Abstract
We performed a case-control study to determine the association of BK plasma viremia with hemorrhagic cystitis (HC) in hematopoietic cell transplant (HCT) recipients. Thirty cases of HC (14 of which occurred after platelet engraftment with documented BK viruria [BK-HC]) were compared with matched controls. Weekly plasma samples were tested for BK virus DNA by polymerase chain reaction (PCR). BK viremia detected before or during the disease was independently associated with HC (adjusted odds ratio = 30, P < .001); BK viremia was even important before clinical symptoms of HC occurred (odds ratio = 11, P < .001). Cases of HC and BK-HC had a significantly higher peak of BK plasma viral load than controls. BK virus was detected by in situ hybridization in bladder biopsies of 2 cases with severe HC and long-lasting BK viremia. BK virus seems to play a role in the development of HC and quantitative detection of BK DNA in plasma appears to be a marker of BK virus disease in HCT recipients.
Introduction
In hematopoietic cell transplant (HCT) recipients, hemorrhagic cystitis (HC) occurring after engraftment has been correlated with presence of BK virus in urine,1,2 and high viral load of BK virus in urine has been associated with a higher risk of BK-associated HC.3 However, viruria is common even in asymptomatic immunocompromised patients, making a direct causative role of BK virus difficult to establish.4 Presence of viral DNA in plasma of latent viruses such as cytomegalovirus (CMV), adenovirus, and Epstein-Barr virus has been shown to be a reliable marker of clinical disease in different transplant settings.5,6 While BK viremia is a sensitive and specific indicator of BK nephritis in solid organ transplants,7 BK viremia has been reported to occur in asymptomatic HCT recipients.8
We conducted a matched case-control study to assess whether plasma BK DNA viral load was associated with HC following hematopoietic cell transplantation.
Study design
All patients who underwent their first allogeneic hematopoietic cell transplantation at the Fred Hutchinson Cancer Research Center (FHCRC) from January 1979 to October 2003 and who had frozen plasma samples available for retrospective testing of BK virus DNA were included in the study. The study received institutional review board approval at FHCRC. Each patient included in the study had previously signed an informed consent form allowing the storage and the use of their blood products for future research. We identified patients with grade 2 to 4 HC from the FHCRC database, reviewed their medical records, and collected the clinical data relevant to hematuria. HC was divided into 4 categories according to the severity of hematuria.4 Patients were required to have at least one sample within 3 weeks before and one week after onset of HC. Each patient except one had plasma samples available before HC. There were 6 patients who had samples available only during HC. At least 2 controls per patient, matched by age (exact) and decade of transplantation, were used. The matched controls had available samples drawn during the corresponding time interval. Patients with HC who underwent a bladder biopsy were retrospectively tested for BK virus by in situ hybridization (ISH). The laboratory records of cases were reviewed for presence of adenovirus (culture or direct fluorescent antibody) and BK virus (polymerase chain reaction [PCR]) in urine. No urine samples were available for retrospective analysis.
The detection of BK virus in tissue bladder was performed by ISH.10 A plasmid-only probe combined with an X-chromosome probe provided negative and positive controls, respectively.
Comparison of categorical data between groups was evaluated by the Fisher exact test. The univariate and multivariable comparisons were performed using the conditional logistic regression model.11 Variables with a significance level less than .1 in univariate analysis were entered into multivariable models and sequentially eliminated in a stepwise backward fashion, with significance level less than .05 as the criterion for inclusion. A trend test using exact conditional logistic regression was performed to assess the association between HC and peak of BK viral load, categorized in 3 groups (0; 1-10 000; and > 10 000 copies DNA/mL). The associations were expressed as estimated relative risks (odds ratio [OR]) with 95% confidence interval (CI). All P values were 2 sided.
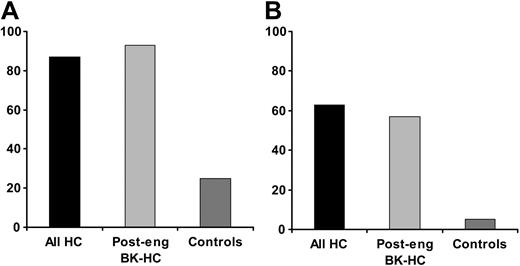
Proportion of patients and controls with BK viremia. (A) The proportion of patients among controls and cases in each subset of HC who developed BK viremia at any level before or during HC. Post-eng indicates postengraftment. (B) The proportion of patients among controls and cases in each subset of HC who developed BK viremia above 104 copies/mL. The sensitivity and the specificity of PCR-based BK viremia at any level either before or at time of HC was 86% and 75%, respectively, for clinical HC, and 92% and 72%, respectively, for postengraftment HC with documented BK viruria. The sensitivity and the specificity of BK viremia before HC was 66% and 85%, respectively, for clinical HC, and 92% and 88%, respectively, for postengraftment HC with documented BK viruria. Plasma BK viral load that measured above 104 copies/mL before or during HC had a sensitivity and specificity of 63% and 95%, respectively, for diagnosing HC; and 57% and 95%, respectively, for diagnosing postengraftment HC with documented BK viruria.
Proportion of patients and controls with BK viremia. (A) The proportion of patients among controls and cases in each subset of HC who developed BK viremia at any level before or during HC. Post-eng indicates postengraftment. (B) The proportion of patients among controls and cases in each subset of HC who developed BK viremia above 104 copies/mL. The sensitivity and the specificity of PCR-based BK viremia at any level either before or at time of HC was 86% and 75%, respectively, for clinical HC, and 92% and 72%, respectively, for postengraftment HC with documented BK viruria. The sensitivity and the specificity of BK viremia before HC was 66% and 85%, respectively, for clinical HC, and 92% and 88%, respectively, for postengraftment HC with documented BK viruria. Plasma BK viral load that measured above 104 copies/mL before or during HC had a sensitivity and specificity of 63% and 95%, respectively, for diagnosing HC; and 57% and 95%, respectively, for diagnosing postengraftment HC with documented BK viruria.
Results and discussion
We identified 46 patients with grade 2 to 4 HC. Thirty patients and 81 matched controls had sera available for retrospective testing. The median age of patients was 43 years (range, 9-63 years). The clinical features, including sex, underlying disease, HLA typing, source of stem cells, CMV serostatus, conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, presence of acute GVHD, and CMV infection (positive CMV antigenemia), were well balanced between the 2 groups except for conditioning regimen (P = .009) and CMV infection (P = .02), which were entered into the multivariable model. There were 307 samples from the 30 patients and 829 samples from the 81 controls that were collected for PCR amplification of BK virus DNA. A median of 10 samples per case (range, 2-20 samples per case) and controls (range, 1-21 controls) was available.
The median onset of HC was day 50 (range, day 6-123) after transplantation. There were 8 (27%) cases of grade 2 HC and 22 (73%) cases of grade 3-4 HC. Twenty-three patients (77%) developed postengraftment HC at a median of 58 days (range, 26-123 days) after transplant. Of 23 patients with postengraftment HC, 14 had documented BK viruria. Those patients were analyzed as an independent subset for 2 reasons. First the restriction of the analysis to patients with postengraftment HC allows us to avoid the potential effect of low platelet count and to decrease the impact of the conditioning regimen on HC. Second, the presence of BK viruria gives support to BK virus–associated HC as a clinical entity.3 The median duration of HC was 25 days (range, 6-80 days) for the 26 patients who survived the event and 19 days (range, 16-36 days) for patients who died with ongoing HC. Seven patients developed renal impairment (median creatinine level 3.4 mg/L) concomitant to HC. No patient underwent kidney biopsy and the etiology of renal failure was deemed to be caused by bladder outlet obstruction in most of the cases.
BK viremia either before or during HC was detected in 26 of 30 (87%) patients and in 20 of 61 (25%) controls (Figure 1A). BK viremia occurred in 20 patients (67%) before the onset of clinical disease. Twenty patients (67%) presented with sustained BK viremia in a median of 15 days (range, 5-80 days) before the onset of HC. Among 26 patients with BK viremia, 17 had been previously tested for BK virus in urine and all were positive (the viremia results were not known at the time of urine testing). Of 24 patients screened for adenovirus in urine, one patient with adenovirus HC, documented by biopsy, was positive for adenovirus in several urine samples.
The analysis of the association between HC and quantitative BK viremia showed that patients with a viral load of more than 104 copies/mL had a significantly higher risk of having HC than patients with a viral load of less than 104 copies/mL (OR = 21; 95% CI 3.3-inf; trend test P < .001). Plasma viral load of more than 104 copies/mL was detected in 63% of patients with HC and 57% of postengraftment BK-HC compared with 5% of the respective controls (Figure 1B).
There were 3 patients who underwent a bladder biopsy at the time of HC. We retrospectively tested the bladder of these patients for BK virus by ISH. BK virus was detected in 2 patients who presented with severe postengraftment HC. Both patients developed long-lasting BK viremia occurring 2 and 4 weeks before the occurrence of HC. No inclusions were seen. BK virus was detected in rare transitional cells in each case.
Univariate analysis demonstrated that sex, CMV serostatus, CMV infection, stem cell source, underlying disease, HLA typing, and acute GVHD were not associated with HC. HC and postengraftment BK-HC were significantly associated with BK viremia occurring before or during HC, BK viremia before HC, and the use of busulfan in conditioning regimen. Multivariable analysis of variables associated with HC and postengraftment BK-HC resulted in the adjusted OR depicted in Table 1.
Risk factors associated with hemorrhagic cystitis on multivariable analysis
Risk factors . | Odds ratio (95% CI) . | P . |
|---|---|---|
| HC* | ||
| BK before or during HC | 30 (5.5-173) | < .001 |
| Busulfan/cyclophosphamide | 8.6 (1.7-44) | .003 |
| BK viremia before HC | 11 (3.3-34) | < .001 |
| Busulfan/cyclophosphamide | 4.3 (1.3-14) | .012 |
| Postengraftment BK-HC† | ||
| BK before or during HC | 20 (3.6-inf) | <.001 |
| Busulfan/cyclophosphamide | 9.2 (1.1-inf) | .037 |
| BK viremia before HC | 17 (2.8-683) | <.001 |
| Busulfan/cyclophosphamide | 9.3 (0.61-678) | .15 |
Risk factors . | Odds ratio (95% CI) . | P . |
|---|---|---|
| HC* | ||
| BK before or during HC | 30 (5.5-173) | < .001 |
| Busulfan/cyclophosphamide | 8.6 (1.7-44) | .003 |
| BK viremia before HC | 11 (3.3-34) | < .001 |
| Busulfan/cyclophosphamide | 4.3 (1.3-14) | .012 |
| Postengraftment BK-HC† | ||
| BK before or during HC | 20 (3.6-inf) | <.001 |
| Busulfan/cyclophosphamide | 9.2 (1.1-inf) | .037 |
| BK viremia before HC | 17 (2.8-683) | <.001 |
| Busulfan/cyclophosphamide | 9.3 (0.61-678) | .15 |
Platelet engraftment was defined as a platelet count greater than 20 × 109/L without transfusion support for 7 consecutive days.
The following factors were entered as covariates: sex, CMV serostatus, CMV infection before HC (antigenemia or plasma PCR positive), source of stem cells (peripheral blood stem cells versus bone marrow), conditioning regimen (busulfan/cyclophosphamide versus others), presence of GVHD (grade = 2) prior to HC, and HLA matching (HLA match versus other). Total body irradiation (TBI) was not entered in the multivariate model due to its high correlation with busulfan and cyclophosphamide regimen (ie, 94% of patients who received busulfan and cyclophosphamide did not receive TBI). Among patients undergoing other regimens, 92% received TBI.
All cases of HC: 30 patients and 81 controls. Variables associated with HC in univariate analysis: BK before or during HC versus no viremia (OR, 20; 95% CI, 4.6-85; P < .001); BK before HC versus no viremia (OR, 10; 95% CI, 3.4- 30; P < .001); use of busulfan and cyclophosphamide regimen versus other regimens (OR, 3.7; 95% CI, 1.4-9.9; P = .006); any busulfan versus no busulfan (OR, 5.6; 95% CI, 1.8-17; P < .001)
Patients who presented with HC after platelet engraftment and had documented BK viruria: 14 patients and 37 controls. Variables associated with postengraftment HC in univariate analysis: BK before or during HC versus no viremia (OR, 20; 95% CI, 2.7-802; P < .001); BK before HC versus no viremia (OR, 13; 95% CI, 3.4-30; P < .001); use of busulfan/cyclophosphamide regimen versus other regimens (OR, 5.0; 95% CI, 0.97-25; P = .03); any busulfan versus no busulfan (OR, 10; 95% CI, 1.2-86; P < .006)
This is the most comprehensive study of HC and its association with PCR-based detection of BK viral DNA in plasma in HCT recipients. Indeed, more than half of the patients presented with BK viremia of more than 104 copies/mL, whereas such titer of BK was rarely observed in controls. In the multivariable model, BK viremia was strongly associated with the risk of developing HC. BK viremia was even important before clinical symptoms of HC occurred and the magnitude of the association was even stronger when the analysis was restricted to cases with documented BK viruria who developed the disease after platelet engraftment. This study was also large enough to control for potential confounding factors such as conditioning regimen, cell source, HLA matching, presence of GVHD, and CMV infection. We were able to demonstrate BK virus in the bladder epithelium of 2 of 3 patients with severe HC who underwent a bladder biopsy. Although small amounts of BK DNA have been detected in bladder tissue of immunocompetent people without active disease by highly sensitive PCR,12 the ISH assay used in this study is fairly insensitive, thus suggesting a high level of viral DNA target.
One theory about the pathogenesis of BK virus HC is that the chemotherapy-induced injury or the cellular regeneration or differentiation after conditioning is not sufficient to cause endorgan BK disease, but makes the target tissue (ie, the bladder epithelium) susceptible to the cytopathic effects of lytic high-level BK infection. An alternative hypothesis for BK virus–associated HC occurring after hematopoietic cell transplantation is that of an immune reconstitution disease.13 According to this theory, HC is the result of an inflammatory process that occurs when antigen-specific T cells recognize BK virus in the bladder. Immune reconstitution disease has been described for JC virus infection in HIV-infected individuals treated with antiretroviral combination therapy.14 However, the immune reconstitution model is probably not sufficient to explain BK virus–associated HC in the hematopoietic cell transplantation setting. Indeed, in the present study, 4 patients developed postengraftment HC with a lymphocyte count in plasma of less than 100 cells/μL. Also, one would expect the immune reconstitution reaction to be suppressed in the presence of high-dose steroids since steroids have been shown to suppress functional T-cell recovery after hematopoietic cell transplantation.15 In our previous study,8 allograft patients who developed postengraftment HC were on high-dose steroids but had high levels of BK viremia. However, despite the strength of the association seen in this study, a causal link between BK virus and HC would ultimately only be established by a prospective study assessing the impact of specific suppression of BK virus after transplantation.
Prepublished online as Blood First Edition Paper, April 21, 2005; DOI 10.1182/blood-2004-12-4988.
Supported by grants from the Swiss National Foundation (PBLAB-100 602), the Joel Meyers Endowment Fund, and the National Institutes of Health (CA15 704, CA18 029).
V.E. designed the research, performed clinical chart reviews, analyzed the data, and wrote the paper; H.W.K. analyzed data and critically reviewed the paper; L.C. contributed to the design of the study, provided resources, and critically reviewed the paper; A.L. provided input on the data analysis and critically reviewed the manuscript; M.-L.H. supervised PCR testing and critically reviewed the manuscript; D.M. performed in situ testing and critically reviewed the manuscript; C.D. wrote computer programs for data extraction and critically reviewed the manuscript; M.B. designed the study, supervised the data collection and analysis, and had overall responsibility for the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Rachael K. Parkin and James Ferrenberg for their technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal