Abstract
Internal tandem duplication mutations of the FLT3 gene (FLT3/ITD mutations) are the most frequent molecular abnormality in acute myeloid leukemia (AML) and are associated with a poor overall survival. While the normal FLT3 receptor is expressed in early hematopoietic progenitor cells, it has not been determined whether FLT3 mutations are present in the leukemic stem cells. In this study, we sorted primary AML samples into stem cell-enriched CD34+/CD38- fractions and then analyzed the sorted and unsorted cells for the FLT3 mutant-wild-type ratio. In each case, the FLT3 mutant-wild-type ratio was not changed by selection of CD34+/CD38- cells, implying that the mutations are present in the leukemic stem cells. We used the stem cell-enriched fraction to engraft nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice and then confirmed that the FLT3/ITD mutation was present in the resultant engrafted marrow. As a final test of the importance of FLT3/ITD signaling in this engraftment model, we used a small molecule FLT3 inhibitor, CEP-701, to inhibit engraftment of FLT3/ITD stem cells. Taken together, these experiments establish that the FLT3/ITD mutations are present in leukemia stem cells, and that FLT3 inhibitors may have activity against these cells. (Blood. 2005;106:673-680)
Introduction
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy that currently requires treatment with intensive chemotherapy for cure. While the majority of patients with AML achieve a complete remission (CR) with induction therapy, greater than half of these subsequently relapse and ultimately die of the disease.1 Relapse is thought to occur because of the failure of chemotherapy to eradicate leukemia stem cells. Human AML stem cells have been characterized as CD34+/CD38- cells with severe combined immunodeficient (SCID)-repopulating ability, which is a reflection of their capacity to self-renew.2,3
The receptor tyrosine kinase FLT3 is expressed in CD34+ hematopoietic stem/progenitor cells and plays an important role in normal hematopoiesis.4-7 FLT3 is also expressed on the leukemic blasts in the majority of cases of acute leukemia, although its expression is no longer tightly coupled to CD34 expression.8-11 Internal tandem duplication mutations of FLT3 (FLT3/ITD mutations) occur in approximately 23% of patients with AML and are associated with an increased relapse rate and reduced overall survival.12-19 These mutations, which consist of in-frame insertions of duplicated sequence localized to the juxtamembrane region of the receptor, constitutively activate the tyrosine kinase function of FLT3.20-22 A number of findings support the participation of constitutively activated FLT3 in leukemogenesis.23-27 The receptor transduces signals that promote proliferation and inhibit apoptosis, and FLT3/ITD expression in murine hematopoietic cell lines blocks differentiation and induces transformation. Interestingly, a high FLT3 mutant-wild-type ratio in AML correlates with a distinctly poor outcome.17,28 A large body of evidence thus indicates that FLT3 is a valid therapeutic target in AML, and, in response to this, several small molecule FLT3 inhibitors are now in development.29,30
In order for any AML therapy to be curative, it needs to be effective against the cells that propagate and maintain the disease, namely the leukemic stem cells. At the present time, there are limited data supporting the presence of FLT3 mutations in leukemia stem cells. In support is the finding that bone marrow cells from patients with AML harboring FLT3/ITD mutations have a greater capacity to engraft nonobese diabetic (NOD)-SCID mice than cells from patients lacking such mutations.31,32 However, several independent studies of paired diagnostic and relapse AML samples have revealed that a small but consistent portion of patients with AML initially harboring FLT3/ITD mutations lack these mutations at relapse.33-35 This would suggest that at least in some cases, the mutations occurred at a later stage of leukemic transformation, and that chemotherapy was successful in eradicating the clones expressing the FLT3 mutations. Further, some samples were actually found to contain multiple different FLT3/ITD mutations, again suggesting that they are present in subclones of cells.15,34,35
In order to better address the issue of whether or not FLT3/ITD mutations are present in leukemia stem cells, we sorted a series of primary AML samples harboring FLT3/ITD mutations into stem cell-enriched fractions, and compared the mutant-wild-type ratios within the sorted and unsorted cells. We then used the stem cell-enriched fractions to try and engraft NOD-SCID mice in order to determine if the mutations were present in the engrafting cells. Finally, we studied the effects of an FLT3 inhibitor on engraftment of 2 AML samples harboring FLT3/ITD mutations. Our data provide the first definitive evidence that FLT3/ITD mutations occur in leukemia stem cells.
Materials and methods
Reagents
Cell culture reagents were from Invitrogen (Carlsbad, CA), except for heat-inactivated fetal bovine serum (FBS), which was obtained from Gemini (Woodland, CA). CEP-701 (supplied by Cephalon, West Chester, PA) was stored at 4 μM in 100% dimethyl sulfoxide (DMSO) for in vitro use. For in vivo use, CEP-701 was formulated in a vehicle of 40% polyethylene glycol 1000, 10% povidone C30, 2% benzyl alcohol in sterile water. All other reagents were from Sigma (St Louis, MO).
Patient samples
Bone marrow and peripheral blood samples from patients who gave informed consent in accordance with the Declaration of Helsinki were obtained according to a protocol approved by The Johns Hopkins University Institutional Review Board. Mononuclear cells were isolated by centrifuging samples over Ficoll-Hypaque (Amersham, Piscataway, NJ) and were comprised of more than 90% blasts as confirmed by light microscopic examination of Wright-stained cytospins. Blasts were stored in liquid nitrogen in FBS plus 10% DMSO. Prior to use, the leukemia samples were thawed into warm cell culture medium (RPMI containing 10% FBS with pen/strep and l-glutamine) and incubated for 12 hours at 37°C in 5% CO2. Typically, 50% to 70% of the cells in the sample were dead from freeze-thaw-induced necrosis. The samples were pelleted and resuspended in warm medium containing 150 U/mL DNAseI (Amersham, Piscataway, NJ) for 5 minutes. They were then recentrifuged over Ficoll to obtain cells with a high fraction that were viable.
Peripheral blood progenitor cell products
Peripheral blood progenitor cell (PBPC) products were excess cells that were to be discarded after 20 × 106 CD34+ cells/kg had been processed for autologous transplantation. All patients gave informed consent for use of these products under an institutional review board-approved protocol. PBPC products were selected for CD34+ cells using a SuperMACS (Miltenyi Biotech, Auburn, CA) selection device according to the manufacturer's recommended procedure. Selection resulted in products with 85% or greater CD34+ purity.
Fluorescence-activated cell sorting analysis
Fluorescence-activated cell sorting (FACS) analysis was performed using a FACScalibur cytometer and CellQuest software from Becton Dickinson (San Jose, CA). Fluorescein isothiocyanate (FITC)-, peridinin-chlorophyll-protein complex (PerCP)-, phycoerythrin (PE)-, and allophycocyanin (APC)-conjugated antibodies to human CD13, CD33, CD34, CD45, CD135, and murine CD45 were obtained from BD Pharmingen (San Jose, CA).
Isolation of CD34+/CD38- cells
Stem cell-enriched fractions of leukemia cells were obtained using immunomagnetic cell sorting with a VarioMACS magnetic sorter (Miltenyi). Leukemia samples were washed in phosphate-buffered saline (PBS) with 2 mM EDTA (ethylenediaminetetraacetic acid) and 0.1% FBS, incubated with APC-conjugated anti-human CD34 and FITC-conjugated anti-human CD38, then incubated with anti-FITC microbeads (Miltenyi). After negative immunomagnetic selection, CD38- cells were incubated with anti-APC microbeads and positively selected through a new column. The unsorted and CD34+/CD38--sorted cells were then analyzed by FACS and used to prepare genomic DNA (QIAamp Mini Kit; Qiagen, Valencia, CA). When sufficient cells were available, they were washed in PBS and injected into mice. For mice injected with only 5000 selected cells, 1 × 106 normal donor lymphocytes irradiated with 3000 rads were used as carrier cells for each injection.
NOD-SCID mice
NOD-SCID, 8-week-old female mice (Jackson Laboratories, Bar Harbor, ME) were maintained 5 per cage under temperature- and humidity-controlled conditions with food and water ad libitum. At 8 hours prior to injection, mice were sublethally irradiated (300 rads) in a cesium irradiator. Cell suspensions were washed twice in PBS, resuspended in PBS, and injected (0.5 mL volume) into lateral tail veins. When treated with CEP-701, mice were injected subcutaneously every 12 hours for 5 days in a row every 28 days for 14 weeks (amounting to 15 days of treatment out of a total of 96 days). The volume of each injection was 0.1 mL, at a dose of 20 mg/kg CEP-701. Control mice were injected with an equal volume of vehicle on the same schedule. Engraftment was analyzed 14 weeks after injection of cells. Immediately after mice were killed, both femurs were removed and flushed with cell culture medium. Harvested cells were washed once in red blood cell lysis buffer (0.155 M NH4Cl, 0.01 M KHCO3, 0.1 mM EDTA), resuspended in cell culture medium, and counted using a hemocytometer.
Polymerase chain reaction for FLT3
For qualitative analysis of FLT3/ITD mutations, genomic DNA was isolated using a QIAamp Mini Kit (Qiagen) and polymerase chain reaction (PCR) was performed using the Platinum PCR Supermix (Invitrogen) with primers flanking the juxtamembrane coding region: 13F (GTAAAACGACGGCCAGTGCAGAACTGCCTATTCCTA) and 15R (CAGGAAACAGCTATGACCTGTCCTTCCACTATACTGT). Thirty-five cycles of amplification were performed at 94°C for 30 seconds for denaturation, at 55°C for 30 seconds for annealing, and at 72°C for 1 minute for extension (DNA iCycler; Bio-Rad, Hercules, CA). PCR products were resolved on 2.5% agarose gels and visualized under ultraviolet light after ethidium bromide staining. For quantitative analysis of FLT3/ITD mutations, the PCR reactions were carried out using Taq Gold (Applied Biosystems, Foster City, CA) with fluorochrome-labeled primers, followed by capillary electrophoresis on an ABI 310 capillary electrophoresis instrument (Applied Biosystems), as described previously.36 All FLT3 mutations were confirmed by DNA sequencing. Sequences were analyzed and allelic ratios calculated using GeneScan software software (Applied Biosystems). For real-time PCR, 200 ng genomic DNA was amplified using the same primers and amplification conditions as for qualitative analysis with QuantiTect SYBR Green MasterMix (Qiagen) in a Bio-Rad iQ Icycler (Bio-Rad).
Immunoblotting
FLT3 expression was analyzed by incubating cells for 1 hour in CEP-701 followed by cell lysis in detergent buffer, immunoprecipitation, gel electrophoresis, and immunoblotting with antiphosphotyrosine antibodies exactly as described previously.37
Colony assays
For leukemia colony-forming unit (CFU-L; colonies defined as having > 40 cells in a cluster) assays, bone marrow cells from engrafted mice were suspended in 0.5 mL cell culture medium (RPMI 1640 with 10% FBS) and added to 4.5 mL warm methylcellulose medium containing granulocyte macrophage-colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), erythropoietin, and stem cell factor (Complete colony assay medium; StemCell Technologies, Vancouver, BC, Canada). Final concentrations were 106, 105, and 104 cells per plate for each marrow sample. The suspension was divided into 4 35-mm dishes and incubated for 4 days at 37°C in a humidified atmosphere with 5% CO2. After 4 days, CFU-Ls were scored manually under light microscopy. For marrow samples from mice engrafted with normal stem cells, granulocyte macrophage-colony-forming units (CFU-GMs) and erythroid-burst-forming units (BFU-Es) were scored after 14 days of incubation. CFU-GMs were defined as colonies of opaque cells and consisted of at least 50 cells. BFU-Es were defined as colonies of at least 50 hemoglobin-containing cells.
Statistics
Differences between experimental groups were calculated using the paired t test.
Analysis of unsorted and CD34+/CD38--selected cells from AML patient samples. Eight AML samples, all harboring FLT3/ITD mutations, were stained with anti-CD34 and anti-CD38 fluorochrome-conjugated antibodies and subjected to immunomagnetic cell separation, as described in “Materials and methods.” The dot plots from the analysis of each sample are displayed above, with each patient represented by a row. The y-axis of all plots represents CD34 staining. The first column represents the unsorted cells and the middle column is the analysis of the CD34+/CD38--selected cells. The column on the right represents the CD135 staining of the selected cells. All analyses shown were performed without gates. The quadrant markers in the middle row were used to obtain the percentages listed in Table 2.
Analysis of unsorted and CD34+/CD38--selected cells from AML patient samples. Eight AML samples, all harboring FLT3/ITD mutations, were stained with anti-CD34 and anti-CD38 fluorochrome-conjugated antibodies and subjected to immunomagnetic cell separation, as described in “Materials and methods.” The dot plots from the analysis of each sample are displayed above, with each patient represented by a row. The y-axis of all plots represents CD34 staining. The first column represents the unsorted cells and the middle column is the analysis of the CD34+/CD38--selected cells. The column on the right represents the CD135 staining of the selected cells. All analyses shown were performed without gates. The quadrant markers in the middle row were used to obtain the percentages listed in Table 2.
Results
The FLT3 mutant-wild-type ratio is the same in sorted versus unsorted cells
The FLT3 mutant-wild-type allelic ratio in AML cells, as determined by a variety of methods, has been found to vary, but all groups have thus far reported that most commonly the mutant is present in heterozygous form.15,17,28 One study, for example, reported a mean mutant-wild-type allelic ratio of 0.78.17 However, it is possible that the FLT3/ITD mutations are present only in a subclone of cells, which have a resultant growth advantage but may lack the capacity for long-term self-renewal. If this were the case, then the mutant-wild-type allelic ratio would decrease in fractions enriched for leukemic stem cells.
To determine whether or not leukemia stem cells have the same FLT3 mutant-wild-type ratio as the majority of blasts, leukemic stem cells were isolated from primary AML samples. The samples consisted of either peripheral blood or bone marrow mononuclear cells, all consisting of more than 90% blasts as confirmed by light microscopy, from patients with AML harboring FLT3/ITD mutations. None of these samples contained FLT3 kinase domain mutations (data not shown). Samples were stored in liquid nitrogen and thawed the day before use, as described in “Materials and methods.” The clinical characteristics of the patients are summarized in Table 1. Immunomagnetic cell selection was used to obtain CD34+/CD38- cells, which have been shown by others to be highly enriched for leukemic stem cells.3 Flow cytometric analysis after selection (Figure 1, Table 2) determined that they were 71.8% to 99.1% CD34+/CD38-. All but 2 of the leukemic stem cell-enriched fractions (samples 7 and 8) expressed CD135 (FLT3). With the exception of a single sample (sample 6), all of CD34+/CD38- fractions amounted to less than 1% of the total cells (Table 2). Genomic DNA from unsorted and sorted cells was isolated and PCR was performed using primers flanking exons 14 and 15 of the FLT3 gene.36,38 The PCR products were then isolated and quantitated by capillary electrophoresis. As summarized in Table 2, in all 8 samples the FLT3/ITD mutation was clearly present in the leukemic stem cell-enriched fraction. In samples 3 and 6, the mutations were most likely present in homozygous form, as less than 5% of the PCR product from the unselected cells represented the wild-type FLT3 gene. After selection of the CD34+/CD38- fraction, no wild-type signal was detectable, probably as a result of eliminating the small percent of normal hematopoietic cells in the sample. In all other cases, the FLT3 mutant-wild-type allelic ratios were very similar between selected and unselected fractions. In no case was the mutant allele depleted by selection for leukemic stem cells, which would have occurred if the mutations were not present in those cells. It is interesting to note that the ratios ranged from 0.67 to 0.9, even in the stem cell-selected fractions. It is possible that this reflects a PCR bias for the shorter (wild-type) molecule, and that this ratio actually represents 100% of cells with a heterozygous mutation. Alternately, these ratios may suggest the presence of either additional subclones of leukemic CD34+/CD38- cells that lacked the mutation, or residual normal (ie, nonleukemic) stem cells within the sample.
Patient characteristics for AML samples
Sample no. . | Age, y . | FAB . | WBC count . | Cytogenetics . | PB/BM . |
|---|---|---|---|---|---|
| 1 | 41 | M1 | 110 170 | Normal | PB |
| 2 | 39 | M4 | 67 530 | Normal | PB |
| 3 | 73 | M5 | 139 852 | Normal | PB |
| 4 | 60 | M0 | 16 130 | Normal | BM |
| 5 | 69 | M1 | 19 120 | Normal | BM |
| 6 | 61 | M2 | 24 700 | t(2; 12) | BM |
| 7 | 61 | MDS/AML | 8 440 | -y | BM |
| 8 | 50 | M2 | 107 530 | Normal | PB |
Sample no. . | Age, y . | FAB . | WBC count . | Cytogenetics . | PB/BM . |
|---|---|---|---|---|---|
| 1 | 41 | M1 | 110 170 | Normal | PB |
| 2 | 39 | M4 | 67 530 | Normal | PB |
| 3 | 73 | M5 | 139 852 | Normal | PB |
| 4 | 60 | M0 | 16 130 | Normal | BM |
| 5 | 69 | M1 | 19 120 | Normal | BM |
| 6 | 61 | M2 | 24 700 | t(2; 12) | BM |
| 7 | 61 | MDS/AML | 8 440 | -y | BM |
| 8 | 50 | M2 | 107 530 | Normal | PB |
FAB indicates French-American-British classification; PB, peripheral blood; BM, bone marrow; MDS, myelodysplastic syndrome.
Summary of results from immunomagnetic cell selection
. | . | . | % CD135 . | . | . | FLT3 ratio . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Sample no. . | Total unsorted cells . | Total yield, CD34+/CD38− cells (% unsorted) . | Unsorted cells . | Sorted cells . | Sorted, % CD34+/CD38− . | Unsorted cells . | Sorted cells . | ||
| 1 | 109 | 4.5 × 106 (0.45) | 96.7 | 97.8 | 98.2 | 0.67 | 0.69 | ||
| 2 | 109 | 3 × 106 (0.30) | 95.2 | 96.5 | 74.6 | 0.70 | 0.73 | ||
| 3 | 8 × 108 | 1.5 × 106 (0.19) | 89.5 | 98.1 | 93.8 | 21.0 | ND | ||
| 4 | 4 × 108 | 1 × 106 (0.25) | 96.4 | 99.5 | 71.8 | 0.77 | 0.70 | ||
| 5 | 2 × 108 | 3.5 × 104 (0.02) | 97.7 | 98.9 | 73.7 | 0.67 | 0.79 | ||
| 6 | 2.5 × 108 | 20 × 106 (8.0) | 91.0 | 92.9 | 95.7 | 9.50 | ND | ||
| 7 | 2.5 × 108 | 2.4 × 106 (0.96) | 10.5 | 0.2 | 98 | 0.80 | 0.90 | ||
| 8 | 3 × 108 | 1.5 × 106 (0.5) | 5.2 | 0.4 | 99.1 | 0.65 | 0.77 | ||
. | . | . | % CD135 . | . | . | FLT3 ratio . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| Sample no. . | Total unsorted cells . | Total yield, CD34+/CD38− cells (% unsorted) . | Unsorted cells . | Sorted cells . | Sorted, % CD34+/CD38− . | Unsorted cells . | Sorted cells . | ||
| 1 | 109 | 4.5 × 106 (0.45) | 96.7 | 97.8 | 98.2 | 0.67 | 0.69 | ||
| 2 | 109 | 3 × 106 (0.30) | 95.2 | 96.5 | 74.6 | 0.70 | 0.73 | ||
| 3 | 8 × 108 | 1.5 × 106 (0.19) | 89.5 | 98.1 | 93.8 | 21.0 | ND | ||
| 4 | 4 × 108 | 1 × 106 (0.25) | 96.4 | 99.5 | 71.8 | 0.77 | 0.70 | ||
| 5 | 2 × 108 | 3.5 × 104 (0.02) | 97.7 | 98.9 | 73.7 | 0.67 | 0.79 | ||
| 6 | 2.5 × 108 | 20 × 106 (8.0) | 91.0 | 92.9 | 95.7 | 9.50 | ND | ||
| 7 | 2.5 × 108 | 2.4 × 106 (0.96) | 10.5 | 0.2 | 98 | 0.80 | 0.90 | ||
| 8 | 3 × 108 | 1.5 × 106 (0.5) | 5.2 | 0.4 | 99.1 | 0.65 | 0.77 | ||
AML samples were stained with anti-CD34 and anti-CD38 antibodies and subjected to immunomagnetic selection as described in “Materials and methods.” Total unsorted cells represents the starting sample size, whereas total yield CD34+/CD38− cells refers to the total number of CD34+/CD38− cells obtained from a given sample after selection. The cells were also stained with anti-CD135 to analyze for cell surface expression of FLT3. Genomic DNA from sorted and unsorted cells was isolated, PCR amplified, and analyzed for the FLT3 mutant-wild-type allelic ratio. ND indicates that no wild-type FLT3 was detected in the sample.
FLT3/ITD mutations are present in the leukemia stem cells that engraft NOD-SCID mice
Engraftment in the NOD-SCID mouse represents one of the best current models for the study of human leukemia stem cells.32 CD34+/CD38- leukemia cells from AML patient samples are capable of causing long-term leukemic engraftment when as few as 5 × 103 are injected into the tail vein of sublethally irradiated NOD-SCID mice.3 We injected NOD-SCID mice, in parallel groups of 4, with 106 unsorted and 5 × 103 CD34+/CD38--sorted cells from 6 of the 8 AML samples (samples 1-4, 7, and 8). After 14 weeks, the mice were killed and bone marrow was isolated from the femurs. The level of human leukemic engraftment was determined using flow cytometric analysis of human CD45 compared with murine CD45 expression. The marrow samples were also analyzed for expression of human CD13, CD33, CD34, and CD135. The results of the engraftment analyses are summarized in Table 3, and representative dot plots are shown in Figure 2. Three of the 6 samples engrafted in the mice, with human CD45+ cells ranging from 4% to 97% of total nucleated cells. In all 3 of the sample groups that successfully engrafted, the FLT3/ITD mutation was present at the same or higher mutant-wild-type allelic ratio seen in the cells used to inject the mice (Table 3, Figure 3A). The FLT3/ITD mutations in the engrafted mice were sequenced to confirm that they represented the identical mutations present in the unsorted original samples (Figure 3B). Adult mice are estimated to have approximately 2 × 108 to 3 × 108 total nucleated cells in the bone marrow.39 Based on this estimate, therefore, engraftment of 4% to 95% in mice injected with 5 × 103 CD34+/CD38--sorted cells represents a 1600- to 38 000-fold expansion. The fact that this expanded population of leukemia cells contained the identical FLT3/ITD mutation at the same mutant-wild-type ratio as was observed in the original unsorted samples constitutes direct evidence that the FLT3/ITD mutations were present, at least in these 3 samples, in leukemia stem cells.
Summary of NOD-SCID engraftment
Sample no.* . | No. engrafted/injected, unsorted . | % human CD45, unsorted . | Engrafted/injected, sorted . | % human CD45, sorted . | Mutant-wild-type ratio of FLT3 . |
|---|---|---|---|---|---|
| 1 | 4/4 | 3/4 | 1.07 | ||
| Mouse 1 | 90 | 22 | |||
| Mouse 2 | 90 | 20 | |||
| Mouse 3 | 90 | 4 | |||
| Mouse 4 | 84 | ||||
| 2 | 0/4 | ND | ND | ND | N/A |
| 3 | 4/4 | 4/4 | All mutant | ||
| Mouse 1 | 97 | 95 | |||
| Mouse 2 | 89 | 60 | |||
| Mouse 3 | 80 | 52 | |||
| Mouse 4 | 70 | 4 | |||
| 4 | 4/4 | 0/4 | ND | 0.81 | |
| Mouse 1 | 20 | ||||
| Mouse 2 | 10 | ||||
| Mouse 3 | 9 | ||||
| Mouse 4 | 5 |
Sample no.* . | No. engrafted/injected, unsorted . | % human CD45, unsorted . | Engrafted/injected, sorted . | % human CD45, sorted . | Mutant-wild-type ratio of FLT3 . |
|---|---|---|---|---|---|
| 1 | 4/4 | 3/4 | 1.07 | ||
| Mouse 1 | 90 | 22 | |||
| Mouse 2 | 90 | 20 | |||
| Mouse 3 | 90 | 4 | |||
| Mouse 4 | 84 | ||||
| 2 | 0/4 | ND | ND | ND | N/A |
| 3 | 4/4 | 4/4 | All mutant | ||
| Mouse 1 | 97 | 95 | |||
| Mouse 2 | 89 | 60 | |||
| Mouse 3 | 80 | 52 | |||
| Mouse 4 | 70 | 4 | |||
| 4 | 4/4 | 0/4 | ND | 0.81 | |
| Mouse 1 | 20 | ||||
| Mouse 2 | 10 | ||||
| Mouse 3 | 9 | ||||
| Mouse 4 | 5 |
Six AML samples were subjected to immunomagnetic selection for CD34+/CD38− cells. The sample numbers shown are the same as in Table 1. For each sample, 4 sublethally irradiated NOD-SCID mice were injected with either 1 × 106 unsorted cells or 5 × 103 CD34+/CD38− cells (thus, 12 groups, of 4 mice each). At 14 weeks, the mice were killed and the bone marrow cells were analyzed for expression of human CD45. Genomic DNA prepared from these marrows were analyzed for FLT3 mutant-wild-type ratio as described in “Materials and methods.” Engrafted/injected refers to the number of mice that engrafted, as defined by having detectable human CD45 staining, versus the number injected within that group.
ND indicates no engraftment detected.
For sample nos. 7 and 8, 0 of 4 mice were engrafted/injected with either sorted or unsorted cells, and thus results are not shown for these groups in the table.
Effects of a FLT3 inhibitor on leukemia cell engraftment
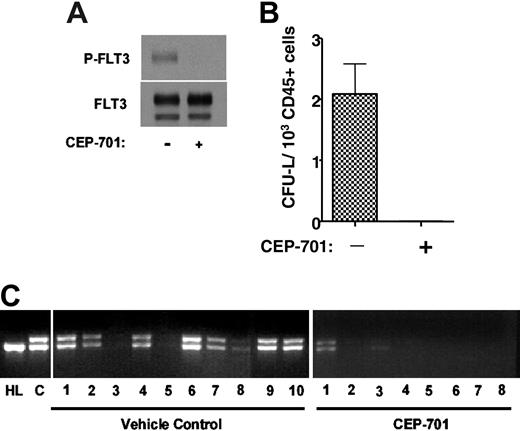
The presence of the FLT3/ITD mutant gene within leukemia stem cells does not prove that the mutant protein is expressed, or that the leukemic stem cell is dependent on FLT3 signaling for survival or engraftment. This issue is particularly pertinent to the development of FLT3 inhibitors for clinical use, where the hope is that such inhibitors will be cytotoxic to leukemia stem cells. Although CD135 expression was observed in 6 of 8 leukemic stem cell-enriched fractions (Figure 1), this doesn't prove that the cells with true self-renewing potential (which may represent only a small fraction of the CD34+/CD38- cells) express or are dependent on FLT3. To address this issue, we tested the ability of an FLT3 inhibitor to affect engraftment of CD34+/CD38- leukemia stem cells in NOD-SCID mice. CEP-701 is an indolocarbazole derivative that potently inhibits FLT3 autophosphorylation in vitro and in vivo.37 Immunoblot analysis of whole-cell extracts confirms that the leukemia cells from sample 1 (unsorted) express autophosphorylated FLT3, and that this phosphorylation is completely inhibited by exposure of the cells for 1 hour to 50 nM CEP-701 (Figure 4A). We isolated CD34+/CD38- cells from another thawed aliquot of sample 1 and injected 2 groups of 10 NOD-SCID mice with 5 × 103 cells each on day 1. One group of mice was treated for 5 consecutive days every 4 weeks, beginning on day 1, with subcutaneous injections of CEP-701 at a dose of 20 mg/kg every 12 hours. A single injection of 20 mg/kg CEP-701 has been shown to inhibit FLT3 autophosphorylation in vivo for 8 to 12 hours.37 The other group of mice was treated on an identical injection schedule with vehicle only. At 14 weeks, the mice were killed, femurs were removed and flushed, and the marrows were analyzed for engraftment. Seven of 10 vehicle control mice had an easily detectable population of cells that stained for human CD45. Meanwhile, no human CD45+ cells were discernible in the marrows from the CEP-701-treated mice. Colony assays on these marrow samples yielded CFU-Ls from all control samples, with a frequency of 2.1 (range, 0.94-3.62) CFU-L/103 human CD45+ cells plated (Figure 4B). When examined using light microscopy, the plucked CFU-Ls had the morphology of blasts, and FACS analysis of the pooled CFU-Ls confirmed that virtually 100% of them expressed human CD45+ (data not shown). No CFU-Ls grew from the marrow samples of the treated mice.
PCR amplification of genomic DNA obtained from the engrafted mice revealed the expected FLT3/ITD mutation when the PCR products were analyzed using ethidium bromide-stained agarose gels (Figure 4B). This assay also revealed the presence of the FLT3/ITD mutation in 2 of the 8 mice treated with CEP-701. This indicated that these mice were indeed engrafted, but the level of engraftment was too low to be detected by the FACS analysis or colony assays. In order to quantify the degree of engraftment in the CEP-701-treated mice, quantitative PCR was performed on genomic DNA from the marrow samples using the same primers as were used in Figure 4B. The results were compared with a standard curve generated by spiking mouse genomic DNA (obtained from the marrow of a NOD-SCID mouse) with serial dilutions of genomic DNA from patient sample 1. The results from this experiment (not shown) confirmed that the DNA from the marrows of these 2 mice contained FLT3/ITD DNA at levels of 0.8% and 0.03%, respectively. The other 6 mice had no detectable FLT3/ITD DNA.
Human leukemia engraftment of NOD-SCID mice. Sublethally irradiated mice were injected with 1 × 106 unsorted cells (top row) or 5 × 103 CD34+/CD38--sorted cells (bottom row) from leukemia sample 1 (Tables 1 and 2). After 14 weeks, mice were killed and the bone marrow cells were analyzed for expression of human and murine CD45 (left), and human CD13 and CD33 (right). Total marrow is shown, with no gates applied.
Human leukemia engraftment of NOD-SCID mice. Sublethally irradiated mice were injected with 1 × 106 unsorted cells (top row) or 5 × 103 CD34+/CD38--sorted cells (bottom row) from leukemia sample 1 (Tables 1 and 2). After 14 weeks, mice were killed and the bone marrow cells were analyzed for expression of human and murine CD45 (left), and human CD13 and CD33 (right). Total marrow is shown, with no gates applied.
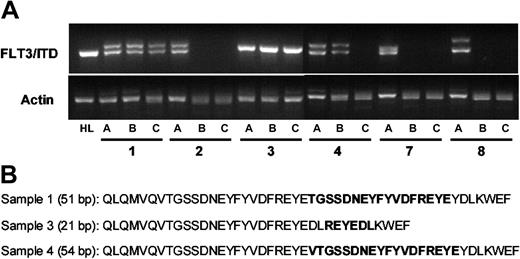
FLT3/ITD mutations in the bone marrow cells of engrafted mice. CD34+/CD38- cells from 6 different patient samples were isolated by immunomagnetic sorting (sorting results displayed in Figure 1). Mice were injected with either 1 × 106 unsorted cells or 5 × 103 CD34+/CD38- cells. After 14 weeks, the mice were killed and bone marrow cells were harvested. (A) For each of the 6 samples (numbers correspond to those in Figure 1), genomic DNA was isolated from the unsorted cells (a), the marrow cells of mice injected with 1 × 106 unsorted cells (b), or those mice injected with 3 × 105 CD34+/CD38- cells (c). PCR was performed using primers flanking the juxtamembrane region of human FLT3 (along with actin controls). The characteristic doublet of the FLT3/ITD mutation is easily visualized after electrophoresis in 2.5% agarose with ethidium bromide staining. Genomic DNA from HL60 cells (HL) was used as a control. (B) The sequences of the FLT3/ITD mutations from the 3 samples that engrafted (1, 3, and 4) are shown. These sequences, obtained from the DNA of the engrafted mice, matched the sequences of the mutations present in the preinjection samples. bp indicates base pair. Boldface sequence refers to the inserted, duplicated material.
FLT3/ITD mutations in the bone marrow cells of engrafted mice. CD34+/CD38- cells from 6 different patient samples were isolated by immunomagnetic sorting (sorting results displayed in Figure 1). Mice were injected with either 1 × 106 unsorted cells or 5 × 103 CD34+/CD38- cells. After 14 weeks, the mice were killed and bone marrow cells were harvested. (A) For each of the 6 samples (numbers correspond to those in Figure 1), genomic DNA was isolated from the unsorted cells (a), the marrow cells of mice injected with 1 × 106 unsorted cells (b), or those mice injected with 3 × 105 CD34+/CD38- cells (c). PCR was performed using primers flanking the juxtamembrane region of human FLT3 (along with actin controls). The characteristic doublet of the FLT3/ITD mutation is easily visualized after electrophoresis in 2.5% agarose with ethidium bromide staining. Genomic DNA from HL60 cells (HL) was used as a control. (B) The sequences of the FLT3/ITD mutations from the 3 samples that engrafted (1, 3, and 4) are shown. These sequences, obtained from the DNA of the engrafted mice, matched the sequences of the mutations present in the preinjection samples. bp indicates base pair. Boldface sequence refers to the inserted, duplicated material.
CEP-701 inhibits engraftment of FLT3/ITD cells in NOD-SCID mice. (A) Immunoblot of leukemia blasts (from patient sample 1) incubated for 1 hour in culture medium with or without 50 nM CEP-701. The blots were probed with antiphosphotyrosine antibody (top blot), then stripped and reprobed with anti-FLT3 antibody (bottom blot). (B) CFU-L assay. NOD-SCID mice injected with 5 × 103 CD34+/CD38- cells obtained using immunomagnetic selection of the sample shown in panel A were treated with vehicle only or with CEP-701. At 14 weeks, the mice were killed. Bone marrow cells harvested from engrafted mice were cultured in methylcellulose medium for CFU-L formation. Each column on the graph represents the average number of colonies obtained from all marrows within a treatment group. The number of colonies is expressed per 1 × 103 human CD45 cells plated. Error bar represents SD. (C) Genomic DNA was prepared from bone marrow cells and PCR was performed to amplify the juxtamembrane region of FLT3, yielding the characteristic doublet of the mutant gene. Following electrophoresis in 2.5% agarose, the ethidium bromide-stained gels were visualized under ultraviolet light. HL indicates control DNA obtained from HL60 cells (which harbor only FLT3 wild type); C, control DNA from the unsorted leukemia sample prior to injection into mice.
CEP-701 inhibits engraftment of FLT3/ITD cells in NOD-SCID mice. (A) Immunoblot of leukemia blasts (from patient sample 1) incubated for 1 hour in culture medium with or without 50 nM CEP-701. The blots were probed with antiphosphotyrosine antibody (top blot), then stripped and reprobed with anti-FLT3 antibody (bottom blot). (B) CFU-L assay. NOD-SCID mice injected with 5 × 103 CD34+/CD38- cells obtained using immunomagnetic selection of the sample shown in panel A were treated with vehicle only or with CEP-701. At 14 weeks, the mice were killed. Bone marrow cells harvested from engrafted mice were cultured in methylcellulose medium for CFU-L formation. Each column on the graph represents the average number of colonies obtained from all marrows within a treatment group. The number of colonies is expressed per 1 × 103 human CD45 cells plated. Error bar represents SD. (C) Genomic DNA was prepared from bone marrow cells and PCR was performed to amplify the juxtamembrane region of FLT3, yielding the characteristic doublet of the mutant gene. Following electrophoresis in 2.5% agarose, the ethidium bromide-stained gels were visualized under ultraviolet light. HL indicates control DNA obtained from HL60 cells (which harbor only FLT3 wild type); C, control DNA from the unsorted leukemia sample prior to injection into mice.
An additional primary AML sample with sufficient cell numbers suitable for a cell sorting and engraftment experiment was subsequently obtained. This sample is designated leukemia sample 9. In contrast to sample 1, which was a diagnostic sample from an untreated patient harboring a heterozygous FLT3/ITD mutation, sample 9 was from a 30-year-old man with refractory M5 AML that harbored a homozygous FLT3/ITD mutation (no wild-type copy present). This patient (and therefore these cells) had been exposed to several different chemotherapy regimens, but they expressed constitutively phosphorylated FLT3 and were sensitive to CEP-701 using an in vitro cytotoxicity assay (not shown; cytotoxicity assay performed as described37 ). We performed immunomagnetic sorting on sample 9, starting with 5 × 108 unsorted cells and obtaining 4 × 106 CD34+/CD38- cells after sorting. For this experiment, in order to compare the engraftment effect of sorted versus unsorted cells, we injected 2 groups of mice with 5 × 106 unsorted cells, and also injected 2 additional groups of mice with 5 × 103 CD34+/CD38- cells, each for treatment with CEP-701 versus vehicle control. There were thus 4 groups of mice in this experiment: unsorted, vehicle-treated; unsorted, CEP-701-treated; sorted, vehicle-treated; and sorted, CEP-701-treated. The mice were treated exactly as with sample 1 for 5 consecutive days every 4 weeks. Over the first 3 days of the eleventh week of the experiment, both groups of vehicle-treated mice became moribund and all mice had to be killed. The CEP-701-treated mice were uniformly healthy appearing at this time. At the time they were killed, the vehicle-treated mice all had splenomegaly (not observed in the sample 1 experiment), with an average spleen size of 1.25 g. We were able to recover roughly 100 × 106 human leukemia cells from each spleen, all of which were essentially identical in profile to the original sample 9 in terms of CD13, CD33, and CD135 staining (not shown). Although it was apparent that survival was prolonged in the CEP-701-treated mice, this was an engraftment study, not a survival study. Therefore, the CEP-701-treated mice (both sorted and unsorted) were killed at 11 weeks, and all had spleens under 0.05 g in size (ie, normal). However, the bone marrows from all mice, both CEP-701- and vehicle-treated, demonstrated significant percentages of human CD45+ cells, and genomic DNA isolated from all marrows contained the same homozygous FLT3/ITD mutation confirmed by PCR (gel not shown; the engraftment percentages for both experiments are summarized in Table 4). There was nonetheless a significant treatment effect (P < .001 for both sorted and unsorted), as the CEP-701-treated mice had normal spleens and engrafted to a lesser degree compared with the vehicle-treated mice (Table 4).
CEP-701 inhibits engraftment of FLT3/ITD leukemia
Leukemia sample 1, % CD45, sorted . | . | Leukemia sample 9, %CD45 . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Sorted . | . | Unsorted . | . | ||||
| Vehicle . | CEP-701 . | Vehicle . | CEP-701 . | Vehicle . | CEP-701 . | ||||
| 3.3 | 0 | 96.1 | 8.7 | 98.3 | 50.4 | ||||
| 3.2 | 0 | 89.0 | 74.6 | 94.2 | 19.1 | ||||
| 4.7 | 0 | 95.5 | 13.2 | 92.6 | 76.9 | ||||
| 4.6 | 0 | 95.6 | 91.2 | 75.7 | 11.7 | ||||
| 11.3 | 0 | 95.0 | 86.9 | 98.0 | 46.5 | ||||
| 19.4 | 0 | 90.2 | 80.0 | 90.0 | — | ||||
| 9.3 | 0 | 94.7 | 1.3 | — | — | ||||
| — | 0 | 95.3 | 78.2 | — | — | ||||
| 8.0* | 0* | 93.9* | 54.2* | 91.5* | 40.9* | ||||
Leukemia sample 1, % CD45, sorted . | . | Leukemia sample 9, %CD45 . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Sorted . | . | Unsorted . | . | ||||
| Vehicle . | CEP-701 . | Vehicle . | CEP-701 . | Vehicle . | CEP-701 . | ||||
| 3.3 | 0 | 96.1 | 8.7 | 98.3 | 50.4 | ||||
| 3.2 | 0 | 89.0 | 74.6 | 94.2 | 19.1 | ||||
| 4.7 | 0 | 95.5 | 13.2 | 92.6 | 76.9 | ||||
| 4.6 | 0 | 95.6 | 91.2 | 75.7 | 11.7 | ||||
| 11.3 | 0 | 95.0 | 86.9 | 98.0 | 46.5 | ||||
| 19.4 | 0 | 90.2 | 80.0 | 90.0 | — | ||||
| 9.3 | 0 | 94.7 | 1.3 | — | — | ||||
| — | 0 | 95.3 | 78.2 | — | — | ||||
| 8.0* | 0* | 93.9* | 54.2* | 91.5* | 40.9* | ||||
CD34+/CD38− cells (5 × 103) obtained from leukemia sample 1 (Table 1) were injected via tail vein into 2 groups of 10 sublethally irradiated NOD-SCID mice. In a subsequent experiment, 5 × 106 unsorted cells and 5 × 103 CD34+/CD38− cells from leukemia sample 9 were likewise injected into 4 groups of 8 mice. Mice were then treated with CEP-701 (20 mg/kg) or vehicle twice daily for 5 days every 4 weeks. Some of the mice in the different groups died of apparent infection in the first month of the experiment; thus, final numbers range from 5 to 8 for each group. At sacrifice, the bone marrow cells were analyzed by flow cytometry for human CD45 staining. The % CD45 refers to the percentage of the total marrow that stained for human CD45 (as compared with murine CD45).
—indicates the animal died.
Indicates the mean value for the mice in that group.
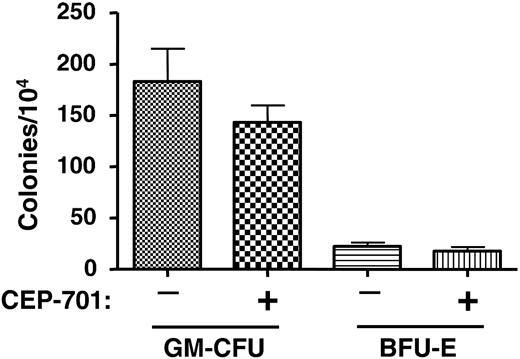
CEP-701 treatment does not significantly affect hematopoietic progenitors from engrafted NOD-SCID mice. Two groups of mice were injected with 6 × 106 CD34+ cells obtained from the mobilized peripheral blood of a normal donor. One group of mice was treated with CEP-701 (20 mg/kg, subcutaneously twice daily for 5 days out of every 4 weeks), the other with vehicle only. After 14 weeks, bone marrow cells were harvested from the mice and assayed for progenitor cells. Each column on the graph represents the average number of colonies obtained from 5 individual marrows. Error bars represent SD.
CEP-701 treatment does not significantly affect hematopoietic progenitors from engrafted NOD-SCID mice. Two groups of mice were injected with 6 × 106 CD34+ cells obtained from the mobilized peripheral blood of a normal donor. One group of mice was treated with CEP-701 (20 mg/kg, subcutaneously twice daily for 5 days out of every 4 weeks), the other with vehicle only. After 14 weeks, bone marrow cells were harvested from the mice and assayed for progenitor cells. Each column on the graph represents the average number of colonies obtained from 5 individual marrows. Error bars represent SD.
The above engraftment studies demonstrated that CEP-701 treatment inhibits leukemic engraftment, at least in the case of these 2 particular patient samples. It is possible, however, that the inhibition occurred in a nonspecific manner; that is, that CEP-701 was acting simply as a general toxin to bone marrow. Alternately, CEP-701 may inhibit engraftment of any cells expressing FLT3, leukemic or normal. As a control experiment, therefore, we injected each of 10 mice with 6 × 106 CD34+ cells that were selected from a PBPC product from a non-AML donor. Five of the mice were treated with CEP-701 for 5 consecutive days every 4 weeks beginning on day 1, as in the previous experiments using the leukemia samples. The other 5 mice were treated with vehicle only. At 14 weeks, femurs were harvested and the marrow was analyzed for human engraftment using human CD45 expression (Table 5) and CFU-GM/BFU-E colony assays (Figure 5). These results show that CEP-701 treatment had, at most, a mild negative effect on engraftment that did not reach statistical significance (P = .4). This control experiment provides evidence that leukemic stem cells harboring FLT3/ITD mutations rely on FLT3 signaling for survival and engraftment capability in NOD-SCID mice more than do normal hematopoietic stem/progenitor cells.
CEP-701 has little effect on engraftment of normal hematopoietic stem cells
Normal donor CD34+ cells . | . | |
|---|---|---|
| Vehicle control, %CD45 . | CEP-701-treated, %CD45 . | |
| 57.1 | 13.6 | |
| 34.5 | 15.6 | |
| 69.9 | 58.0 | |
| 9.5 | 14.2 | |
| 44.2 | ||
| 43.0* | 25.4* | |
Normal donor CD34+ cells . | . | |
|---|---|---|
| Vehicle control, %CD45 . | CEP-701-treated, %CD45 . | |
| 57.1 | 13.6 | |
| 34.5 | 15.6 | |
| 69.9 | 58.0 | |
| 9.5 | 14.2 | |
| 44.2 | ||
| 43.0* | 25.4* | |
CD34+ cells (6 × 106) obtained from the mobilized peripheral blood of a healthy human donor were injected into 2 groups of 5 irradiated NOD-SCID mice. Mice were then treated with CEP-701 (20 mg/kg) or vehicle twice daily for 5 days out of each month. One mouse in the CEP-701 group died of apparent infection in the first month of the experiment (CEP-701, given subcutaneously, acts as a considerable skin irritant in these mice, which may have predisposed them to infection). At 14 weeks, mice were killed and the bone marrow cells were analyzed by flow cytometry for human CD45 staining. The % CD45 refers to the percentage of the total marrow that stained for human CD45 (as compared with murine CD45).
Indicates the mean value for the mice in that group.
Discussion
This study was undertaken to investigate whether or not FLT3/ITD mutations are present in leukemia stem cells. This issue is important because several lines of evidence suggest that these mutations can occur relatively late in the development of leukemia. Three separate studies have demonstrated that in a small proportion of cases, FLT3/ITD mutations are lost at relapse.33-35 This suggests that, in these few cases, the mutations occurred in a subclone that was eliminated by treatment. The relatively high concordance between FLT3/ITD mutations and partial tandem duplications of the MLL gene suggest that both mutations may arise from a defect in DNA repair mechanisms that develops even earlier in leukemogenesis.40 When mice expressing a promyelocyte-retinoic acid receptor α (PML/RARα) transgene receive a transplant of bone marrow cells transduced with an FLT3/ITD gene, acute leukemia develops with increased frequency and reduced latency.41 Given that numerous small molecule FLT3 inhibitors are in development, it is of utmost importance to establish that the target of these new agents is a target within the cell responsible for initiating and maintaining the disease.
In the 8 samples studied, there was no evidence to suggest that the FLT3/ITD mutations were not present within leukemia stem cells. There were no significant differences in the FLT3 mutant-wild-type ratios between unsorted and CD34+/CD38- cells. Although the sorted cells in most of the samples comprised less than 1% of the unsorted population, it is certainly possible that the true leukemia stem cells represented an even smaller fraction of the total CD34+/CD38- group. If this were the case, then the mutant-wild-type ratio of the sorted cells would still approximate that of the bulk, unsorted cells. However, the fact that leukemia cells from the marrows of the engrafted NOD-SCID mice contained the identical FLT3/ITD mutations, at the same mutant-wild-type allelic ratios as were present in the unsorted samples, represents more definitive evidence that the mutations are present in the leukemia stem cells. In all, therefore, our evidence would suggest that, at least at the rate at which the samples can engraft in NOD-SCID mice (3 of 8 in this study), the FLT3/ITD mutations occur at the level of the leukemia stem cell. If the CD34+/CD38- subset contains a significant fraction of stem cells, then the FLT3 mutant-wild-type ratio data imply that most of these mutations are in cells capable of self-renewal.
How, then, to explain the occasional loss of the FLT3/ITD mutations at relapse? It still seems likely that in at least a subset of AML, the FLT3/ITD mutations are present only in subclones derived from the original leukemic stem cell, subclones that lack the capacity for self-renewal. It may be that our sample size was simply not large enough to uncover such cases. In particular, the group of leukemia samples we studied did not include any with a low mutant-wild-type ratio. These may represent the cases in which the mutation arose as a relatively late hit in leukemogenesis, and may also be the cases in which the mutation is lost at relapse. We are currently attempting to collect some of these low-mutant-ratio samples for study. Alternately, FLT3 mutations could always be present in leukemia stem cells, but occasionally chemotherapy succeeds in eradicating the FLT3/ITD samples, whereas other leukemic stem cells that lack the mutation are resistant. In other words, the pool of leukemia stem cells may be quite heterogeneous in nature, with only the first of several genes contributing to leukemogenesis present in all leukemia stem cells.
Treatment with the FLT3 inhibitor CEP-701 blocked or significantly reduced engraftment in the NOD-SCID mice. In light of the fact that CEP-701 did not significantly affect engraftment of normal human stem cells, the inhibition of engraftment of an FLT3/ITD leukemia specimen strongly suggests that the mutation is not only in the leukemic stem cell, but also that the leukemic stem cell is highly dependent on signaling from the mutant receptor for survival. In one specimen engraftment was completely blocked, whereas in another engraftment was only reduced or delayed. This may reflect heterogeneity between AML samples, but probably highlights the lack of efficacy of FLT3 inhibition as monotherapy—as clinical trials with these agents suggest. It may be that the best use of FLT3 inhibitors will be as an “adjuvant” to chemotherapy, in which FLT3 inhibitors are used to suppress or kill residual leukemia stem cells still present after induction of remission.
Prepublished online as Blood First Edition Paper, March 29, 2005; DOI 10.1182/blood-2004-05-1902.
Supported by grants from the National Cancer Institute (CA70 970, CA90 668, CA100 632 [D.S.]; K23 CA81 262-01A1 [B.D.S.]; CA095 600 [M.L.]), Leukemia and Lymphoma Society (D.S.), Sidney Kimmel Foundation (M.L.), and the American Society of Clinical Oncology (M.L.). D.S. is the Douglas Kroll Research Foundation Translational Researcher of the Leukemia and Lymphoma Society and the recipient of the Kyle Hadock Professorship of Oncology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Drs Bruce Ruggieri and Susan Bolin-Jones of Cephalon Inc for technical assistance with CEP-701, and Dr Richard Jones of Johns Hopkins Oncology for helpful discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal