The immunosuppressive drug mycophenolate mofetil (MMF) is used after nonmyeloablative hematopoietic cell transplantation (HCT); however, limited pharmacodynamic data are available. We evaluated plasma concentrations of mycophenolic acid (MPA), the active metabolite of MMF, and outcomes in 85 patients with hematologic malignancies conditioned with fludarabine and 2 Gy total body irradiation followed by HLA-matched unrelated-donor HCT and postgrafting cyclosporine and MMF. The first 38 patients received MMF 15 mg/kg twice daily; the next 47 patients received MMF 3 times daily. MPA pharmacokinetics were determined on days 7 and 21. Comparing the twice-daily and 3-times-daily MMF groups, the mean total MPA concentration steady state (Css) was 1.9 and 3.1 μg/mL; the unbound Css was 18 and 36 ng/mL, respectively (P < .001). Sixteen patients with a total MPA Css less than 3 μg/mL had low (< 50%) donor T-cell chimerism (P = .03), and 6 patients with MPA Css less than 2.5 μg/mL had graft rejection. An elevated unbound Css was associated with cytomegalovirus reactivation (P = .03). There were no significant associations between MPA pharmacokinetics and acute graft-versus-host disease (GVHD) or relapse. We conclude that increased MPA Css's predicted higher degrees of donor T-cell chimerism after unrelated donor nonmyeloablative HCT and suggest that targeting MPA Css's greater than 2.5 μg/mL could prevent graft rejection.
Introduction
Nonmyeloablative conditioning regimens for allogeneic hematopoietic cell transplantation (HCT) have recently been developed with the goal of reducing treatment-related toxicities, thereby increasing the number of patients who can receive an allogeneic HC transplant.1-9 Following nonmyeloablative HCT, mycophenolate mofetil (MMF) is used in combination with cyclosporine as immunosuppressive treatment to prevent both graft rejection and graft-versus-host disease (GVHD). Mycophenolic acid (MPA), the active metabolite of MMF, interferes with cell proliferation by inhibiting inosine monophosphate dehydrogenase type II, which blocks de novo purine synthesis in T and B lymphocytes.10
MPA pharmacokinetics has been extensively studied in solid organ transplantation patients, primarily after kidney transplantation.11-14 Several studies have evaluated the pharmacodynamic relationships between MPA and clinical outcomes after solid organ transplantation including organ rejection, hematologic toxicity, gastrointestinal symptoms, and infections.14-17 To decrease graft rejection and MMF-associated toxicities, some investigators have proposed that optimal MMF treatment with concomitant cyclosporine after renal transplantation should include targeting either (1) the total MPA area under the plasma concentration-versus-time curve (AUC) between 30 and 60 μg · h/mL or (2) the predose MPA between 1 and 3.5 μg/mL.18 MPA is highly bound to human serum albumin, and the unbound fraction is the pharmacologically active form.19,20 Recent studies in renal transplantation patients suggested that hematologic toxicity was more closely associated with the unbound fraction of MPA than with total MPA.21 Thus, both the total and unbound MPA concentrations should be considered in pharmacodynamic analysis in HCT patients.
Pharmacokinetic data in allogeneic HC transplant recipients after either myeloablative or nonmyeloablative conditioning suggested that the plasma MPA half-life ranged from 1.5 to 3 hours following oral or intravenous administration, which is shorter than that reported in patients after solid organ transplantation.6 This resulted in MPA concentrations below the therapeutic range recommended for solid organ transplantation.22-26 It was recently suggested that an increased incidence of acute GVHD after allogeneic HCT was associated with low unbound MPA AUC in adults and total predose MPA concentration in children.26,27 We report an analysis of pharmacokinetic parameters of MPA in 2 groups of patients treated on 2 consecutive protocols that gave MMF orally twice daily or 3 times daily, respectively, after nonmyeloablative conditioning and unrelated donor HCT. The only difference between the twice-daily MMF and 3-times-daily MMF protocols was the dosing frequency of MMF and the exclusive use of peripheral blood stem cells (PBSCs) in the 3-times-daily MMF protocol in an effort to reduce the 21% incidence of graft rejection observed in the first protocol.6 A pharmacodynamic analysis was then conducted examining the association of total and unbound MPA with reactivation of cytomegalovirus (CMV), donor chimerism in CD3+ cells, graft rejection, acute GVHD, and relapse.
Patients, materials, and methods
Patient characteristics and treatment plan
MPA plasma concentrations were examined in 2 sequential cohorts of patients who underwent unrelated donor HCT for a variety of hematologic malignancies between March 2000 and September 2003 at the Fred Hutchinson Cancer Research Center (Seattle, WA). The 85 patients who participated in this MPA pharmacokinetic study were a subset of patients treated in 2 sequential multicenter clinical trials. Written informed consent was obtained from all patients, and the study protocols were approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center and monitored for safety by an independent Data Safety Monitoring Board. Patient characteristics are summarized in Table 1.
Patient characteristics
. | Twice-daily MMF . | 3-times-daily MMF . |
|---|---|---|
| No. of patients | 38 | 47 |
| Mean age, y (range) | 52 (18-70) | 52 (17-67) |
| Bone marrow source (%) | 6 (16) | 0 |
| Recipients CMV seropositive (%)* | 20 (53) | 29 (62) |
| Disease, no. of patients | ||
| AML CR1 | 1 | 5 |
| AML > CR1 | 6 | 7 |
| MDS RA | 0 | 2 |
| MDS AML | 4 | 3 |
| ALL CR1 | 1 | 0 |
| ALL > CR1 | 2 | 0 |
| CML CP1 | 6 | 1 |
| CML > CP1 | 3 | 0 |
| CLL | 3 | 4 |
| Myelofibrosis | 2 | 2 |
| MM | 3 | 6 |
| NHL | 6 | 11 |
| HD | 1 | 6 |
. | Twice-daily MMF . | 3-times-daily MMF . |
|---|---|---|
| No. of patients | 38 | 47 |
| Mean age, y (range) | 52 (18-70) | 52 (17-67) |
| Bone marrow source (%) | 6 (16) | 0 |
| Recipients CMV seropositive (%)* | 20 (53) | 29 (62) |
| Disease, no. of patients | ||
| AML CR1 | 1 | 5 |
| AML > CR1 | 6 | 7 |
| MDS RA | 0 | 2 |
| MDS AML | 4 | 3 |
| ALL CR1 | 1 | 0 |
| ALL > CR1 | 2 | 0 |
| CML CP1 | 6 | 1 |
| CML > CP1 | 3 | 0 |
| CLL | 3 | 4 |
| Myelofibrosis | 2 | 2 |
| MM | 3 | 6 |
| NHL | 6 | 11 |
| HD | 1 | 6 |
MMF indicates mycophenolate mofetil; CMV, cytomegalovirus; AML, acute myeloid leukemia; CR1, first complete remission; MDS, myelodysplastic syndrome; RA, refractory anemia; ALL, acute lymphocytic leukemia; CML, chronic myelogenous leukemia; CP1, first chronic phase; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; and HD, Hodgkin disease.
For the CMV-seropositive patients with twice-daily MMF, 8 donors were CMV seropositive and 12 were seronegative. For the 29 CMV-seropositive 3-times-daily MMF patients, 7 donors were CMV seropositive and 22 were seronegative
The conditioning regimen consisted of fludarabine (30 mg/m2/d intravenously) from day -4 to day -2 (total cumulative dose 90 mg/m2) and a single fraction of 2 Gy total body irradiation (TBI) on day 0.6 For postgrafting immunosuppression, all patients received cyclosporine 6.25 mg/kg orally every 12 hours (twice daily) from day -3 to day +100, followed by taper to day +177. Unless clinically intolerable, cyclosporine doses were adjusted to maintain blood trough levels of 500 ng/mL through the first month after HCT. In addition, MMF was given at 2 different dose frequencies. Patients in the first treatment group (n = 38) received MMF 15 mg/kg every 12 hours (twice daily) orally, whereas patients in the second treatment group (n = 47) received MMF 15 mg/kg every 8 hours (3 times daily) orally. The MMF doses were based on adjusted ideal body weights and rounded to the nearest 250-mg dose. Patients were asked to take MMF at the same time daily. There was no adjustment of MMF dose based upon MPA plasma concentrations. Both groups received MMF treatment from day 0 to day 40 and, if there was no GVHD, a scheduled dose reduction of 10% per week over 10 weeks occurred. All grafts were from unrelated donors who were matched for HLA-A, -B, and -C at intermediate-resolution DNA typing and for HLA-DRB1 and -DQB1 by high-resolution techniques.28 In the twice-daily MMF group, either bone marrow (BM) or PBSCs were used as the source of the hematopoietic cell graft. Because the graft rejection rate was higher in the BM recipients,6 only PBSCs were used in the 3-times-daily MMF group.
Pharmacokinetic analysis
Blood samples were collected in EDTA (ethylenediaminetetraacetic acid) tubes on days 7 and 21 after transplantation. In patients treated with twice-daily MMF, blood samples were obtained before the morning dose and at 1, 2, 4, 6, 8, and 10 hours after the morning dose. Patients who received 3-times-daily MMF had blood samples drawn before the morning dose and at 1, 2, 4, 6, and 8 hours after the morning dose. The MPA was excluded if less than 5 plasma samples were available for one area under the plasma concentration time curve (AUC).
Total MPA plasma levels were quantified by reverse-phase high-performance liquid chromatography (HPLC) with UV detection (adapted from Tsina et al).29,30 Plasma samples (500 μL) and the internal standard (20 μL of diphenylacetic acid 0.54 μg/mL) were diluted with 1.5 mL of 150 mM HCl, vortexed, and subsequently placed on their respective solid-phase extraction cartridges. Solid-phase extraction cartridges (C18, Waters no. WAT043410; Milford, MA) were preconditioned with 2 mL methanol followed by 2 mL deionized water. The column was allowed to drip dry and then eluted with 1 mL methanol. The compounds were collected in vials and 10 μL of the eluate was loaded onto the HPLC column. Mobile phase consisted of 55% phosphate buffer 20 mM and 45% acetonitrile with a flow rate of 1 mL/min. The UV detector was set at 220 nm. The relationship between the peak area and height ratio of MPA concentration was analyzed by linear least squares regression to determine the slope, intercept, and correlation coefficient of the standard calibration curve. The dynamic range was 0.2 to 30 μg/mL and the interday coefficient of variation was less than 10%.
The unbound fraction of MPA was separated from the protein-bound MPA through equilibrium dialysis20 and measured as described. Briefly, 200 μL of plasma was placed in a water-tight Teflon dialysis chamber separated by dialysis membranes (Spectrapor 4; 14-14k molecular weight cutoff; Spectrum Laboratories, Los Angeles, CA). The samples were dialyzed for 2 hours against an equal volume of 10% phosphoric acid buffer at 37°C in a water bath. Then, 120 μL dialysate was mixed with 500 μL acetonitrile, vortexed, dried, and reconstituted in 20 mM phosphate buffer (pH 3.2). The reconstituted sample was analyzed as described. The total MPA and the fraction of MPA bound to plasma protein (bMPA) was quantitated and the percentage of unbound drug was calculated as follows: unbound MPA = 100 (1-bMPA).
After quantitation, noncompartmental analyses of concentration time data were conducted to estimate the area under the plasma concentration time curve (AUC). In the 3-times-daily MMF group, 26 patients on day 7 and 23 patients on day 21 had their blood drawn over 6 hours instead of 8 hours, but since AUC0-6h correlates well to AUC0-8h (r2 = 0.92 and 0.96, respectively), these patients were included in the analyses. The estimation of concentration at steady state (Css) was calculated by dividing the AUC by the dosing interval,31 specifically, AUC/12 hours in the twice-daily MMF group and AUC/8 hours in the 3-times-daily MMF group, in order to compare the 2 groups of patients. Total MPA Css was evaluated on day 7 in 34 patients in the twice-daily group and 41 patients in the 3-times-daily group. On day 21, 33 patients in the twice-daily MMF group and 42 patients in the 3-times-daily MMF group were evaluable. Two patients who received intravenous MMF on day 21 were excluded from analysis. Additional pharmacokinetic parameters of total plasma MPA included maximal concentration (Cmax), time to maximum concentration (Tmax), the last concentration time point obtained from pharmacokinetic sampling for Css determination (Ctrough), and half-life (t½). Half-life was determined in patients with at least 3 declining concentration time points. The number of evaluable patients for MPA half-life was 18 and 19 patients on days 7 and 21, respectively, in the twice-daily MMF group and 11 and 21 patients on days 7 and 21, respectively, in the 3-times-daily MMF group. The elimination half-life was calculated as the reciprocal of the slope of the last 3 log concentration time points. The unbound fraction was evaluated on day 7 in 30 patients in the twice-daily MMF group and 32 in the 3-times-daily MMF group, and on day 21 in 30 and 31 patients in the twice-daily MMF and 3-times-daily MMF groups, respectively. The unbound AUC and unbound Css were calculated by multiplying the unbound fraction of MPA by total AUC and Css, respectively. The MPA parameters evaluated for pharmacodynamic relationships were total MPA Css, total MPA Ctrough, and unbound MPA Css. Serum albumin levels and total bilirubin levels were obtained from day 0 to day 28.
Toxicity
Hematologic toxicity was evaluated from day 0 to day 28 after HCT by assessment of daily complete blood counts with differential and assessment of absolute neutrophil count (ANC). This time point was chosen because many confounding variables (such as corticosteroid therapy, viral infection, or reactivation) could affect the leukocyte counts beyond day 28 after HCT. CMV reactivation was evaluated as a measure of toxicity. CMV serologic status was assessed in each patient before HCT.32 The CMV antigenemia assay to detect CMV pp65 antigen was performed on a weekly basis for the first 3 months after HCT.33
Chimerism and graft rejection
The percentages of donor CD3+ T cells in the peripheral blood on days 28, 56, and 84 after HCT were determined in all patients. CD3+ cells were sorted by flow cytometry, and chimerism was detected using fluorescent in situ hybridization for sex-mismatched HC transplant recipients and polymerase chain reaction of polymorphic microsatellite regions in sex-matched HC transplant recipients.34 Graft rejection was defined as donor CD3+ cells 5% or less at any of the assessed time points after HCT. Two patients with impending rejection were included: autologous stem cells or donor lymphocytes were infused, respectively, before formal rejection occurred to avoid prolonged aplasia. Due to the greater risk of graft rejection among BM recipients compared with PBSC recipients in the twice-daily MMF protocol,6 patients who received BM as the source of stem cells were excluded from the pharmacodynamic analyses of donor chimerism and graft rejection.
Acute GVHD and disease relapse
Acute GVHD was graded as previously described.35 Hematologic diseases were classified as low risk of relapse (chronic myelogenous leukemia in chronic phase, myelodysplastic syndrome refractory anemia, acute myeloid leukemia, and acute lymphoblastic leukemia in first remission) or high risk of relapse (all other diseases) to uniformly evaluate relapse rate.6 Disease relapse or disease progression was defined as recurrence of disease after complete remission or progression of persistent disease. The median follow-up among all patients was 359 days (range, 49-1397 days).
Statistical analysis
Linear regression was used to quantify the magnitude of correlation between various pharmacokinetic parameters (eg, MPA Css and Ctrough). The 2-sample t test was used to compare means of pharmacokinetic parameters in situations where patients contributed only a single pharmacokinetic value to the analysis. The chi-square test was used to evaluate the relationship between neutropenia and dosing frequency of MMF. The associations between MPA pharmacokinetics and chimerism and between pharmacokinetics and frequency of dosing of MMF were assessed using generalized estimating equations (GEEs). This allowed the use of chimerism levels on multiple days as well as pharmacokinetic values on each of days 7 and 21. Dependence of associations on day of chimerism and day of pharmacokinetic analysis was assessed by fitting appropriate interaction terms in the GEE models. The dependence of relevant associations on the frequency of MMF dosing was also assessed by fitting appropriate interaction terms in the GEE models. Cox regression was used to assess the association between pharmacokinetics and the hazard of relapse. All reported P values were 2-sided, and those estimated from regression models were derived from the Wald test. No adjustments were made for multiple comparisons.
Results
Pharmacokinetic results
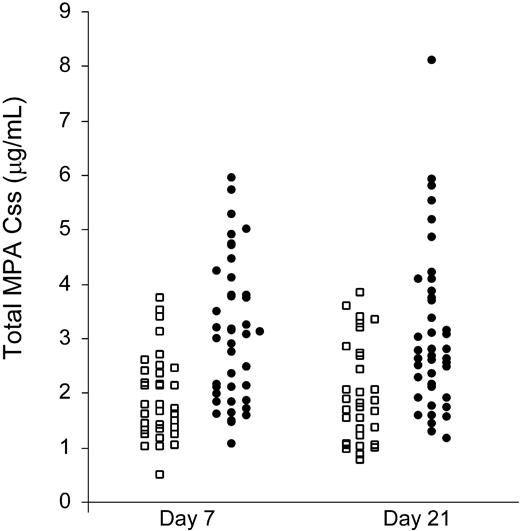
Despite using an adjusted ideal body weight dosage of MMF, large interindividual variations of MPA plasma levels were found in both the twice-daily and 3-times-daily MMF patient groups. The total MPA AUC ranged 8-fold, from 5.8 to 46.1 μg · h/mL, in the twice-daily MMF group, and 7.6-fold, from 8.5 to 64.8 μg · h/mL, in the 3-times-daily MMF group (Figure 1; Table 2). As shown in Tables 2 and 3, pharmacokinetic data obtained on days 7 and 21 within each MMF treatment group were similar. Comparing the mean values between the twice-daily and 3-times-daily MMF groups (days 7 and 21 combined), the total MPA Ctrough was 0.8 and 2.5 μg/mL, the total MPA Css was 1.9 and 3.1 μg/mL, and the unbound Css was 19 and 38 ng/mL, respectively (P < .001, each comparison). These results are consistent with the hypothesis that MPA has linear pharmacokinetics. The total MPA Cmax on days 7 and 21 ranged from 1.0 to 29.3 μg/mL, and the mean values were not statistically significantly different between the twice-daily and 3-times-daily MMF groups (P = .14). The total MPA Cmax occurred at a mean of 2 hours (range, 1-10 hours) after MMF administration. The mean MPA half-lives (t½) were 3.4 hours and 2.7 hours in the twice-daily and 3-times-daily MMF groups, respectively, with a trend toward a shorter t½ in the 3-times-daily MMF group (P = .08).
Plasma total MPA pharmacokinetic data
. | No. of patients . | AUC0-8, μg · h/mL . | AUC0-12, μg · h/mL . | Css, μg/mL* . | Ctrough, μg/mL . | Cmax, μg/mL . | t 1/2, h† . |
|---|---|---|---|---|---|---|---|
| Day 7 | |||||||
| Twice-daily MMF | 34 | — | 23.0 (5.8-44.8) | 1.9 (0.5-3.7) | 0.8 (0.1-1.9) | 6.9 (1.0-15.7) | 3.0 (1.2-7.9) |
| 3-times-daily MMF | 41 | 24.7 (8.5-47.7) | — | 3.1 (1.1-6.0) | 2.5 (0.4-19.3) | 8.9 (2.3-24.7) | 2.8 (1.0-6.6) |
| Day 21 | |||||||
| Twice-daily MMF | 33 | — | 23.5 (9.2-46.1) | 2.0 (0.8-3.8) | 0.9 (0.2-3.2) | 7.1 (2.1-21.2) | 3.8 (1.8-7.7) |
| 3-times-daily MMF | 42 | 25.0 (9.2-64.8) | — | 3.1 (1.2-8.1) | 2.7 (0.6-22.2) | 7.7 (2.9-29.3) | 2.7 (1.0-6.8) |
| P | — | — | — | < .001 | < .001 | .14 | .08 |
. | No. of patients . | AUC0-8, μg · h/mL . | AUC0-12, μg · h/mL . | Css, μg/mL* . | Ctrough, μg/mL . | Cmax, μg/mL . | t 1/2, h† . |
|---|---|---|---|---|---|---|---|
| Day 7 | |||||||
| Twice-daily MMF | 34 | — | 23.0 (5.8-44.8) | 1.9 (0.5-3.7) | 0.8 (0.1-1.9) | 6.9 (1.0-15.7) | 3.0 (1.2-7.9) |
| 3-times-daily MMF | 41 | 24.7 (8.5-47.7) | — | 3.1 (1.1-6.0) | 2.5 (0.4-19.3) | 8.9 (2.3-24.7) | 2.8 (1.0-6.6) |
| Day 21 | |||||||
| Twice-daily MMF | 33 | — | 23.5 (9.2-46.1) | 2.0 (0.8-3.8) | 0.9 (0.2-3.2) | 7.1 (2.1-21.2) | 3.8 (1.8-7.7) |
| 3-times-daily MMF | 42 | 25.0 (9.2-64.8) | — | 3.1 (1.2-8.1) | 2.7 (0.6-22.2) | 7.7 (2.9-29.3) | 2.7 (1.0-6.8) |
| P | — | — | — | < .001 | < .001 | .14 | .08 |
Levels are expressed as mean (range). P refers to the comparison between twice-daily MMF and 3-times-daily MMF and is derived by combining day 7 and day 21 data.
AUC indicates area under the plasma concentration-versus-time curve; Css, concentration at steady state; Ctrough, last concentration time point obtained from pharmacokinetic sampling for AUC determination; Cmax, maximum concentration; t½, half-life; and -, not applicable.
Css = AUC/time interval (h) between each dose
The number of patients evaluable for t½ is indicated in “Pharmacokinetic analysis”
Unbound MPA pharmacokinetic data
. | No. of patients . | Unbound fraction, % . | Unbound AUC, ng · h/mL . | Unbound Css, ng/mL . |
|---|---|---|---|---|
| Day 7 | ||||
| Twice-daily MMF | 30 | 1.0 (0.5-2.4) | 211 (42-533) | 18 (4-44) |
| 3-times-daily MMF | 32 | 1.3 (0.6-3.8) | 285 (82-833) | 36 (10-104) |
| Day 21 | ||||
| Twice-daily MMF | 30 | 1.1 (0.5-3.5) | 251 (70-1024) | 21 (6-85) |
| 3-times-daily MMF | 31 | 1.3 (0.8-4.4) | 317 (112-1308) | 40 (14-163) |
| P* | — | — | — | < .001 |
. | No. of patients . | Unbound fraction, % . | Unbound AUC, ng · h/mL . | Unbound Css, ng/mL . |
|---|---|---|---|---|
| Day 7 | ||||
| Twice-daily MMF | 30 | 1.0 (0.5-2.4) | 211 (42-533) | 18 (4-44) |
| 3-times-daily MMF | 32 | 1.3 (0.6-3.8) | 285 (82-833) | 36 (10-104) |
| Day 21 | ||||
| Twice-daily MMF | 30 | 1.1 (0.5-3.5) | 251 (70-1024) | 21 (6-85) |
| 3-times-daily MMF | 31 | 1.3 (0.8-4.4) | 317 (112-1308) | 40 (14-163) |
| P* | — | — | — | < .001 |
Levels are expressed as mean (range).
AUC indicates area under the serum concentration-versus-time curve of MPA; Css, concentration at steady state; and -, not applicable.
P value refers to the comparison between twice-daily and 3-times-daily MMF groups and is derived by combining day 7 and day 21 data
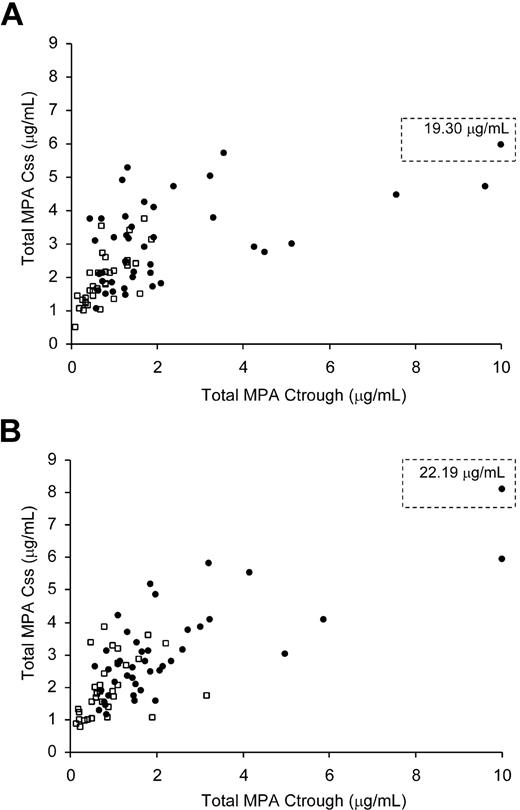
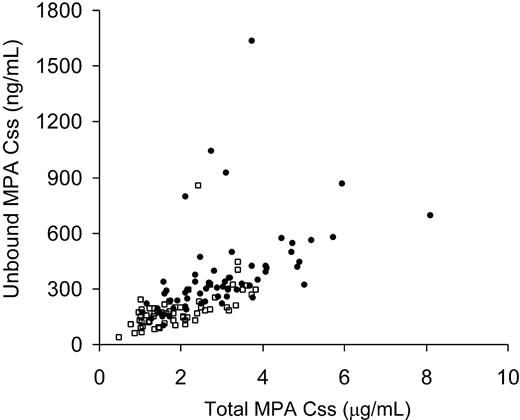
Since the MPA Css is more cumbersome to determine than Ctrough, we determined if Ctrough could closely predict Css. On day 7, the estimated correlation coefficient between Ctrough and Css was 0.57 (P < .001) and on day 21 the estimate was 0.70 (P < .001). On day 7, Ctrough increased by 1.19 units for each increase in Css of 1 unit, whereas on day 21, Ctrough increased by 1.58 units for each Css increase of 1 unit (Figure 2). The association between Ctrough and Css did not appear to depend on the frequency of MMF administration (P = .52). The correlation between total MPA Css and unbound Css was .34 (r = .58; Figure 3).
Mycophenolic acid plasma concentration steady state (MPA Css) on days 7 and 21 after HCT. The twice-daily MMF group is represented by squares and 3-times-daily MMF group by circles.
Mycophenolic acid plasma concentration steady state (MPA Css) on days 7 and 21 after HCT. The twice-daily MMF group is represented by squares and 3-times-daily MMF group by circles.
Next, we examined if pharmacokinetic data obtained on days 7 and 21 were similar. Among patients who had MPA data available from both days 7 and 21 there was a suggestion that the correlation between data collected at both time points was influenced by frequency of MMF administration for total MPA Css (P = .15) and unbound Css (P = .02). The correlation between Ctrough on days 7 and 21 did not differ between the twice-daily and 3-times-daily groups (P = .66). For total MPA Css there was a statistically significant positive correlation between day-7 and day-21 values for both the twice-daily and 3-times-daily groups (r = .78, P < .001 for twice daily; r = .45, P = .007 for 3 times daily). For unbound Css, there was also a positive correlation between day-7 and day-21 values, but the magnitude of association was not as strong as for total MPA Css (r = .31, P = .13 for twice daily; r = .64, P = .001 for 3 times daily). By combining twice-daily data with 3-times-daily for Ctrough, a statistically significant positive correlation was also seen (r = .74, P < .001).
Pharmacokinetic relationship to albumin level, liver function, and disease status
An increase in total MPA Css of one unit was accompanied by an increase in serum albumin level of 1.07 units (P < .001) on days of pharmacokinetic assessment, but there was no such association seen with total bilirubin level (P = .64). No associations were seen between unbound MPA Css and albumin (P = .52) or bilirubin levels (P = .29). There was no association between disease status or the number of prior therapies and total MPA Css, Ctrough, and unbound MPA Css (data not shown).
Neutropenia
The number of patients who had an ANC below 1 × 109/L (1000/μL) before HCT was similar in both groups (5/38 versus 9/47 in the twice-daily and 3-times-daily MMF groups, respectively; P = .46, chi-square test). The majority of patients (40/66) who experienced neutropenia (defined as ANC below 0.5 × 109/L [500/μL]) after HCT developed this toxicity before day 7, and thus it was not possible to assess the pharmacodynamic relationship between MPA and neutropenia.
Gastrointestinal toxicity
Seven patients developed transient grades III-IV nausea and vomiting within 14 days after HCT that was not temporally related to a diagnosis of acute GVHD. Six of the 7 patients received 3-times-daily MMF, and symptoms resolved with antiemetic therapy. The mean value of day-7 total MPA Css was 0.48 μg/mL higher among these patients compared with patients without gastrointestinal toxicity, but this difference was not significant (P = .46).
CMV reactivation
We evaluated the incidence of CMV reactivation among the 43 patients who were CMV seropositive before HCT and had MPA pharmacokinetic data available. There were no statistically significant interactions between CMV reactivation and frequency of MMF administration, so the analyses combined patients in the twice-daily and 3-times-daily MMF groups. There was an interaction between MPA levels measured on days 7 or 21 and CMV reactivation. The 13 patients who did not show CMV reactivation had a mean day-7 total MPA Css of 1.8 μg/mL compared with 2.2 μg/mL among the 30 patients who had subsequent CMV reactivation (P = .38). CMV reactivation was not associated with total MPA Ctrough on day 7 (P = .20). The mean unbound MPA Css determined on day 7 in 10 patients who did not develop CMV reactivation was 20 ng/mL versus 31 ng/mL for the 28 patients who had subsequent CMV reactivation (P = .03).
Correlation between MPA Ctrough and total MPA Css. The twice-daily MMF group is represented by squares and 3-times-daily MMF group by circles. (A) MPA assessments on day 7. Seventy-five patients are represented (r = 0.57; P < .001). (B) MPA assessments on day 21. Seventy-five patients are represented (r = 0.70; P < .001). The values off the x-axis scale are shown in the dotted line box.
Correlation between MPA Ctrough and total MPA Css. The twice-daily MMF group is represented by squares and 3-times-daily MMF group by circles. (A) MPA assessments on day 7. Seventy-five patients are represented (r = 0.57; P < .001). (B) MPA assessments on day 21. Seventy-five patients are represented (r = 0.70; P < .001). The values off the x-axis scale are shown in the dotted line box.
Among patients with no CMV reactivation by day 21, 12 without subsequent reactivation had a mean day-21 Css of 2.4 μg/mL compared with 2.6 μg/mL among the 27 with subsequent CMV reactivation (P = .67). Total MPA Ctrough on day 21 was also not associated with CMV reactivation (P = .21). The mean day-21 unbound Css for the 10 patients without CMV reactivation was 21 ng/mL compared with 32 ng/mL among the 22 patients with subsequent CMV reactivation (P = .07). Consideration of donor serostatus did not alter the qualitative associations.
Donor chimerism and graft rejection
There were 6 patients in the twice-daily MMF group who received bone marrow as the source of hematopoietic cells. These 6 patients were excluded from the chimerism and rejection analyses since patients who received bone marrow were identified to have an increased risk of graft rejection.6 Of the remaining 79 patients, the graft rejection rate was similar between the 2 MMF treatment groups for those receiving PBSC grafts (3/32 in the twice-daily MMF group and 3/47 in the 3-times-daily MMF group, P = .68).
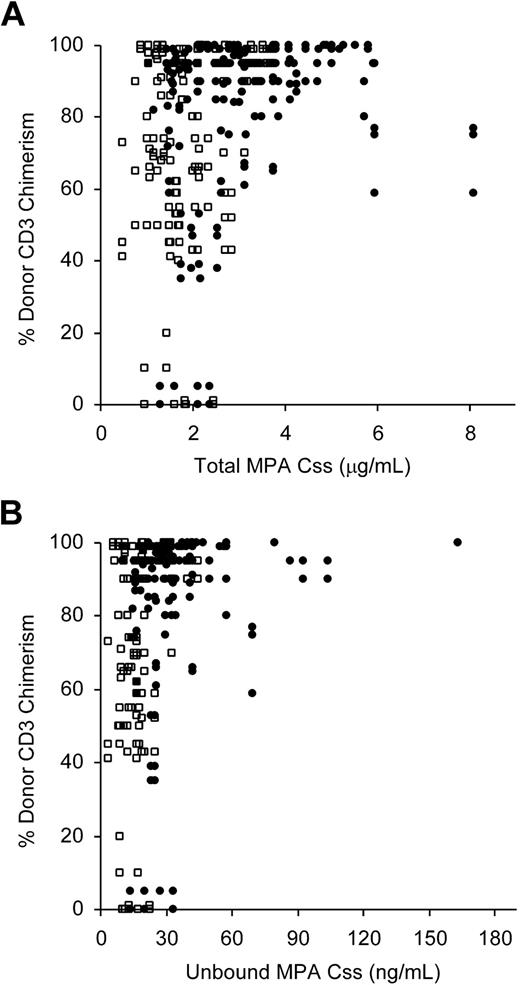
Since a low level of donor chimerism in the CD3+ T cells early after nonmyeloablative HCT has been highly predictive of subsequent graft rejection,1,6,36 we evaluated if total or unbound MPA Css was associated with the subsequent degree of donor CD3+ T-cell chimerism. The association between MPA Css on day 7 and subsequent donor chimerism was similar to the association between MPA Css on day 21 and donor chimerism. Moreover, the association between MPA Css and chimerism did not appear to depend on the day of chimerism assessment or the frequency of MMF administration. Therefore, the data comparing total MPA Css from both days 7 and 21 and the degree of donor CD3+ chimerism at all time points (days 28, 56, 84) from both twice-daily and 3-times-daily MMF treatment groups were analyzed together using a GEE model. After adjusting for the frequency of MMF administration and the days of pharmacokinetic and chimerism assessment, there was a statistically significant positive correlation between total MPA Css and percent donor T-cell chimerism (P = .04). As shown in Figure 4A, only patients who had a total MPA Css below 3 μg/mL on at least one occasion (day 7 or day 21) also had donor CD3+ chimerism values below 50% after HCT. Sixteen of 63 patients with total MPA Css values less than 3 μg/mL compared with 0 of 16 whose Css exceeded 3 μg/mL had low donor chimerism values (P = .03). However, there was no suggestion of such an association between total MPA Ctrough and chimerism (P = .87). A statistically significant positive association was also observed between unbound MPA Css and chimerism (P = .02; Figure 4B). Among the 16 patients with donor CD3+ chimerism levels below 50%, the total MPA Ctrough ranged from 0.10 to 3.16 μg/mL, and unbound Css (evaluated in 14 of 16 patients) ranged from 3.5 to 33 ng/mL. Of the 16 patients with less than 50% donor T-cell chimerism levels, 6 progressed to graft rejection or impending graft rejection.
Correlation between total MPA Css and unbound MPA Css. The twice-daily MMF group is represented by squares and 3-times-daily MMF group by circles. A total of 75 patients (35 from twice-daily MMF group and 40 from 3-times-daily MMF group) and 123 MPA assessments are represented.
Correlation between total MPA Css and unbound MPA Css. The twice-daily MMF group is represented by squares and 3-times-daily MMF group by circles. A total of 75 patients (35 from twice-daily MMF group and 40 from 3-times-daily MMF group) and 123 MPA assessments are represented.
Correlation between the degree of CD3+ donor chimerism and MPA Css. Data from the day-7 and day-21 MPA Css evaluation and day 28, 56, 84 chimerism analyses are shown (6 recipients of bone marrow were excluded). The twice-daily MMF group is represented by squares and the 3-times-daily MMF group by circles. (A) Total MPA Css, 79 patients (P = .04). (B) Unbound MPA Css, 70 patients (P = .02).
Correlation between the degree of CD3+ donor chimerism and MPA Css. Data from the day-7 and day-21 MPA Css evaluation and day 28, 56, 84 chimerism analyses are shown (6 recipients of bone marrow were excluded). The twice-daily MMF group is represented by squares and the 3-times-daily MMF group by circles. (A) Total MPA Css, 79 patients (P = .04). (B) Unbound MPA Css, 70 patients (P = .02).
Low plasma levels of MPA were associated with graft rejection, but because of the low overall incidence of graft rejection, the difference between patients with low and high MPA Css's was not statistically significant. Six of 63 patients (10%) with a low total Css rejected the PBSC graft, whereas none of the patients with Css above 3 μg/mL had graft rejection (P = .34). Among the 63 patients with total MPA Css value less than 3 μg/mL measured at least once on days 7 or 21, 29 were in the twice-daily MMF group and 34 in the 3-times-daily MMF group (Figure 4A). Notably, the total MPA Css was below 2.5 μg/mL at both time points in all 6 patients with graft rejection, whereas there was considerable variability in the other pharmacokinetic parameters and type of disease.
Graft-versus-host disease
The median onset of acute GVHD among those with grades II-IV was day 29. Eighteen patients developed acute GVHD on or before day 21, so assessment of associations with pharmacodynamics was performed using data only from day 7. Of the patients without onset of acute GVHD by day 7, 21 patients with subsequent grade 0-I acute GVHD had a mean day-7 total MPA Css of 2.9 μg/mL compared with 2.5 μg/mL among 52 patients who developed grade II-IV acute GVHD (P = .25). The 21 patients with subsequent grade 0-I acute GVHD had an average day-7 Ctrough of 2.0 μg/mL compared with 1.6 μg/mL among 52 patients with grades II-IV (P = .64). The mean unbound MPA Css for 17 patients with subsequent grade 0-I GVHD was 27 ng/mL compared with 28 ng/mL for 43 patients who developed grade II-IV GVHD (P = .88). Similarly, there was no statistically significant association between MPA plasma levels and subsequent grade III-IV GVHD.
Table 4 summarizes the distribution and stage of skin, gut, or liver involvement in patients with GVHD among the twice-daily and 3-times-daily MMF recipients. The proportion of patients with stages 1+ to 4+ gut GVHD was suggestively higher in the 3-times-daily group (P = .05); otherwise, there was no statistically significant difference between the twice-daily and 3-times-daily groups.
Incidence of acute graft-versus-host disease, severity, and organ distribution
. | No. of patients (%) . | . | |
|---|---|---|---|
| Acute GVHD . | Twice-daily MMF, n = 38 . | 3-times-daily MMF, n = 47 . | |
| Grades II-IV | 26 (68) | 32 (68) | |
| Grades III-IV | 4 (11) | 8 (17) | |
| Stages 2+ to 4+ | |||
| Skin | 21 (55) | 27 (57) | |
| Gut* | 3 (8) | 8 (17) | |
| Liver | 1 (3) | 3 (6) | |
. | No. of patients (%) . | . | |
|---|---|---|---|
| Acute GVHD . | Twice-daily MMF, n = 38 . | 3-times-daily MMF, n = 47 . | |
| Grades II-IV | 26 (68) | 32 (68) | |
| Grades III-IV | 4 (11) | 8 (17) | |
| Stages 2+ to 4+ | |||
| Skin | 21 (55) | 27 (57) | |
| Gut* | 3 (8) | 8 (17) | |
| Liver | 1 (3) | 3 (6) | |
Additionally, there were 11 and 20 patients in the twice-daily and 3-times-daily MMF group, respectively, with stage 1+ gut GVHD. Considering all patients with stage 1+ to 4+ gut GVHD, there was a suggestion of an increased incidence of gut GVHD in the 3-times-daily MMF group, P = .05. Twenty-eight patients (33%) underwent gut biopsy to evaluate symptoms consistent with acute GVHD between 14 to 126 (median, 39) days after transplantation. Twenty-five of 28 gut biopsies (89%) had histologic evidence diagnostic for acute GVHD. The 3 patients with gut biopsies that showed nonspecific changes subsequently developed and were treated for histologically confirmed acute GVHD of the skin at 3, 9, and 24 days after gut biopsy. There was no endoscopic evidence of MMF toxicity documented in any of the 85 patients in the study.
P = .33
Relapse
There was no association between day-7 total MPA Css or unbound MPA Css and the hazard of relapse (P = .35, P = .28, respectively). There was a suggestion of a negative association between the day-21 total MPA Css and relapse (P = .09; increasing Css was associated with a decreased hazard of relapse). However, no association was observed for day-21 unbound MPA Css and relapse (P = .79). Neither day-7 nor day-21 Ctrough was associated with relapse (P = .49 and P = .21, respectively).
Discussion
We report our findings of MPA pharmacokinetics and pharmacodynamics in 85 consecutive patients who were given nonmyeloablative conditioning and unrelated donor HC transplants for treatment of hematologic malignancies. All patients were treated with the same conditioning and similar postgrafting immunosuppressive regimens, and the criteria for HLA typing were uniform. The first 38 patients received twice-daily MMF, and the subsequent 47 patients received 3-times-daily MMF. Total and unbound MPA Css's were 1.5-fold increased in the 3-times-daily MMF group, suggesting that MPA has linear pharmacokinetics within this dose range. The last MPA concentration (Ctrough) was also higher with 3-times-daily dosing of MMF, as expected. The maximum plasma concentration, time to Cmax, and t½ were similar between the twice-daily and 3-times-daily groups. The pharmacodynamic analyses showed an important association between an increase in total MPA Css and increased donor CD3+ T-cell chimerism, as well as an increase in unbound Css and CMV reactivation. Regardless of twice-daily or 3-times-daily MMF treatment, patients with total MPA Css less than 3 μg/mL were at a statistically significant increased risk of low (< 50%) donor CD3 chimerism. Patients with less than 50% donor CD3 chimerism were at increased risk for graft rejection. Graft rejection was seen exclusively in those patients with total MPA Css less than 2.5 μg/mL. MPA plasma concentrations were not associated with the incidence of acute GVHD or disease relapse.
After conventional myeloablative conditioning and HCT with oral administration of twice-daily MMF, other investigators have reported total MPA Ctrough ranging between 0.1 and 0.5 μg/mL and Cmax less than 5 μg/mL.23 Similarly, following myeloablative conditioning and oral MMF administered twice daily, our group has reported a mean total MPA AUC of 11.9 μg · h/mL and Cmax of 3.3 μg/mL.24 Similar to previous reports in both conventional and nonmyeloablative HCT,6,22-24,26,37,38 the t½ of MPA was markedly reduced compared with healthy volunteers (3 versus 16 hours).19
The association between MPA Ctrough and Css is of particular clinical interest because Ctrough is more convenient to obtain. Ctrough only explains 32% and 49% of the variability in Css on days 7 and 21, respectively. Similarly, it was recently shown that total MPA Ctrough after HCT did not accurately describe MPA AUC.37 In the current study, Ctrough did not appear to be clinically useful. Further work is needed to develop limited sampling schemas to estimate AUC or Css after oral MMF administration in HCT patients, as has been described in solid organ transplantation patients.11,18
Among patients receiving the 3-times-daily MMF regimen, higher MPA Ctrough and Css were achieved compared with patients receiving twice-daily MMF. The total and unbound MPA Ctrough and Css obtained in patients enrolled in the 3-times-daily MMF group appeared to be similar to those described in solid organ transplant recipients at comparable time points after transplantation. The suggested therapeutic range for AUC0-12 between 30 and 60 μg · h/mL12,14,15 corresponds to a Css of 2.5 to 5 μg/mL, which has been achieved on at least one day of measurement in 10 of 38 patients in the twice-daily MMF group and in 34 of 47 patients in the 3-times-daily MMF group. The total MPA Cmax levels were similar to those described in adult solid organ transplantation39 for both twice-daily and 3-times-daily MMF groups. Drug interaction between cyclosporine and MMF12 did not affect our analyses, as the cyclosporine dose was adjusted to obtain the same therapeutic cyclosporine Ctrough range in all patients.
Although neutropenia has been associated with total and free MPA AUC in renal transplantation patients,16,21 we were unable to examine the association between neutropenia and MPA pharmacokinetics because a majority of patients developed neutropenia before the first MPA measurement (day 7). We did not observe moderate-severe gastrointestinal toxicity associated with elevated MPA Css. Another potential measure of MMF toxicity, the incidence of CMV reactivation, was significantly affected by unbound MPA Css but not by total MPA Css or Ctrough.
Both total and unbound MPA Css's appeared to influence the degree of donor CD3+ T-cell chimerism. In particular, all patients with less than 50% donor peripheral blood CD3+ chimerism also had a total MPA Css below 3 μg/mL, and all patients who subsequently rejected their grafts had a total MPA Css less than 2.5 μg/mL. Our current findings are consistent with what has been reported in the renal transplantation setting concerning the relationship between MPA concentration and acute graft rejection, since the recommended therapeutic total MPA AUC0-12 of at least 30 μg · h/mL12 corresponds to a Css of 2.5 μg/mL.
In contrast to other reports involving fewer numbers of patients,26,27,40 our results suggest that the incidence of acute GVHD was not influenced by MPA concentration. This observation in the current study may be confounded by the overall high incidence of grade II-IV acute GVHD (71% of patients). Finally, we did not observe a statistically significant association between MPA concentrations and the hazard of relapse or disease progression after transplantation. The conclusions of this study are limited to patients receiving the fludarabine and 2-Gy TBI conditioning regimen with cyclosporine-based GVHD prophylaxis.
In summary, this study suggests significant pharmacokinetic differences in the metabolism of MMF after nonmyeloablative HCT compared with either myeloablative conditioning and HCT or solid organ transplantation. Nonmyeloablative conditioning allowed for improved oral absorption of MMF compared with reported Cmax and AUC following conventional HCT. Treatment with 3-times-daily MMF significantly enhanced the MPA minimum and steady-state concentrations in plasma. MPA Ctrough was not sufficiently predictive of MPA Css; therefore, further pharmacokinetic studies are needed to determine if there is a more efficient and convenient method to measure MPA concentrations than by formal AUC assessment.
The most important pharmacodynamic observation of this study was that increased total MPA Css predicted a higher degree of donor T-cell chimerism after HCT, whereas increased unbound MPA Css predicted a higher risk of CMV reactivation. These 2 associations suggest that a narrow therapeutic range of MPA should be achieved to maximize donor chimerism while minimizing the risk of CMV reactivation. However, further data are needed to confirm these observations. Achieving total MPA Css levels greater than 3 μg/mL will increase the likelihood of achieving a high degree of donor T-cell chimerism and decrease the risk of graft rejection. The overall low incidence of graft rejection limited the statistical power of the analysis. Nonetheless, the observation that all patients with graft rejection had total MPA Css less than 2.5 μg/mL regardless of whether they received twice-daily or 3-times-daily MMF suggests that real-time pharmacokinetic monitoring of patients may be useful to guide MMF dose escalation or dose frequency increases after unrelated donor nonmyeloablative HCT.
Prepublished online as Blood First Edition Paper, September 6, 2005; DOI 10.1182/blood-2005-06-2217.
Supported in part by grants CA18029, CA78902, DK02753, HL36444, CA15704, and CA92058; G&P Foundation for Cancer Research; and Associazione Italiana Ricerca Cancro (AIRC), Consiglio Nazionale Ricerche, San Paolo Foundation.
L.G. and G.E.G. designed the research, performed the clinical aspects of the research, analyzed the data, and wrote or substantially edited the manuscript; J.S.M. designed the research, performed the pharmacologic analyses in the research, analyzed the data, and wrote or substantially edited the manuscript; M.B.M., B.M.S., R.A.N., and R.F.S. designed the research and performed the clinical aspects of the research; T.A.G. analyzed the data and wrote or substantially edited the manuscript; and J.T.S. and S.C. performed the pharmacologic analyses in the research and developed new analytic tools.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are very grateful to the patients who participated in this study. In addition, the authors wish to thank the research nurses Mary Hinds, Steve Minor, and John Sedgwick and the data coordinator Debbie Bassuk for their invaluable help in making the study possible. The authors also wish to thank Helen Crawford and Bonnie Larson for manuscript preparation and all physicians, nurses, and support personnel for their care of patients on this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal