Abstract
Using a cohort of C57BL/6 (B6) × (NZB × B6)F1 backcross male mice bearing the Yaa (Y-linked autoimmune acceleration) mutation, we mapped and characterized the NZB-derived susceptibility loci predisposing to the development of autoimmune hemolytic anemia (AHA). Our analysis identified 2 major loci on NZB chromosome 7 and chromosome 1 linked with Coombs antierythrocyte autoantibody production, and their contributions were confirmed by the analysis of B6.Yaa mice (B6 mice bearing the Yaa mutation) congenic for each NZB-derived susceptibility interval. A newly identified Aia3 (autoimmune anemia 3) locus present on NZB chromosome 7 selectively regulated Coombs antibody responses, while the second locus, directly overlapping with Nba2 (NZB autoimmunity 2) on chromosome 1, promoted the development of AHA, likely as part of its effect on overall production of lupus autoantibodies. A higher incidence of Coombs antibody production in B6.Aia3 congenic mice (B6 mice bearing the NZB-Aia3 locus) than B6.Nba2 mice (B6 mice bearing the NZB-Nba2 locus) indicated a major role for Aia3 in AHA. Notably, lack of expansion of B1 cells in B6.Aia3 congenic mice argued against the involvement of this subset in AHA. Finally, our analysis of BC mice also demonstrated the presence of a B6-derived H2-linked locus on chromosome 17 that apparently regulated the production of Coombs antibodies as a result of its overall autoimmune promoting effect.
Introduction
Autoimmune hemolytic anemia (AHA) is the oldest recognized autoimmune disease in humans. It is characterized by the destruction of red blood cells (RBCs) due to production of Coombs antierythrocyte autoantibodies. Since the discovery of the spontaneous development of AHA in the New Zealand black (NZB) strain of mice,1 this model has been widely used in studies aiming to define the immunologic mechanisms underlying this disease. Although the molecular nature of RBC autoantigens responsible for the induction of this autoimmune response has not been well characterized, it is clear that anti-RBC Coombs antibodies specific for exposed surface determinants of intact RBCs are of primary importance in the development of AHA.2 Studies of anti–mouse RBC monoclonal autoantibodies derived from NZB mice revealed that immunoglobulin G (IgG) Fc receptor– and complement receptor–dependent erythrophagocytosis by Kupffer cells, but not complement-mediated hemolysis, is the major pathogenic mechanism for the development of AHA.3-5 Furthermore, it has been suggested that CD5+ B1 cells in NZB mice may be involved in AHA because of a marked expansion of this subset in NZB mice6 and because of the production of Coombs antibodies by B1 cells in 4C8 anti-RBC transgenic mice.7,8 Although B1 cells are known to produce several types of natural autoantibodies,9,10 it is still unclear whether these cells are indeed responsible for the spontaneous production of pathogenic Coombs antibodies in NZB mice.
The pathogenesis of AHA is a complex process in which many factors apparently play essential roles. Among these, the importance of genetic factors in determining the incidence, onset, and severity of AHA has become clear. Notably, NZB mice have proved to be a powerful tool to define the genetic mechanism of the disease. Previous studies have provided evidence that AHA is under some form of polygenic control, with several genetic factors contributing to the overall susceptibility and progression of the disease.11-15 Early genetic analyses involving the NZB and NZC strains suggested the presence of 2 dominant or codominant AHA-susceptibility genes, Aia1 (autoimmune anemia 1) and Aia2.14 Although the Aia1 locus was approximately mapped to chromosome 4 based on linkage to the b (black/brown) coat color locus, classic progeny studies have provided only limited information on the number and chromosomal location of AHA-susceptibility genes. A recent genomewide linkage analysis using polymorphic microsatellite markers in (C57BL/6 × NZB)F1 × NZB backcross (BC) mice suggested that Coombs antibody production was negatively regulated by 2 dominant modifying genes present on C57BL/6 (B6) chromosomes 7 and 10.16 In contrast, the precise chromosomal location of NZB-derived susceptibility loci has never been defined.
The BXSB Y chromosome–linked mutant gene Yaa (Y-linked autoimmune acceleration) promotes the accelerated development of systemic lupus erythematosus (SLE) in BXSB mice and in their F1 hybrids with autoimmune-prone NZB, NZW, and MRL mice.17 Yaa is able to accelerate the spontaneous production of various autoantibodies, including Coombs antibodies, through interaction with autoimmune susceptibility genes present in different lupusprone mice, which by themselves are not sufficient to trigger lupuslike autoimmune responses.18,19 In contrast, the effect of the Yaa mutation is minimal in mice that are not predisposed to autoimmune diseases. Thus, genetic analyses involving Yaa represent a useful approach for unraveling the susceptibility loci implicated in murine AHA.
In the present study, we first determined whether (NZB × B6.Yaa)F1 male mice were able to develop Coombs antibodies in the presence of the Yaa mutation. Then, we used B6 × (NZB × B6.Yaa)F1 BC male mice and congenic mice bearing mapped susceptibility intervals to identify critical NZB-derived AHA-susceptibility loci. Here we report the mapping of 2 major quantitative trait loci (QTL) contributing to Coombs antibody production on NZB chromosome 7, designated Aia3 (autoimmune anemia 3), and on NZB chromosome 1 corresponding to the Nba2 (NZB autoimmunity 2) locus, which is known to control the overall production of lupus autoantibodies.20,21 The contribution of these 2 loci to AHA was confirmed by the analysis of congenic B6 mice bearing either of the NZB-derived susceptibility intervals. Furthermore, our results showed a lack of association of Coombs antibody production with expansion of B1 cells in the development of AHA.
Materials and methods
Mice
NZB mice (H2d) were purchased from the Jackson Laboratory (Bar Harbor, ME). B6 mice (H2b) bearing the Yaa mutation (B6.Yaa) were established by repeated backcrossing (more than 20 times), as described previously.19 (NZB × B6.Yaa)F1 and B6 × (NZB × B6.Yaa)F1 BC mice were obtained by local breeding in our animal facility. B6.NZB-Nba2 (B6.Nba2 [B6 mice bearing the NZB-Nba2 locus]) congenic mice were generated as described previously.20 B6 mice bearing the NZB-Aia3 locus (B6.Aia3) on chromosome 7 were generated by backcrossing an approximately 23 centiMorgan (cM) NZB-derived interval encompassing markers D7Mit154 and D7Mit194 onto the B6 background using marker-assisted selection, as described previously.22 After 6 generations of backcrossing, siblings were intercrossed to generate congenic mice homozygous for the NZB chromosome 7 intervals. Males of all congenic mice used in the present study carry the Yaa mutation. Blood samples were collected by orbital sinus puncture.
Detection of Coombs antibodies
A flow cytometric assay was used to detect Coombs antibodies using biotinylated rat anti–mouse k chain monoclonal antibody (mAb) (H139.52.1.5), followed by phycoerythrin (PE)–conjugated streptavidin, as described previously.23 The results are expressed as mean fluorescence intensity (MFI), analyzed with a FACSCalibur (BD Biosciences, San Jose, CA). Analysis of circulating RBCs from 4-month-old B6 male mice in multiple tests (10 mice in each assay) yielded consistent values of MFI, which were in the range of 4.0 to 4.5, and means + 3 SD never exceeded more than 9.0. Therefore, a positive Coombs test was defined as more than 9.0.
Determination of hematocrit (Ht)
Blood samples were collected into heparinized microhematocrit tubes and centrifuged in a microfuge, as described previously.3 The percentage of packed RBC volume was directly measured after centrifugation. Mean hematocrit (Ht) value (±SD) of 4-month-old B6 male mice (n = 30) was 44.9 (±1.7). Abnormal Ht values were defined as lower than 39.8 (mean –3 SD).
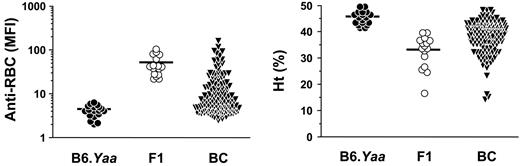
Coombs antibody activities and Ht values in B6.Yaa, (NZB × B6.Yaa)F1, and B6 × (NZB × B6.Yaa)F1 BC male mice. Coombs antibody activities and Ht values were determined at 10 months of age, and results are expressed as MFI for Coombs antibodies and as a percentage for Ht. Mean values are indicated by horizontal lines. Each symbol represents an individual animal of B6.Yaa (n = 18), (NZB × B6.Yaa)F1 (n = 16), and BC Yaa males (n = 144).
Coombs antibody activities and Ht values in B6.Yaa, (NZB × B6.Yaa)F1, and B6 × (NZB × B6.Yaa)F1 BC male mice. Coombs antibody activities and Ht values were determined at 10 months of age, and results are expressed as MFI for Coombs antibodies and as a percentage for Ht. Mean values are indicated by horizontal lines. Each symbol represents an individual animal of B6.Yaa (n = 18), (NZB × B6.Yaa)F1 (n = 16), and BC Yaa males (n = 144).
Genotyping and statistical analysis
Genotypes were determined by polymerase chain reaction (PCR) using 95 selected microsatellite markers either purchased from Research Genetics (Huntsville, AL) or Invitrogen (Carlsbad, CA). DNA from NZB, B6, (NZB × B6)F1, and BC mice was extracted from tail samples kept at –70°C before use. PCR amplification was conducted with RED Taq DNA polymerase (Sigma-Aldrich, Saint Louis, MO) using a GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, CA), as described.22 The positions of the microsatellite markers with respect to the centromere were obtained from the Mouse Genome Database.24 The linkage program MAPMAKER/QTL was used to identify QTL.25 Coombs antibody activities were log10 transformed. A threshold for suggestive linkage was set at log-likelihood of the odds (LOD) more than 1.9, and for significant linkage at LOD more than 3.3 based on the recommendation of Lander and Kruglyak.26 Coombs antibody activities and Ht values were correlated by Spearman rank correlation method, and P values were calculated using StatView (SAS Institute, Cary, NC). Coombs antibody levels among groups of BC mice with different combinations of AHA-susceptibility alleles were compared using nonparametric Dunn procedure of Kruskal-Wallis test. Probability values above 5% were considered insignificant.
Flow cytometric analysis
Flow cytometry was performed for the enumeration of B1 cells in peritoneal cavity using a FACSCalibur. The following antibodies were used: anti-B220 (RA3-6B2), anti-CD5 (53-7.3), and anti-CD11b (M1/70) mAb. Staining was performed in the presence of a saturating concentration of 2.4G2 anti-FcγRII/III mAb, as described previously.27
Results
Development of Coombs antibodies in autoimmune-prone (NZB × B6.Yaa)F1 Yaa male mice but not in B6.Yaa male mice
We assessed the possible spontaneous production of Coombs antibodies and the development of anemia in (NZB × B6.Yaa)F1 male mice, which develop severe SLE, with a 50% mortality rate at 14 months of age.21 At 10 months of age, all (NZB × B6.Yaa)F1 Yaa males (n = 16) displayed positive Coombs test, as determined by flow cytometric analysis (MFI ± SD, 51.9 ± 27.5; Figure 1). Notably, their Coombs antibody activities were comparable to those of 10-month-old NZB female mice (MFI of 10 mice ± SD, 55.4 ± 36.6). When Ht values were determined as a marker of AHA, 14 of 16 F1 male mice had abnormally low Ht values (mean values ± SD, 33.2 ± 6.4; Figure 1). In contrast, B6.Yaa mice (n = 18) remained negative for Coombs antibodies (4.5 ± 1.4; P < .001) and had normal Ht values (45.8 ± 2.5; P < .001).
Development of Coombs antibodies in B6 × (NZB × B6.Yaa)F1 BC mice bearing the Yaa mutation
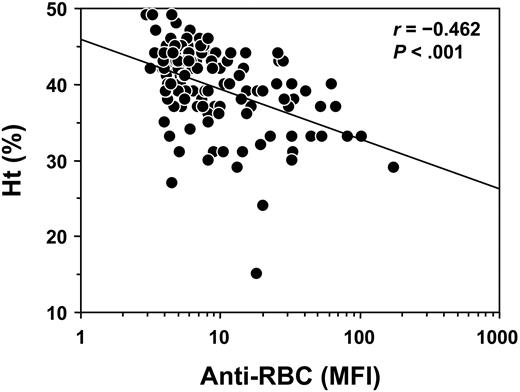
To determine the NZB genetic contribution to the Yaa-induced production of Coombs antibodies in (NZB × B6.Yaa)F1 male mice, a total of 144 B6 × (NZB × B6.Yaa)F1 male BC male mice bearing the Yaa mutation were produced and followed for the development of Coombs antibodies. At 6 months of age, 16% (23 of 144) of BC mice exhibited positive Coombs test, and cumulative incidence of Coombs antibodies reached 44% (64 of 144) at 10 months of age (Figure 1). In parallel, 19% (28 of 144) and 49% (70 of 144) of BC male mice had Ht values below 40 at 6 and 10 months of age, respectively (Figure 1). Notably, Coombs antibody titers and Ht values in individual mice at 10 months of age were inversely correlated (r =–0.462; P < .001; Figure 2).
Inverse correlation of Coombs antibody activities and Ht values in B6 × (NZB × B6.Yaa)F1 BC male mice bearing the Yaa mutation. Coombs antibody activities and Ht values were determined at 10 months of age, and results are expressed as MFI for Coombs antibodies and as a percentage for Ht. Each symbol represents an individual animal of BC Yaa males (n = 144). Linear regression line is indicated.
Inverse correlation of Coombs antibody activities and Ht values in B6 × (NZB × B6.Yaa)F1 BC male mice bearing the Yaa mutation. Coombs antibody activities and Ht values were determined at 10 months of age, and results are expressed as MFI for Coombs antibodies and as a percentage for Ht. Each symbol represents an individual animal of BC Yaa males (n = 144). Linear regression line is indicated.
Linkage of marker loci to Coombs antibodies in B6 × (NZB × B6.Yaa)F1 BC mice
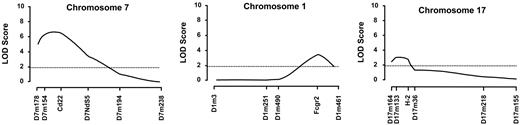
To map the chromosomal loci responsible for the regulation of Coombs antibody production, B6 × (NZB × B6.Yaa)F1 male BC mice were genotyped for microsatellite markers polymorphic between B6 and NZB mice. The analysis of serologic data at 10 months of age, using the program MAPMAKER/QTL, revealed significant or suggestive linkage with 3 chromosomal regions (Figure 3). The strongest linkage was obtained with an interval on NZB chromosome 7, peaking in the vicinity of the locus Cd22 at 9.0 cM from the centromere, with a LOD score of 6.62 (P = 3.33 × 10–8). This locus is close but distinct from a recently identified NZB-derived lupus-susceptibility locus, Nba5, peaking at the D7Nds5 marker (23.0 cM from the centromere), which is associated with the formation of gp70–anti-gp70 immune complexes (gp70 ICs) but not antichromatin and anti-DNA autoantibodies.21 This new NZB-derived AHA-susceptibility locus was designated Aia3. The second locus localized to an interval of NZB chromosome 1 directly overlapping with the Nba2 locus (about 90 to 98 cM from the centromere), which controls the overall production of lupus autoantibodies.20,21 In this interval, the Fcgr2 marker (92.3 cM from the centromere) gave a maximal LOD score of 3.45 (P = 6.66 × 10–5). The third locus peaked in the H2 region on chromosome 17, with a LOD score of 3.03 (P = 1.86 × 10–4). However, as opposed to Aia3 and Nba2, this susceptibility allele was inherited from the B6 strain, because H2b B6 homozygosity but not H2b/d heterozygosity was predisposed to Coombs antibody production. Notably, B6 mice carrying the H2b haplotype express I-Ab but not I-E molecules, because of the deletion of the promoter region of the Ea gene encoding I-E α-chains,28 while H2d-bearing NZB mice express both I-Ad and I-Ed molecules.
Linkage of chromosome 1, 7, and 17 markers with Coombs antibody activities in B6 × (NZB × B6.Yaa)F1 BC male mice bearing the Yaa mutation. Coombs antibody activities were determined at 6 and 10 months of age. LOD scores were generated with MAPMAKER/QTL, and the highest scores obtained at 10 months of age are shown. The horizontal dotted line represents the threshold for suggestive linkage.
Linkage of chromosome 1, 7, and 17 markers with Coombs antibody activities in B6 × (NZB × B6.Yaa)F1 BC male mice bearing the Yaa mutation. Coombs antibody activities were determined at 6 and 10 months of age. LOD scores were generated with MAPMAKER/QTL, and the highest scores obtained at 10 months of age are shown. The horizontal dotted line represents the threshold for suggestive linkage.
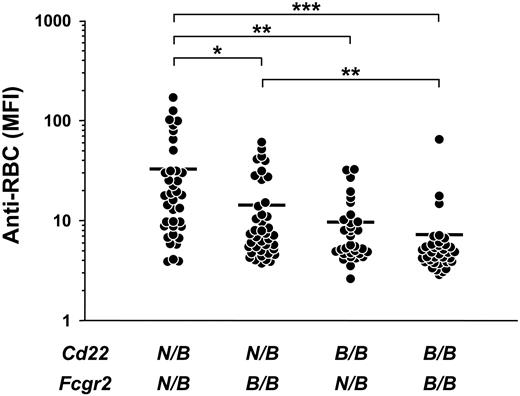
To further examine the effect of Aia3 and Nba2 on Coombs antibody production, Coombs antibody activities were plotted based on the genotype at the Cd22 and Fcgr2 loci, corresponding to Aia3 and Nba2, respectively, in BC mice. Among different groups of mice classified according to the combination of genotypes, mice heterozygous for both loci (ie, bearing the NZB allele) had the highest Coombs antibody levels (MFI ± SD, 32.8 ± 40.2), which significantly differed from those in mice heterozygous at only Cd22 (14.3 ± 15.2; P < .01) or Fcgr2 (9.7 ± 8.1; P < .005) and in mice homozygous for the B6 alleles at both loci (7.3 ± 10.7; P < .001) (Figure 4). These data suggested an additive effect of both Aia3 and Nba2 to the production of Coombs antibodies. Notably, levels of Coombs antibodies in mice heterozygous at Cd22 only were significantly higher than those of mice homozygous for the B6 alleles at both loci (P < .005), which was not the case for mice heterozygous at Fcgr2 only, consistent with the stronger linkage of Coombs antibody production with the Aia3 locus than with the Nba2 locus.
Development of Coombs antibodies in B6 congenic mice bearing the NZB-Aia3 locus
To confirm the contribution of the Aia3 locus to the development of AHA, B6.Aia3 congenic mice homozygous for an NZB-derived interval flanked by markers D7Mit154 and D7Mit194 (about 23 cM interval) were produced and analyzed for the spontaneous production of Coombs antibodies. As expected from the results obtained from BC mice, most (14 of 18) B6.Aia3 male mice bearing the Yaa mutation spontaneously developed increased Coombs antibody activities at 10 months of age (Figure 5; Table 1). However, their titers were significantly lower than those observed with (NZB × B6.Yaa)F1 Yaa male mice (P < .001). Although the incidence of positive Coombs antibody tests was less than that of their Yaa male counterparts (P < .005), 39% of B6.Aia3 females exhibited significant Coombs antibody activities, as compared with 10-month-old B6.Yaa male and female mice (P < .01 and P < .001, respectively). Ht values were also decreased in 61% of B6.Aia3 Yaa male mice, while only 1 of 18 female congenic mice displayed a significantly decreased Ht value at 10 months of age (P < .001; Figure 5; Table 1). Notably, none of the 10-month-old B6.Yaa male and female mice displayed abnormally reduced Ht values.
Coombs antibody activities and Ht values in B6.Aia3, B6.Nba2, and B6 mice
. | . | Coombs . | . | Ht . | . | ||
|---|---|---|---|---|---|---|---|
| Mice . | No. mice . | Mean MFI ± SD . | No. Coombs-positive mice* . | Mean Ht ± SD . | No. anemic mice† . | ||
| B6.Aia3 Yaa, male | 18 | 20.6 ± 14.5 | 14 | 37.4 ± 4.0 | 11 | ||
| B6.Aia3, female | 18 | 8.9 ± 4.3 | 7 | 45.0 ± 3.1 | 1 | ||
| B6.Nba2 Yaa, male | 15 | 9.3 ± 4.8 | 6 | 40.8 ± 5.5 | 3 | ||
| B6.Nba2, female | 17 | 5.8 ± 2.1 | 2 | 45.5 ± 1.9 | 0 | ||
| B6.Yaa, male | 15 | 5.4 ± 1.4 | 0 | 44.1 ± 2.0 | 0 | ||
| B6, female | 10 | 4.0 ± 1.0 | 0 | 44.7 ± 2.5 | 0 | ||
. | . | Coombs . | . | Ht . | . | ||
|---|---|---|---|---|---|---|---|
| Mice . | No. mice . | Mean MFI ± SD . | No. Coombs-positive mice* . | Mean Ht ± SD . | No. anemic mice† . | ||
| B6.Aia3 Yaa, male | 18 | 20.6 ± 14.5 | 14 | 37.4 ± 4.0 | 11 | ||
| B6.Aia3, female | 18 | 8.9 ± 4.3 | 7 | 45.0 ± 3.1 | 1 | ||
| B6.Nba2 Yaa, male | 15 | 9.3 ± 4.8 | 6 | 40.8 ± 5.5 | 3 | ||
| B6.Nba2, female | 17 | 5.8 ± 2.1 | 2 | 45.5 ± 1.9 | 0 | ||
| B6.Yaa, male | 15 | 5.4 ± 1.4 | 0 | 44.1 ± 2.0 | 0 | ||
| B6, female | 10 | 4.0 ± 1.0 | 0 | 44.7 ± 2.5 | 0 | ||
Coombs antibody activities and Ht values were determined at 10 months of age. *Defined as MFI > 9.0. †Defined as hematocrit < 39.8.
Additive effect of the Aia3 and Nba2 loci on the production of Coombs antibodies in B6 × (NZB × B6.Yaa)F1 BC male mice bearing the Yaa mutation. Coombs antibody activities were determined at 10 months of age. BC mice were subdivided into 4 groups according to genotype combinations at markers Cd22 and Fcgr2, corresponding to the Aia3 and Nba2 loci, respectively. N/B indicates NZB/B6 heterozygous genotype; B/B, B6/B6 homozygous genotypes. Each symbol represents an individual animal in each group: mice heterozygous at both loci (n = 37), mice heterozygous at only Cd22 (n = 40), mice heterozygous at only Fcgr2 (n = 32), and mice homozygous for the B6 alleles at both loci (n = 35). Mean values are indicated by horizontal lines. Significant differences between the groups are shown by asterisks (*P < .01, **P < .005, ***P < .001).
Additive effect of the Aia3 and Nba2 loci on the production of Coombs antibodies in B6 × (NZB × B6.Yaa)F1 BC male mice bearing the Yaa mutation. Coombs antibody activities were determined at 10 months of age. BC mice were subdivided into 4 groups according to genotype combinations at markers Cd22 and Fcgr2, corresponding to the Aia3 and Nba2 loci, respectively. N/B indicates NZB/B6 heterozygous genotype; B/B, B6/B6 homozygous genotypes. Each symbol represents an individual animal in each group: mice heterozygous at both loci (n = 37), mice heterozygous at only Cd22 (n = 40), mice heterozygous at only Fcgr2 (n = 32), and mice homozygous for the B6 alleles at both loci (n = 35). Mean values are indicated by horizontal lines. Significant differences between the groups are shown by asterisks (*P < .01, **P < .005, ***P < .001).
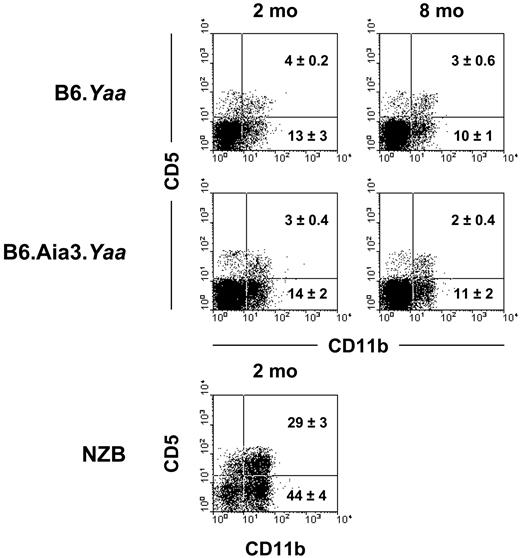
A unique cellular abnormality in NZB mice is the expansion of CD5+ B1 cells in the peritoneal cavity.6 In addition, studies in 4C8 Coombs anti-RBC transgenic mice have demonstrated that B1 cells present in this compartment can produce pathogenic Coombs antibodies.7,8 Therefore, we determined whether the selective association of the Aia3 locus with Coombs antibody production was related to the expansion of the B1 subset. The analysis of peritoneal cavity cells from 2- and 8-month-old B6.Aia3 Yaa male mice showed no increases in percentages of CD5+ B1a and CD5– B1b subsets, as compared with B6.Yaa male mice (Figure 6). Notably, as reported previously,6 the B1 subset was greatly expanded in 2-month-old NZB mice.
Development of Coombs antibodies in B6 congenic mice bearing the NZB-Nba2 locus
We have recently shown that B6.Yaa male mice bearing the NZB-Nba2 locus spontaneously produce increased levels of IgG antinuclear autoantibodies and gp70 ICs and develop severe glomerulonephritis.21 Because Coombs antibody production in B6 mice was also linked to Nba2, we assessed the spontaneous production of Coombs antibodies in B6 mice bearing an Nba2 interval flanked by markers D1Mit47 and D1Mit461 (about 23 cM interval). The presence of Coombs antibodies was indeed detectable in B6.Nba2 Yaa male mice at 10 months of age (Figure 5; Table 1). Although their incidence (40%) was much lower than that of B6.Aia3 Yaa males (P < .01), it was significantly elevated as compared with B6.Yaa male mice (P < .005). Moreover, 20% of them had abnormally low Ht values (Figure 5; Table 1). As expected, at 10 months of age, B6.Nba2 female mice lacking the Yaa mutation rarely produced Coombs antibodies (2 of 17 mice), and none of them displayed Ht values lower than 40.
Discussion
Using a cohort of B6 × (NZB × B6.Yaa)F1 BC mice expressing the Yaa mutation, we mapped and characterized 2 NZB-derived AHA-susceptibility loci. The significance of these loci in the development of AHA was further confirmed by the analysis of B6.Yaa mice bearing either of the NZB-derived susceptibility intervals. The newly identified Aia3 locus present on NZB chromosome 7 specifically regulates Coombs antibody responses and subsequent development of AHA, while the second locus, directly overlapping with Nba2 on chromosome 1, promotes the development of AHA, likely as a result of its effect on overall production of lupus autoantibodies.20,21
Earlier genetic studies using a few selected markers claimed that Aia1 is a dominant NZB AHA-susceptibility allele, and these studies suggested that it is located on NZB chromosome 4.14 However, the existence of this locus was not confirmed in the present study as well as in a previous genomewide linkage analysis of (B6 × NZB)F1 × NZB BC mice.16 If Aia1 is indeed of a dominant nature, this would explain why this locus was not revealed by the analysis of (B6 × NZB)F1 × NZB BC mice. However, the presence of such a locus should be apparent in our B6 × (NZB × B6.Yaa)F1 BC mice. Nevertheless, we cannot exclude a possible role of gender dimorphism for the expression of Aia1 and hence the Coombs antibody production, because we have analyzed only Yaa male BC mice. Instead, our results identified 2 novel NZB AHA-susceptibility loci on proximal chromosome 7 and distal chromosome 1. Study of B6.Yaa mice congenic for each NZB susceptibility interval, together with the analysis of BC mice, clearly showed that the contribution of the chromosome 7 Aia3 locus to spontaneous production of Coombs antibodies was more significant than that of the second locus corresponding to chromosome 1 Nba2. This conclusion was based on the findings that in B6 × (NZB × B6.Yaa)F1 BC mice Coombs antibody production was most strongly linked with the Aia3 locus and that B6.Aia3 congenic mice displayed a higher incidence of AHA than B6.Nba2 congenic mice.
Coombs antibody activities and Ht values in B6.Aia3, B6.Nba2, and B6 mice. Coombs antibody activities and Ht values were determined at 10 months of age, and results are expressed as MFI for Coombs antibodies and as a percentage for Ht. Mean values are indicated by horizontal lines. Each symbol represents an individual animal (10 to 18 mice in each group). • indicates B6.Aia3, B6.Nba2, and B6 male mice bearing the Yaa mutation (Aia3.Yaa, Nba2.Yaa, and Yaa, respectively); ○, B6.Aia3, B6.Nba2, and B6 female mice lacking the Yaa mutation (Aia3, Nba2, and wild type [WT], respectively).
Coombs antibody activities and Ht values in B6.Aia3, B6.Nba2, and B6 mice. Coombs antibody activities and Ht values were determined at 10 months of age, and results are expressed as MFI for Coombs antibodies and as a percentage for Ht. Mean values are indicated by horizontal lines. Each symbol represents an individual animal (10 to 18 mice in each group). • indicates B6.Aia3, B6.Nba2, and B6 male mice bearing the Yaa mutation (Aia3.Yaa, Nba2.Yaa, and Yaa, respectively); ○, B6.Aia3, B6.Nba2, and B6 female mice lacking the Yaa mutation (Aia3, Nba2, and wild type [WT], respectively).
Of importance, the proximal region of NZB chromosome 7 contains not only Aia3 but also the recently identified Nba5 locus, which selectively regulates the production of nephritogenic gp70 ICs.21 However, the Nba5 locus, peaking at the D7Nds5 marker, was located more than 10 cM distal to Aia3, peaking at the locus Cd22. Because current B6.Aia3 congenic mice carry an NZB-derived interval including both Aia3 and Nba5, we cannot formally prove that these 2 loci are distinct and differentially regulate anti-RBC and anti-gp70 autoantibody responses. However, this region is unable to promote the development of anti-DNA and antichromatin autoantibody production.21 Thus, the candidate genes for Aia3 and Nba5 are most likely to be different from those promoting general hyperresponsiveness of B and/or T cells, thereby enhancing overall autoimmune responses in NZB mice. It should also be stressed that the Aia3 locus is distinct from a B6 modifying locus for Coombs antibody production, reported by recent studies in (B6 × NZB)F1 × NZB BC mice,16 because the latter was mapped on mid chromosome 7, peaking at the D7Mit30 marker (about 20 cM distal to Aia3).
The selective effect of Aia3 on Coombs antibody responses is intriguing, especially when compared with the effect of Nba2 and the B6 chromosome 17 loci on overall autoantibody production. The LOD score curve revealing the Aia3 locus peaked in the vicinity of the Cd22 gene, which encodes a B cell–restricted adhesion molecule that binds α2,6-linked sialic acid and functions as a negative regulator of B-cell receptor (BCR) signaling.29 Significantly, NZB mice carry a defective Cd22a allele, and CD22 expression on Cd22a B cells is lower at steady state and less up-regulated following B-cell activation than that of B cells from B6 mice bearing the Cd22b allele.30 In addition, Cd22a B cells appear to express aberrant forms of CD22, which differ in the N-terminal sequences constituting the ligand-binding site, due to the synthesis of abnormally processed Cd22 mRNA.30 Indeed, we have recently observed that CD22 molecules expressed on Cd22a B cells are less efficient in the binding to CD22 ligand (CD22L) than their Cd22b counterparts (Nitschke L, Lajaunias F, T.M., et al; submitted for publication). It has been proposed that simultaneous engagement of BCR by autoantigen and of CD22 by CD22L could be a mechanism to prevent the activation of autoreactive B cells specific against membrane antigens on self-target cells, if the latter coexpress CD22L.31 Notably, CD22L is abundantly expressed on RBCs.32 Thus, the activation of anti-RBC–reactive B cells could be more specifically controlled through the CD22-CD22L interaction. Consequently, alterations of CD22 expression on NZB B cells may favor the selective activation of anti-RBC autoreactive B cells by reducing the BCR signaling threshold.
Lack of expansion of B1 cells in B6.Aia3 congenic mice. Peritoneal cavity cells from 2- and 8-month-old B6 and B6.Aia3 Yaa male mice and 2-month-old NZB female mice were stained with a combination of anti-B220, anti-CD5, and anti-CD11b mAb. Representative staining profiles for the CD5 and CD11b on B220+ B cells from the indicated mice (3 to 5 mice in each group) are shown. Mean percentages (±SD) of B1a (CD5+CD11b+) and B1b (CD5–CD11b+) subsets are indicated.
Lack of expansion of B1 cells in B6.Aia3 congenic mice. Peritoneal cavity cells from 2- and 8-month-old B6 and B6.Aia3 Yaa male mice and 2-month-old NZB female mice were stained with a combination of anti-B220, anti-CD5, and anti-CD11b mAb. Representative staining profiles for the CD5 and CD11b on B220+ B cells from the indicated mice (3 to 5 mice in each group) are shown. Mean percentages (±SD) of B1a (CD5+CD11b+) and B1b (CD5–CD11b+) subsets are indicated.
In addition to the Aia3 locus, the present study has shown that B6.Yaa males bearing the Nba2 locus were able to develop Coombs antibodies. However, in contrast to the selective effect of Aia3, this production of Coombs antibodies is likely to be a result of the general autoimmune potentiating effect of Nba2, which is also able to promote the formation of antinuclear autoantibodies and gp70 ICs.20,21 The Fcgr2 allele of the NZB strain has been suggested as one possible candidate gene located within the Nba2 interval. The NZB allele has been associated with defective expression of FcγRIIB on germinal center B cells.33,34 Our preliminary analysis has shown an increased production of Coombs antibodies in FcγRIIB haploinsufficient B6.Yaa mice, suggesting the role of Fcgr2 polymorphism in the development of AHA in NZB mice. In addition, another candidate gene, Ifi202 (interferon-inducible p202), could be implicated in AHA, because its expression is markedly increased in NZB mice, as compared with B6 mice,20 in agreement with the demonstration of a critical role of type I interferon in the pathogenesis of AHA in NZB mice.35 More recently, lupus susceptibility has been shown to be associated with polymorphism of the signaling lymphocytic activation molecule (SLAM)/CD2 gene family (Cd244, Cd229, Cs1, Cd48, Cd150, Ly108, and Cd84).36 Because these genes encode cell-surface molecules that play a role in the modulation of activation and signaling of immune cells, they are also good candidates for predisposition to lupuslike autoimmune disease. Analysis of Nba2 subcongenic mice will help define the respective contributions of the Fcgr2, Ifi202, and SLAM/CD2 genes to Nba2-linked autoimmune traits.
It has long been claimed that CD5+ B1 cells could be the major subset involved in the production of Coombs antibodies in NZB mice. This is based on the findings that NZB mice display an expansion of B1 cells6 and that the depletion of B1 cells via intraperitoneal injection of distilled water delayed the onset of AHA in NZB mice.37 Furthermore, it has been shown that the 4C8 anti-RBC transgenic B cells are enriched in the B1 population of the peritoneal cavity and secrete 4C8 Coombs antibodies.7,38,39 However, our present and previous analysis of B6.Aia3 and B6.Nba2 mice failed to show any significant expansion of B1 cells.40 These results indicate that the NZB-determined B1-cell expansion is not required for the production of Coombs antibodies in NZB mice and is probably due to a genetic contribution distinct from Nba2 and Aia3.
Our present BC study also revealed an AHA-susceptibility locus of B6 origin on chromosome 17. This locus overlapped with the H2 complex and promoted various lupus autoimmune responses.21 The production of various autoantibodies in our BC mice was associated with H2b/b homozygosity (versus H2b/d heterozygosity, with H2b and H2d of B6 and NZB origin, respectively), in agreement with the previous observation that lupus susceptibility is more closely linked to the H2b haplotype than to the H2d haplotype in different lupusprone mice.41-43 The autoimmune inhibitory effect of H2d may in part be related to the expression of I-E molecules, because transgenic I-E efficiently inhibits the development of SLE in I-E–deficient H2b-bearing lupusprone mice.44-46 More precisely, formation of I-E α-chain–derived peptides, which display high affinity binding to I-A molecules, could decrease the use of I-A for the presentation of pathogenic self-peptides, thereby limiting the activation of autoreactive T and B cells.47
It is significant that the incidence of Coombs antibody production in BC mice was increased in mice bearing the NZB allele for both Aia3 and Nba2, as compared with that in mice bearing either AHA-susceptibility locus alone, indicating an additive effect of both Aia3 and Nba2 to the development of AHA. This is consistent with the fact that Coombs antibody activities in (NZB × B6.Yaa)F1 males were higher than those of B6.Yaa congenics bearing either Aia3 or Nba2. Clearly, more detailed analysis of phenotypes in monocongenic and bicongenic strains carrying these susceptibility loci should help define key genetic mechanisms in the development of AHA. Moreover, the construction of additional subcongenic B6.Yaa mice carrying different intervals of the Aia3 and Nba2 loci should allow fine mapping and positional cloning of the gene(s) underlying the NZB predisposition to murine AHA. The future identification of mouse AHA-susceptibility genes will have important implications for diagnostic, prognostic, and therapeutic approaches in AHA.
Prepublished online as Blood First Edition Paper, April 28, 2005; DOI 10.1182/blood-2005-02-0558.
Supported by a grant from the Swiss National Foundation for Scientific Research and grant AR 37070 from the National Institutes of Health. L.F.-J. is a recipient of a fellowship from the Arthritis Research Campaign, United Kingdom.
S.K., H.A., and E.A. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr L. Reininger for his critical reading of the manuscript, Dr S. Hirose and Dr T. Winkler for providing us with information on microsatellite markers polymorphic between NZB and B6 mice, and Mr G. Brighouse, Mr S. Jacquier, and Mr G. Celetta for their excellent technical assistance.

![Figure 5. Coombs antibody activities and Ht values in B6.Aia3, B6.Nba2, and B6 mice. Coombs antibody activities and Ht values were determined at 10 months of age, and results are expressed as MFI for Coombs antibodies and as a percentage for Ht. Mean values are indicated by horizontal lines. Each symbol represents an individual animal (10 to 18 mice in each group). • indicates B6.Aia3, B6.Nba2, and B6 male mice bearing the Yaa mutation (Aia3.Yaa, Nba2.Yaa, and Yaa, respectively); ○, B6.Aia3, B6.Nba2, and B6 female mice lacking the Yaa mutation (Aia3, Nba2, and wild type [WT], respectively).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/4/10.1182_blood-2005-02-0558/7/m_zh80160582580005.jpeg?Expires=1767905753&Signature=rh-eQfi7NC18cK9oMps3i3Ei0pW1Wbt5Zrie-SHP~hw0pOiMPETCNzwlR47kX8FSDYYzn2e7VZGcjDK2-e4JsTg0AVCWVYixsdiuewwanX2xXtn43Ann89Q1cY9iTkIOL2PzQ8WsP8qSEtHAxsBNP4POcnSPhg9iIJgl3OwkgkHBT~IP30019EXaqNW~2KmmyKR95sQ1StACwJcVl2jKYH5ABTbD1m0gNnjEbSUVSzZLSvhetzLK7UaE8uysdO4kiURkLQz4mii~q-1HRQStBHfC7jBvypefpjZ4beeniBtU1nw-lgBn8ePL7FNB~rMiSRPwPc3iyf1y5GA4FhCyUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal