLs, ABC-like DLBCLs did not exhibit consistent nuclear localization of c-REL-containing heterodimers, prompting speculation that additional NFκB heterodimers may be active in these tumors. Consistent with this possibility, ABC-like DLBCL cell lines were previously reported to have p50/RELA DNA binding activity in addition to p50/c-REL heterodimers.13 Given the unique characteristics of the ABC-like NFκB target gene signature, these data suggest that NFκB activation may be associated with different combinations of NFκB heterodimers and signaling molecules in discrete LBCL subtypes.
In addition to characterizing the NFκB target gene signatures of primary MLBCL and ABC-type DLBCLs, we identified a robust NFκB target gene signature in HR DLBCLs. HR DLBCLs and primary MLBCLs share certain features including a brisk host inflammatory infiltrate and increased expression of associated NFκB target genes (chemokine [c-c motif] ligand 2 [CCL2, MCP1], chemokine [c-c motif] ligand 3 [CCL3, MIP1α], syndican 4 [SDC4],42 platelet/endothelial cell adhesion molecule 1 [PECAM1, CD31],43 SOD234 ; Figure 3D). Like primary MLBCLs and ABC-like DLBCLs, HR DLBCLs also had increased expression of c-FLIP, underscoring its potential importance in each tumor type. Consistent with the specific characteristics of the T/NK-cell-rich inflammatory infiltrate in HR DLBCLs, these tumors also had increased expression of NFκB targets potentially derived from infiltrating activated T/NK cells (IL15R44 and NK4; Figure 3D).
Given the prominent host inflammatory responses in primary HR DLBCLs and MLBCLs, it is of interest that the LBCLs express NFκB targets that may limit the effectiveness of host immune responses (HLA-F and CCL22; Figure 3D). Although major histocompatibility complex, class I, F (HLA-F) has limited tissue distribution, this atypical HLA class 1 molecule is expressed on B-lymphoblastoid and monocytoid cell lines.45 Recent reports suggest that HLA-F interacts with the inhibitory counterreceptors, ILT2 and ILT4, potentially limiting associated T- and NK-cell responses.46,47 The chemokine, (c-c motif) ligand 22 (CCL22, macrophage-derived chemokine [MDC]), which is expressed by macrophages, dendritic cells, and certain tumors (including Hodgkin lymphoma), attracts chemokine (c-c motif) receptor 4 (CCR4)+ T-regulatory cells into secondary lymphoid organs and areas of inflammation and limits host antitumor immune responses.48-50
After implicating the NFκB-cell survival pathway in primary MLBCLs and characterizing the NFκB target gene signatures in primary MLBCLs and ABC-like and HR DLBCLs, we assessed the role of cREL amplification in these tumors. Consistent with recent reports,4 we found that cREL amplification was more common in GC-like DLBCLs than in LCL subtypes with evidence of NFκB activation. In our series, only 1 of 34 primary MLBCLs had amplification of the cREL locus, although all analyzed tumors had nuclear accumulation of c-REL and NFκB target gene upregulation. Taken together, these data suggest that amplification of the cREL locus is not the pathogenetic mechanism associated with NFκB activity in LBCL subtypes and that additional gene(s) at 2p12-16 may contribute to the observed GC subtype.
The current studies define a role for NFκB-mediated tumor-cell survival in primary MLBCL and identify an overlapping NFκB target gene signature in the newly characterized HR DLBCLs. In addition, the studies delineate important differences between the shared NFκB target gene signature in primary MLBCLs and HR tumors and the more restricted, potentially developmental NFκB signature in ABC-type DLBCLs. In addition to providing specific insights regarding similarities and differences in NFκB signaling in these tumors, the data will guide our attempts to pharmacologically manipulate the NFκB pathway in these diseases.51
Prepublished online as Blood First Edition Paper, May 3, 2005; DOI 10.1182/blood-2004-12-4901.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal