Abstract
Chemotherapy- or radiation-induced myelosuppression results in apoptosis of cycling hematopoietic cells and induces regression of bone marrow (BM) sinusoidal vessels. Moreover, timely regeneration of BM neovessels is essential for reconstitution of hematopoiesis. However, the identity of angiogenic factors that support reconstitution of BM's vasculature is unknown. Here, we demonstrate that angiopoietin/tyrosine kinase with immunoglobulin and epidermal growth factor homology domains-2 (Tie2) signaling contributes to the assembly and remodeling of BM neovessels after myelosuppression. Using transgenic mice where the Tie2 promoter drives the reporter LacZ gene (Tie2-LacZ), we demonstrate that at steady state, there was minimal expression of Tie2 in the BM vasculature. However, after 5-fluorouracil (5-FU) treatment, there was a rapid increase in plasma vascular endothelial growth factor A (VEGF-A) levels and expansion of Tie2-positive neovessels. Inhibition of Tie2 resulted in impaired neoangiogenesis, leading to a delay in hematopoietic recovery. Conversely, angiopoietin-1 (Ang-1) stimulated hematopoiesis both in wild-type and thrombopoietin-deficient mice. In addition, Ang-1 shortened the duration of chemotherapy-induced neutropenia in wild-type mice. Exogenous VEGF-A and Ang-1 stimulated Tie2 expression in the BM vasculature. These data suggest that VEGF-A–induced up-regulation of Tie2 expression on the regenerating vasculature after BM suppression supports the assembly of sinusoidal endothelial cells, thereby promoting reconstitution of hematopoiesis. Angiopoietins may be clinically useful to accelerate hemangiogenic recovery after myelosuppression.
Introduction
Within the bone marrow (BM), hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) reside in defined microenvironments or “niches,” where they receive molecular and cellular instructions for proliferation, differentiation, and mobilization to the circulation.1-7 The majority of HSCs are believed to be localized to the periendosteal region referred to as the osteoblastic niche.1-6 We have shown that another dynamic BM microenvironment demarcated by the sinusoidal BM endothelial cells (BMECs), identified as the vascular niche,8-11 supports differentiation of HPCs and their mobilization to the circulation.12-19
Through activation of vascular endothelial growth factor receptor-1 (VEGFR1, fms-like tyrosine kinase-1 [Flt-1]), angiogenic factors induce expression of metalloproteinase-9 (MMP-9) and the release of bioactive soluble Kit-ligand (sKitL),20 which in turn induces the cycling of hibernating VEGFR1+ HSCs and HPCs,1 translocating them to the BM's vascular niche.8 We have previously shown that chemokine-mediated translocation of HPCs to the vascular niche in thrombopoietin knock-out (Thpo–/–) mice restores thrombopoiesis. Indeed, hematopoietic-active chemokines, including stromal derived factor-1 (SDF-1) and fibroblast growth factor-4 (FGF-4), were able to support platelet production in thrombopoietin- and thrombopoietin receptor–(Mpl–/–) deficient mice, suggesting that the BM vascular niche is not only a conduit for mobilization, but also a cellular platform for the differentiation of HPCs and HSCs.8,21,22
BM also contains a population of CD133+VEGFR2+23-25 vascular progenitors that has been shown to contribute to neoangiogenesis.26-31 Subsets of proangiogenic VEGFR1+ hematopoietic cells facilitate differentiation of vascular progenitors and their assembly into neovessels.32 However, the precise identity of angiogenic pathways that are involved in functional assembly and remodeling of the BM neovessels is unknown. Since (tyrosine kinase with immunoglobulin and epidermal growth factor homology domains-2) (Tie2) is expressed on hemangiogenic stem cells33-36 and angiopoietins have been shown to support the remodeling of neovessels,37,38 we hypothesized that expression of Tie2 may also contribute to the interplay between regenerating BM neovessels and hematopoietic progenitors, leading to the rapid reconstitution of hematopoiesis after myelosuppression.
Tie2 (tek) is a tyrosine kinase receptor that is primarily expressed on vascular endothelium and a small subset of hematopoietic cells.39-41 It has been demonstrated that Tie2-positive cells in the aorta-gonad-mesonephros (AGM) region contain hemangioblasts capable of differentiating into both hematopoietic and endothelial lineages. Targeted deletion of the Tie2 gene results in early embryonic lethality because of vascular abnormalities.40 Taken together, these findings suggest that the angiopoietin/Tie2 signaling plays a crucial role in the remodeling and maturation/stabilization of the embryonic vasculature. However, there is an increasing body of evidence for the importance of angiopoietin/Tie2 signaling in adult vasculature maintenance and repair as well as adult hematopoiesis. Recently, activation of Tie2 has been shown to play a role in the retention and quiescence of HSCs within the BM's osteoblastic niche.42
Chemotherapy and irradiation induce both apoptosis of hematopoietic cells and regression of the BM's sinusoidal neovessels. It has long been known that the function and physical integrity of the BM endothelial cells are impaired after chemotherapy.43-46 However, the identification of angiogenic factors, such as VEGF-A,47-49 has opened up the door for studying the mechanisms for the regeneration and reconstruction of BM microvasculature.7,8 We have observed that angiogenic reconstitution occurs concomitantly with hematopoietic reconstitution, and at this stage their interdependence is unclear.8 However, preliminary studies show that destabilizing the BM vascular niche via inhibition of vascular endothelial (VE)–cadherin homotypic endothelial-cell interactions greatly impairs hematopoietic reconstitution.8 In conclusion, these data suggest that the proper assembly of sinusoidal endothelial cells directly contributes to the differentiation of hematopoietic cells and reconstitution of hematopoiesis in addition to providing a conduit for the mobilization of hematopoietic stem cells. However, the identity of hemangiogenic factors and the mechanism by which these factors contribute to the reconstruction of functional sinusoidal vessels after chemotherapy and irradiation are not fully known.
Here, we describe a role for Tie2 in the functional regeneration of the BM vascular niche after chemotherapy in adult mice. Our results support the hypothesis that Tie2 is an important maintenance and repair receptor in adult microvasculature as well as the notion that reconstitution of the BM vascular niche is the decisive step in hematopoietic regeneration after chemotherapy.
Materials and methods
Administration of 5-FU, cyclophosphamide, and adenoviral vectors
The sex-matched, 6- to 10-week-old FVB/NJ, FVB/N-TG(Tie2-lacZ)182Sato/J, Thpo–/–, and C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) used in these experiments were maintained in air-filtered units. Animal experiments were performed with the authorization of the institutional review board and Animal Care and Use Committee of Weill Medical College of Cornell University. Some experimental animals received adenoviral vectors expressing angiopoietin-1 (AdAng1), VEGF-A (AdVEGF-A), the soluble decoy Tie2 receptor (AdTie2Fc), kindly provided by Regeneron Pharmaceuticals (Tarrytown, NY), or empty vector (AdNull) in 100 μL phosphate-buffered saline (PBS), administered by single tail-vein injection on day 0. AdVEGF-A was used at a 1.5 × 108 plaque-forming units (pfu); the other vectors were used at a 109 pfu.
For myelosuppression experiments, mice received a single tail-vein injection of 5-fluorouracil (5-FU, 250 mg per kg body weight, in 100 μL) or cyclophosphamide (CTX, 450 mg per kg body weight, in 100 μL). After 24 hours, mice were injected with a single intravenous dose of AdTie2Fc, AdAng1, and AdNull as indicated.
Total-body irradiation
There were 4 mice irradiated with a dose of 4.5 Gy from a 137Cs γ-ray source at a dose rate of approximately 0.90 Gy/min. At days 4, 7, and 10 after irradiation, 2 mice were killed and their femurs obtained for bone marrow histology.
Peripheral-blood analysis
Retro-orbital blood was collected on indicated days after chemotherapy with capillary pipettes (Fisher Scientific, Hampton, NH). Differential blood counts were obtained using an automated Bayer Advia 120 MultiSpecies Hematology Analyzer (Bayer HealthCare, Tarrytown, NY) with multispecies software (Bayer, Tarrytown, NY). Peripheral blood counts are depicted as average values plus or minus SEM.
VEGF-A ELISA
Platelet-poor plasma samples were obtained by retro-orbital or cardiac puncture of 1 mL peripheral blood. Plasma was taken after a first centrifugation step of 10 minutes at 1000g. The supernatant was centrifuged for another 30 minutes at 18 000g to obtain platelet-poor plasma. Samples were immediately frozen down at –20°C if they were to be measured later. In order to measure plasma levels of VEGF-A, we used the VEGF enzyme-linked immunosorbent assay (ELISA) kit according to the instructions of the manufacturer (Quantikine Immunoassays by R&D Systems, Minneapolis, MN).
Bone marrow histology and X-Gal staining
FVB and FVB/Tie2LacZ mice (n = 16, each group) were injected intravenously with 250 mg/kg 5-FU. Mice of each group (n = 3) were killed on the specified days after myelosuppression. Femurs were harvested, washed in ice-cold PBS, and fixed in a 2% paraformaldehyde solution (PFA) in PBS at 4°C for 4 hours. After fixation, tissue was washed briefly in PBS and allowed to react with the chromogenic substrate (β-galactosidase [β-Gal] staining set; Roche Diagnostics, Indianapolis, IN). After decalcification (Protocol Decalcifier A; Fisher Diagnostics, Middletown, VA), the tissue was embedded in paraffin and sectioned (9 μm). A counterstain of nuclear fast red (NFR) was used. Selected tissues were processed for hematoxylin and eosin (H&E) staining.
Bone marrow immunohistochemistry
Femurs were harvested, fixed, paraffin-embedded, and sectioned as described in the previous section. For immunohistochemistry, slides were deparaffinized and rehydrated. After antigen retrieval (DAKO Target Retrieval Solution; DAKOCytomation, Carpinteria, CA) and blocking endogenous peroxidases as well as unspecific binding, slides were incubated with a primary rat anti–mouse panendothelial cell antigen monoclonal antibody (MECA-32) antibody (Pharmingen, San Diego, CA). Using a secondary anti–rat immunoglobulin G (IgG)–horseradish peroxidase (HRP) antibody (Jackson ImmunoResearch Laboratories, West Grove, Pa), antibody binding was made visible with DAKO Liquid DAB (DAKOCytomation). We counterstained with hematoxylin.
Histologic image acquisition and analysis
Images of BM sections were taken with a digital camera (Retiga EX; Qimaging, Burnaby, BC) mounted on an Olympus BX51 microscope (Olympus America, Melville, NY). UPLFLN 10X/0.25, 20X/0.50, and 40X/0.75 objective lenses were used. Images were recorded using Mac Qcapture Acquisition software (Version 2.68.6; Qimaging).
Digital recordings of the tissue slides were taken and analyzed for differential chromogen content.50 Briefly, Adobe Photoshop software (Version 7.0; Adobe Systems, San Jose, CA) was used for color selection. Using Photoshop-based image analysis as described previously, we were able to quantify the blue chromogen by counting the number of blue pixels as a measure of spatial distribution.
Transgenic mice and mouse strains
The derivation of Thpo–/– (C57BL/6 background) has been described previously.51,52 C57BL/6 mice and FVB/N-Tg(TIE2-lacZ)182Sato/J mice were purchased from Jackson Laboratories. FVB/NJ mice (Jackson Laboratories) were used as controls.
FVB/N-Tg(TIE2-lacZ)182Sato/J transgenic mice carry a β-gal reporter gene under the control of the murine Tie2 promoter.
Statistical analysis
Results were statistically analyzed using a 2-tailed Student t test. The results are expressed as mean value plus or minus standard error of the mean (SEM). P less than .05 was considered significant.
Results
Myelosuppression results in the regression of sinusoidal vessels followed by rapid regeneration of the vascular niche
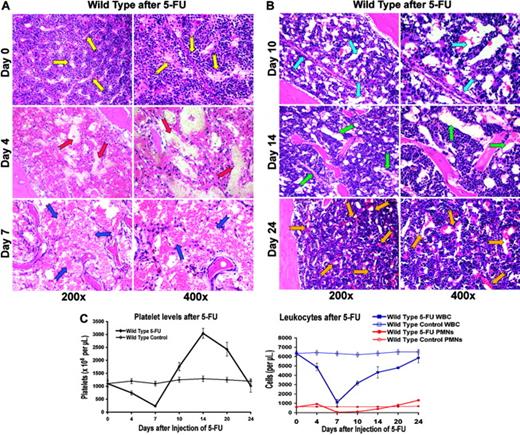
5-FU–induced myelosuppression not only led to the depletion of the hematopoietic cells, but also induced regression of the majority of BM sinusoidal vasculature. This resulted in a profound hypocellularity and disruption of BM cytoarchitecture with diffuse intracavitary hemorrhage and a concomitant peripheral pancytopenia (Figure 1A). Within 7 to 10 days after BM suppression, there was a rapid proliferation of hematopoietic cells and simultaneous regeneration of sinusoidal vasculature in the BM (Figure 1B).
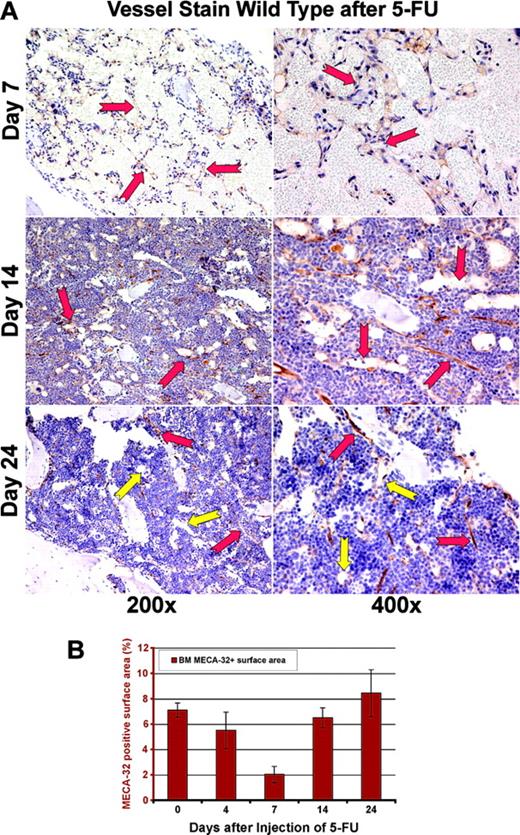
In order to obtain a precise assessment of the neovessels, BM sections were stained with an endothelial-specific marker, MECA-32 (Figure 2A). By day 7 after myelosuppression, there was a significant decrease in MECA-32–positive, red-blood-cell (RBC)–loaded sinusoids. The quantification of MECA-32–positive cells provided further evidence that BM's vascular surface area significantly decreases after myelosuppression, plummeting to 2% of the total hematopoietic BM surface area (Figure 2B). After day 7, there was a time-dependent regeneration of the neovasculature as reflected by a profound increase in the vascular surface area demarcated by the Meca-32–positive cells (Figure 2B, P < .05 on day 7). This process was associated with a hyperproliferative state, resulting in a transient rebound thrombocytosis 10 to 21 days after induction of myelosuppression (Figure 1C). The rebound effect was readily observed in platelet levels with a 2-fold increase (2800 × 109/L) over baseline 14 days after myelosuppression (n = 10). White blood counts revealed no overt rebound leukocytosis, but showed a more prolonged recovery, reaching baseline levels only after 4 weeks of therapy (n = 10). These results suggest that myelosuppression results in vascular regression followed by regeneration of BM neovessels and therefore provides an instructive model to identify the angiogenic factors that contribute to the reconstruction of the BM's vascular niche.
5-FU–induced regression of hemangiogenesis in wild-type mice. (A-B) Chemotherapy-mediated disruption of the BM vascular niche is followed by regeneration of neovessels. On days 4, 7, 10, 14, and 24 after injection, 3 mice were killed, and their femurs were collected and processed for histologic analysis. Paraffin sections of the BM were stained by H&E. Representative sections at steady state and after 4, 7, 10, 14, and 21 days are shown. Arrows point to the BM vascular sinusoids. At baseline, there are small and narrow sinusoids, decorated by hematopoietic cells (yellow arrows). On day 4, there is a complete disruption of vasculature with plasma leakage (red arrows). On day 7, marrow hypocellularity and regression of BM vasculature with areas of hemorrhage are readily evident (blue arrows). Simultaneous with the regeneration of BM vasculature, which manifests itself in the formation of early, dilated nascent neosinusoids (light blue arrows), there is a rapid increase in the platelet counts. By day 14, the newly reconstructed sinusoids undergo remodeling (green arrows), a process that is completed by day 24 after myelosuppression (orange arrows). (C) Recovery of hematopoiesis parallels the histologic changes observed after myelosuppression. Wild-type mice were intravenously injected with one single dose of 250 mg/kg 5-FU (n = 16). Complete blood counts, including a differential white-blood-cell (WBC) count, were obtained using the Advia 120 Multi-Species Hematology Analyzer after a single intravenous injection with 5-FU. (Left) After initial pancytopenia, there is a rebound thrombocytosis up to 3.0 × 106/μL that lasts for several days (n = 16, P < .01 at day 14, 250 mg/kg 5-FU) before platelet counts revert to normal levels around 4 weeks after injection. (Right) Similarly, white blood cells reach their nadir around day 7 to revert more slowly to normal 4 weeks after myelosuppression without displaying rebound leukocytosis. Polymorphonuclear neutrophils (PMNs) take the same course as total white blood cells with neutropenia lasting about 18 days (average ± SE).
5-FU–induced regression of hemangiogenesis in wild-type mice. (A-B) Chemotherapy-mediated disruption of the BM vascular niche is followed by regeneration of neovessels. On days 4, 7, 10, 14, and 24 after injection, 3 mice were killed, and their femurs were collected and processed for histologic analysis. Paraffin sections of the BM were stained by H&E. Representative sections at steady state and after 4, 7, 10, 14, and 21 days are shown. Arrows point to the BM vascular sinusoids. At baseline, there are small and narrow sinusoids, decorated by hematopoietic cells (yellow arrows). On day 4, there is a complete disruption of vasculature with plasma leakage (red arrows). On day 7, marrow hypocellularity and regression of BM vasculature with areas of hemorrhage are readily evident (blue arrows). Simultaneous with the regeneration of BM vasculature, which manifests itself in the formation of early, dilated nascent neosinusoids (light blue arrows), there is a rapid increase in the platelet counts. By day 14, the newly reconstructed sinusoids undergo remodeling (green arrows), a process that is completed by day 24 after myelosuppression (orange arrows). (C) Recovery of hematopoiesis parallels the histologic changes observed after myelosuppression. Wild-type mice were intravenously injected with one single dose of 250 mg/kg 5-FU (n = 16). Complete blood counts, including a differential white-blood-cell (WBC) count, were obtained using the Advia 120 Multi-Species Hematology Analyzer after a single intravenous injection with 5-FU. (Left) After initial pancytopenia, there is a rebound thrombocytosis up to 3.0 × 106/μL that lasts for several days (n = 16, P < .01 at day 14, 250 mg/kg 5-FU) before platelet counts revert to normal levels around 4 weeks after injection. (Right) Similarly, white blood cells reach their nadir around day 7 to revert more slowly to normal 4 weeks after myelosuppression without displaying rebound leukocytosis. Polymorphonuclear neutrophils (PMNs) take the same course as total white blood cells with neutropenia lasting about 18 days (average ± SE).
Regression of MECA-32–positive vessels in BM after 5-FU. (A) Regression and regeneration of marrow's vasculature after myelosuppression. In order to quantitate the degree of vascular regression after myelosuppression with 5-FU, the number of sinusoidal vessels was determined using the panendothelial marker MECA-32. At day 7 after myelosuppression, there is a profound regression and disruption of the BM vasculature with dilated and leaky vessels, displaying local disintegration (red arrows). Remarkably, by day 14 there is a gradual regeneration of large nascent vessels (red arrows at days 14 and 24). By day 24 after 5-FU myelosuppression, the large dilated nascent neovessels remodel into small functional fully RBC-loaded vessels decorated by hematopoietic cells, including megakaryocytes (yellow arrows). (B) Quantification of BM vasculature by determination of MECA-32–positive neovessel surface area. MECA-32/hematoxylin-stained BM sections were obtained at different time points after injection of 5-FU. The MECA-32–positive BM surface area was measured by quantification of the brown chroma pixels compared with the total number of pixels in the digital image as described in “Materials and methods.” At steady-state conditions, the surface area of MECA-32–positive cells as normalized to the percentage of total BM hematopoietic cell-surface area is about 7%. Myelosuppression diminishes the surface area index of MECA-32 positive to 2%, reminiscent of the vasculotoxic effects of 5-FU. All results are shown as mean ± standard deviation (n = 4, P < .01 at day 7).
Regression of MECA-32–positive vessels in BM after 5-FU. (A) Regression and regeneration of marrow's vasculature after myelosuppression. In order to quantitate the degree of vascular regression after myelosuppression with 5-FU, the number of sinusoidal vessels was determined using the panendothelial marker MECA-32. At day 7 after myelosuppression, there is a profound regression and disruption of the BM vasculature with dilated and leaky vessels, displaying local disintegration (red arrows). Remarkably, by day 14 there is a gradual regeneration of large nascent vessels (red arrows at days 14 and 24). By day 24 after 5-FU myelosuppression, the large dilated nascent neovessels remodel into small functional fully RBC-loaded vessels decorated by hematopoietic cells, including megakaryocytes (yellow arrows). (B) Quantification of BM vasculature by determination of MECA-32–positive neovessel surface area. MECA-32/hematoxylin-stained BM sections were obtained at different time points after injection of 5-FU. The MECA-32–positive BM surface area was measured by quantification of the brown chroma pixels compared with the total number of pixels in the digital image as described in “Materials and methods.” At steady-state conditions, the surface area of MECA-32–positive cells as normalized to the percentage of total BM hematopoietic cell-surface area is about 7%. Myelosuppression diminishes the surface area index of MECA-32 positive to 2%, reminiscent of the vasculotoxic effects of 5-FU. All results are shown as mean ± standard deviation (n = 4, P < .01 at day 7).
Tie2 expression is up-regulated on the regenerating BM endothelial cells after myelosuppression
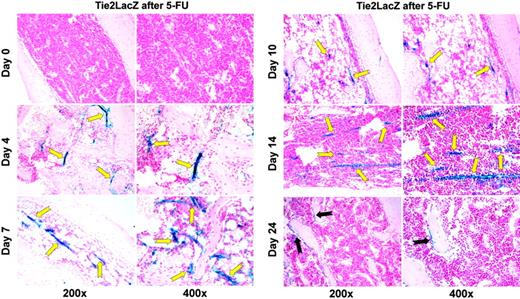
Tie2/angiopoietin signaling is known to contribute to the remodeling and stabilization of neovessels. Therefore, we hypothesized that activation of Tie2 plays a role in the regeneration of BM sinusoidal vessels and restoration of hematopoiesis after myelosuppression. To this end, in order to track the cellular itinerary of regenerating vascular cells expressing Tie2, we took advantage of transgenic mice, where the Tie2 promoter drives the expression of the reporter gene LacZ (β-gal).
Surprisingly, under steady-state conditions, only a few LacZpositive cells could be detected in the proximity of the endosteal/osteoblastic region on both ends of the femur bone (compare with Figure 3B, day 24). Conversely, under these steady-state conditions, vascular endothelial expression of Tie2 was undetectable by histochemistry (Figure 3A). However, 4 to 7 days after a myelosuppressive dose of 5-FU, there was a significant up-regulation of the expression of LacZ in the regenerating BM sinusoidal vessels (Figure 3A). The expression of Tie2 on the regenerating neovasculature reached a maximum at day 10 and reverted to steady-state levels after 24 days, the time at which the majority of the neovessels was regenerated (Figure 3B). Similar results were observed when wild-type mice were treated with a myelosuppressive dose of cyclophosphamide or received a sublethal dose of 4.5 Gy whole-body irradiation (n = 4 animals each, data not shown). These results indicate that generation of Tie2-positive vascular cells after myelosuppression may support reconstruction and functional remodeling of the BM vascular niche.
Disruption of Tie2 signaling inhibits hematopoietic recovery
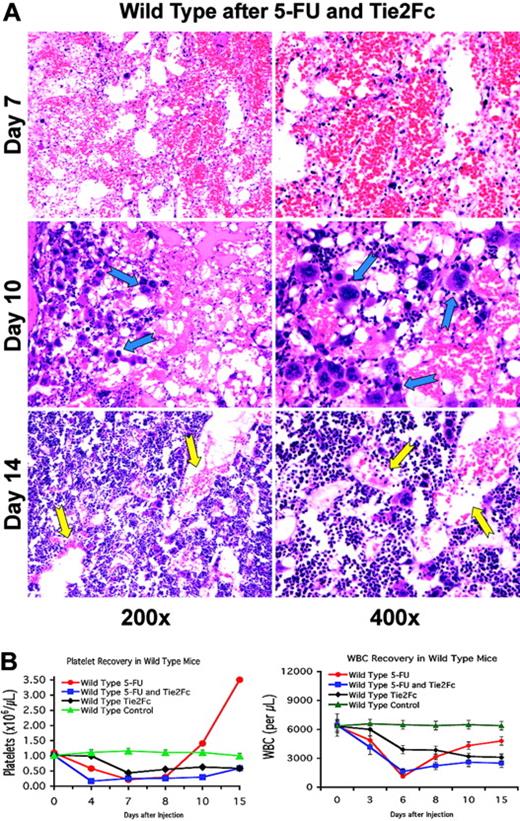
To determine the role of angiopoietins in the regeneration of the BM's vascular niche, the soluble decoy receptor Tie2-Fc was used to trap angiopoietins, thereby inhibiting Tie2 signaling during BM regeneration. To this end, 5-FU–treated wild-type mice were infected with the adenoviral vector that expresses the extracellular domain of Tie2, fused in frame with an IgG-Fc fragment. Intravenous injection of adenoviral vectors allows continuous expression of the recombinant protein for approximately 10 days before adenoviral transduced cells are immunologically neutralized. Wild-type mice that were treated with AdTie2-Fc alone showed thrombocytopenia, which reached nadir levels at day 7 after injection, plummeting to approximately 60% compared with untreated mice (n = 6; Figure 4B). Injection of AdTie2-Fc immediately after 5-FU administration inhibited both vascular reconstitution and platelet recovery. Soluble Tie2-Fc resulted in an early platelet nadir (day 4 as opposed to day 7) and prolonged thrombocytopenia without rebound thrombocytosis (n = 6; Figure 4B), suggesting that the addition of Tie2-Fc effectively inhibited platelet production.
Indeed, the resulting platelet decline is similar to the drop in platelet counts in thrombopoietin receptor knock-out mice (c-Mpl–/–). Therefore, the rapid decrease in platelets observed in the mice that were treated with 5-FU and Tie2Fc is unlikely to be due to an additional effect on platelet clearance. Introduction of Tie2Fc also delayed white-blood-cell recovery, with a failure to regenerate peripheral leukocytes compared with the control animals (n = 6, Figure 4B). BM histology revealed that Tie2-Fc, given after myelosuppression, inhibited regeneration of the BM sinusoidal neovessels (Figure 4A). During the prolonged pancytopenic phase, there was a paradoxic accumulation of megakaryocytes in the BM. As we have previously shown,8 this phenomenon may be due to disruption of the vascular niche and inability of megakaryocytes to interact with sinusoidal endothelial cells, which is a critical step in platelet formation. This will result in diminished thrombopoiesis and accumulation of megakaryocytes in the vicinity of the sinusoidal endothelial cells. These data suggest that Tie2 signaling contributes to the proper assembly and functional remodeling of sinusoidal endothelial cells, a process that contributes to the reconstitution of hematopoiesis.
Vascular Tie2 expression is increased in a time-dependent manner after myelosuppression.Tie2-LacZ mice received a single tail-vein injection of 250 mg/kg 5-FU. Groups of mice were killed before the injection and 4, 7, 10, 14, and 24 days thereafter. Femurs were collected and prepared for histologic analysis by an X-Gal staining procedure. Paraffin sections were counterstained with NFR. Representative LacZ-NFR–stained sections at steady state and after 4, 7, 10, 14, and 24 days are shown. Regenerating neovessels consist of LacZ-positive endothelial cells, which are easily identifiable by their blue stain (yellow arrows). On day 4 after injection, these intensely LacZ-stained neovessels emerge at different sizes and shapes throughout the reconstituting BM (n = 15). The × 400 magnification clearly demonstrates that perivascular hematopoietic cells are not stained, but that LacZ positivity is limited to endothelial cells and their surroundings due to limited diffusion of the blue dye. By day 24, the only LacZ-expressing cells in the femur can be found in the epiphyseal region, consisting of few spindle-shaped endosteal cells (black arrows).
Vascular Tie2 expression is increased in a time-dependent manner after myelosuppression.Tie2-LacZ mice received a single tail-vein injection of 250 mg/kg 5-FU. Groups of mice were killed before the injection and 4, 7, 10, 14, and 24 days thereafter. Femurs were collected and prepared for histologic analysis by an X-Gal staining procedure. Paraffin sections were counterstained with NFR. Representative LacZ-NFR–stained sections at steady state and after 4, 7, 10, 14, and 24 days are shown. Regenerating neovessels consist of LacZ-positive endothelial cells, which are easily identifiable by their blue stain (yellow arrows). On day 4 after injection, these intensely LacZ-stained neovessels emerge at different sizes and shapes throughout the reconstituting BM (n = 15). The × 400 magnification clearly demonstrates that perivascular hematopoietic cells are not stained, but that LacZ positivity is limited to endothelial cells and their surroundings due to limited diffusion of the blue dye. By day 24, the only LacZ-expressing cells in the femur can be found in the epiphyseal region, consisting of few spindle-shaped endosteal cells (black arrows).
Angiopoietin-1 stimulates platelet production independent of thrombopoietin and shortens chemotherapy-induced neutropenia
If Tie2 signaling is critical for the functional assembly of the BM neovessels, then overexpression of Ang-1 may accelerate the functional assembly of sinusoidal vessels and hematopoietic reconstitution. To test this hypothesis, we delivered Ang-1 by using an adenoviral vector expressing Ang-1 (AdAng-1). Intravenous delivery of AdAng-1 is followed by localization of the adenoviral vector to the liver. This results in plasma elevation of Ang-1 for approximately 10 days. Thereafter, immunologic clearance of the adenoviral vectors causes a decline in Ang-1 levels.
Injection of AdAng-1 resulted in a delayed, but sustained, increase in leukocytes and platelets in wild-type mice (n = 6, Figure 5A). The initial discrete cytopenia and the delay in the described effect may be due to remodeling of the sinusoidal endothelial cells or up-regulation of other cytokines that may promote hematopoiesis. Indeed, one mechanism by which Ang-1 could induce thrombocytosis may be through up-regulation of thrombopoietin production.
To test this hypothesis, we took advantage of Thpo-deficient mice (Thpo–/–), which have a profound defect in platelet production, but no apparent hemostatic defect. The number of platelets in the peripheral blood and megakaryocytes in the BM are decreased by approximately 80% in Thpo–/– mice.8 Remarkably, Ang-1 induced a delayed augmentation of platelet production in Thpo–/– mice, increasing platelets from approximately 280 × 109/L to more than 700 × 109/L (n = 6, Figure 5A). As in wild-type mice, a delayed response to AdAng-1 was observed in Thpo–/– mice.
Remarkably, AdAng-1 also shortened chemotherapy-induced neutropenia when given one day after 5-FU. Similar to granulocyte colony-stimulating factor (G-CSF), the nadir of aplasia was not affected quantitatively or temporally, but the duration of neutropenia was significantly shorter in mice treated with both 5-FU and Ang-1 compared with 5-FU alone (Figure 5B).
VEGF-A is released after myelosuppression and stimulates Tie2 expression in the BM vascular niche
Because we have previously shown that angiogenic cytokines such as VEGF-A can stimulate hematopoiesis,8 we hypothesized that the Ang-1/Tie2 signaling pathway may contribute to the BM's revascularization by remodeling of regenerating dilated neovessels observed during days 7 to 14 of BM regeneration. This might also explain the delayed contribution of Ang-1 in inducing hematopoiesis. Indeed, we found that the introduction of AdAng-1 resulted in a consistent increase of plasma VEGF-A levels on day 10 after injection with a 100-fold increase up to 200 pg/mL (n = 3, Figure 6A, P < .001 on day 10). Myelosuppressive doses of 5-FU were followed by an increase in plasma VEGF-A levels as well. Similarly, the injection of AdVEGF-A was followed by an intense overexpression of LacZ in the Tie2/LacZ knock-in mice (n = 3, Figure 6B).
Angiopoietin trapping delays hemangiogenic recovery. (A) Disruption of the angiopoietin signal disturbs BM vascular remodeling after chemotherapy. Wild-type mice received a single tail-vein injection of AdTie2-Fc one day after a single tail-vein dose of 250 mg/kg 5-FU (n = 12). At the indicated time points, 3 mice were killed, and BM was obtained for histologic analysis. Hematoxylin and eosin–stained paraffin sections highlight vascular changes during hemangiogenic reconstitution. These histologic studies demonstrate that the inhibition of angiopoietin signaling after myelosuppression results in impaired assembly of neovessels with a delayed reconstruction of the BM microarchitecture. On day 10, there is an accumulation of polyploid megakaryocytes entrapped within the BM, dissociated from the sinusoidal endothelial cells, resulting in thrombocytopenia (blue arrows). In addition, on day 14, the newly formed sinusoids were still dilated and irregular, displaying features of unstabilized neovessels (yellow arrows). (B) Introduction of Tie2-Fc diminishes peripheral blood-cell concentration both at steady state and after myelosuppression. (Left) AdTie2-Fc results in a decrease of platelet levels by 60% to that observed at steady state (n = 6, P < .01 on day 7). Compared with control mice, AdTie2-Fc inhibits rebound thrombocytosis and delays thrombopoietic recovery after myelosuppression (n = 6, P < .02 at day 15). All data are expressed as the mean ± SEM. (Right) Similar changes are seen in the white blood cells (n = 6).
Angiopoietin trapping delays hemangiogenic recovery. (A) Disruption of the angiopoietin signal disturbs BM vascular remodeling after chemotherapy. Wild-type mice received a single tail-vein injection of AdTie2-Fc one day after a single tail-vein dose of 250 mg/kg 5-FU (n = 12). At the indicated time points, 3 mice were killed, and BM was obtained for histologic analysis. Hematoxylin and eosin–stained paraffin sections highlight vascular changes during hemangiogenic reconstitution. These histologic studies demonstrate that the inhibition of angiopoietin signaling after myelosuppression results in impaired assembly of neovessels with a delayed reconstruction of the BM microarchitecture. On day 10, there is an accumulation of polyploid megakaryocytes entrapped within the BM, dissociated from the sinusoidal endothelial cells, resulting in thrombocytopenia (blue arrows). In addition, on day 14, the newly formed sinusoids were still dilated and irregular, displaying features of unstabilized neovessels (yellow arrows). (B) Introduction of Tie2-Fc diminishes peripheral blood-cell concentration both at steady state and after myelosuppression. (Left) AdTie2-Fc results in a decrease of platelet levels by 60% to that observed at steady state (n = 6, P < .01 on day 7). Compared with control mice, AdTie2-Fc inhibits rebound thrombocytosis and delays thrombopoietic recovery after myelosuppression (n = 6, P < .02 at day 15). All data are expressed as the mean ± SEM. (Right) Similar changes are seen in the white blood cells (n = 6).
Consistent with previously published papers, our data suggest that VEGF-A is a potent inducer of Tie2 expression53,54 on the regenerating marrow vessels.
Therefore, the release of VEGF-A after myelosuppression may not only initiate the assembly of dilated sinusoidal neovessels detected 7 to 10 days after myelosuppression, but also activate the expression of Tie2, thereby supporting the remodeling of dilated nascent sinusoidal neovessels into functional stabilized BM vasculature. In this regard, VEGF-A and Ang-1 are the angiogenic hubs, which link myelosuppression to hematopoietic regeneration.
Alterations of the levels of Tie2 expression precede analogous changes in platelet levels
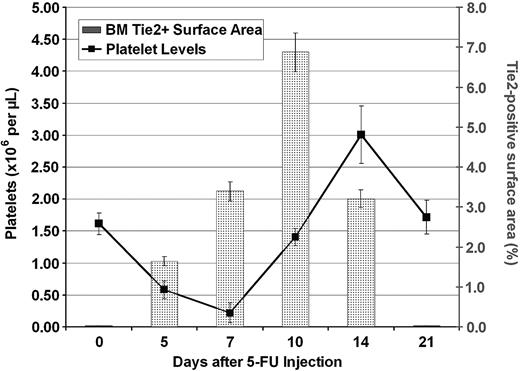
In order to identify the mechanism by which Tie2-mediated vasculogenesis supports thrombopoiesis, we sought to quantify the number and surface area of regenerating Tie2-positive neovasculature in the BM cavity at different time points during hematopoietic reconstitution after myelosuppression. The surface area of lacZ-expressing neovessels was determined by quantifying the chroma (blue) pixels from the digitally acquired image. The overall surface area occupied by the neovessels was normalized by calculating the percentage of the surface area of blue pixels relative to the overall surface area of BM demarcated by hematopoietic cells, stromal cells, and blood vessels.50 The Tie2-positive vascular surface area index reached a maximum at 14 days after induction of BM suppression. The neovessel surface area demarcated by LacZ/Tie2-positive endothelial cells increased rapidly from 0.02% to 6.8% of total BM surface area (Figure 7). This rapid expansion of Tie2-positive vasculature was followed by a rapid burst of platelet production. Indeed, the maximum surface area of Tie2-positive neovessels preceded platelet recovery. Similarly, the rebound overcompensation of thrombopoiesis was preceded by vascular regeneration by 4 days. In addition, down-regulation of Tie2 expression and stabilization of the vascular surface area was followed closely by the return of platelets to steady state. These data introduce the novel notion that the surface area of the fully remodeled and functional BM vascular niche correlates with the rate and level of thrombopoiesis.
Discussion
The BM sinusoidal vessels define a dynamic microenvironment, which supports the proliferation and differentiation of the hematopoietic cells.8 Here, we show that myelosuppression-induced up-regulation of angiogenic factors, such as VEGF-A and Ang-1, supports the emergence of Tie2-positive BM neovasculature, thereby contributing to the reconstitution of hematopoiesis. We demonstrate that under normal conditions, Tie2 is minimally expressed on BM sinusoidal endothelial cells. However, myelosuppression not only induces expression of VEGF-A, but is also followed by the regeneration of Tie2-positive neovessels in the BM. Expression of Tie2 was upregulated predominantly in capillaries, and to a smaller extent in the sinusoidal vessels. Remarkably, Ang-1 stimulates hematopoiesis, including thrombopoiesis in Thpo–/– mice, suggesting that remodeling of the vascular niche directly supports thrombopoiesis. Treatment of 5-FU–myelosuppressed animals with AdAng-1 enhanced late phases of platelet recovery and shortened neutropenia in these animals. Collectively, our data support the concept that angiogenic factors through reconstruction of the BM vascular niche generate a permissive microenvironment for the maturation of hematopoietic progenitors and reconstitution of hematopoiesis as well as a conduit for mobilization to the peripheral circulation.
Myelosuppression causes rapid regression of the BM's sinusoidal vasculature. Regeneration of sinusoidal vessels is a highly orchestrated process and requires the generation of new endothelial cells that assemble into dilated irregular sinusoidal nascent neovessels. By day 24 after BM suppression, these neovessels remodel into the typical functional RBC-loaded sinusoidal BM vasculature. Functional assembly of the regenerating vessels is most likely driven by multiple angiogenic signaling pathways. VEGF-A through interaction with VEGFR2 and VEGFR1 supports the initial assembly of blood neovessels.49 However, reorganization and remodeling of neovessels are mediated through platelet-derived growth factor (PDGF)/PDGF receptor (PDGF-R), angiopoietin/Tie2, and Edg/Sphingosine-1-phosphate (S1P) pathways, which support the formation of fully functional stabilized neovessels.49,55-57 PDGF/PDGF-receptor and Edg/S1P (endothelial differentiation G-protein–coupled receptor/sphingosine-1-phosphate) pathways58,59 seem to be critical for stabilization of neovessels through recruitment of smooth muscle cells.56 The fact that sinusoidal BM neovessels are stabilized mostly by surrounding hematopoietic cells and not by organized pericytic mural cells44 led us to hypothesize that angiopoietin/Tie2 may play a role in the functional stabilization of BM sinusoidal neovasculature. This notion was corroborated by our finding that myelosuppression results in the emergence of Tie2-positive neovessels. Interestingly, Tie2 expression reverts to an undetectable level when steady-state hematopoiesis is reestablished after myelosuppression. These data indicate that Tie2 plays a permissive role in supporting stress hematopoiesis.
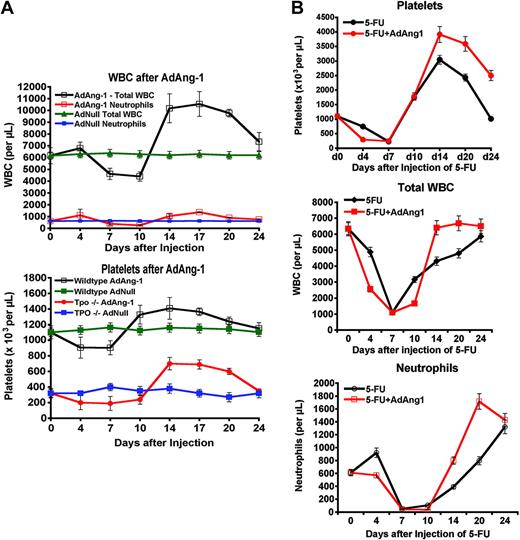
Impact of Ang-1 signaling on peripheral-blood counts. (A) Enforced Ang-1 expression stimulates hematopoiesis and restores platelet counts in Thpo–/– mice. Both wild-type and Thpo–/– mice received a single intravenous dose of AdAng-1 (109 pfu) or AdNull (109 pfu). Ang-1 induced a delayed, but sustained, leukocytosis and thrombocytosis with an almost 2-fold increase in total white blood cell counts (top) over baseline levels in wild-type mice (n = 4, P < .05 on day 17). This effect is reproducible independent of thrombopoietin, as injection of AdAng1 into Thpo–/– mice enhanced platelet production (bottom; n = 4, P < .05 at day 17). Data are expressed as the mean ± SEM. (B) Ad-Ang1 rescues neutropenia after 5-FU. Wild-type mice (n = 4 in each group) were injected with 250 mg/kg 5-FU and received either a single tail-vein injection of AdAng-1 (109 pfu) or AdNull (109 pfu). Whereas the nadirs of platelets (top), total white blood cells (middle), and neutrophils (bottom) were not changed, Ad-Ang1 lead to a significantly accelerated recovery of total white blood cells and neutrophils (P < .05 at day 14 after 5-FU). Platelet levels showed an exaggerated rebound thrombocytosis.
Impact of Ang-1 signaling on peripheral-blood counts. (A) Enforced Ang-1 expression stimulates hematopoiesis and restores platelet counts in Thpo–/– mice. Both wild-type and Thpo–/– mice received a single intravenous dose of AdAng-1 (109 pfu) or AdNull (109 pfu). Ang-1 induced a delayed, but sustained, leukocytosis and thrombocytosis with an almost 2-fold increase in total white blood cell counts (top) over baseline levels in wild-type mice (n = 4, P < .05 on day 17). This effect is reproducible independent of thrombopoietin, as injection of AdAng1 into Thpo–/– mice enhanced platelet production (bottom; n = 4, P < .05 at day 17). Data are expressed as the mean ± SEM. (B) Ad-Ang1 rescues neutropenia after 5-FU. Wild-type mice (n = 4 in each group) were injected with 250 mg/kg 5-FU and received either a single tail-vein injection of AdAng-1 (109 pfu) or AdNull (109 pfu). Whereas the nadirs of platelets (top), total white blood cells (middle), and neutrophils (bottom) were not changed, Ad-Ang1 lead to a significantly accelerated recovery of total white blood cells and neutrophils (P < .05 at day 14 after 5-FU). Platelet levels showed an exaggerated rebound thrombocytosis.
The rapid plasma elevation of VEGF-A after myelosuppression provided important clues to angiogenic cascades regulating the assembly of marrow neovessels. We propose a model in which myelosuppression leads to a complete loss of microarchitectural integrity with regression of both hematopoietic and vascular tissue. As a consequence, angiogenic factors such as VEGF-A and Ang-1 are produced and reconstruct a functional vasculature.
The increase in the number of endothelial cells expressing Tie2 as a consequence of myelosuppression may also be explained by recruitment of Tie2-positive hemangiogenic stem cells that reside within the BM microenvironment. In fact, few Tie2-positive cells could be detected within the BM osteoblastic niche at steady state. Therefore, the majority of the Tie2-positive cells was most likely originating from proliferating Tie2-positive endothelial precursor cells. However, we cannot rule out the possibility that Tie2-positive vascular cells may also originate from pre-existing mature endothelial cells, such as those lining the arterioles that are preserved during chemotherapy- or radiation-induced BM suppression.
The precise mechanism by which angiopoietin-mediated remodeling of the sinusoidal endothelial cells enhances the physical localization of regenerating progenitors to the BM vascular niche is not well defined. Studies have shown that angiopoietins could potentially alter the adhesion molecule repertoire of endothelial cells.42,60 Thus, Ang-1 may support thrombopoiesis even in the absence of thrombopoietin by supporting the haptotactic localization of megakaryocytic progenitors to the BM's vascular niche.
We have previously shown that SDF-1 (CXC ligand 12 [CXCL12])–and FGF-4–mediated localization of (CXC receptor 4 [CXCR4])–positive progenitors to the vascular niche is sufficient to restore thrombopoiesis in Thpo–/– or Mpl–/– mice.8 Targeted disruption of the BM vascular niche by monoclonal antibodies to VE-cadherin inhibited thrombopoiesis at both steady state and after myelosuppression. Similarly, interference with Tie2 signaling resulted in impaired assembly and remodeling of sinusoidal endothelial cells, leading to delayed thrombopoietic recovery as a result of disturbed interaction of megakaryocytes with a functional neovasculature. Together, these data suggest that proper reconstruction of the BM vascular niche is essential for the reconstitution of hematopoiesis. Therefore, proangiogenic factors such as Ang-1 may promote hematopoiesis through enhancing the regeneration and remodeling of a permissive neoangiogenic niche. One recent report has demonstrated that Ang-1 may stabilize the adhesion of hematopoietic stem cells to the osteoblastic niche.42 Our data provide evidence for another dimension of angiopoietin/Tie2 signaling, suggesting that this pathway may also contribute to hematopoiesis through timely regeneration of the BM vascular niche, thereby accelerating the maturation of the hematopoietic progenitor cells.
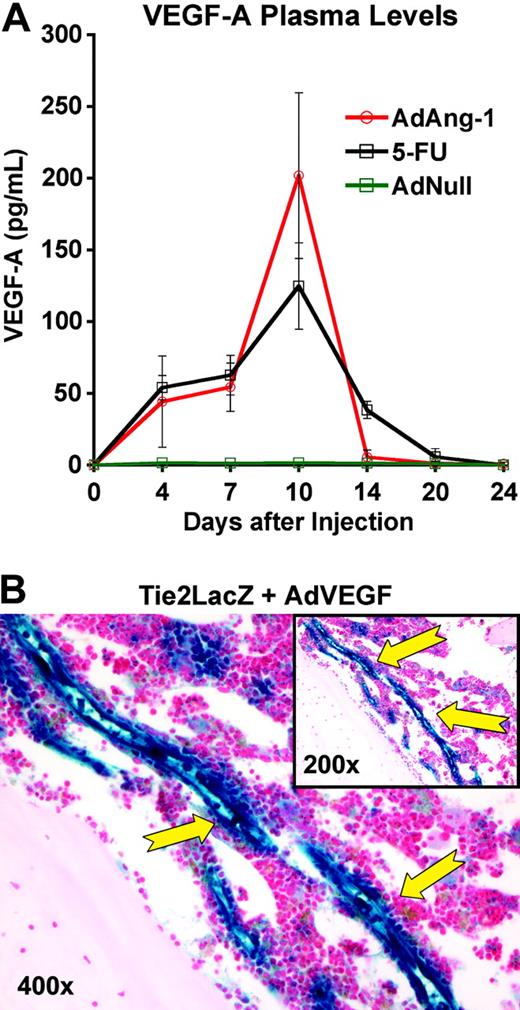
Role of VEGF-A. (A) Both 5-FU and Ang-1 induce VEGF-A expression in wild-type mice. Wild-type mice received a single intravenous injection of AdAng-1 (109 pfu), AdNull (109 pfu), or 5-FU (250 mg/kg). Introduction of Ang-1 and myelosuppression induced by 5-FU were followed by a robust increase in plasma VEGF-A levels, with maximum levels detected at day 10 after treatment with Ang-1 or 5-FU (n = 3, P < .001 on day 10). After 20 days, at the time where steady-state hematopoiesis was reached, VEGF-A levels reverted to normal. Data are displayed as mean ± standard deviation. (B) Introduction of VEGF-A induces up-regulation of Tie2 expression in the BM vasculature. Tie2-LacZ animals were injected with a single tail-vein injection of AdVEGF-A (1.5 × 108 pfu per injection, n = 4). At day 4 after injection, mice were killed and femurs obtained for histology. β-Gal staining showed exceptionally strong LacZ activity in the BM vasculature of treated animals, demonstrating that VEGF-A is a primary factor supporting the emergence of Tie2-positive endothelial cells.53,54
Role of VEGF-A. (A) Both 5-FU and Ang-1 induce VEGF-A expression in wild-type mice. Wild-type mice received a single intravenous injection of AdAng-1 (109 pfu), AdNull (109 pfu), or 5-FU (250 mg/kg). Introduction of Ang-1 and myelosuppression induced by 5-FU were followed by a robust increase in plasma VEGF-A levels, with maximum levels detected at day 10 after treatment with Ang-1 or 5-FU (n = 3, P < .001 on day 10). After 20 days, at the time where steady-state hematopoiesis was reached, VEGF-A levels reverted to normal. Data are displayed as mean ± standard deviation. (B) Introduction of VEGF-A induces up-regulation of Tie2 expression in the BM vasculature. Tie2-LacZ animals were injected with a single tail-vein injection of AdVEGF-A (1.5 × 108 pfu per injection, n = 4). At day 4 after injection, mice were killed and femurs obtained for histology. β-Gal staining showed exceptionally strong LacZ activity in the BM vasculature of treated animals, demonstrating that VEGF-A is a primary factor supporting the emergence of Tie2-positive endothelial cells.53,54
Increase in surface area of Tie2-positive vessels after myelosuppression precedes increase in platelet levels. LacZ/NFR-stained BM sections were obtained at different time points after injection of 5-FU as described. The LacZ-positive BM surface area was measured by quantification of the blue chroma pixels compared with the total number of pixels in the digital image. The surface area demarcated by LacZ-positive vessels was normalized to the percentage of total BM hematopoietic surface area. At steady state, there was a minimal number of LacZ-positive cells (0.02% of total pixels were blue). At 10 days after myelosuppression, Tie2 expression was found to be maximal (6.8% of depicted BM surface area pixels were blue), which was followed closely by large increases in peripheral platelet counts (n = 4). All results are shown as mean ± SEM (n = 6, P < .01 at day 10).
Increase in surface area of Tie2-positive vessels after myelosuppression precedes increase in platelet levels. LacZ/NFR-stained BM sections were obtained at different time points after injection of 5-FU as described. The LacZ-positive BM surface area was measured by quantification of the blue chroma pixels compared with the total number of pixels in the digital image. The surface area demarcated by LacZ-positive vessels was normalized to the percentage of total BM hematopoietic surface area. At steady state, there was a minimal number of LacZ-positive cells (0.02% of total pixels were blue). At 10 days after myelosuppression, Tie2 expression was found to be maximal (6.8% of depicted BM surface area pixels were blue), which was followed closely by large increases in peripheral platelet counts (n = 4). All results are shown as mean ± SEM (n = 6, P < .01 at day 10).
It remains to be determined whether in BM failure states there is dysregulation of the expression of Tie2/angiopoietin. Nonetheless, our data demonstrate that Ang-1 alone or in combination with other angiogenic factors may promote hematopoiesis and may be used in the clinical setting for the treatment of BM failure states, where there is an impairment in the integrity of the BM's vascular niche.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2004-11-4269.
H.-G.K. is supported by a grant from Deutsche Krebshilfe, Bonn, Germany. S.T.A. is supported by National Institutes of Health Medical Scientist Training Program (MSTP) grant GM07739 (Cornell/Rockefeller/Sloan-Kettering MD-PhD program). S.R. is supported by the National Institutes of Health (R01 grants HL075234, HL59312, HL67839, HL61849, and HL66592), the American Cancer Society, and the Leukemia and Lymphoma Society.
H.-G.K. and S.T.A. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal