Abstract
Sphingosine-1-phosphate (S1P) and its receptor S1P1 control T-cell egress from thymus and secondary lymphoid organs (SLOs). To further define the role of S1P1 in lymphocyte trafficking, we performed adoptive transfer experiments and intravital microscopy (IVM) using both S1P1–/– lymphocytes and recipient wild-type (WT) mice treated with FTY720, an immunosuppressant that downmodulates S1P receptors. S1P1 deficiency and FTY720 caused rapid disappearance of T cells from blood, prolonged retention in SLOs, and accumulation in bone marrow, but did not alter interstitial T-cell motility in peripheral lymph nodes (PLNs) as assessed by multiphoton IVM. However, S1P1–/– lymphocytes displayed reduced short-term homing to PLNs due to attenuated integrin-mediated firm arrest in high endothelial venules (HEVs). By contrast, S1P1–/– T cells homed normally to Peyer patches (PPs), whereas S1P1–/– B cells had a marked defect in homing to PPs and arrested poorly in PP HEVs. Therefore, S1P1 not only controls lymphocyte egress from SLOs, but also facilitates in a tissue- and subset-specific fashion integrin activation during homing. Interestingly, FTY720 treatment enhanced accumulation of both S1P1 sufficient and S1P1–/– T cells in PPs by enhancing integrin-mediated arrest in HEVs. Thus, FTY720 exerts unique effects on T-cell traffic in PPs that are independent of T-cell–expressed S1P1.
Introduction
Most immunosuppressive drugs that are currently in clinical use for the prevention of organ graft rejection and autoimmune diseases interfere with discrete events during T- and B-cell activation. FTY720 is a relatively novel immunosuppressant with a different mechanism of action1-3 ; it neither affects the induction and expansion of lymphocyte responses in secondary lymphoid organs (SLOs) nor does it induce T-cell death at clinically effective concentrations.4 Instead, FTY720 induces rapid disappearance of T cells from peripheral blood PBL and lymph, and sequestration in SLOs, thereby preventing T-cell migration to sites of inflammation.4-7 Furthermore, FTY720 blocks mature T-cell emigration from the thymus.8-11
The exact mechanism(s) by which FTY720 induces immunosuppression is not completely understood, but recent findings explain some of its effects on lymphocyte trafficking. A phosphate ester metabolite of FTY720, FTY-P (formerly called Compound A7 ), is a ligand for 4 of the 5 known G-protein–coupled S1P receptors (S1PRs; S1P1, S1P3, S1P4, S1P5).7,12 Similarly to FTY-P, administration of S1P induces lymphopenia.7 Besides its involvement in lymphocyte trafficking, S1P, which has a micromolar plasma concentration, also mediates diverse cellular processes including growth, survival, morphogenesis, and migration.13 Moreover, recent reports have shown that S1P acts as a T-cell chemoattractant11,14,15 and that both FTY720 and S1P can induce down-regulation of S1PRs on lymphocytes.11,16-18
Since selective agonists or antagonists for S1PRs are only beginning to emerge,19 it has been difficult to dissect the physiologic role of individual S1PRs. S1P1 and S1P4 are the main S1PRs on T and B cells,14,16 but endothelial cells (ECs) also express S1P1 and other S1PRs.7 It is still unclear whether the effects of S1P and FTY720 on lymphocyte trafficking are mediated solely by S1PRs on lymphocytes or also on other cell types. Recent data from S1P1–/– fetal liver (FL) chimeras and conditional T-cell–specific S1P1–/– mice have shown that S1P1 is required for the emigration of mature single positive (SP) cells from the thymus and SLOs.11,15 However, the specific role of S1P1 during T-cell entry from PBLs into SLOs has not been examined.
Here, we studied the effect of FTY720 and FTY-P on trafficking of wild-type (WT) and S1P1–/– T and B cells. FTY720 and FTY-P reduced the recruitment of adoptively transferred WT T cells to peripheral lymph nodes (PLNs) and mesenteric lymph nodes (MLNs), while accelerating their homing to Peyer patches (PPs). Accordingly, adoptively transferred S1P1–/– T and B cells had a significant defect in homing to PLNs and MLNs. However, the effects of FTY720 differed from those of S1P1 deficiency in PPs, where T cells homed normally, while B-cell homing was reduced. Intravital microscopy (IVM) of T cells in PLN high endothelial venules (HEVs) and of B and T cells in PP HEVs revealed that S1P1–/– lymphocytes were compromised in their ability to undergo firm adhesion in HEVs. This suggests a novel role for the S1P-S1P1 pathway in amplifying lymphocyte responses to integrin-activating stimuli in HEVs. These results establish that the absence of S1P1 induces a T-cell trafficking phenotype similar to that observed after FTY720 treatment with one notable exception: IVM comparison of T-cell adhesiveness in PP HEVs showed that FTY720 treatment enhanced firm arrest of both WT and S1P1–/– T cells, suggesting that FTY720-induced modulation of T-cell trafficking to PPs is, in part, independent of T-cell–expressed S1P1.
Materials and methods
Mice
BALB/c, FVB, C57BL/6, and B6.SJL-PtprcaPep3b/BoyJ (C57BL/6 [CD45.1+]) mice were purchased from Taconic Farms (Germantown, NY). Transgenic mice expressing green fluorescent protein (GFP) in naive T cells (T-GFP)20 and S1P1+/– mice (CD45.2+)21 have been described. Mice were housed and bred in a specific pathogen-free (SPF)/viral antibody–free (VAF) animal facility. Experiments complied with National Institutes of Health (NIH) guidelines and were approved by the committees on animals of Harvard Medical School and The CBR Institute for Biomedical Research (CBRI).
Reagents
Monoclonal antibodies (mAbs) against the following antigens were obtained from BD Biosciences (Franklin Lakes, NJ): CD3ϵ, CD4, CD8α, CD8β, CD25, CD24, CD44, CD45.1, CD45.2, CD62L, and TCRβ. To detect CCR7, recombinant human CCL19-immunoglobulin (Ig) chimera was used.22 Anti–l-selectin mAb Mel-14 was purified from hybridoma cell cultures (ATTC, Manassas, VA). FTY720 was kindly provided by Dr Volker Brinkmann (Novartis Pharma AG, Basel, Switzerland). FTY-P was synthesized at Merck Research Laboratories (Rahway, NJ).
FL chimeras
FL chimeras were generated by lethal irradiation (2 doses of 625 rads) of C57BL/6 (CD45.1+) mice. Donor cells (CD45.2+) were isolated from embryonic day 13 (E13) FL of S1P1–/– and S1P1+/+ or S1P1+/– embryos (cells from the latter 2 were pooled and are referred to as S1P1+/). Phenotypic distinction of embryos was based on the presence of intraembryonic hemorrhages in S1P1–/– embryos and subsequently confirmed by polymerase chain reaction (PCR).21 FLs were digested with 0.05% collagenase type 2 (Worthington Biochemical, Lakewood, NJ). Cells (3 × 105-2 × 106) were injected intravenously into irradiated recipients. Mice were screened for CD45.2+ donor leukocytes in PBL samples prior to use. Reconstitution was always more than 80% after 8 to 12 weeks.
Homing assays
Homing assays were performed as described.23 T-GFP splenocytes and PLN cells were fluorescently labeled with tetramethylrhodamine-5-isothiocyanate (TRITC; Molecular Probes, Eugene, OR) yielding GFP+TRITC+ T cells and GFPnegTRITC+ B cells. These cells were injected intravenously into BALB/c mice 2.5 hours after FTY720 (1 mg/kg orally by gavage) or FTY-P (1 mg/kg intraperitoneally) treatment. At time-points indicated, mice were anesthetized by intraperitoneal injection of 10 mL/kg saline containing xylazine (1 mg/mL) and ketamine (5 mg/mL), and PBL (1 mL) was obtained by cardiac puncture. Single-cell suspensions were generated from spleens, PLNs (2 brachial, 2 axilary, 2 inguinal), MLNs (n = 3), PPs (n = 6), livers, and lungs. Bone marrow (BM) cells were harvested from 1 limb (corresponding to 10% of total BM24 ) and their numbers were normalized to total BM. For competitive homing experiments, S1P1–/– and S1P1+/ thymocytes or splenic B cells from FL chimeras were labeled with TRITC or carboxy-fluorescein diacetate, succinimidyl ester (CFSE; Molecular Probes) as described25 and coinjected intravenously into recipient mice. Single-cell suspensions were stained with anti–CD4–peridinin chlorophyll protein (PerCP) and anti–CD8α-allophycocyanin (APC) or anti-B220-PerCP and analyzed by fluorescence activated cell sorting (FACS). Lymphocyte counts in PBL were determined with a Drew Hemavet (CDC Technologies, Oxford, CT).
IVM and image analysis
IVM of mouse inguinal LNs and PPs was performed as described.26,27 S1P1–/– and S1P1+/ CD4+ SP thymocytes were purified (> 90%) by depleting CD8α+, CD8β+, HSA+, B220+, and CD25+ cells using biotinylated antibodies (BD Biosciences) and streptavidin-conjugated magnetic beads (Dynal Biotech, Oslo, Norway). Purified CD4+ SP cells were labeled with calcein AM (Molecular Probes) and small boli of cells were injected intraarterially. S1P1–/– and S1P1+/ B cells were immunomagnetically purified from spleens (Miltenyi Biotec, Auburn, CA), calcein-labeled, and injected intraarterially. In some experiments, T cells were purified from mice treated with FTY720 18 hours earlier. Lymphocyte-HEV interactions were recorded and analyzed offline as described.26 Rolling fraction is defined as the percentage of fluorescent cells interacting with an HEV in the total number of cells passing through the vessel. The sticking fraction is defined as the percentage of rolling cells that arrested for at least 30 seconds.
Multiphoton intravital microscopy (MP-IVM)
S1P1–/– and S1P1+/ CD4+ SP cells or naive CD4+ T cells from FTY720- and saline-treated C57BL/6 mice were labeled with either 10 μM 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR; Molecular Probes) or 5 μM 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CMFDA, SE; Molecular Probes) and injected intravenously into C57BL/6 mice. At various time-points thereafter, mice were anesthetized and the right popliteal LN was prepared for MP-IVM as described.28 Four-dimensional analysis of cell migration was performed using Volocity software (Improvision, Lexington, MA) as described.28
Statistical analysis
Data are shown as the mean plus or minus the standard error of the mean (SEM), unless otherwise noted. IVM and homing data were analyzed with a Student t test or one-way analysis of variance (ANOVA). Differences were considered statistically significant when P values are less than .05.
Results
FTY720 and FTY-P induce T-cell accumulation in PPs and BM but not in PLNs
Several reports have demonstrated that FTY720, by means of its phosphorylated form FTY-P, induces disappearance of lymphocytes from PBL and sequestration in SLOs, especially in LNs and PPs.2-4,7,29 This has been attributed to both prolonged retention and enhanced homing. However, while the former mechanism is well documented,7,11 the latter effect is less well established. Therefore, we studied the effect of FTY720 and FTY-P treatment on homing of adoptively transferred naive T and B cells. Mice were treated either with FTY720 or FTY-P, and 2.5 hours later TRITC-labeled lymphocytes from T-GFP mice were injected intravenously. Mice were killed after 2.5 hours or 16 hours. This strategy allowed us to track both B cells (GFPnegTRITC+) and T cells (GFP+TRITC+)23 and to distinguish between drug effects on immigration (2.5 hours) versus recirculation (16-20 hours). This is because the number of homed cells recovered late after adoptive transfer is determined by the equilibrium of immigration and emigration, whereas early cell counts predominantly reflect the rate of entry from the PBLs, as evidenced by the paucity and abundance of adoptively transferred lymphocytes in thoracic duct lymph at the early and late time-point, respectively (Figure S1; see the Supplemental Materials link at the top of the online article, at the Blood website). Indeed, as will be shown in Figure S4, there is an approximately 90% turnover of the intranodal T-cell pool within a 20-hour period.
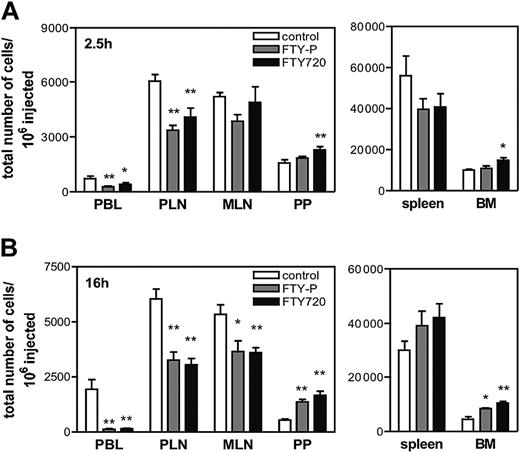
At 2.5 hours after injection, donor T cells were decreased in PBLs of both FTY-P– and FTY720-treated mice (Figure 1A). Unexpectedly, the number of T cells recovered from PLNs was also significantly reduced after FTY-P and FTY720 treatment. In contrast, T-cell homing to MLNs and spleen remained unchanged and was increased in PPs and the BM after FTY720 treatment. Recipient lungs and livers contained similar numbers of transferred T cells in all groups (C.H. and M.L.S., unpublished data, March 2004).
At 16 hours after adoptive transfer, 95% of injected T cells had disappeared from PBL of FTY-P– and FTY720-treated mice, and homed T-cell numbers in PLNs remained significantly reduced (Figure 1B). At this late time-point, homed cells were also significantly fewer in MLNs. By contrast, drug-induced T-cell accumulation in PPs and BM had become more pronounced. These effects were equivalent in magnitude for CD4+ and CD8+ cells (C.H. and M.L.S., unpublished data, March 2004). Furthermore, the observed reduction in T-cell homing to PLNs was not specific for BALB/c mice since similar results were observed in FTY720-treated C57BL/6 recipients (data not shown). In contrast, neither FTY-P nor FTY720 altered B-cell trafficking to PLNs, MLNs, or PPs. However, B-cell homing to the spleen of FTY-P–treated mice was significantly reduced at 2.5 hours, while at 16 hours the number of homed B-cells in the BM (Figure S2) of both FTY-P and FTY720 was more than doubled.
Effect of FTY720 and FTY-P on homing and tissue distribution of adoptively transferred T cells. (A-B) TRITC-labeled splenocytes and PLN cells from T-GFP mice were injected intravenously into WT mice (n = 6 mice/group), which had been pretreated 2.5 hours earlier with FTY720, FTY-P, or saline. At different time-points, PBL (1 mL), SLOs, and BM were harvested and single-cell suspensions were analyzed by FACS for the presence of TRITC+GFP+ T cells. At 2.5 hours after adoptive transfer (A), homed T cells were reduced in PLNs by 44% and 30% in FTY-P– and FTY720-treated mice, respectively, and were increased in PPs by 46% and in BM by 48% after FTY720 treatment. At 16 hours after transfer (B), homed T cells in PLNs remained reduced by approximately 46%. T-cell homing to PPs increased over control: 2.8-fold for FTY-P; 3.9-fold for FTY720; and in BM 3.7-fold for FTY-P; 4.3-fold for FTY720. Data are shown as total number of homed cells per million injected. Error bars indicate SEM. *P < .05; **P < .01 (versus control).
Effect of FTY720 and FTY-P on homing and tissue distribution of adoptively transferred T cells. (A-B) TRITC-labeled splenocytes and PLN cells from T-GFP mice were injected intravenously into WT mice (n = 6 mice/group), which had been pretreated 2.5 hours earlier with FTY720, FTY-P, or saline. At different time-points, PBL (1 mL), SLOs, and BM were harvested and single-cell suspensions were analyzed by FACS for the presence of TRITC+GFP+ T cells. At 2.5 hours after adoptive transfer (A), homed T cells were reduced in PLNs by 44% and 30% in FTY-P– and FTY720-treated mice, respectively, and were increased in PPs by 46% and in BM by 48% after FTY720 treatment. At 16 hours after transfer (B), homed T cells in PLNs remained reduced by approximately 46%. T-cell homing to PPs increased over control: 2.8-fold for FTY-P; 3.9-fold for FTY720; and in BM 3.7-fold for FTY-P; 4.3-fold for FTY720. Data are shown as total number of homed cells per million injected. Error bars indicate SEM. *P < .05; **P < .01 (versus control).
No significant accumulation of endogenous lymphocytes was detected in PLNs or MLNs of FTY720- and FTY-P–treated mice at either time-point (Table S1). However, similar to our homing experiments and consistent with other studies,4,7 endogenous lymphocytes were markedly reduced in PBL and elevated in PPs 16 hours after drug treatment. Thus, FTY720 and FTY-P have similar effects on the migratory behavior of adoptively transferred lymphocytes; namely, in the case of T cells, depletion from PBL, decreased homing to PLNs, and increased homing to PPs and BM, while drug effects on B-cell distribution were only apparent in spleen and BM.
S1P1 is required for optimal lymphocyte homing to SLOs
FTY-P binds 4 of the 5 known S1PRs,7 but recent findings have pointed to functional antagonism of S1P1 as the most likely mechanism for the drug's effect on lymphocyte trafficking.11 Therefore, we focused on this receptor. S1P1 deficiency is embryonic lethal, but mature S1P1–/– lymphocytes can be obtained from FL chimeras.21,30 Consistent with a recent report using this strategy,11 S1P1–/– B cells were markedly reduced in PBL, but a sizeable B-cell population was present in SLOs. By contrast, S1P1–/– T cells were virtually absent from PBL and SLOs, while significantly larger numbers of CD4+ and CD8+ SP cells were present in the thymus (Figure S3A). All SP cells of both genotypes expressed LFA-1 at equivalent levels to mature T cells from WT PLNs, and both populations contained equivalent fractions of cells expressing l-selectin and CCR7, the essential receptors for PLN homing. Moreover, as reported previously,11 both subsets migrated vigorously to the CCR7 agonists, CCL19 and CCL21 (data not shown). Analogously, S1P1–/–, S1P1+/+, and S1P1+/– B cells (the latter 2 were pooled and are referred to as S1P1+/) expressed equivalent levels of l-selectin, α4β7, and LFA-1 and migrated similarly to CXCL12 (CXCR4 agonist), CXCL13 (CXCR5 agonist), and CCL21 (data not shown).
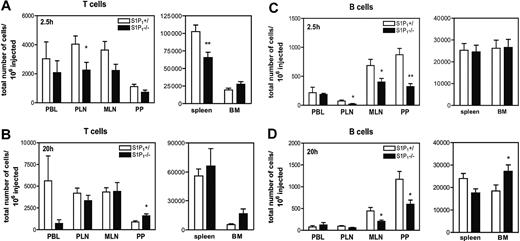
To test the role of S1P1 in T-cell trafficking, S1P1–/– and S1P1+/ thymocytes were fluorescently labeled and injected intravenously into WT mice. The number of fluorescent SP cells was assessed in SLOs, BM, and PBL 2.5 hours or 20 hours later. As expected, both SP cells as well as immature CD4+CD8+ double-positive (DP) cells were found in recipient PBL (Figure S3B), but DP cells were excluded from PLNs, MLNs, and PPs, presumably because they expressed little or no l-selectin (Figure S3C and data not shown). At 2.5 hours after injection, significantly more S1P1+/ than S1P1–/– SP cells were recovered from PLNs and spleen, and this tendency was also apparent in MLNs, but not in PPs or BM (Figure 2A). At 20 hours after transfer, S1P1–/– SP cells were selectively depleted from PBL, but their number in SLOs had reached levels similar to S1P1+/ SP cells, except in PPs where S1P1–/– SP cells outnumbered their S1P+/ counterparts (Figure 2B). Furthermore, S1P1–/– SP cells displayed a tendency for enhanced accumulation in BM (P = .07 versus S1P1+/). The magnitude of these effects was equivalent for CD4+ and CD8+ SP cells. These data are consistent with our findings with FTY720 and FTY-P and reveal a previously unrecognized supportive function of S1P1 during short-term T-cell homing to SLOs. The homing defect of S1P1–/– T cells becomes masked after longer time intervals, presumably due to their prolonged retention in SLO (Figure S4).
After adoptive transfer of B cells, significantly fewer S1P1–/– than S1P1+/ B cells homed to PLNs, MLNs, and PPs at 2.5 hours (Figure 2C). This defect persisted until 20 hours (Figure 2D), but equal numbers of S1P1–/– and S1P1+/ B cells were found in SLOs at 48 hours (C.H. and M.L.S., unpublished data, March 2004). B cells were not depleted from PBL at any time-point. No significant defect in B-cell homing to SLOs was observed in FTY720-treated mice at either time-point (Figure S2). However, both FTY720 and S1P1 deficiency induced B-cell accumulation in BM at 16 to 20 hours.
Role of S1P1 in T- and B-cell trafficking to SLOs. (A-B) Thymocytes from S1P1+/ or S1P1–/– FL chimeras were differentially labeled with TRITC or CFSE, mixed, and adoptively transferred into WT mice (n = 6 mice/time-point). After 2.5 hours (A) or 20 hours (B), PBL, SLOs, and BM were harvested and single-cell suspensions were stained for CD4 and CD8α and analyzed by FACS for the presence of TRITC+ and CFSE+ SP T cells. (C-D) Differentially labeled splenocytes from S1P1+/ and S1P1–/– FL chimeras were adoptively transferred into WT mice (n = 4 mice/time-point).After 2.5 hours (C) and 20 hours (D), PBL, single-cell suspensions from SLOs, and BM were stained for B220 and analyzed by FACS for the presence of TRITC+ and CFSE+ B cells. Data are shown as total number of homed cells per million injected. Error bars indicate SEM. *P < .05; **P < .01.
Role of S1P1 in T- and B-cell trafficking to SLOs. (A-B) Thymocytes from S1P1+/ or S1P1–/– FL chimeras were differentially labeled with TRITC or CFSE, mixed, and adoptively transferred into WT mice (n = 6 mice/time-point). After 2.5 hours (A) or 20 hours (B), PBL, SLOs, and BM were harvested and single-cell suspensions were stained for CD4 and CD8α and analyzed by FACS for the presence of TRITC+ and CFSE+ SP T cells. (C-D) Differentially labeled splenocytes from S1P1+/ and S1P1–/– FL chimeras were adoptively transferred into WT mice (n = 4 mice/time-point).After 2.5 hours (C) and 20 hours (D), PBL, single-cell suspensions from SLOs, and BM were stained for B220 and analyzed by FACS for the presence of TRITC+ and CFSE+ B cells. Data are shown as total number of homed cells per million injected. Error bars indicate SEM. *P < .05; **P < .01.
Both S1P1 deficiency and FTY720 treatment lead to T-cell retention in PLNs, but do not affect intranodal T-cell motility
Retention in PLNs is a hallmark of both FTY720 treatment and S1P1 deficiency.7,11 We have independently confirmed these findings in adoptive transfer experiments that enabled us to assess the fate and dwell time of LN-resident T cells (Figure S4). For this, intravenously injected T cells were allowed to home (4 hours) before further cell entry into LNs was blocked with anti–l-selectin mAb Mel-14.28 Using this approach, we estimate that at least 90% of homed S1P1+/ T cells exit from PLNs within 18 hours after homing, whereas virtually no S1P1–/– T cells were lost from PLNs during this interval. Similarly, FTY-P treatment induced significant retention of transferred T cells in LNs (Figure S5).
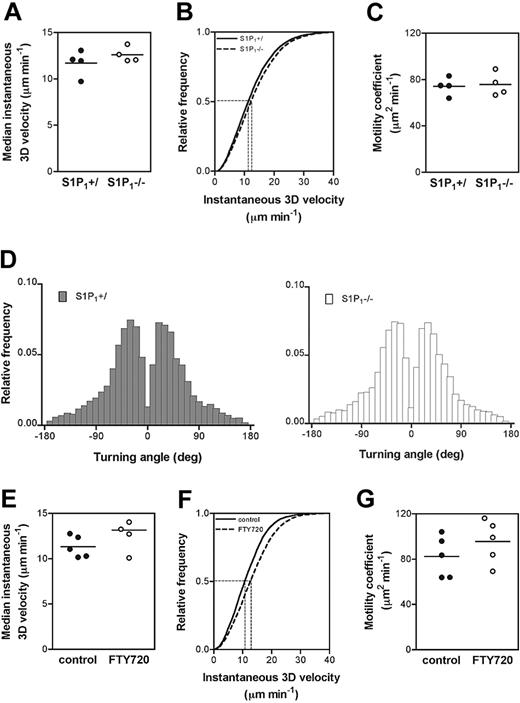
It was recently suggested that S1P1–/– T-cell retention in SLOs could be caused by the cells' inability to sense and chemotax toward the high concentrations of S1P in lymph (and PBL).11 Alternatively, S1P1 deficiency might also negatively affect cell motility within LNs. To resolve this issue, we performed MP-IVM in mouse popliteal LNs28 and compared the migratory behavior of homed S1P1–/– and S1P1+/ CD4+ SP cells (Video S1). There were no detectable differences in instantaneous 3D velocity (Figure 3A-B), motility coefficient (Figure 3C), or turning angle distribution (Figure 3D). Similar results were obtained when comparing T cells from untreated and FTY720-treated mice (Figure 3E-G). Instantaneous velocities and motility coefficients shown here were higher than previous measurements in explanted LNs and in vivo.28,31 This is because cells that were stationary over the entire 30-minute observation interval were excluded, since stationary behavior probably reflects cellular damage caused by ex vivo handling and labeling (T.R.M., unpublished data, March 2004). Overall, these results demonstrate that S1P1-dependent retention in SLOs cannot be explained by impaired cell motility, at least within the part of the cortex that can be visualized in our MP-IVM setup (up to 400 μm below the LN capsule opposite to the hilar pole).
S1P1–/– T cells are defective in their ability to undergo integrin-mediated arrest in PLN HEVs
B- and T-cell homing to PLNs, MLNs, and PPs occurs in HEVs and depends on a series of consecutive adhesion steps, which are characteristic for each organ and cell type.32 For T-cell homing to PLNs, the initial adhesion step is mediated by l-selectin binding to peripheral node addressin.32 Subsequent signaling through binding of CCL21/CCL19 to lymphocyte-expressed CCR7 triggers activation of the integrin LFA-1, allowing rolling cells to adhere firmly (stick).32-34
To further characterize the role of S1P1 in lymphocyte homing to SLOs, we studied the behavior of S1P1–/– and S1P1+/ T cells during intravascular adhesion in PLN HEVs. To this end, purified fluorescently-labeled CD4+ SP thymocytes were injected intraarterially and observed in inguinal LN HEVs26 (branching orders III-V) by epifluorescence video IVM. S1P1+/ and S1P1–/– SP cells rolled equally well in HEVs (Figure 4A), but the sticking fraction of S1P1–/– cells was 40% lower than that of S1P1+/ cells (Figure 4B). These results are in excellent agreement with our short-term homing experiments and suggest that S1P1 is required for optimal integrin activation and sticking of T cells in PLN HEVs.
S1P1 deficiency affects integrin-mediated arrest of B cells but not of T cells in PP HEVs
Since our adoptive transfer experiments revealed that S1P1 deficiency causes a defect in short-term homing of B but not T cells to PPs (Figure 2A,C), we examined the adhesive behavior of both subsets by IVM in PP HEVs. Rolling of both subsets in PP HEVs is initiated by l-selectin and maintained by α4β7, while firm adhesion requires activation of LFA-1 and/or α4β7.27,35 Integrin activation on T cells is triggered by activation of CCR7, whereas B-cell sticking can be induced by ligands for CCR7, CXCR4, and CXCR5.
IVM of PP HEVs revealed that both the rolling and sticking fractions of S1P1–/– B cells were significantly reduced compared with S1P1+/ B cells (Figure 5A-B). By contrast, consistent with our short-term adoptive transfer experiments with T cells, there was no difference in the rolling and sticking fractions of S1P1+/ and S1P1–/– T cells (Figure 5C-D).
S1P1 deficiency does not affect T-cell motility within the popliteal LN. (A-D) Differentially fluorescently labeled S1P1+/ and S1P1–/– CD4+ SP cells were adoptively transferred into WT mice and their migratory behavior was analyzed in the same LN after 24 hours and 48 hours using MP-IVM (pooled data, n = 2 mice/time-point). Parameters analyzed are (A) the median instantaneous 3D velocity (S1P1+/: 12.0 ± 0.7 μm minute–1 versus S1P1–/–: 12.4 ± 0.4 μm minute–1), (B) the cumulative instantaneous 3D velocity, (C) motility coefficients (S1P1+/: 73.5 ± 5.1 μm2 minute–1 versus S1P1–/–: 74.8 ± 4.0 μm2 minute–1), and (D) turning angles. (E-G) Differentially fluorescently labeled untreated (control) or CD4+ T cells from FTY720-treated WT donors were adoptively transferred into WT recipients (n = 2 mice, 2-3 recordings/mouse). After 3 hours, cell motility was analyzed in the popliteal LN. (E) Median instantaneous 3D velocity (control: 11.3 ± 0.5 μm minute–1 versus FTY720: 13.2 ± 0.9μm minute–1), (F) cumulative instantaneous 3D velocity, (G) motility coefficients (control: 82.3 ± 8.2 μm2 minute–1 versus FTY720: 95.6 ± 8.5 μm2 minute–1). Horizontal bars in panels A, C, E, G represent the mean of the measurements. Dashed horizontal lines in panels B and F indicate a frequency of 0.5, corresponding to the median. Vertical dashed lines connect these data points to the x-axis, indicating the median values for instantaneous 3D velocity.
S1P1 deficiency does not affect T-cell motility within the popliteal LN. (A-D) Differentially fluorescently labeled S1P1+/ and S1P1–/– CD4+ SP cells were adoptively transferred into WT mice and their migratory behavior was analyzed in the same LN after 24 hours and 48 hours using MP-IVM (pooled data, n = 2 mice/time-point). Parameters analyzed are (A) the median instantaneous 3D velocity (S1P1+/: 12.0 ± 0.7 μm minute–1 versus S1P1–/–: 12.4 ± 0.4 μm minute–1), (B) the cumulative instantaneous 3D velocity, (C) motility coefficients (S1P1+/: 73.5 ± 5.1 μm2 minute–1 versus S1P1–/–: 74.8 ± 4.0 μm2 minute–1), and (D) turning angles. (E-G) Differentially fluorescently labeled untreated (control) or CD4+ T cells from FTY720-treated WT donors were adoptively transferred into WT recipients (n = 2 mice, 2-3 recordings/mouse). After 3 hours, cell motility was analyzed in the popliteal LN. (E) Median instantaneous 3D velocity (control: 11.3 ± 0.5 μm minute–1 versus FTY720: 13.2 ± 0.9μm minute–1), (F) cumulative instantaneous 3D velocity, (G) motility coefficients (control: 82.3 ± 8.2 μm2 minute–1 versus FTY720: 95.6 ± 8.5 μm2 minute–1). Horizontal bars in panels A, C, E, G represent the mean of the measurements. Dashed horizontal lines in panels B and F indicate a frequency of 0.5, corresponding to the median. Vertical dashed lines connect these data points to the x-axis, indicating the median values for instantaneous 3D velocity.
FTY720 enhances S1P1–/– T-cell accumulation in PPs
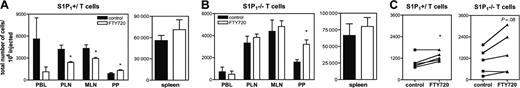
FTY720 induces down-regulation and partial degradation of S1P1 in lymphocytes.11,16 While this explains the similarities in trafficking behavior of S1P1–/– and WT T cells exposed to FTY720, it remained possible that FTY720 exerts additional effects on ECs, stromal cells, or on lymphocyte-expressed S1PRs other than S1P1. To examine this possibility, we compared the effect of FTY720 in homing of S1P1–/– and S1P1+/ SP cells. WT mice were pretreated with either saline or FTY720 and differentially labeled S1P1–/– or S1P1+/ thymocytes were injected intravenously 2.5 hours later. Consistent with our findings using peripheral T cells (Figure 1B), S1P1+/ SP thymocytes were reduced in PBL, PLNs, and MLNs, and significantly increased in PPs of FTY720-treated mice 20 hours after injection (Figure 6A). At this time-point, levels of S1P1–/– SP cells in PBL, spleen, and LNs were equivalent in untreated and FTY720-treated mice. However, FTY720 significantly enhanced also the accumulation of S1P1–/– SP cells in PPs at 20 hours (Figure 6B), and this effect was even apparent in 4 of 5 mice as early as 2.5 hours after cell transfer (Figure 6C). Thus, the effect of FTY720 on T-cell homing to PPs is independent of S1P1 on T cells, while its effect on homing to LNs is adequately explained by functional inhibition of T-cell–expressed S1P1.
S1P1–/– T cells display a defect in integrin-mediated firm arrest in PLN HEVs. The intravascular behavior of calcein-labeled CD4+ SP cells from S1P1+/ or S1P1–/– FL chimeras was analyzed by IVM in inguinal LN of WT mice (n = 2 mice). (A) Rolling and (B) sticking fractions (44.1% ± 5.6% of S1P1+/ versus 26.5% ± 6.2% of S1P1–/–; *P < .05) of cells were determined in venular orders III, IV, and V. Error bars indicate SEM.
S1P1–/– T cells display a defect in integrin-mediated firm arrest in PLN HEVs. The intravascular behavior of calcein-labeled CD4+ SP cells from S1P1+/ or S1P1–/– FL chimeras was analyzed by IVM in inguinal LN of WT mice (n = 2 mice). (A) Rolling and (B) sticking fractions (44.1% ± 5.6% of S1P1+/ versus 26.5% ± 6.2% of S1P1–/–; *P < .05) of cells were determined in venular orders III, IV, and V. Error bars indicate SEM.
FTY720 treatment enhances T-cell sticking in PP HEVs independent of T-cell–expressed S1P1
In vitro studies have documented that FTY720-induced effects on lymphocytes require prolonged incubation.16,36 Consistent with these findings, there was no significant change in PBL or PLNs when T cells were injected into FTY720-treated mice and examined 30 minutes thereafter (data not shown). However, the 30-minute transfer interval was sufficient to cause significantly increased T-cell accumulation in PPs (Figure 7A). Since these rapid kinetics suggested an effect on T-cell entry via HEVs, we performed IVM in PP HEVs by injecting WT and S1P1–/– T cells into mice that were treated 2.5 hours earlier with saline or FTY720. WT T cells showed no difference in rolling (Figure 7B), but the sticking fraction in HEVs of FTY720-treated mice was increased by 53% compared with untreated animals. No difference in rolling or sticking was observed upon injection of FTY720-treated or control T cells into untreated recipients (data not shown). In excellent agreement with our findings in short-term homing experiments (Figure 6C), sticking of S1P1–/– T cells in PP HEVs in FTY720-treated mice was significantly enhanced (Figure 7C). Taken together, these findings identify a novel mode of action for FTY720; the drug promotes T-cell accumulation in PPs by boosting integrin-dependent arrest of rolling cells in HEVs through a mechanism that is independent of T-cell–expressed S1P1.
S1P1–/– B cells but not S1P1–/– T cells display a defect in integrin-mediated firm arrest in PP HEVs. (A-B) The intravascular behavior of calcein-labeled B cells from S1P1+/ or S1P1–/– FL chimeras was analyzed by IVM in PP HEVs (n = 3 mice). (A) Rolling (32.1% ± 4.1% of S1P1+/ versus 24.2% ± 3.6% of S1P1–/–; **P < .01) and (B) sticking fractions (11.3% ± 1.8% of S1P1+/ versus 5.9% ± 1.3% of S1P1–/–;* P < .05). (C-D) Calcein-labeled CD4+ SP cells from S1P1+/ or S1P1–/– FL chimeras were analyzed by IVM in PP HEVs (n = 3 mice). No difference was observed in (C) rolling (37.4% ± 4.4% of S1P1+/ versus 28.4% ± 3.5% of S1P1–/–) and (D) sticking fractions (12.5% ± 2.5% of S1P1+/ versus 11.5% ± 2.8% of S1P1–/–). Error bars indicate SEM.
S1P1–/– B cells but not S1P1–/– T cells display a defect in integrin-mediated firm arrest in PP HEVs. (A-B) The intravascular behavior of calcein-labeled B cells from S1P1+/ or S1P1–/– FL chimeras was analyzed by IVM in PP HEVs (n = 3 mice). (A) Rolling (32.1% ± 4.1% of S1P1+/ versus 24.2% ± 3.6% of S1P1–/–; **P < .01) and (B) sticking fractions (11.3% ± 1.8% of S1P1+/ versus 5.9% ± 1.3% of S1P1–/–;* P < .05). (C-D) Calcein-labeled CD4+ SP cells from S1P1+/ or S1P1–/– FL chimeras were analyzed by IVM in PP HEVs (n = 3 mice). No difference was observed in (C) rolling (37.4% ± 4.4% of S1P1+/ versus 28.4% ± 3.5% of S1P1–/–) and (D) sticking fractions (12.5% ± 2.5% of S1P1+/ versus 11.5% ± 2.8% of S1P1–/–). Error bars indicate SEM.
FTY720 promotes accumulation of S1P1–/– T cells in PPs. TRITC- or CFSE-labeled thymocytes from S1P1+/ (A) or S1P1–/– (B) FL chimeras were adoptively transferred into control or FTY720-treated WT recipients (n = 4 mice/group). After 20 hours, mice were killed and PBL and SLOs were harvested. Single-cell suspensions were stained for CD4 and CD8α and analyzed by FACS for the presence of TRITC+ and CFSE+ donor SP cells. (C) TRITC- or CFSE-labeled thymocytes from S1P1+/ or S1P1–/– FL chimeras were adoptively transferred into control or FTY720-treated WT recipients (n = 5). After 2.5 hours mice were killed and PPs were harvested and single-cell suspensions were stained for CD4 and CD8α and analyzed by FACS. Error bars indicate SEM. *P < .05.
FTY720 promotes accumulation of S1P1–/– T cells in PPs. TRITC- or CFSE-labeled thymocytes from S1P1+/ (A) or S1P1–/– (B) FL chimeras were adoptively transferred into control or FTY720-treated WT recipients (n = 4 mice/group). After 20 hours, mice were killed and PBL and SLOs were harvested. Single-cell suspensions were stained for CD4 and CD8α and analyzed by FACS for the presence of TRITC+ and CFSE+ donor SP cells. (C) TRITC- or CFSE-labeled thymocytes from S1P1+/ or S1P1–/– FL chimeras were adoptively transferred into control or FTY720-treated WT recipients (n = 5). After 2.5 hours mice were killed and PPs were harvested and single-cell suspensions were stained for CD4 and CD8α and analyzed by FACS. Error bars indicate SEM. *P < .05.
Discussion
Here, we have examined how treatment with FTY720 and S1P1 deficiency impact the equilibrium in T-cell distribution between PBL and lymphoid tissues. A summary of our findings and their possible meaning for the role of the S1P/S1P1 pathway in lymphocyte trafficking is presented in Figure 8. Consistent with recent reports,11,15 we found that S1P1 is required for T-cell exit from SLOs other than the spleen. While T-cell retention in SLOs is a well-established consequence of FTY720 treatment and S1P1 deficiency,7,8,11 the role of S1P1 in lymphocyte homing has been less clear. We now show that S1P1 accelerates lymphocyte exit from PBL by facilitating integrin-dependent arrest in HEVs.
In contrast to our current findings, it had been assumed that S1P1 attenuates T-cell homing to SLOs. This interpretation was based in part on observations that FTY720 causes marked lymphopenia.37 However, it is difficult to pinpoint the precise mechanism(s) of drug-induced lymphopenia by studying endogenous T cells whose local and systemic distribution is determined by a dynamic equilibrium of numerous factors, including the production and release of newly formed T cells in the thymus; the rate of homing and exit to and from SLOs and PBL; the size and turnover of the intra- and extravascular T cell pools in nonlymphoid organs, especially lung and liver; and the rate of T-cell loss by death or permanent sequestration. Interference with any of these factors may result in pronounced effects on homeostatic T-cell concentrations. Given these considerations, adoptive transfer experiments of a defined number of tagged T cells are advantageous because the many confounding factors can be more precisely controlled. Furthermore, the time interval during which transferred T cells are allowed to home allows us to distinguish effects of S1P1 and FTY720 on homing versus tissue retention.
Treatment of mice with FTY720 induces T-cell sticking in PP HEVs. (A) Splenocytes and PLN cells from T-GFP mice were injected intravenously into WT mice (n = 6 mice/group), which had been pretreated 2.5 hours earlier with FTY720 or saline. After 30 minutes, PPs were harvested and single-cell suspensions were analyzed by FACS for the presence of GFP+ T cells. Homed T cells were increased in PPs of FTY720-treated mice by 62%; *P < .05. (B-C) The intravascular behavior of calcein-labeled WT and S1P1–/– T cells in PP HEVs of untreated or FTY720-treated recipients was analyzed by IVM (n = 4 mice/group). (B) WT T-cell rolling and sticking fractions (9.4% ± 2.2% in control mice versus 14.4% ± 2.0% in FTY720-treated mice; P = .05) and (C) S1P1–/– T-cell rolling and sticking fractions (11.5% ± 2.8% in control mice versus 26.0% ± 5.0% in FTY720-treated mice. Error bars indicate SEM. *P < .05).
Treatment of mice with FTY720 induces T-cell sticking in PP HEVs. (A) Splenocytes and PLN cells from T-GFP mice were injected intravenously into WT mice (n = 6 mice/group), which had been pretreated 2.5 hours earlier with FTY720 or saline. After 30 minutes, PPs were harvested and single-cell suspensions were analyzed by FACS for the presence of GFP+ T cells. Homed T cells were increased in PPs of FTY720-treated mice by 62%; *P < .05. (B-C) The intravascular behavior of calcein-labeled WT and S1P1–/– T cells in PP HEVs of untreated or FTY720-treated recipients was analyzed by IVM (n = 4 mice/group). (B) WT T-cell rolling and sticking fractions (9.4% ± 2.2% in control mice versus 14.4% ± 2.0% in FTY720-treated mice; P = .05) and (C) S1P1–/– T-cell rolling and sticking fractions (11.5% ± 2.8% in control mice versus 26.0% ± 5.0% in FTY720-treated mice. Error bars indicate SEM. *P < .05).
Several earlier investigations have used adoptive transfer experiments to study the effect of FTY720 and/or S1P1 on T-cell trafficking in WT mice.2,11,29,38 However, so far all data reported in mice have examined homing intervals lasting at least 18 hours without discerning between effects on T-cell entry and exit from SLOs.11,29,38 Short-term homing experiments have only been performed in rats in which 30 minutes after adoptive transfer significantly more lymphocytes were recovered from LNs and PPs of FTY720-treated animals than from SLOs of a control group.2 Employing the same protocol in mice, we only detected a statistically significant increase in T-cell homing to PPs (Figure 7A) of FTY720-treated animals, but no differences in other SLOs or PBL (C.H. and M.L.S., unpublished data, March 2004). The reason for this discrepancy remains unexplained, but might reflect subtle species differences in initial T-cell responses to S1P agonists.
A complicating factor in homing experiments comparing lymphocyte trafficking with and without functional S1P1 is the profound lymphopenia observed in the latter setting. The reduced number of homed cells in SLOs (Figure 1) was probably, at least in part, a consequence of decreased lymphocyte supply via the PBL. Therefore, it was important to conduct IVM experiments, which allowed us to assess at the single-cell level the role of S1P1 in lymphocyte-HEV interactions. These experiments revealed that S1P1–/– T and B cells were compromised in their ability to stick in HEVs of PLNs and PPs, respectively (Figures 4 and 5). T-cell arrest in PLN HEVs requires the functional activation of LFA-1,33 whereas both α4β7 and LFA-1 mediate sticking of B cells in PP HEVs.27
Summary of the effects of S1P1 deficiency or FTY720 treatment on the trafficking of adoptively transferred lymphocytes. The effects of S1P1 deficiency and FTY720 treatment on cell entry or cell exit to various lymphoid organs are presented for T cells (top) and B cells (bottom). Conclusions on “cell entry” are derived from our short-term homing and IVM experiments, whereas “cell exit” summarizes our findings in emigration experiments, as well as observations made by others. “Cell content in organ” refers to our results in long-term (16 to 20 hours) homing experiments and provides a measure of the cell numbers of adoptively transferred cells found in a particular organ. Open arrows within the organ drawings symbolize the entry and exit routes of naive lymphocytes for each organ. Other symbols and abbreviations are explained as follows: upward arrows indicate enhancement; downward arrows, reduction; lateral arrows, indicate no effect; and ND, not determined. Arrows in parentheses indicate a pronounced, although not statistically significant, tendency. Footnotes: a, based on the fact that cell exit from PP is reduced; b, occurs independently of T cell–expressed S1P1; c, the profound lymphopenia in blood suggests a reduction in lymphocyte exit from spleen; d, although not addressed experimentally, it remains possible that T-cell accumulation in BM is increased not only due to enhanced cell entry but also due to decreased cell exit; e, based on the analysis of endogenous single-positive CD4+ or CD8+ T cells in thymus of S1P1–/– chimeras and FTV720-treated mice8,11,15 ; f, suggested by thoracic duct lymph canulation experiments, which have revealed a substantial reduction of S1P1-deficient11 or FTY720-treated T and B cells7 in lymph; g, as shown by Cinamon et al17 ; h, the fact that B-cell entry is unaffected, but S1P1 deficiency or FTY720 treatment leads to accumulation of cells in BM after long-term adoptive transfer, suggests that B-cell exit from BM is reduced.
Summary of the effects of S1P1 deficiency or FTY720 treatment on the trafficking of adoptively transferred lymphocytes. The effects of S1P1 deficiency and FTY720 treatment on cell entry or cell exit to various lymphoid organs are presented for T cells (top) and B cells (bottom). Conclusions on “cell entry” are derived from our short-term homing and IVM experiments, whereas “cell exit” summarizes our findings in emigration experiments, as well as observations made by others. “Cell content in organ” refers to our results in long-term (16 to 20 hours) homing experiments and provides a measure of the cell numbers of adoptively transferred cells found in a particular organ. Open arrows within the organ drawings symbolize the entry and exit routes of naive lymphocytes for each organ. Other symbols and abbreviations are explained as follows: upward arrows indicate enhancement; downward arrows, reduction; lateral arrows, indicate no effect; and ND, not determined. Arrows in parentheses indicate a pronounced, although not statistically significant, tendency. Footnotes: a, based on the fact that cell exit from PP is reduced; b, occurs independently of T cell–expressed S1P1; c, the profound lymphopenia in blood suggests a reduction in lymphocyte exit from spleen; d, although not addressed experimentally, it remains possible that T-cell accumulation in BM is increased not only due to enhanced cell entry but also due to decreased cell exit; e, based on the analysis of endogenous single-positive CD4+ or CD8+ T cells in thymus of S1P1–/– chimeras and FTV720-treated mice8,11,15 ; f, suggested by thoracic duct lymph canulation experiments, which have revealed a substantial reduction of S1P1-deficient11 or FTY720-treated T and B cells7 in lymph; g, as shown by Cinamon et al17 ; h, the fact that B-cell entry is unaffected, but S1P1 deficiency or FTY720 treatment leads to accumulation of cells in BM after long-term adoptive transfer, suggests that B-cell exit from BM is reduced.
Recent work has shown that S1P1 is expressed on extravascular T cells in SLOs, where S1P concentrations are low, but becomes down-regulated on circulating T cells in PBL or lymph.18 However, we still observed a significant decrease (∼25%) in short-term (2.5 hours) homing to PLNs in FTY720-treated mice after adoptive transfer of PBL-derived, rather than SLO-derived, T cells (data not shown). Thus, S1P supports T-cell homing to PLNs even at the low S1P1 receptor levels present on circulating T cells. However, the mechanism by which S1P1 contributes to integrin-dependent arrest remains unclear at this point. Of interest, it has been reported that S1P can amplify lymphocyte chemotaxis to certain chemokines14,38 such as the CCR7 agonist CCL21, which is highly expressed in HEVs and induces LFA-1 activation on rolling T cells.34,39 Therefore, S1P1 might facilitate T-cell sticking in PLN HEVs by amplifying CCR7-dependent integrin activation. However, we cannot exclude that S1P1 promotes T-cell homing through additional mechanisms, since FTY720 has been reported to enhance T-cell accumulation in PLNs even in the absence of CCR7 or its chemokine agonists,29 although this CCR7-independent effect is relatively small.38
It should be emphasized that T-cell sticking in HEVs and homing to PLNs was reduced, but not abolished in the absence of S1P1, whereas T-cell egress from LNs and PPs is apparently entirely dependent on S1P17,11 (and data not shown). It is inevitable, therefore, that the paralysis of T-cell recirculation by inhibition of S1P1 causes progressive T-cell depletion from PBL. However, the disappearance of circulating T cells is apparently not solely dependent on sequestration in SLOs, since T cells injected into FTY720-treated mice were depleted from the circulation even when they were prevented from homing to LNs and PPs. This drop in circulating lymphocyte counts very likely reflects a redistribution of cells within the body, rather than enhanced cell death, since the total sum of transferred cells that was recovered from recipient mice at the 16-hour time-point was similar between all treatment groups. Indeed, both FTY720 and FTY-P enhanced the accumulation of adoptively transferred T and B cells in BM, and S1P1–/– lymphocytes also accumulated much more in BM, suggesting that lymphocyte retention in this organ is also regulated by S1P1 (Figures 1, 2, S2). It is tempting to speculate that the BM may function as a sink for circulating lymphocytes that do not express functional S1P1, which could explain the pronounced lymphopenia induced by FTY720.
Although the present findings together with recent work by others firmly establish that S1P1 is required for T-cell exit from numerous tissues, the precise mechanism for this effect is still unknown. It has been proposed that low concentrations of S1P in the extravascular compartment may permit S1P1-mediated chemotaxis toward high S1P concentrations present in PBL or lymphatics.11 Alternatively, it is possible that S1P1 regulates lymphocyte diapedesis across endothelial barriers and/or controls lymphocyte motility in the interstitial space. However, given that there was no difference in T-cell motility with or without S1P1 (Figure 3), the latter explanation seems less likely. However, it should be cautioned that our popliteal LN preparation for MP-IVM does not allow imaging of very deep cortical areas or the cortico-medullary junction. Therefore, it is still possible that differences in cell motility occur in these regions.
The mechanism(s) by which S1P controls lymphocyte traffic appear(s) to be distinct in PPs compared with PLNs and MLNs (Figure 8). Homing to PLNs and MLNs was consistently reduced at 2.5 hours after adoptive transfer of S1P1–/– T cells into untreated mice or of WT T cells into FTY720- or FTY-P–treated mice. By contrast, drug treatment enhanced T-cell homing to PPs, whereas this process was unaffected by S1P1 deficiency (Figures 1 and 2). Interestingly, the effects of FTY720 and S1P1 deficiency were more dissimilar for B cells than T cells; drug treatment did not significantly alter B-cell homing to SLOs at any time-point analyzed, but S1P1–/– B cells displayed a profound homing defect to all SLOs, including PPs (Figures 2 and S2). The latter findings, in combination with our IVM data (Figure 5), suggest that S1P1 is required for B cells but not T cells to interact with PP HEVs. The reason for this difference is unclear but might involve the known differences in chemokine requirements for B-versus T-cell homing to PPs.39 Additionally, unlike in PLNs, PP HEVs are segmentally specialized to support selective integrin-dependent sticking of either B or T cells, whereas both lymphocyte populations are recruited across the same HEVs in PLNs.35
FTY720 treatment significantly enhanced S1P1–/– T-cell accumulation in PPs, but not in other SLOs (Figure 6), and the drug also increased sticking of both WT and S1P1–/– T cells in PP HEVs (Figure 7). These findings indicate that FTY720 modulates T-cell traffic in PPs through pathways that are independent of lymphocyte-expressed S1P1. Furthermore, the fact that no difference in rolling or sticking was observed upon injection of FTY720-treated or untreated T cells into untreated mice, suggests that FTY720 exerts its effects by acting on resident cells in PPs, possibly ECs. In fact, ECs are known to express S1PRs, including S1P1, and FTY720 effects on endothelial S1PRs have been implicated in various processes such as angiogenesis, migration, integrin-mediated adhesion,40 and the formation of adherens junctions.41-43
In summary, our homing experiments and IVM analysis reveal that FTY720 treatment and S1P1 deficiency not only affect lymphocyte exit from SLOs, but also modulate integrin-dependent lymphocyte homing to PLNs and PPs via HEVs. Both FTY720 and S1P1 deficiency also induce retention of B and T cells in BM. Although S1P1 deficiency and FTY720 treatment exert similar effects on T-cell trafficking in most organs, we now show that FTY720-induced enhancement of T-cell homing to PPs is not a consequence of the drug's effect on T-cell–expressed S1P1. As such, our data provide the first evidence that FTY720 affects T-cell trafficking not only via its action on S1P1 on lymphocytes, but also by exerting indirect effects on traffic molecules within target organs. The relative contribution of these distinct modes of action to the immunosuppressive activity of FTY720 and its analogues remains to be determined.
Prepublished online as Blood First Edition Paper, May 3, 2005; DOI 10.1182/blood-2004-09-3687.
Supported in part by fellowships from the Swiss National Science Foundation and from the Novartis Foundation, Switzerland (C.H.), and National Institutes of Health grants HL48675, HL54936, and HL56949 and a grant from Merck Research Laboratories (U.H.v.A.).
C.H. and M.L.S. contributed equally to this study.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal