Abstract
Osteopontin (OPN) has been shown to have T helper 1 (Th1) cytokine functions in cell-mediated immunity. Deficiency of OPN is linked to a reduced Th1 immune response in autoimmunity, infectious disease, and delayed-type allergy. Dendritic cells (DCs) are central for the induction of T-cell–mediated immunity, when initially flexible DCs are instructed by priming signals and tissue-derived factors to adopt Th1, Th2, or regulatory T-cell–inducing phenotypes. Although OPN influences the cytokine secretion of T cells and macrophages, its effects on DC polarization remain an important missing link in the understanding of OPN functions in Th1 immunity. Here we demonstrate that OPN promotes the emigration of human DCs from the epidermis and functionally activates myeloid-type DCs, augmenting their expression of HLA-DR, costimulatory, and adhesion molecules. OPN induces their Th1-promoting tumor necrosis factor α (TNF-α) and interleukin-12 (IL-12) secretion, and enhances their allostimulatory capacity. In mixed lymphocyte reactions (MLRs), OPN stimulates IL-12 secretion by DCs, inducing elevated interferon-γ (IFN-γ) production by T cells. Naive Th cells stimulated by OPN-activated DCs show a Th1-polarized cytokine production. Our findings identify OPN as an important tissue-derived factor that DCs encounter when traveling from peripheral sites of activation to secondary lymphatic organs, which induces DC maturation toward a Th1-promoting phenotype.
Introduction
Osteopontin (OPN) is a secreted phosphoprotein that contains an integrin-binding arginine-glycine-aspartate sequence (RGD) motif. OPN has proinflammatory cytokine and chemokine functions in cell-mediated immunity.1-4 A number of studies have demonstrated that OPN critically contributes to the development of T helper 1 (Th1)–mediated immunity and disease.5,6 Among other reports, OPN-deficient mice develop disseminated infection and have a delayed ability to clear disease when infected with Mycobacterium bovis (BCG).7 In murine models of autoimmune encephalomyelitis, a model of human multiple sclerosis that critically depends upon the balance of Th-shaping cytokines such as interleukin-10 (IL-10) and IL-12, OPN-deficient mice develop milder disease.8,9 Corneal infection of OPN-deficient mice with herpes simplex virus 1 (HSV-1) is followed by a reduced delayedtype hypersensitivity to HSV.6 We have shown that OPN-deficient mice have an impaired allergic contact hypersensitivity (CHS) response against trinitrochlorobenzene, which is accompanied by a reduced ability to attract dendritic cells (DCs) from the periphery into draining lymph nodes.10
DCs are the most potent antigen-presenting cells (APCs). Their function and polarizing capacities are decisive for the outcome of Th-mediated immunity.11-13 Although, it is known that OPN plays a central role for the initiation of Th1-mediated immune responses, it is unknown whether it is involved in DC instruction to induce Th1-mediated responses. In their immature state, DCs are situated in peripheral nonlymphoid tissues.14,15 For example, Langerhans cells (LCs) are located in the epidermis. Upon stimulation, LCs/DCs undergo a maturation process resulting in the down-regulation of their antigen uptake and processing capacity; up-regulation of major histocompatibility complex (MHC) II and costimulatory molecules; and a switch in their expression pattern of chemokines and adhesion molecules.14,15 As a consequence of this reprogramming, DCs migrate into lymphoid organs.16,17 In the T-cell zone of lymph nodes, they function as APCs, which prime naive antigen-specific T cells and drive their differentiation toward Th1, Th2, or regulatory T cells.11-13
The initial activation stimulus in concert with tissue environmental factors encountered by migrating DCs instruct DCs to polarize toward a phenotype that initiates Th1, Th2, or regulatory T effector cells.11,12,18,19 Numerous viral and microbial factors, among them lipopolysaccharide (LPS), Staphylococcus aureus Cowan strain I, bacterial DNA and dsRNA,20-24 and Pertussis toxin in the context of IL-1β and tumor necrosis factor α (TNF-α),25 have been described as IL-12– and Th1-prompting factors. In contrast, substances that increase intracellular cyclic adenosine monophosphate (cAMP), such as cholera toxin26 or soluble extract of Schistosoma mansoni eggs,25,27 initiate DC differentiation toward a Th2 phenotype.
Incomplete knowledge exists regarding tissue factors that are encountered by DCs on their way to antigen presentation.11 Such factors may be decisively involved in fine-tuning DC polarization. An example for a Th1-inducing tissue factor is interferon-γ (IFN-γ), the most important enhancer of IL-12 secretion, which is produced at inflammatory sites following the invasion of intracellular bacteria or viruses.28 Recently, we have shown that in the sensitization phase of CHS, within 6 hours after hapten application, OPN is up-regulated within the skin and the skin-draining lymph nodes.10 Furthermore, OPN is highly expressed at sites of mycobacterial infection.29 Immunohistochemical double staining of skin specimen in CHS revealed colocalization of migrating LCs with high OPN expression within the dermis, passed by LCs on their way to lymph nodes and upon their entrance in lymph nodes.10 These findings prompted us to investigate the function of OPN as a DC-instructing tissue factor that is encountered by DCs at sites of inflammation and at sites passed on their way to secondary lymphatic organs, which may decisively affect the polarization of an immune response through DCs.
Materials and methods
Media and reagents
RPMI 1640 was supplemented (complete RPMI [c-RPMI]) with 10% heat-inactivated fetal calf serum (FCS), 1 mM nonessential amino acids, 45 μg/mL penicillin and streptomycin, and 2 mM l-glutamine (all from Gibco, Eggenstein, Germany). For Th polarization assays, c-RPMI was supplemented with 5 × 10–5 M mercaptoethanol (Sigma-Aldrich, Taufkirchen, Germany).
Recombinant fully mature human OPN was obtained from Chemicon (Hofheim, Germany).
Monoclonal antibodies (mAbs) specific for human leukocyte antigen DR (HLA-DR) (G46-6 (L243)), CD1a (HI149), CD80 (BB1), and CD86 (2331) were purchased from BD-Pharmingen (Heidelberg, Germany); and mAb for CD14 (RMO52), CD40 (MAB89), and CD54 (84H10), from Immunotech (Marseille, France). The anti–human integrin antibodies αvβ3 (LM609), αvβ5 (P1FG), and β1 (P4G11) were obtained from Chemicon. Isotype control antibodies (mouse immunoglobulin M [IgM, G155-228], mouse IgG1 [107.3], and mouse IgG2a [MOPC-21]) were from BD-Pharmingen.
Split thickness skin organ culture
The 1 cm × 2 cm split thickness skin (2-4 mm) was prepared by dermatome (Aesculap, Tuttlingen, Germany)30 and floated on c-RPMI in 6-well plates (Greiner, Frickenhausen, Germany) in the presence or absence of OPN (0.5 μg/mL). After 24 hours, cells that had migrated into the culture medium were collected and counted microscopically after lysis of erythrocytes. Migrated cells were stained with fluorescein isothiocyanate (FITC)–conjugated mAbs against CD1a, and for double staining additionally with phycoerythrin (PE)–conjugated anti–HLA-DR mAb or appropriate isotype controls, and were analyzed by fluorescence-activated cell sorter (FACS). The percentage of CD1a+ cells that migrate from split thickness skin, predominantly LCs, was calculated by CellQuest software (BD-Pharmingen). The number of LCs that had migrated was calculated by the following formula: LCs = absolute cell number (counted microscopically) × percentage of CD1a+ emigrated cells. Skin specimens were obtained from patients with elective plastic surgery, following informed consent. Approval for this study was obtained from the institutional review board of the University of Freiburg.
Generation of human myeloid DCs
Monocyte-derived DCs were prepared as described.31 In brief, CD14+ cells from peripheral blood mononuclear cells (PBMCs) were enriched with a bead-labeled anti-CD14 mAb (Miltenyi Biotec, Bergisch-Gladbach, Germany) using the MACS magnetic cell sorting system (Miltenyi Biotec). CD14+ cells (mean purity, 89.6%; SD ± 9.14%) (1 × 106/mL) were cultured for 5 days in C-RPMI containing granulocyte-macrophage colony-stimulating factor (GM-CSF, 1000 U/mL Leucomax; Novartis, Basel, Switzerland) and IL-4 (1000 U/mL; Promocell, Heidelberg, Germany) in 24-well culture plates to generate DCs. Cells were identified as immature DCs by positive expression of CD1a and the lack of CD14 (purity, 84.21%; SD ± 8.5%) and low expression of HLA-DR, CD40, CD80, and CD86.14
DC maturation/stimulation
To study DC activation by OPN, immature DCs were harvested at day 5, extensively washed, and cultured (1 × 106) for 48 hours in c-RPMI in the presence or absence of 10 μg/mL LPS (Serotype O111:B4; Sigma-Aldrich), or 0.5 μg/mL OPN (Chemicon). Supernatants and cells were obtained after 24 and 48 hours. The chosen concentration was optimized in titration experiments for DC migration10 and stimulation of HLA-DR, CD40, and CD54 expression (Table 1).
Dose-dependent OPN-induced DC activation
. | HLA-DR . | CD40 . | CD54 . |
|---|---|---|---|
| 0 h | 154.3 | 68.2 | 86.8 |
| Unstimulated | 217.1 | 84.0 | 112.5 |
| OPN, 0.05 μg | 224.0 | 151.8 | 183.1 |
| OPN, 0.5 μg | 243.2 | 280.4 | 244.7 |
. | HLA-DR . | CD40 . | CD54 . |
|---|---|---|---|
| 0 h | 154.3 | 68.2 | 86.8 |
| Unstimulated | 217.1 | 84.0 | 112.5 |
| OPN, 0.05 μg | 224.0 | 151.8 | 183.1 |
| OPN, 0.5 μg | 243.2 | 280.4 | 244.7 |
CD14+ derived DCs were stimulated on day 5 with the indicated concentrations of OPN. The expression of HLA-DR, CD40, or isotype control was analyzed by flow cytometry after 24 hours. Data indicate the corrected mean fluorescence intensity, after subtraction of the fluorescence of the isotype control. Results from one of 2 dose-finding experiments are shown.
Adhesion slides and immunostaining
Stimulated DCs were adhered to BioRad adhesion slides (BioRad, Munich, Germany) according to the manufacturer's protocol. The slides were incubated with the primary anti–HLA-DR antibody followed by a biotinylated antimouse mAb and streptavidin peroxidase, both from LSAB-Kit (DAKO, Hamburg, Germany). The peroxidase was developed by 3-amino-9-ethyl carbazole according to the manufacturer's protocol.
Flow cytometry
Surface receptor expression on DCs was determined on days 5, 6, and 7. Cells were stained (4°C, 30 minutes) with FITC- or PE-labeled mAb. To exclude dead cells by appropriate gating, 2.5 μg/mL 7-aminoactinomycin D (7-AAD; Sigma-Aldrich) was added. Viable cells (5-10 × 103 per sample) were analyzed and mean fluorescence intensities (MFIs) determined by CellQuest software.
Generation of human T cells
PBMCs were isolated from blood of healthy donors by Ficoll gradient centrifugation.32 T cells for mixed lymphocyte reactions (MLRs) were enriched (purity, > 95%) by immunomagnetic negative-depletion using pan T-cell separation Kit and MACS columns (Miltenyi Biotec) according to the manufacturer's instructions. CD4+ cells for Th-cell polarization assays were isolated with the CD4 isolation Kit (Miltenyi Biotec) from PBMCs. For separation of CD45RA+ cells, CD4+ cells were incubated with PE-labeled anti-CD45R0 mAb, followed by incubation with anti-PE beads (Miltenyi Biotec) and separated by MACS.
Mixed lymphocyte reaction (MLR)
MLRs were performed at 2 different time points of DC culture. In setting one, day-5 immature DCs were used and OPN was present or absent throughout the MLR. In setting 2, day-5 DCs were left untreated for control, or stimulated with 0.5 μg/mL OPN or with 10 μg/mL LPS for 48 hours and then used for MLR. In both settings, MLRs were performed with a DC/T-cell (Tc) ratio of 1 × 104:1 × 105/200 μL in U-bottom 96-well plates (Greiner) using allogeneic pan T cells. For both MLR settings, allogeneic DCs and T cells were combined from the same donor pair. At both time points, maximum T-cell proliferation was induced by addition of phytohemagglutinin (PHA, 0.5 μg/mL) as positive control.
Supernatants were obtained after 48 hours of MLR to determine IL-12p70 and IL-10 secreted from DCs or IFN-γ, IL-13, and IL-4 secreted from T cells. After 96 hours, T-cell proliferation was measured by [3H]-thymidine incorporation within the last 18 hours of the experiment. Plates were harvested with a Canberra Packard Filter-Mate, and [3H]-thymidine incorporation was determined by liquid scintillation spectroscopy using a TopCount (Canberra Packard, Dreieich, Germany). If fewer than 1 × 105 T cells/200 μL were available for MLR on day 7, the measured T-cell proliferation was normalized by the following formula: [3H]-thymidine incorporation after PHA stimulation on day 5/[3H]-thymidine incorporation after PHA stimulation on day 7 × determined [3H]-thymidine incorporation after specific stimulation.
Th-cell polarization assay
Naive CD45RA+ Th cells (20 × 103/200 μL) were enriched by immunomagnetic negative selection. They were then cocultured with DCs (5 × 103/200 μL) that had been prematured in the presence or absence of OPN (0.5μg/mL) or IFN-γ (1000 U/mL; R&D Systems, Wiesbaden, Germany) alone or combined with IFN-γ, IL-1β (2000 U/mL; R&D Systems), and TNF-α (5000 U/mL; Promocell),20,28 pulsed with staphylococcus enterotoxin B (SEB, 100 pg/mL; Sigma-Aldrich). On day 5, IL-2 (50 U/mL; R&D Systems) was added. The cytokine secretion was induced by phorbol myristate acetate (PMA, 10–7 M) and ionomycin (1 μg/mL; both from Sigma-Aldrich) on day 9, and supernatants were obtained on day 10 of coculture.
Cytokine measurement
Supernatants from DCs, MLR, and Th-cell polarization assays were harvested at the indicated time points and stored at –80°C. Cytokines were quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions and measured at an extinction of 450 nm (MR5000 ELISA-reader and Bio-Linx Software; Dynatech, Chantilly, VA). The following ELISA kits were used (sensitivity): OPN (5 ng/mL) from IBL (Takasaki, Japan); IL-4 (7.8 pg/mL), IL-5 (7.8 pg/mL), IL-6 (4.7 pg/mL), IL-10 (7.8 pg/mL), IL-13 (2.1 pg/mL), IFN-γ (4.7 pg/mL), and TNF-α (all Opteia) from BD-Pharmingen; IL-12p40 (> 15 pg/mL) and IL-12p70 (7.8 pg/mL) from Quantikine (R&D Systems) or Opteia (BD-PharMingen).
Results
OPN activates human Langerhans cells, inducing their emigration from the epidermis and their phenotypical maturation
Recently, we have demonstrated that OPN induces LC migration in vitro and in vivo in a murine CHS model.10 Whether OPN promotes LC/DC activation remains unclear. To explore whether OPN influences emigration of human LCs, thereby inducing their activation, we used a skin explant model in which LCs migrate from split thickness skin floated on culture medium with or without recombinant OPN.33,34 We found that the addition of OPN strongly induces the emigration of CD1a+ LCs from the epidermis (Figure 1A). LCs that had migrated upon OPN stimulation in skin explant cultures consistently showed up-regulated MHC II molecules compared with cells that had migrated into medium that was not OPN supplemented (Figure 1B). Our findings indicate that OPN promotes LC emigration from human skin and simultaneously induces signals promoting their maturation.
OPN induces the emigration of human Langerhans cells from the epidermis and their activation with up-regulated expression of MHC class II molecules. Split thickness sheets of human skin (2 cm2) were floated on c-RPMI supplemented with (closed symbols) or without (open symbols) OPN (0.5 μg/mL). Sheets were removed after 24 hours. Emigrated cells were counted microscopically following lyses of contaminating erythrocytes. Cells were double stained by FITC-labeled antibodies against CD1a- and PE-labeled antibodies against HLA-DR. (A) The relative number of LCs was determined by flow cytometry by positive CD1a staining. The absolute number of emigrated LCs per cm2 of skin was determined by the following formula: percentage of CD1a+ cells × microscopically counted cells/cm2 of skin. Data are expressed as absolute number of LCs that had emigrated from 1 cm2 skin. The mean of 6 independent experiments ± SEM is shown. Each pair of closed and corresponding open symbols represents data from the same donor. In 5 of 6 donors, OPN stimulated LC migration. When calculating the percentage of OPN-induced migration for each donor and performing a Wilcoxon signed rank test, a P value of .094 was obtained for this set of experiments. (B) In FACS analysis CD1a and HLA-DR, double-stained emigrated cells were gated for CD1a expression and HLA-DR expression of CD1a+ cells was analyzed. The dotted curves give the isotype control staining. Shown is 1 representative experiment of 5, each with tissues from different donors.
OPN induces the emigration of human Langerhans cells from the epidermis and their activation with up-regulated expression of MHC class II molecules. Split thickness sheets of human skin (2 cm2) were floated on c-RPMI supplemented with (closed symbols) or without (open symbols) OPN (0.5 μg/mL). Sheets were removed after 24 hours. Emigrated cells were counted microscopically following lyses of contaminating erythrocytes. Cells were double stained by FITC-labeled antibodies against CD1a- and PE-labeled antibodies against HLA-DR. (A) The relative number of LCs was determined by flow cytometry by positive CD1a staining. The absolute number of emigrated LCs per cm2 of skin was determined by the following formula: percentage of CD1a+ cells × microscopically counted cells/cm2 of skin. Data are expressed as absolute number of LCs that had emigrated from 1 cm2 skin. The mean of 6 independent experiments ± SEM is shown. Each pair of closed and corresponding open symbols represents data from the same donor. In 5 of 6 donors, OPN stimulated LC migration. When calculating the percentage of OPN-induced migration for each donor and performing a Wilcoxon signed rank test, a P value of .094 was obtained for this set of experiments. (B) In FACS analysis CD1a and HLA-DR, double-stained emigrated cells were gated for CD1a expression and HLA-DR expression of CD1a+ cells was analyzed. The dotted curves give the isotype control staining. Shown is 1 representative experiment of 5, each with tissues from different donors.
OPN induces DC differentiation
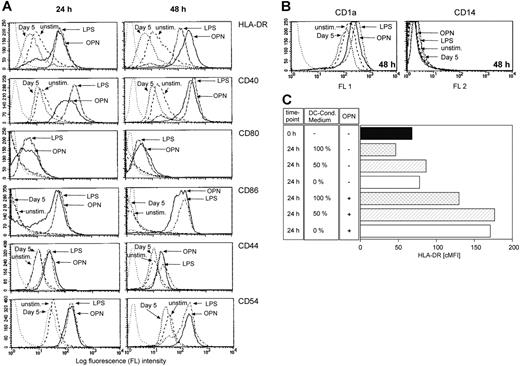
DCs have been shown to express OPN mRNA.35,36 We therefore investigated the OPN production of DCs generated from peripheral blood CD14+ monocytes. During their GM-CSF– and IL-4–driven differentiation from CD14+ monocytes into immature DCs on day 5, cells secreted 460.3 ng/mL (SD ± 214.2 ng/mL; n = 4, 1 × 106 cells/mL) OPN. To quantify the OPN secretion of the immature DCs after day 5, cells were extensively washed and recultured in fresh medium without cytokines. Within 24 hours, immature DCs secreted 313 ng/mL (SD ± 208.1 ng/mL; n = 4, 1 × 106 cells/mL) OPN. To investigate the effects of recombinant OPN on phenotypical myeloid DC maturation, washed DCs were stimulated with 0.5 μg/mL OPN. DCs were analyzed by light microscopy and flow cytometry at different time points of in vitro maturation.31 Immature DCs were either cultured without stimulus (Figure 2A-C) or treated with OPN (Figure 2D-F) or LPS as an established factor that initiates terminal DC maturation (Figure 2G-I) from culture days 5 to 7. In Figure 2, images were taken on days 6 (A,D,G) and 7 (B-C,E-F,H-I) of culture. When analyzing DC cultures by light microscopy, it was remarkable that OPN induced the formation of DC clusters (Figure 2). In contrast to unstimulated cultures (Figure 2A-B), addition of OPN induced DC clustering beginning 24 hours after stimulation (Figure 2D), which was further enhanced after 48 hours (Figure 2E). DCs cultured in the presence of OPN developed the characteristic dendrites of activated DCs (Figure 2F). Interestingly, they did not display the veils of DCs terminally matured by LPS treatment (Figure 2I).37 FACS analyses on days 5, 6, and 7 revealed a strong expression of CD1a, while CD14 was not detectable (Figure 3B), classifying these cells as DCs.31 In contrast to unstimulated cells, OPN treatment induced the expression of HLA-DR, CD40, CD80, CD86, CD44, and CD54 within 48 hours, in a manner comparable with that observed with LPS (Figure 3A; Table 1). Viability of DCs in the absence of OPN was 90.5% (SD ± 4.8%) on day 6 and 91.8% (SD ± 6.3%) on day 7. Addition of OPN did not affect DC viability (91% ± 3.7% on day 6, n = 6; 92.3% ± 5.5% on day 7, n = 4). DCs express the OPN receptors CD44 and the integrins αvβ3, αvβ5, and β1. While CD44 was up-regulated by OPN and LPS, the expression of the analyzed integrins was not modulated (data not shown). To investigate whether in our system the OPN secreted by DCs influences the stimulatory capacity of the recombinant OPN, DCs were cultured in different concentrations of DC-conditioned medium obtained on day 5 of CD14+ cell culture and were stimulated with the recombinant protein. Independent of the concentration of DC-conditioned medium, the exogenous OPN was stimulatory (Figure 3C). Our findings demonstrate that OPN not only acts as a chemoattractant for DCs but, as determined by morphology, also induces a state of LC/DC maturation distinct from the terminal maturation induced by LPS.
OPN-stimulated DCs show morphologic signs of activation. DCs derived from peripheral blood monocytes were left untreated (A-C), or stimulated with 0.5 μg/mL OPN (D-F) or 10 μg/mL LPS (G-I) on day 5 of culture. Photographs of cultures were taken 24 hours (A,D,G) and 48 hours (B,E,H) after addition of the stimulus. Bar in panel A for magnification in panels A-B, D-E, G-H: 150 μM. For morphologic evaluation of DCs, cells were cultured as described in “Materials and methods,” and after 48 hours of treatment, unstimulated DCs (C), OPN-stimulated DCs (F), or LPS-stimulated DCs (I) were fixed on adhesion slides and stained immunohistochemically with antibodies against HLA-DR. Bar in panel C for magnification in panels C,F,I: 15 μM. Panels A-B, D-E, G-H: Cell cultures were analyzed with an Axiovert microscope (Zeiss, Göttingen, Germany) using a Zeiss 10.0 objective lens providing a 10-fold magnification. Panels C, F, I: Adhesion slides were analyzed with an Axioskop microscope (Zeiss) with oil immersion using a 100× objective lens (Plan Neofluar objective, Zeiss). Photos were taken with a Canon EOS 300V (Canon, Krefeld, Germany) used as an onboard camera without additional optical lenses. All slides were scanned with CanoScan (Canon), and images were assembled with CorelDraw 11.
OPN-stimulated DCs show morphologic signs of activation. DCs derived from peripheral blood monocytes were left untreated (A-C), or stimulated with 0.5 μg/mL OPN (D-F) or 10 μg/mL LPS (G-I) on day 5 of culture. Photographs of cultures were taken 24 hours (A,D,G) and 48 hours (B,E,H) after addition of the stimulus. Bar in panel A for magnification in panels A-B, D-E, G-H: 150 μM. For morphologic evaluation of DCs, cells were cultured as described in “Materials and methods,” and after 48 hours of treatment, unstimulated DCs (C), OPN-stimulated DCs (F), or LPS-stimulated DCs (I) were fixed on adhesion slides and stained immunohistochemically with antibodies against HLA-DR. Bar in panel C for magnification in panels C,F,I: 15 μM. Panels A-B, D-E, G-H: Cell cultures were analyzed with an Axiovert microscope (Zeiss, Göttingen, Germany) using a Zeiss 10.0 objective lens providing a 10-fold magnification. Panels C, F, I: Adhesion slides were analyzed with an Axioskop microscope (Zeiss) with oil immersion using a 100× objective lens (Plan Neofluar objective, Zeiss). Photos were taken with a Canon EOS 300V (Canon, Krefeld, Germany) used as an onboard camera without additional optical lenses. All slides were scanned with CanoScan (Canon), and images were assembled with CorelDraw 11.
OPN induces terminal DC differentiation with up-regulated expression of MHC class II, costimulatory, and adhesion molecules. DCs (1 × 106) were cultured in the presence or absence of either OPN or LPS from days 5 to 7 of culture. Cells were stained with antibodies against HLA-DR, CD40, CD80, CD86, CD44, and CD54 (A) and with antibodies against CD1a and CD14 to determine their DC phenotype (B) and were analyzed by FACS after 24 and 48 hours of stimulation. FACS analysis of 1 representative of 5 experiments is shown. The dotted line on the left in panels A and B represents isotype control staining of unstimulated DCs. (C) To demonstrate that the OPN added for DC activation induces HLA-DR expression independent of the endogenous OPN, DCs from day 5 (▪) were cultured for 24 hours in 100% (▩) or 50% (▨) DC-conditioned medium or in fresh medium without GM-CSF or IL-4 (0%; □), with (+) or without (–) exogenous OPN. Corrected mean fluorescence (cMFI) of HLA-DR expression from 1 representative of 3 independent experiments is given.
OPN induces terminal DC differentiation with up-regulated expression of MHC class II, costimulatory, and adhesion molecules. DCs (1 × 106) were cultured in the presence or absence of either OPN or LPS from days 5 to 7 of culture. Cells were stained with antibodies against HLA-DR, CD40, CD80, CD86, CD44, and CD54 (A) and with antibodies against CD1a and CD14 to determine their DC phenotype (B) and were analyzed by FACS after 24 and 48 hours of stimulation. FACS analysis of 1 representative of 5 experiments is shown. The dotted line on the left in panels A and B represents isotype control staining of unstimulated DCs. (C) To demonstrate that the OPN added for DC activation induces HLA-DR expression independent of the endogenous OPN, DCs from day 5 (▪) were cultured for 24 hours in 100% (▩) or 50% (▨) DC-conditioned medium or in fresh medium without GM-CSF or IL-4 (0%; □), with (+) or without (–) exogenous OPN. Corrected mean fluorescence (cMFI) of HLA-DR expression from 1 representative of 3 independent experiments is given.
OPN induces the secretion of TNF-α and IL-12 by DCs. DCs were cultured in the presence or absence of OPN from days 5 to 7 of culture. Supernatants were analyzed by ELISA after 24 hours and 48 hours of OPN stimulation for the secretion of TNF-α (A), IL-12p40 (B), bioactive IL-12p70 (C, ▪), and IL-10 (C, □). Cytokine secretion of 1 × 106 DCs in pg/mL ± SEM of 8 (A,C) or 6 (B) independent experiments is shown.
OPN induces the secretion of TNF-α and IL-12 by DCs. DCs were cultured in the presence or absence of OPN from days 5 to 7 of culture. Supernatants were analyzed by ELISA after 24 hours and 48 hours of OPN stimulation for the secretion of TNF-α (A), IL-12p40 (B), bioactive IL-12p70 (C, ▪), and IL-10 (C, □). Cytokine secretion of 1 × 106 DCs in pg/mL ± SEM of 8 (A,C) or 6 (B) independent experiments is shown.
OPN stimulates the secretion of TNF-α and IL-12 by DCs
In different systems, OPN has been shown to be central for the induction of an adequate Th1-type response.3,6,8,9,38 Because DCs play the key role for shaping T-cell–mediated immune responses through their secretion of specific cytokine patterns, we went on to determine the effect of recombinant OPN on the cytokine release of DCs.
Day-5 immature DCs (1 × 106/mL) were cultured in the presence or absence of OPN and supernatants were tested after 24 hours and 48 hours. Upon OPN stimulation, DCs secreted high amounts of TNF-α (Figure 4A). Unstimulated DCs secreted IL-12p40 (Figure 4B) but no, or only low amounts of, IL-12p70 (Figure 4C). OPN as a single stimulus induced the secretion of bioactive IL-12p70 (Figure 4C) but did not significantly affect IL-12p40 secretion (Figure 4B). In contrast, the secretion of IL-10 (Figure 4C), an antagonist of IL-12–induced Th1-cell polarization, was not significantly modulated by addition of OPN.15,39 LPS contamination effects can be excluded because OPN preparations and fresh culture medium both contained less than 0.01 EU/mL LPS (Limulus amebocyte lysate analysis). Furthermore, OPN induced similar amounts of TNF-α in Toll-like receptor 4 (TLR-4)–defective DCs from C3H/HeJ or wild-type C3H/HeN mice (Table 2), thus excluding the theory that DC activation by OPN is due to LPS contamination. These findings give strong evidence, that OPN affects T-cell–mediated responses by provoking DCs to release Th1-promoting cytokines.
Recombinant OPN induces TNF-α in DCs from TLR-4 wild-type C3H/HeN and TLR-defective C3H/eJ mice
. | C3H/HeN, pg/mL ± SD . | C3H/eJ, pg/mL ± SD . |
|---|---|---|
| Unstimulated | 7.2 ± 2.3 | 9.8 ± 2 |
| LPS, 29.7 EU/mL | 25.2 ± 0 | 12.9 ± 3.1 |
| OPN, 0.5 μg/mL | 17.9 ± 1.8 | 40.9 ± 24.5 |
. | C3H/HeN, pg/mL ± SD . | C3H/eJ, pg/mL ± SD . |
|---|---|---|
| Unstimulated | 7.2 ± 2.3 | 9.8 ± 2 |
| LPS, 29.7 EU/mL | 25.2 ± 0 | 12.9 ± 3.1 |
| OPN, 0.5 μg/mL | 17.9 ± 1.8 | 40.9 ± 24.5 |
Bone marrow DCs from TLR-4 wild-type C3H/HeN and TLR-defective C3H/eJ mice were generated as previously described10 and stimulated by the indicated concentrations of LPS or OPN for 48 hours. TNF-α secretion in supernatants was analyzed by ELISA. Mean of duplicate measurements is given in pg/mL ± SD. Results from one representative experiment of 3 independent experiments are shown.
OPN enhances the allostimulatory capacity of DCs
Having demonstrated that OPN induces DC activation, we went on to show that such activation is functional for the allostimulatory capacity of DCs. We had previously shown that OPN is up-regulated in the paracortical T-cell regions of skin-draining lymph nodes during the sensitization phase of CHS when DCs encounter T cells for activation.10 We now simulated this in vivo situation by adding recombinant OPN directly to DCs interacting with T cells in MLR. To address this, OPN was first added directly into the MLR with immature day-5 DCs. In this setting, OPN in contrast to control significantly augmented allogeneic T-cell proliferation (Figure 5A). Interestingly, if DCs were prestimulated by OPN from days 5 to 7 of culture, an even more pronounced increase in T-cell proliferation was detected (Figure 5B). These results were confirmed when the percentage of OPN-induced T-cell proliferation was calculated for each individual experiment and statistical comparison with untreated day-5 DCs (Figure 5C) or more mature day-7 DCs (Figure 5D) was performed. Comparing T-cell proliferation induced by day-5 DCs (open diamonds), addition of OPN to MLR significantly increased their potential to stimulate T-cell proliferation (Figure 5C open circles, mean: 1.33-fold, median: 1.25-fold). Percent induction of T-cell proliferation was even higher when DCs were OPN pretreated for 48 hours from days 5 to 7 of culture prior to MLR (Figure 5C closed circles, mean: 4.06-fold, median: 3.13-fold increase in T-cell proliferation). Although DCs cultured until day 7 without additional stimulus are more potent inducers of T-cell proliferation than immature day-5 DCs, as would be expected (Figure 5B), OPN treatment for the last 48 hours of culture further significantly enhanced their T-cell stimulatory capacity (Figure 5D closed circles; mean: 1.55-fold, median: 1.39-fold increase in T-cell proliferation). Similar results were obtained by direct addition of LPS to MLR or addition of LPS-prestimulated effector DCs (data not shown). Together with our findings that OPN induces changes in morphologic phenotype, cell surface protein expression, and cytokine secretion, these data suggest that OPN is a potent factor inducing functional DC activation.
In the presence of allogeneic T cells, OPN augments the production of IL-12p70 by DCs and of IFN-γ by T cells
In addition to the capacity of OPN-treated DCs to induce T-cell proliferation, we next analyzed whether Th cells were also polarized in their pattern of cytokine expression. IL-12 and IL-10 were measured to evaluate Th-polarizing cytokines secreted by the DCs and IFN-γ, IL-13, IL-5, and IL-4, to detect the state of Th-cell polarization. Speculating that OPN could function as a cofactor in the initiation of IL-12 secretion by DCs, we first performed MLR with immature DCs in the presence of recombinant OPN. Under these conditions, DCs secreted high amounts of IL-12p70 (Figure 6A). At the same time IFN-γ production by T cells increased (Figure 6C), while the production of IL-10 (Figure 6A), IL-13 (Figure 6C), and IL-5 (data not shown) was low and was not influenced by added OPN. Likewise, IL-4 secretion was not detectable and was not modulated by OPN (data not shown). When OPN was added to T cells without additional second signal, which could be provided (eg, by DCs), it had no influence on cytokine secretion by T cells (data not shown), which is in accordance to previous findings by O'Regan et al.38
OPN-stimulated DCs exhibit enhanced T-cell allostimulatory capacity. Mixed lymphocyte reaction (MLR) was performed with allogeneic T cells (10 × 104/200μL) and immature DCs (10 × 103/200 μL) on day 5 of DC culture in the presence or absence of OPN, added throughout the MLR (A). Alternatively (B), DCs were stimulated with OPN from day 5 to day 7 of DC culture (48 h DC OPN) or left untreated for control (48 h DC wo OPN), prior to their addition to MLR. T-cell proliferation was measured by [H3]-thymidine incorporation in the last 18 hours of the experiment. (A-B) One representative experiment with T cells and DCs from the same donor pair is shown. This is representative of 7 independent experiments. Data are expressed as the mean counts per minute ± SD of quadruplicate cultures. (C) MLR was performed with DCs cultured for 5 days and OPN was added throughout the experiment (○). Alternatively, DCs were prestimulated with OPN for 48 hours prior to addition to MLR (•). The allostimulatory capacity in both settings was compared with that of immature DCs from day 5 (open gray diamonds). For statistical analysis of all experiments, the percent induction of T-cell proliferation by OPN was calculated by the following formula: T-cell proliferation induced by prestimulated DCs or DCs in the presence of OPN (CPM)/T-cell proliferation induced by immature DCs of day 5 (CPM) × 100%. Statistical analysis of 7 independent experiments ± SEM (*P < .05, one-way analysis of variance [ANOVA], Student-Neuman-Keuls test). (D) The stimulatory capacity of 48-hour OPN-stimulated DCs (•) is compared with the T-cell stimulatory capacity of unstimulated DCs from day 7 of DC culture (closed gray diamonds). Statistical analysis of 10 independent experiments ± SEM (#P < .05, Kruskal-Wallis one-way ANOVA on Ranks, Student-Neuman-Keuls test) is shown.
OPN-stimulated DCs exhibit enhanced T-cell allostimulatory capacity. Mixed lymphocyte reaction (MLR) was performed with allogeneic T cells (10 × 104/200μL) and immature DCs (10 × 103/200 μL) on day 5 of DC culture in the presence or absence of OPN, added throughout the MLR (A). Alternatively (B), DCs were stimulated with OPN from day 5 to day 7 of DC culture (48 h DC OPN) or left untreated for control (48 h DC wo OPN), prior to their addition to MLR. T-cell proliferation was measured by [H3]-thymidine incorporation in the last 18 hours of the experiment. (A-B) One representative experiment with T cells and DCs from the same donor pair is shown. This is representative of 7 independent experiments. Data are expressed as the mean counts per minute ± SD of quadruplicate cultures. (C) MLR was performed with DCs cultured for 5 days and OPN was added throughout the experiment (○). Alternatively, DCs were prestimulated with OPN for 48 hours prior to addition to MLR (•). The allostimulatory capacity in both settings was compared with that of immature DCs from day 5 (open gray diamonds). For statistical analysis of all experiments, the percent induction of T-cell proliferation by OPN was calculated by the following formula: T-cell proliferation induced by prestimulated DCs or DCs in the presence of OPN (CPM)/T-cell proliferation induced by immature DCs of day 5 (CPM) × 100%. Statistical analysis of 7 independent experiments ± SEM (*P < .05, one-way analysis of variance [ANOVA], Student-Neuman-Keuls test). (D) The stimulatory capacity of 48-hour OPN-stimulated DCs (•) is compared with the T-cell stimulatory capacity of unstimulated DCs from day 7 of DC culture (closed gray diamonds). Statistical analysis of 10 independent experiments ± SEM (#P < .05, Kruskal-Wallis one-way ANOVA on Ranks, Student-Neuman-Keuls test) is shown.
In the presence of allogeneic T cells, OPN augments the production of IL-12p70 by DCs and IFN-γ by T cells. MLR was performed with immature DCs (10 × 103/200 μL), cultured for 5 days prior to MLR and allogeneic T cells (10 × 104/200 μL) in the presence or absence of OPN, added throughout the MLR (A,C). (B,D) DCs were prestimulated with OPN from day 5 to day 7 of DC culture or left untreated as controls prior to addition to MLR. Supernatants were obtained after 48 hours of coculture and analyzed for their content of IL-12p70 and IL-10 (A-B), or IFN-γ and IL-13 (C-D) by ELISA. Data are given as mean pg/mL ± SD of duplicate measurements. Shown is 1 representative of 7 independent experiments.
In the presence of allogeneic T cells, OPN augments the production of IL-12p70 by DCs and IFN-γ by T cells. MLR was performed with immature DCs (10 × 103/200 μL), cultured for 5 days prior to MLR and allogeneic T cells (10 × 104/200 μL) in the presence or absence of OPN, added throughout the MLR (A,C). (B,D) DCs were prestimulated with OPN from day 5 to day 7 of DC culture or left untreated as controls prior to addition to MLR. Supernatants were obtained after 48 hours of coculture and analyzed for their content of IL-12p70 and IL-10 (A-B), or IFN-γ and IL-13 (C-D) by ELISA. Data are given as mean pg/mL ± SD of duplicate measurements. Shown is 1 representative of 7 independent experiments.
Interestingly, when DCs were exposed to OPN or LPS for 48 hours prior to adding them to the MLR (Figure 6B,D), IL-10 secretion was reduced and only minimal amounts of IL-12 remained detectable (Figure 6B). When adding such DCs to MLR, OPN-prestimulated cells are still able to induce an increase of IFN-γ secretion but have lost their maximal polarizing capacity (Figure 6D). These findings provide strong evidence that OPN polarizes DC maturation toward a DC1 phenotype that enhances Th1 polarization by augmenting DC secretion of IL-12,18 thereby initiating Th1-cell induction. Furthermore, OPN-stimulated DCs only transiently produce IL-12 upon OPN encounter and become refractory to further T-cell–mediated stimulation.
OPN-activated DCs polarize naive T cells toward a Th1-, IFN-γ–secreting phenotype
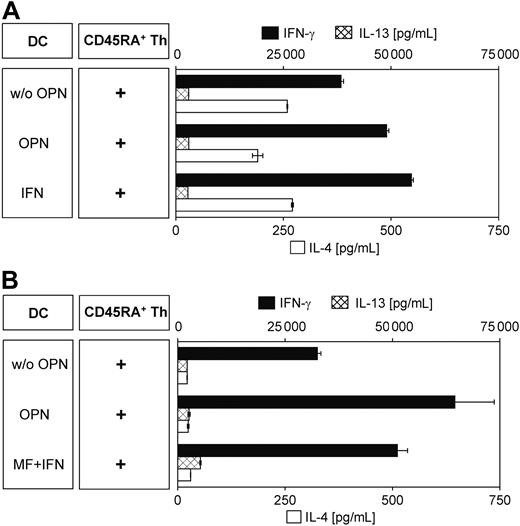
It is the unique ability of DCs to induce a primary immune response through activation and polarization of naive T cells. Therefore, we determined the ability of OPN-activated DCs to induce a Th1 phenotype in naive (CD4+CD45RA+) Th cells. Enriched naive Th cells were cocultured with DCs that had been prestimulated for 12 hours prior to MLR in the presence of the superantigen SEB for 9 days. Experiments were performed either with allogeneic (Figure 7A) or autologous (Figure 7B) T cells. When cytokine secretion was induced by addition of PMA and ionomycin, we found that naive Th cells secreted substantially elevated amounts of the Th1 cytokine IFN-γ, while IL-4 and IL-13 secretion was less affected or even reduced, indicating their polarization toward a Th1 phenotype. Such secretion was independent of the allogeneic or autologous origin of the Th cells used. Interestingly, DCs stimulated for more than 36 hours failed to induce this IFN-γ–secreting phenotype (data not shown), indicating their state of exhaustion.18,39
Discussion
Although OPN has been shown to have Th1 cytokine effects on T cells and macrophages in different in vivo and in vitro systems, it remained unknown whether OPN could exert its Th1-polarizing effects by influencing DCs. Here we demonstrate that OPN induces DC migration and activation, and polarizes them into Th1-promoting effector DCs. Our findings are important since they demonstrate that OPN as an endogenous environmental signal shapes the outcome of the immune response by DCs conditioned in peripheral tissues and during their encounter as APCs with naive T cells in secondary lymphatic tissues.
OPN-activated DCs polarize T cells toward a Th1- and IFN-γ–secreting phenotype. Naive CD45RA+ Th cells (10 × 103/200 μL) isolated by immunomagnetic negative depletion were polarized by coculture with 12-hour ± OPN-stimulated DCs (5 × 103/200 μL). On day 5 of coculture, IL-2 (50 U/mL) was added. Cytokine secretion was induced on day 9 by the addition of PMA and ionomycin. Supernatants were obtained after 24 hours and analyzed by cytokine-specific ELISA. Experiments were performed with allogeneic (A) or autologous (B) DCs and Th cells. As positive control for DC-induced Th1 differentiation, DCs were polarized in the allogeneic system by IFN-γ (A); and in the autologous model, with a combination of IL-1β, TNF-α (maturation factor, MF), plus IFN-γ.20,28 Shown is 1 representative experiment of 2 (A) and 4 (B) independent experiments. Data are expressed as mean pg/mL ± SD of duplicate measurements. Absolute cytokine amounts in the autologous and the allogeneic system cannot be compared because no crossover experiments with matched donor pairs were performed.
OPN-activated DCs polarize T cells toward a Th1- and IFN-γ–secreting phenotype. Naive CD45RA+ Th cells (10 × 103/200 μL) isolated by immunomagnetic negative depletion were polarized by coculture with 12-hour ± OPN-stimulated DCs (5 × 103/200 μL). On day 5 of coculture, IL-2 (50 U/mL) was added. Cytokine secretion was induced on day 9 by the addition of PMA and ionomycin. Supernatants were obtained after 24 hours and analyzed by cytokine-specific ELISA. Experiments were performed with allogeneic (A) or autologous (B) DCs and Th cells. As positive control for DC-induced Th1 differentiation, DCs were polarized in the allogeneic system by IFN-γ (A); and in the autologous model, with a combination of IL-1β, TNF-α (maturation factor, MF), plus IFN-γ.20,28 Shown is 1 representative experiment of 2 (A) and 4 (B) independent experiments. Data are expressed as mean pg/mL ± SD of duplicate measurements. Absolute cytokine amounts in the autologous and the allogeneic system cannot be compared because no crossover experiments with matched donor pairs were performed.
We have shown that murine DCs are guided to lymphatic tissues by OPN.10 Here we confirm that OPN induces the migration and maturation of human LCs as demonstrated in explant cultures of keratome skin. Human DCs express the OPN receptors CD44, αvβ3, αvβ5, and β1 integrin. Interestingly, OPN, like LPS, induces the expression of the standard form of CD44 (CD44s), while the constitutive integrin expression was not further augmented. The OPN-induced CD44 expression by DCs may positively affect their migration to lymphatic tissues; as we have previously demonstrated, certain CD44 isoforms play a central role for LCs/DCs to migrate to and adhere in T-cell zones of lymph nodes.34,40
When further characterizing the OPN-induced DC phenotype, we found that OPN and LPS had a comparable potential to up-regulate MHC class II, costimulatory, and adhesion molecules (Figure 3). Importantly, although potently activating DC expression of these molecules, our morphologic observations (Figure 2) indicate that OPN induces a phenotype that probably represents a preterminal state of the LPS-driven myeloid-DC maturation pathway. However, we cannot exclude that OPN activation of the same CD14–CD1a+ immature DCs may induce a different pathway of DC maturation. Thus, OPN alone or in the context of other endogenous and exogenous factors enhances a distinct type of DC activation with T-cell stimulatory function.
Recently Kawamura et al investigated the autocrine effects of OPN produced by DCs, neutralizing OPN with OPN-specific antibodies.41 OPN-deprived DCs had a reduced viability and expression of MHC class II and costimulatory molecules, which is in accordance to our findings that recombinant OPN induces DC activation (Figure 3A). Comparable with this work, we found that OPN is secreted during the GM-CSF– and IL-4–driven differentiation of CD14+ monocytes into DCs. Immature DCs washed and cultured in the absence of GM-CSF and IL-4 secreted OPN in a range of 300 ng/mL within 24 hours. In our system, following elimination of endogenous OPN, the addition of recombinant OPN induced DC maturation that was more pronounced than that of washed DCs cultured in the absence of OPN containing DC-conditioned medium (Figure 3C). Most likely in this setting the immediate increase in OPN concentration initiates the activation signal. By using different systems, both studies indicate that OPN is an important autocrine maturation stimulus for DCs. Furthermore, we found that additional exogenous OPN in the presence of self-produced OPN (Figure 3C) induces DC activation. This may be explained by an additive effect. However, it cannot be excluded that the recombinant OPN, due to missing posttranslational modifications, has different receptor avidity.
IL-12 produced by DCs is the critical Th1-polarizing cytokine for CD4+ T cells and stimulates the secretion of IFN-γ by unsensitized NK and T cells.42 A wide variety of toxins and microbial components induce the secretion of IL-12 by DCs.20-24,43,44 Important other modulators of DC polarization are tissue factors, such as IFN-γ, as the most prominent factor initiating Th1 polarization.11 Tissue factors known to shape DCs toward a Th2-polarizing phenotype are histamine,45 prostaglandin E2 (PGE2),46 and adenosine triphosphate (ATP).47 IL-10 and TGF-β have both been associated with modulation toward the induction of regulatory T cells.48
Inspired by the concept that the IL-12–producing capacity of DCs, which migrate into the regional lymphatic tissue, is preestablished in the tissue of their origin and instructed by tissue environmental factors,28,49 we investigated whether OPN influences the Th-polarizing capacity of DCs. Indeed OPN enhanced the Th1-driving phenotype of DCs, because we found that OPN induced the secretion of IL-12p70 to amounts within the range of 0.03 to 1.0 ng/mL, a concentration that has been described by Snijders et al to direct the development of naive Th cells into Th1 cells.20 In our experiments, the level of IL-12p70 showed some variation among different donors. This may be explained by donor variability, for example a Th2-primed state of DCs. Although for all experiments only DCs from healthy donors were used, one cannot exclude a latent atopic disposition of some donors, which is accompanied by a Th2-polarized immune response.25
CD40 ligand (CD40L) and IFN-γ were shown to be key molecules in the induction of IL-12 secretion by DCs.20,50 They act synergistically or even additively, and none of the mentioned factors share the unique capacity of IFN-γ to induce stably polarized effector DCs with enhanced IL-12–producing capacity.28 In different experimental cell systems, OPN was able to induce IL-12 secretion both dependently or independently of CD40L or IFN-γ. While OPN induces IL-12 secretion by macrophages6 independent of CD40L or IFN-γ, the secretion of IL-12 by cultures with nylon-wool nonadherent human T cells is CD40L and IFN-γ dependent,38 as OPN induces T-cell IFN-γ secretion and CD40L expression.3,38 In our experimental setting, we found that in immature DCs, OPN is able to induce low-level IL-12p70 secretion independently of IFN-γ or CD40L. However, in the presence of potentially IFN-γ– or CD40L-expressing allogeneic CD3+ T cells, the IL-12 secretion of DCs was further increased. Although IL-12 plays a central role in Th1 polarization, other factors such as a strong and long-lasting T-cell receptor engagement and an increased antigen dose contribute to the predominant Th1 differentiation.11,39 Specific costimulatory events, especially, contribute to the differentiation of T-helper cells both in vitro and in vivo.51,52 Interaction of intercellular adhesion molecule-1 (ICAM-1) and leukocyte function antigen-1 (LFA-1) under low cytokine conditions induces a Th1 polarization in humans and at least prevents Th2 development in the mouse.49,51,53 The effect of the B7/CD28 costimulation pathway in Th-cell polarization is more complex, leading to different polarization results depending on the conditions.51,52 We demonstrate that OPN strongly up-regulates MHC II, members of the B7 family (CD80 and CD86), and ICAM-1. In concert with IL-12, these molecules may further enhance the Th1-polarizing capacity of OPN-stimulated DCs.
In contrast to IFN-γ, the IL-12–inducing effect of OPN seems to be more transient, because 48-hours OPN-matured DCs lose their ability to secrete high amounts of IL-12, even in the presence of allogeneic T cells. A synergistic transient and antigen-specific modulated IL-12 gene expression during DC activation and maturation has been shown after the activation with bacterial, viral, or fungal pathogens and antigens.54 Our findings are in accordance with reports from Langenkamp et al39 and Kalinski et al18 who observed that myeloid-derived DCs stimulated with LPS or TNF-α/IL-1β produce IL-12 mRNA only within a narrow time window of 3 to 24 hours. Furthermore, their studies show that DCs prestimulated with LPS or TNF-α/IL-1β for at least 24 hours, although still able to up-regulate their CD80 and CD86 molecules, are refractory to IL-12 induction upon stimulation with IFN-γ, CD40L, or memory CD4+ T cells.18,39 Kalinski et al described that induction of the refractory state is accompanied by a downmodulation of the IFN-γ receptor.18 These findings are confirmed by an in vivo model in which the intravenous injection of microbial antigens of Toxoplasma gondii initiates an extremely rapid but transient IL-12 production by spleen interdigitating DCs in a CD40L-independent manner.55
In peritoneal macrophages, OPN engagement with β3 integrin up-regulates IL-12 and dampens IL-10 upon OPN CD44 interaction.6 DCs constitutively express CD44 isoforms and αv integrins. While CD44 isoforms are up-regulated on activated DCs, αvβ3 and αvβ5 expression is unchanged (Figure 3; data not shown).10,34 We can only speculate which signaling pathways induced by OPN regulate this cytokine secretion. Studies analyzing the antiapoptotic effect of OPN found OPN to stimulate the phosphatidylinositol 3-kinase/Akt signaling pathway by CD44 binding56 or activate nuclear factor κB (NF-κB) downstream of αvβ3.57 From Toll-like receptor signaling cascades, NF-κB activation is known to be involved in the induction of several cytokines, IL-12 among them.58,59 We hypothesize that signal transduction through αvβ3 modulates cytokine secretion in a NF-κB–dependent manner. Additionally, CD44 may augment signaling via other receptors by its platform or coreceptor functions.60 Interestingly, we observed that OPN-treated DCs from certain donors decrease IL-10 secretion when up-regulating CD44, indicating that OPN may downmodulate IL-10 through a CD44 pathway (Figure 6B).
In several disease models with OPN-deficient mice the role of OPN as a Th1-driving cytokine has been established.6,61-64 OPN-deficient mice show a milder course and improved clinical outcome of an experimental autoimmune encephalitis.8,9 These studies compared Th-polarizing cytokines from OPN-deficient or wild-type mice in mixed cell populations generated from regional lymph nodes or spleen. In such cell populations, immune cells from OPN-deficient mice secreted fewer Th1-driving cytokines, while increased levels of antagonistic Th2 cytokines (eg, IL-10) were detected. In the study by Ashkar et al, OPN was shown to differentially regulate IL-12 secretion by peritoneal macrophages.6 However, although macrophages shape the type of immune response at the site of inflammation, they are not the primary cells to leave such sites to modulate the immune response in secondary lymphatic organs.65 In contrast to macrophages, DCs are able to encounter the antigen at the site of inflammation and thereafter invade regional secondary lympoid organs to initiate antigen-specific cell-mediated immune response. Here we show that OPN early during DC activation induces polarization of DCs toward a Th1-inducing phenotype. We therefore propose that OPN-polarized DCs may be of central importance for the initiation of the Th1-modulated cytokine milieu detected in lymph nodes of the mentioned disease models.
In conclusion, our findings suggest that OPN is a tissue factor expressed during inflammatory responses that, through its ability to activate DCs, in concert with other cytokines, shapes T-cell–mediated immune responses. Furthermore, OPN may decisively influence T-cell polarization by its presence when DC–T-cell interaction occurs after DCs have entered lymph nodes. This concept is underlined by the deficiency of Th1 immunity in OPN-deficient mice, as in these mice the absence of OPN may lead to a lack in priming of DCs toward the Th1-initiating phenotype.
Prepublished online as Blood First Edition Paper, April 26, 2005; DOI 10.1182/blood-2004-08-3228.
Supported by grants from the Deutsche Forschungsgemeinschaft (DFG WE 1919/2-3 and SFB 620, TP B5).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Christine Bernardi for excellent technical assistance.




![Figure 5. OPN-stimulated DCs exhibit enhanced T-cell allostimulatory capacity. Mixed lymphocyte reaction (MLR) was performed with allogeneic T cells (10 × 104/200μL) and immature DCs (10 × 103/200 μL) on day 5 of DC culture in the presence or absence of OPN, added throughout the MLR (A). Alternatively (B), DCs were stimulated with OPN from day 5 to day 7 of DC culture (48 h DC OPN) or left untreated for control (48 h DC wo OPN), prior to their addition to MLR. T-cell proliferation was measured by [H3]-thymidine incorporation in the last 18 hours of the experiment. (A-B) One representative experiment with T cells and DCs from the same donor pair is shown. This is representative of 7 independent experiments. Data are expressed as the mean counts per minute ± SD of quadruplicate cultures. (C) MLR was performed with DCs cultured for 5 days and OPN was added throughout the experiment (○). Alternatively, DCs were prestimulated with OPN for 48 hours prior to addition to MLR (•). The allostimulatory capacity in both settings was compared with that of immature DCs from day 5 (open gray diamonds). For statistical analysis of all experiments, the percent induction of T-cell proliferation by OPN was calculated by the following formula: T-cell proliferation induced by prestimulated DCs or DCs in the presence of OPN (CPM)/T-cell proliferation induced by immature DCs of day 5 (CPM) × 100%. Statistical analysis of 7 independent experiments ± SEM (*P < .05, one-way analysis of variance [ANOVA], Student-Neuman-Keuls test). (D) The stimulatory capacity of 48-hour OPN-stimulated DCs (•) is compared with the T-cell stimulatory capacity of unstimulated DCs from day 7 of DC culture (closed gray diamonds). Statistical analysis of 10 independent experiments ± SEM (#P < .05, Kruskal-Wallis one-way ANOVA on Ranks, Student-Neuman-Keuls test) is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/3/10.1182_blood-2004-08-3228/6/m_zh80150581830005.jpeg?Expires=1763596952&Signature=5IGOHq8EuZUARsIUy3VBboZjNOtB3mkcslFaqHPCmgnoIMfvjLJxTfQnFPsD4zx0xSYmTuaWQ-~3BHJOhLdqLSftU2LPKdWn9jSaG~o0N8~geOnWyP4~tWKQnK4tY3eUI3vaZfkxnSF1U7QoUPHUW5y4-pIvS5PPnjoAiQgskRWjrShkNRhYoHe7AtygoFfPgtGrAEtvTKLjql8IrqEdw16YHQJnYO2asNRuTc1EHXWP3oBVtA0gEP~MLWsEnCaWtuhN0HgkGKElIOG2rHlSLgQRWO9hkOchNkZQE9HCVxtxP5mmJFqBWLuAHeulqoGjOaS3FRZ52fqMZviLuHo-1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal