Abstract
Thrombotic thrombocytopenic purpura (TTP) is a rare disorder of small vessels that is associated with deficiency of the von Willebrand factor–cleaving protease, ADAMTS13. The presence of anti-ADAMTS13 autoantibodies is considered a factor predisposing to relapses. Despite close monitoring and intensive plasma treatment, in these patients acute episodes are still associated with substantial morbidity and mortality rates, and the optimal therapeutic option should be prevention of relapses. This study was conducted in a patient with recurrent TTP due to high titers of ADAMTS13 inhibitors, who used to have 2 relapses of TTP a year. The study compared the standard treatment plasma exchange with rituximab. Results documented that plasma exchange had only a small transient effect on ADAMTS13 activity and inhibitors; on the contrary, prophylaxis with rituximab was associated with disappearance of anti-ADAMTS13 antibodies, a progressive recovery of protease activity, and it allowed the patient to maintain a disease-free state during a more than 2-year follow-up.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a potentially life-threatening disease characterized by microangiopathic hemolytic anemia, thrombocytopenia, and formation of microthrombi in different organs.1,2 The disease may manifest once in the lifetime or it may relapse even after complete recovery of the initial episode; in these recurrent cases death and neurologic sequelae are common final outcomes.
Deficiency of the von Willebrand factor (VWF)–cleaving protease, ADAMTS13, has been reported in 60% to 70% of patients with TTP3-6 ; the defect may be constitutive or acquired due to the presence of circulating inhibitory antibodies that may predispose to disease recurrence. Several therapies have been used to limit the production of these autoantibodies, including nonselective immunosuppression with corticosteroids, cyclophosphamide, vincristine, cyclosporine A, and azathioprine, with variable results.7
Rituximab, a humanized monoclonal antibody directed against the B-cell antigen CD20,8 is widely used in the treatment of B-cell lymphoproliferative disorders, and accumulating data suggest that it may prove an optimal treatment in various diseases related to autoantibody production. Recently, some patients with chronic TTP refractory to any therapy achieved complete remission of the disease after rituximab administration,7,9-13 thus providing the rationale for the use of this drug in ADAMTS13 autoantibody-associated TTP. In the recent reports, patients also received treatments other than rituximab, so it is hard to dissect the specific effect of each drug on ADAMTS13 autoantibodies and activity. In addition, no data are available on the possible effect of rituximab when given in remission to prevent disease recurrencies.
This study was undertaken in a patient with chronic relapsing TTP and ADAMTS13 autoantibodies to address a number of unsolved issues on the efficacy of rituximab in such patients. First, we formally compared the effect of plasma exchange with that of rituximab on ADAMTS13 inhibitors and activity. A characterization and quantification of ADAMTS13 autoantibodies and VWF multimers was also done before and after each treatment. Second, we evaluated whether prophylactic rituximab administration during remission prevented disease recurrence. Finally, we tested the possibility that retreatment with rituximab was safe and effective in maintaining long-term remission.
Study design
ADAMTS13 activity, inhibitor assays, and ADAMTS13 antigen
ADAMTS13 activity was assessed using the residual VWF–collagen binding assay.6 The inhibitor titer was measured using a procedure based on the Bethesda method.14 The characterization of anti-ADAMTS13 antibodies as well as the immunoglobulin G (IgG) and IgM titer were done with an enzyme-linked immunosorbent assay (ELISA) as described.15 The presence of IgG was confirmed by immunoblots with recombinant ADAMTS13 (kindly provided by Prof P.M. Mannucci and Dr F. Peyvandi, Milan, Italy) reacted with serum samples and detected with goat anti–human IgG antibodies (Sigma Chemical, St Louis, MO). Plasmatic ADAMTS13 antigenic levels were evaluated with an ELISA using recombinant human ADAMTS13 as standard.16
Analysis of VWF antigen and multimers
CD20 cell count
The absolute number of CD20 cells was determined by fluorescence-activated cell sorting (FACS) analysis as described.20
Results and discussion
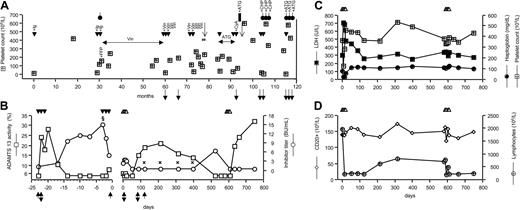
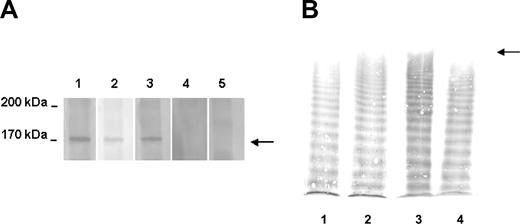
This study was approved by the institutional review board of the Azienda Sanitaria Locale di Bergamo and is in accordance with the Helsinki Declaration. Informed written consent was obtained from the patient before treatment. The patient, a 60-year-old male, developed his first episode of TTP in 1992 at the age of 48 years, presenting with thrombocytopenia, hemolytic anemia, headache, aphasia, and seizures. Remission was achieved with plasma exchange and intravenous immunoglobulins. Between 1994 and 2000, the patient experienced 8 relapses treated with plasma exchanges, vincristine, corticosteroids, antiplatelets agents, cyclophosphamide, and cyclosporine A (Figure 1A). During a relapse in 1997, a severe deficiency of ADAMTS13 activity due to high levels of ADAMTS13 inhibitor was first detected,6 and the defect was not corrected by any of the aforementioned treatments. In 2000, the patient underwent splenectomy, reaching a temporary stabilization of his platelet count (Figure 1A). However, during the next 2 years he continued to have relapses (approximately twice a year) preceded by viral infections; ADAMTS13 activity was undetectable and inhibitors were present (Figure 1A). During the last relapse, the ADAMTS13 inhibitor titer was 12 BU/mL. Characterization and quantification of anti-ADAMTS13 autoantibodies by ELISA revealed a high titer of anti–ADAMTS13 IgG (1:1600), but no IgM. Western blot analysis (Figure 2A) showed that patient serum reacted with a band of about 170 kDa corresponding to recombinant ADAMTS13 (rADAMTS13), confirming the presence of anti–ADAMTS13 IgG. ADAMTS13 antigen plasma levels were below the detection limit (< 100 ng/mL).
History of the patient, ADAMTS13 activity, and inhibitors and laboratory parameters. (A) Timeline history of the patient. Treatments: plasma exchange (▾); immunoglobulins (Ig); corticosteroids (circles with vertical lines); fresh frozen plasma (FFP); vincristine (Vin); antiplatelet agents (ATG); cyclosporine A (CyA); cyclophosphamide (CHP); plasma exchange, vincristine, antiplatelet agents, and corticosteroids (#). Filled rectangle indicates splenectomy; ↓, TIA; filled down arrow, ADAMTS13 activity less than 6% (normal range, 50%-150%), ADAMTS13 inhibitors present; and ( ), platelet count. (B) ADAMTS13 activity (□) and anti-ADAMTS13 inhibitor titer (○) in the 2 courses of plasma exchange (▾), during rituximab (▵) treatment and in the follow-up period. § indicates IgG titer, 1:1600; IgM, negative.
), platelet count. (B) ADAMTS13 activity (□) and anti-ADAMTS13 inhibitor titer (○) in the 2 courses of plasma exchange (▾), during rituximab (▵) treatment and in the follow-up period. § indicates IgG titer, 1:1600; IgM, negative.  : IgG titer, negative; IgM, negative. The upward-pointing filled arrowhead indicates time of sampling for immunoblot (see Figure 2A); and the vertical double-headed arrow, time of sampling for VWF multimer analysis (see Figure 2B). (C) Platelet count (
: IgG titer, negative; IgM, negative. The upward-pointing filled arrowhead indicates time of sampling for immunoblot (see Figure 2A); and the vertical double-headed arrow, time of sampling for VWF multimer analysis (see Figure 2B). (C) Platelet count ( ; normal range, 140 × 109/L-440 × 109/L), LDH (▪; normal range, 240 U/L-460 U/L), and haptoglobin (•; normal range, 49 mg/dL-246 mg/dL). (D) Lymphocytes count (⊕; normal range, 1.000-5.000 × 106/L) and CD20 count (⋄; normal range, 5%-15% of lymphocytes count).
; normal range, 140 × 109/L-440 × 109/L), LDH (▪; normal range, 240 U/L-460 U/L), and haptoglobin (•; normal range, 49 mg/dL-246 mg/dL). (D) Lymphocytes count (⊕; normal range, 1.000-5.000 × 106/L) and CD20 count (⋄; normal range, 5%-15% of lymphocytes count).
History of the patient, ADAMTS13 activity, and inhibitors and laboratory parameters. (A) Timeline history of the patient. Treatments: plasma exchange (▾); immunoglobulins (Ig); corticosteroids (circles with vertical lines); fresh frozen plasma (FFP); vincristine (Vin); antiplatelet agents (ATG); cyclosporine A (CyA); cyclophosphamide (CHP); plasma exchange, vincristine, antiplatelet agents, and corticosteroids (#). Filled rectangle indicates splenectomy; ↓, TIA; filled down arrow, ADAMTS13 activity less than 6% (normal range, 50%-150%), ADAMTS13 inhibitors present; and ( ), platelet count. (B) ADAMTS13 activity (□) and anti-ADAMTS13 inhibitor titer (○) in the 2 courses of plasma exchange (▾), during rituximab (▵) treatment and in the follow-up period. § indicates IgG titer, 1:1600; IgM, negative.
), platelet count. (B) ADAMTS13 activity (□) and anti-ADAMTS13 inhibitor titer (○) in the 2 courses of plasma exchange (▾), during rituximab (▵) treatment and in the follow-up period. § indicates IgG titer, 1:1600; IgM, negative.  : IgG titer, negative; IgM, negative. The upward-pointing filled arrowhead indicates time of sampling for immunoblot (see Figure 2A); and the vertical double-headed arrow, time of sampling for VWF multimer analysis (see Figure 2B). (C) Platelet count (
: IgG titer, negative; IgM, negative. The upward-pointing filled arrowhead indicates time of sampling for immunoblot (see Figure 2A); and the vertical double-headed arrow, time of sampling for VWF multimer analysis (see Figure 2B). (C) Platelet count ( ; normal range, 140 × 109/L-440 × 109/L), LDH (▪; normal range, 240 U/L-460 U/L), and haptoglobin (•; normal range, 49 mg/dL-246 mg/dL). (D) Lymphocytes count (⊕; normal range, 1.000-5.000 × 106/L) and CD20 count (⋄; normal range, 5%-15% of lymphocytes count).
; normal range, 140 × 109/L-440 × 109/L), LDH (▪; normal range, 240 U/L-460 U/L), and haptoglobin (•; normal range, 49 mg/dL-246 mg/dL). (D) Lymphocytes count (⊕; normal range, 1.000-5.000 × 106/L) and CD20 count (⋄; normal range, 5%-15% of lymphocytes count).
Immunoblot of anti–ADAMTS13 IgG and VWF multimeric pattern. (A) Immunoblot showing the presence of IgG reacting with recombinant (r) ADAMTS13. Serum samples were reacted with human rADAMTS13 previously transblotted to a nitrocellulose membrane. The presence of IgG was detected by a peroxidase conjugate goat anti–human IgG antibody. Lane 1: patient serum collected during an acute phase; lane 2: patient serum collected at remission before plasma (day –23); lane 3: patient serum collected at day 0 before rituximab treatment; lane 4: patient serum at day 123; and lane 5: serum from a healthy subject. The time of sampling is indicated by the upward-pointing filled arrowhead in Figure 1B. In samples containing an inhibitor, a reactive band of about 170 kDa corresponding to rADAMTS13 was found (arrow). (B) Representative images of VWF multimers in patient and control plasma. The origin of the gel is on the top; the arrow indicates the position of UL VWF multimers. Lane 1: control plasma pool; lane 2: patient sample collected before plasma at day –23; lane 3: patient sample collected before rituximab infusion (day 0); and lane 4: patient sample collected at day 88. The time of sampling is indicated by the vertical double-headed arrow in Figure 1B.
Immunoblot of anti–ADAMTS13 IgG and VWF multimeric pattern. (A) Immunoblot showing the presence of IgG reacting with recombinant (r) ADAMTS13. Serum samples were reacted with human rADAMTS13 previously transblotted to a nitrocellulose membrane. The presence of IgG was detected by a peroxidase conjugate goat anti–human IgG antibody. Lane 1: patient serum collected during an acute phase; lane 2: patient serum collected at remission before plasma (day –23); lane 3: patient serum collected at day 0 before rituximab treatment; lane 4: patient serum at day 123; and lane 5: serum from a healthy subject. The time of sampling is indicated by the upward-pointing filled arrowhead in Figure 1B. In samples containing an inhibitor, a reactive band of about 170 kDa corresponding to rADAMTS13 was found (arrow). (B) Representative images of VWF multimers in patient and control plasma. The origin of the gel is on the top; the arrow indicates the position of UL VWF multimers. Lane 1: control plasma pool; lane 2: patient sample collected before plasma at day –23; lane 3: patient sample collected before rituximab infusion (day 0); and lane 4: patient sample collected at day 88. The time of sampling is indicated by the vertical double-headed arrow in Figure 1B.
After the patient achieved remission, ADAMTS13 activity remained undetectable due to persistent anti-ADAMTS13 inhibitors, and ADAMTS13 antigen levels (351 ng/mL) were below the normal range (680-1350 ng/mL). In September 2002, a course of plasma exchange (2 volume exchange, daily for 3 consecutive days) was started (day –23) with the aim to remove ADAMTS13 inhibitors and prevent relapse. ADAMTS13 activity, which was undetectable before plasma exchange (Figure 1B), increased during plasma exchange and the inhibitors became undetectable. However, one week after the end of treatment the protease activity fell below 6% and the inhibitors were again detectable (Figure 1B) and antigen levels (226 ng/mL) were below normal range. At day –3, a second cycle of plasma exchange was started, but during this course the protease activity remained undetectable and the inhibitor did not disappear. At day 0, ADAMTS13 was undetectable, and inhibitors and IgG titers were 2.3 BU/mL and 1:400, respectively (Figure 1B). Rituximab (MabThera; Roche, Milan, Italy) treatment was started, at the dose of 375 mg/m2 once weekly for a total of 4 intravenous infusions at days 0, 7, 14, and 21. Before each dose, the patient received paracetamol and diphenhydramine. The initial infusion rate was 50 mg/hour, and was progressively increased up to 100 mg/hour according to drug tolerability.
ADAMTS13 inhibitors disappeared starting from one month follow-up (day 50) after rituximab and remained undetectable thereafter. ELISA assay for anti–ADAMTS13 IgG was negative at the 3-, 6-, 9-, and 12-month follow-ups (Figure 1B), a result confirmed by immunoblot (Figure 2A) at day 123. Inhibitors again became detectable at the 16-month follow-up (Figure 1B). Protease activity was measurable (15%) from the second month of follow-up (day 88), reached a peak at the sixth month (day 214), then decreased slowly and at 16 months it was undetectable. From the 6- to the 12-month follow-up, ADAMTS13 antigen levels were in the lower normal range (day 214, 695 ng/mL; day 315, 624 ng/mL; day 399, 683 ng/mL). A second course of rituximab was started (day 587), which was followed by a faster recovery of ADAMTS13 activity as compared with the first course. Indeed, 25 days after the last rituximab infusion protease activity was again detectable and inhibitors disappeared, a finding confirmed at 661 days follow-up (Figure 1B).
VWF antigen levels were always in the normal range. Unusually large (UL) VWF multimers were found in plasma either before any treatment (day –23) or after plasma exchange (day 0). By contrast, at day 88 the VWF multimeric pattern was normal and no UL VWF forms were detected (Figure 2B).
Before rituximab (day 0) platelet count, lactate dehydrogenase (LDH), haptoglobin (Figure 1C), hemoglobin, and red and white cell counts were normal. No adverse reaction, but a transient increase in LDH (peak at day 7: 704 IU/L) and a transient decrease in haptoglobin (nadir at day 14: 19 mg/100 mL) and platelet count (nadir at day 7: 126 × 109/L), were observed in association with the first but not with the second course of rituximab. By day 21 all the above parameters completely normalized (Figure 1C). The patient had no signs or symptoms of TTP, with a normal platelet count, stable hematocrit, normal LDH, and normal haptoglobin during the entire (748 days) follow-up period (Figure 1C). Total lymphocyte count did not change during treatment and follow-up; as expected, B lymphocytes (CD20) decreased during rituximab treatment, slightly increased from the 6-month follow-up, but remained lower than normal for the entire follow-up period (Figure 1D).
Anti-ADAMTS13 inhibitory antibodies during remission have been reported in 30% to 40% of patients with recurrent TTP,21 although no data are available on their long-term persistence. However, the relapsing clinical course of our patient would indicate that the presence of anti-ADAMTS13 antibodies may be a risk of relapse. Here we present the case of one patient in whom we characterized the nature and titer of ADAMTS13 inhibitor and searched for the best prophylactic strategy to prevent recurrences. In agreement with a recent study,21 we found that plasma exchange alone had a small transient effect on ADAMTS13 inhibitor. We also found that this procedure did not modify the abnormal VWF multimeric pattern, thus suggesting that plasma is not the ideal treatment for preventing relapses in such patients. By contrast, rituximab treatment was followed by a progressive disappearance of inhibitors with a subsequent increase of protease activity, which was associated with normalization of VWF pattern. The partial recovery of B cells was followed by a decline in ADAMTS13 activity and reappearance of the inhibitor after 16 months, which is in line with a previous report showing a gradual decrease in ADAMTS13 activity from 14 months after rituximab.7 However, here we document that a second course of rituximab—as sole therapy—is very effective in inducing a prompt recovery of ADAMTS13 activity and disappearance of the inhibitor. Of note, the peak value of ADAMTS13 activity recorded after rituximab was 32%, which is below the normal range. However, it is known that ADAMTS13 activity values over 5% to 10% are sufficient to protect from disease recurrences.22 This possibility is confirmed by the fact that the patient, who used to have 2 disease relapses a year, experienced no recurrence during the 2-year follow-up period after rituximab. The partial recovery of ADAMTS13 activity could possibly be due to persistence of a very low titer of antibodies that were not detected by our assays. Alternatively, some kind of hereditary partial ADAMTS13 deficiency could be suspected, although normal antigen levels after rituximab would exclude the presence of common disease-associated mutations that usually cause an impaired secretion.23
Chronic relapsing TTP is a debilitating and life-threatening disease. Current management of patients with TTP essentially relies on treatment of the relapse as soon as hematologic and neurologic symptoms manifest. However, acute episodes are still associated with high morbidity and mortality rates, with coma and death being recorded in 20% to 30% of patients carrying anti-ADAMTS13 antibodies.21 The ideal treatment should be the one capable of retarding or even preventing relapses, which occur so frequently in these patients. Splenectomy has been shown to induce sustained remission in some patients with anti-ADAMTS13 antibodies,24 but was of no benefit in other similar cases,7,25 as was the case in our patient. Here we provide the evidence that prophylaxis with rituximab may be effective in maintaining a long-term, relapse-free condition in patients with a diagnosis of relapsing TTP and anti-ADAMTS13 antibodies in whom other treatments failed to maintain a sustained remission. Retreatment with rituximab should be considered when ADAMTS13 inhibitors reappear into the circulation, to avoid a new relapse.
A prospective clinical trial is needed to prove the efficacy of rituximab prophylaxis in these patients, as compared with other currently used treatments such as steroids, and to define clinical parameters (such as the rise of CD20 population) or laboratory tests (ADAMTS13 activity and inhibitors) that may predict a relapse and provide the indication for retreatment.
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2004-12-4885.
C.C. is a recipient of Helsinn Healthcare Lugano fellowship through the Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR).
For the International Registry of Recurrent and Familial HUS/TTP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal