1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), the activated vitamin D3 hormone, is a key regulator of calcium homeostasis and thereby indispensable for bone metabolism. In addition, 1α,25(OH)2D3 is known to mediate predominantly immunosuppressive responses in vitro and in vivo. It has been demonstrated that macrophages can produce 1α,25(OH)2D3 on activation with interferon γ (IFN-γ), although little is understood about the biologic significance of this response. We show here that 1α,25(OH)2D3 can selectively suppress key effector functions of IFN-γ–activated macrophages. Among these are the suppression of listericidal activity, the inhibition of phagocyte oxidase-mediated oxidative burst, and the suppression of important IFN-γ–induced genes, including Ccl5, Cxcl10, Cxcl9, Irf2, Fcgr1, Fcgr3, and Tlr2. The deactivation of IFN-γ–stimulated macrophages is dependent on a functional vitamin D receptor and 1α,25(OH)2D3 acts specifically on IFN-γ–activated macrophages, whereas the steroid has no effects on resting macrophages. Therefore, the 1α,25(OH)2D3–mediated suppression of macrophage functions is distinct from previously described macrophage deactivation mechanisms. In conclusion, our data indicate that the production of 1α,25(OH)2D3 by IFN-γ–stimulated macrophages might be an important negative feedback mechanism to control innate and inflammatory responses of activated macrophages.
Introduction
The steroid hormone 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) is known for its important role in regulating calcium homeostasis and bone mineralization.1 1α,25(OH)2D3 acts through a nuclear receptor, the vitamin D receptor (Vdr), which is a member of the steroid and thyroid hormone receptor superfamily. More recently, evidence has accumulated that the hormone can have important functions in the immune system. Expression of Vdr was found in different immune effector cells of the myeloid and lymphoid lineage under resting and activating conditions.2,3 These findings contributed to the hypothesis that locally produced 1α,25(OH)2D3 may perform regulatory functions on those cells. Indeed, over the past few years it has been demonstrated that 1α,25(OH)2D3 can act as an important immunosuppressive modulator. 1α,25(OH)2D3 has been shown to suppress T-cell proliferation4 and to decrease the production of the T helper type 1 (Th1) cytokines interleukin 2, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α), leading to the inhibition of Th1 cell development.5 Besides its direct effects on T cells, 1α,25(OH)2D3 and its analogs are potent inhibitors of dendritic cell (DC) differentiation and maturation and can impair the capacity of DCs to induce alloreactive T-cell activation.6,7 In line with this, Vdr-deficient mice have been shown to have an increased frequency of mature DCs in lymph nodes.8 Additional support for the immunomodulatory role of 1α,25(OH)2D3 in vivo came from studies of autoimmune diseases in several different animal models. It has been demonstrated that 1α,25(OH)2D3 can prevent or suppress experimental autoimmune encephalomyelitis,9 rheumatoid arthritis,10 systemic lupus erythematosus,11 type 1 diabetes,12 and inflammatory bowel disease,13,14 further supporting its potent suppressive effects on the immune system.
In contrast to its well-characterized effects on adaptive immune responses, much less is known about the effects of 1α,25(OH)2D3 on effectors of innate immunity, especially on macrophages. It has been shown that 1α,25(OH)2D3 can induce the differentiation of myeloid progenitors into macrophages.15,16 However, the effects of 1α,25(OH)2D3 on mature and activated macrophages that are involved in inflammatory reactions have not been characterized yet. Such possible effects might be of especial importance since it was demonstrated that macrophages can release biologically active 1α,25(OH)2D3 on activation with IFN-γ.17,18 The production of 1α,25(OH)2D3 by activated macrophages is regulated by the IFN-γ–mediated induction of 1α-hydroxylase expression, the enzyme controlling the last step of 1α,25(OH)2D3 synthesis.17,18 In granulomatous diseases, such as sarcoidosis and tuberculosis, dysregulated production of 1α,25(OH)2D3 by activated macrophages can lead to hypercalcemia due to elevated levels of circulating 1α,25(OH)2D3 in the serum of patients.19,20 Because the differentiation of myeloid precursors into mature macrophages is associated with a down-regulation of Vdr expression, it has been hypothesized that the macrophage-derived 1α,25(OH)2D3 acts principally on other immune effector cells such as T cells, DCs, and monocytes but not on mature macrophages themselves.21,22
Here, we show that treatment of mature, primary murine macrophages with IFN-γ and 1α,25(OH)2D3 induces a synergistic up-regulation of Vdr mRNA expression and a subsequent accumulation of the Vdr protein in the cell nucleus. Under these conditions, 1α,25(OH)2D3 exerts strong suppressive effects on IFN-γ–stimulated macrophages, which include the inhibition of listericidal activity and suppression of oxidative burst. The effects depend on the 1α,25(OH)2D3 concentration and on a functional Vdr but are not present in nonactivated or lipopolysaccharide (LPS)–activated macrophages. Moreover, 1α,25(OH)2D3 treatment of IFN-γ–stimulated macrophages inhibits the expression of important IFN-γ–induced genes (eg, Ccl5, Cxcl16, Cxcl10, Cxcl9, Irf2, Ifi203, Fcgr1, Fcgr3, Tlr2). These findings demonstrate a new negative feedback mechanism of 1α,25(OH)2D3 on inflammatory macrophage reactions and have a potential application for the future design of anti-inflammatory therapies.
Materials and methods
Reagents
Recombinant murine IFN-γ was purchased from PeproTech (London, United Kingdom). Recombinant murine macrophage colony-stimulating factor (M-CSF), 1α,25(OH)2D3, 4-nitroblue-tetrazoliumchlorid (NBT), phorbol 12-myristate 13-acetate (PMA), and safranin O were from Sigma-Aldrich (Munich, Germany); gentamicin was obtained from Invitrogen (Karlsruhe, Germany). IFN-γ was diluted in phosphate-buffered saline (PBS) and used at a final concentration of 500 U/mL. 1α,25(OH)2D3 was initially dissolved in ethanol and added to the cell culture medium at a dilution of 1:1000 (final concentrations of 0.04, 0.4, 4, 40 nM), whereas the controls included the respective amounts of ethanol.
Mice and bacteria
C57BL/6 mice were purchased from Harlan (Borchen, Germany) and bred in the animal facilities of the German Research Center for Biotechnology (GBF). Vdr tm1 Rge knockout mice23 were obtained from Reinhold Erben (Vienna, Austria) and maintained on a C57BL/6 background. Listeria monocytogenes (L monocytogenes) and the listeriolysin (hly)–deficient listeriolysin mutant24 were grown in brain-heart infusion broth (BHI; Difco, Becton Dickinson, Baltimore, MD).
Macrophage isolation
Bone marrow-derived macrophages (BMDMs) were differentiated from bone marrow cells of 8- to 14-week-old mice. Briefly, femora and tibia of the hind legs were flushed with cold Dulbecco modified Eagle medium (DMEM; Invitrogen) containing 10% heat-inactivated fetal calf serum (FCS; Biowest, Nuaillé, France), 200 mM l-glutamine, and 10 000 U/mL penicillin/streptomycin (P/S; Invitrogen). This complete medium was supplemented with M-CSF (50 ng/mL) and bone marrow cells were cultured for 7 days. Thioglycolate-elicited macrophages were obtained 4 days after the intraperitoneal injection of sterile 3% thioglycolate medium. Cells were harvested by peritoneal lavage with cold PBS and 1% FCS and were cultured in RPMI 1640 (Invitrogen) including 10% heat-inactivated FCS, l-glutamine, and P/S.
Determination of listericidal activity
Macrophages were plated at 8 × 104/well in 96-well plates and cultured in P/S-free medium in the presence or absence of IFN-γ,1α,25(OH)2D3, or both for 24 and 48 hours. Cells were infected with L monocytogenes at a multiplicity of infection (MOI) of 0.1 for 15 minutes. When specifically indicated, infection experiments were performed with L monocytogenes that were opsonized in 10% mouse serum from C57BL/6J mice for 10 minutes. Extracellular growth of L monocytogenes after infection was prevented by the addition 10 μg/mL gentamicin and numbers of intracellular bacteria were determined after 1 and 3 hours. Macrophages were lysed in PBS containing 1% saponin and intracellular bacteria were quantified by counting the number of colony forming units (CFUs) in the lysate on BHI plates. Alternatively, macrophages grown on coverslips were infected with L monocytogenes and fixed with 4% paraformaldehyde (PFA) in PBS for 15 minutes on ice. Coverslips were then stained using the Hemacolor kit (Merck, Darmstadt, Germany) according to the supplied protocol.
Measurements of reactive oxygen species by the NBT reduction assay
BMDMs were plated at 2.5 × 105/well in 24-well plates or grown on coverslips. Cells were cultured in the presence or absence of IFN-γ,1α,25(OH)2D3, or both for 48 hours, washed, and cultured for 1 hour in serum-free DMEM supplemented with 0.1% NBT in the presence or absence of PMA (170 ng/mL). Cells grown on coverslips were fixed for 15 minutes with 4% PFA and nuclei were counterstained for 30 seconds by safranin O (0.1% in PBS). For photometrical quantification, cells were fixed in 100% methanol (15 minutes) and washed twice in 70% methanol and plates were dried at room temperature. The fixed cells were homogenized in 62.5 μL 2 M KOH and 75 μL dimethyl sulfoxide per well, and the OD650 of 100 μL of the cell lysate was determined.
RNA isolation, gene expression profiling, and quantitative RT-PCR
RNA was isolated using TRIzol reagent (Invitrogen). For biotin-labeled target synthesis starting from 3 μg total RNA, reactions were performed using standard protocols supplied by the manufacturer (Affymetrix; Santa Clara, CA). Briefly, 3 μg total RNA was converted to dsDNA using 100 pmol of a T7T23V primer (Eurogentec; Seraing, Belgium) containing a T7 promoter. The cDNA was then used directly in an in vitro transcription reaction in the presence of biotinylated nucleotides. The concentration of biotin-labeled cRNA was determined by UV absorbance. In all cases, 12.5 μg of each biotinylated cRNA preparation was fragmented and placed in a hybridization cocktail containing 4 biotinylated hybridization controls (BioB, BioC, BioD, and Cre) as recommended by the manufacturer. Samples were hybridized to an identical lot of Affymetrix MOE430A for 16 hours. Analysis was done with gene expression software (MAS5, MicroDB and Data Mining Tool 3.0, all Affymetrix) at the Array Facility of the German Research Center for Biotechnology. The Genesis software package was applied for the generation of heat maps and cluster analysis (http://genome.tugraz.at).25 Gene expression profiling data were deposited at the GEO repository under the accession number GSE2421.
For reverse transcription-polymerase chain reaction (RT-PCR), 1 μg RNA was reverse transcribed using random hexamers (Amersham Bioscience, Freiburg, Germany) and Superscript II RNase H- Reverse Transcriptase (Invitrogen). Real-time quantitative PCR was performed on an Applied Biosystems RT-PCR System (PRISM T 7000) using the Brilliant SYBR Green QPCR Core Reagent Kit (Stratagene, La Jolla, CA). Expression was normalized to housekeeping genes (Rps9 or Gapdh) and to unstimulated wild-type controls. Oligonucleotide primers used for amplification are listed in the Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Immunoblotting and immunohistochemistry
For Western blot analysis, macrophages were plated at 1.5 × 106/well in 6-well plates and lysed with 50 mM Tris (tris(hydroxymethyl)aminomethane)/HCl, pH7.5, 150 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 1% Triton X-100, 0.5% NP-40, 1 mM Na3VO4, 1 mM NaF, and protease inhibitor cocktail (CompleteMini; Roche, Mannheim, Germany). Lysates were cleared by centrifugation at 1 500g for 5 minutes at 4°C. Equal amounts of total protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Schwalbach, Germany). Membranes were blocked in 20 mM Tris/HCl, pH 7.5, 137 mM NaCl, and 10% FCS for 4 hours at room temperature followed by an overnight incubation simultaneously with 2 rabbit anti–phospho signal transducer and activator of transcription 1 (Stat1) antibodies (Cell Signaling, Upstate, Dundee, United Kingdom). After incubation with secondary horseradish peroxidase-conjugated antirabbit antibody (Amersham), blots were developed using the enhanced chemiluminescence system (ECLPlus; Amersham).
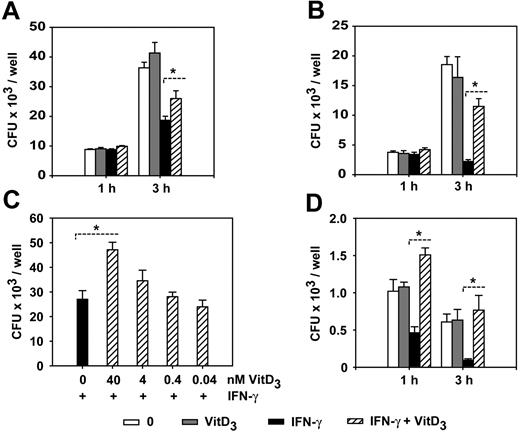
1α,25(OH)2D3 inhibits the listericidal activity in IFN-γ–activated macrophages. (A) BMDMs were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3 for 24 hours and infected with opsonized L monocytogenes (MOI = 0.1) for 1 hour and 3 hours. Extracellular growth of L monocytogenes was prevented by the addition of gentamicin to the medium and intracellular bacteria were quantified by counting the number of CFUs in the cell lysates on BHI plates. (B) BMDMs were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3 for 48 hours and infected and analyzed as described in panelA. The data are representative of at least 5 independent experiments. Similar results were obtained when nonopsonized L monocytogenes was used in the infection experiments. (C) BMDMs were cultured with IFN-γ and different concentrations of 1α,25(OH)2D3 and infected with L monocytogenes for 3 hours. (D) BMDMs were cultured with IFN-γ and 1α,25(OH)2D3 and infected with L monocytogenes mutants deficient for listeriolysin (del hly). Experiments were repeated twice with similar results (A,C-D). Data are depicted as the mean ± SEM calculated from triplicate wells (plating was carried out in duplicate). *P < .05; Wilcoxon-signed rank test. VitD3 indicates 1α,25-dihydroxycholecalciferol. IFN-γ = 500 U/mL, VitD3 = 40 nM, and treatment was performed for 48 hours except as otherwise indicated.
1α,25(OH)2D3 inhibits the listericidal activity in IFN-γ–activated macrophages. (A) BMDMs were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3 for 24 hours and infected with opsonized L monocytogenes (MOI = 0.1) for 1 hour and 3 hours. Extracellular growth of L monocytogenes was prevented by the addition of gentamicin to the medium and intracellular bacteria were quantified by counting the number of CFUs in the cell lysates on BHI plates. (B) BMDMs were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3 for 48 hours and infected and analyzed as described in panelA. The data are representative of at least 5 independent experiments. Similar results were obtained when nonopsonized L monocytogenes was used in the infection experiments. (C) BMDMs were cultured with IFN-γ and different concentrations of 1α,25(OH)2D3 and infected with L monocytogenes for 3 hours. (D) BMDMs were cultured with IFN-γ and 1α,25(OH)2D3 and infected with L monocytogenes mutants deficient for listeriolysin (del hly). Experiments were repeated twice with similar results (A,C-D). Data are depicted as the mean ± SEM calculated from triplicate wells (plating was carried out in duplicate). *P < .05; Wilcoxon-signed rank test. VitD3 indicates 1α,25-dihydroxycholecalciferol. IFN-γ = 500 U/mL, VitD3 = 40 nM, and treatment was performed for 48 hours except as otherwise indicated.
For Vdr antibody staining, macrophages were grown on glass coverslips and fixed in ice-cold methanol for 5 minutes and permeabilized in PBS, 0.5% Tween-20 for 5 minutes. Staining was performed using the rat anti–vitamin D receptor antibody (Research Diagnostics, Concord, MA) and reagents included in the Ready-to-Use Vectastain Kit (Vector Labs, Peterborough, United Kingdom) according to the manufacturer's instructions with the following modifications. The incubation with the primary antibody was performed overnight at 4°C and washes were done with PBS, 0.1% Tween-20. Avidin-fluorescein isothiocyanate (Vector) diluted in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonice acid, 150 mM NaCl was used for detection. Imaging analysis of confocal microscopy (Figure 4C) was performed with a Zeiss (Göttingen, Germany) LSM 510 inverted confocal laser scanning microscope using a Plan-Neofluar 100 ×/1.4 numerical aperture (NA) oil immersion lens. Fixed wild-type and Vdr-KO cells were excited with an argon laser at 488 nm at low laser intensity (2.5%), and emission was collected using a 505- to 550-nm bandpass filter to monitor signals from the immunostaining. The scanning for all images shown in Figure 4C was done with a pinhole of 203 μm and 12-bit data depth. Light microscopy of Hemacolor- or NBT-stained macrophages (Figures 2, 3A-B, and 4D) was performed using an inverted Zeiss Axiovert 100 microscope using either an LD Acroplan 40 ×/0.6 NA objective lens or a Plan-Neofluar 100 ×/1.3 NA oil immersion lens, as indicated by the magnification in the figure legends. A digital AxioCam HRc camera (Zeiss) was used for documentation.
Results
1α,25(OH)2D3 specifically inhibits the listericidal activity of IFN-γ–stimulated macrophages by suppression of oxidative burst
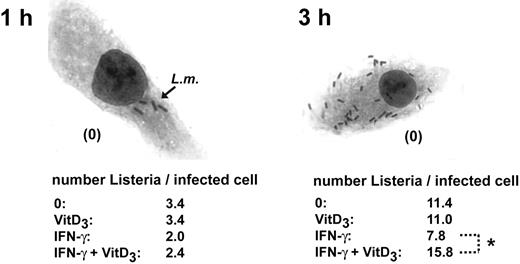
Previously, it was demonstrated that exposure of the myeloblastic cell line HL-60 to 1α,25(OH)2D3 can modulate its bactericidal activity.26 To investigate how 1α,25(OH)2D3 might modulate the bacterial killing activity of mature, primary macrophages, we examined the effects of 1α,25(OH)2D3 on the antimicrobial response of murine BMDMs. In addition, we also investigated the effect of 1α,25(OH)2D3 on the bactericidal activity of IFN-γ–activated macrophages. To evaluate bactericidal activity, macrophages were infected with the intracellular, Gram-positive bacterium L monocytogenes. IFN-γ is known to be essential for the control of the intracellular growth of L monocytogenes27,28 BMDMs were left untreated or treated with 1α,25(OH)2D3, IFN-γ, or both for 24 and 48 hours and infected with L monocytogenes at an MOI of 0.1. After infection for 1 and 3 hours, we quantified the listericidal activity of macrophages by plating and counting surviving intracellular bacteria. Treatment of macrophages with 1α,25(OH)2D3 alone showed no influence on the listericidal activity of mature macrophages. As expected, IFN-γ activation of BMDMs led to decreased amounts of intracellular L monocytogenes. Remarkably, even though we were not able to detect any influence of 1α,25(OH)2D3 on the listericidal activity of nonstimulated macrophages, we found that combined treatment with IFN-γ and 1α,25(OH)2D3 resulted in a significant inhibition of IFN-γ–induced listericidal activity (Figure 1). This diminished bacterial killing activity was observed when macrophages were pretreated with IFN-γ and 1α,25(OH)2D3 for 24 hours (Figure 1A), but was more pronounced after pretreatment for 48 hours (Figure 1B). The increased numbers of intracellular bacteria in 1α,25(OH)2D3/IFN-γ–treated macrophages were also confirmed at the single-cell level (Figure 2). We found that 1α,25(OH)2D3 suppressed the listericidal activity of IFN-γ–stimulated BMDMs in a concentration-dependent manner (Figure 1C), but did not interfere with phagocytotic L monocytogenes uptake (quantified 20 minutes after infection; data not shown). Taken together, these results demonstrate that 1α,25(OH)2D3 is able to specifically inhibit the listericidal activity of IFN-γ–stimulated macrophages, whereas 1α,25(OH)2D3 has no obvious effect on resting macrophages. It is known that after phagocytosis L monocytogenes evades the killing by escaping from vacuoles into the cytoplasm. To investigate if the 1α,25(OH)2D3 treatment affected the ability of the macrophages to kill the bacteria within the phagosomal vacuole rather than allowing an enhanced growth of Listeria in the cytoplasm, we infected BMDMs with a mutant L monocytogenes strain, which is deficient for the listeriolysin (hly) gene. The del hly mutants of Listeria are unable to disrupt the phagosomal membrane because they lack the pore-forming listeriolysin O, a key factor for host cytosol entry.29 Usually, del hly mutants are rapidly killed by macrophages due to their inability to escape from the phagosome. We found that 1α,25(OH)2D3 treatment of IFN-γ–activated macrophages markedly suppressed the killing of the attenuated del hly mutant (Figure 1D), thus demonstrating that the 1α,25(OH)2D3-mediated inhibition of the listericidal activity is acting on the phagosome.
1α,25(OH)2D3 inhibits the antilisterial activity in IFN-γ–activated macrophages, single-cell analysis. BMDMs were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3 and infected with L monocytogenes (MOI = 0.1) for 1 hour and 3 hours. Cells were stained using Hemacolor and analyzed microscopically and the number of intracellular bacteria per infected cell was quantified by counting (original magnification × 400). Data are presented as the mean calculated from 50 cells analyzed per condition. *P < .05; Mann-Whitney U test. The data are representative of 3 independent experiments.
1α,25(OH)2D3 inhibits the antilisterial activity in IFN-γ–activated macrophages, single-cell analysis. BMDMs were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3 and infected with L monocytogenes (MOI = 0.1) for 1 hour and 3 hours. Cells were stained using Hemacolor and analyzed microscopically and the number of intracellular bacteria per infected cell was quantified by counting (original magnification × 400). Data are presented as the mean calculated from 50 cells analyzed per condition. *P < .05; Mann-Whitney U test. The data are representative of 3 independent experiments.
We also examined if the 1α,25(OH)2D3-mediated suppression of the IFN-γ–induced microbicidal activity was specific for macrophages differentiated from the bone marrow. Using peritoneal, thioglycolate-elicited macrophages we found that 1α,25(OH)2D3 was also able to inhibit IFN-γ–induced bacterial killing in these primary cells (data not shown), demonstrating that the 1α,25(OH)2D3-mediated suppression is functional in different populations of mature macrophages. The effect was observed using nonopsonized and opsonized Listeria. Under opsonizing conditions the induced killing activity by IFN-γ and the inhibition of bactericidal activity in cells treated with IFN-γ and 1α,25(OH)2D3 was even more pronounced (Figure 1A-B and data not shown).
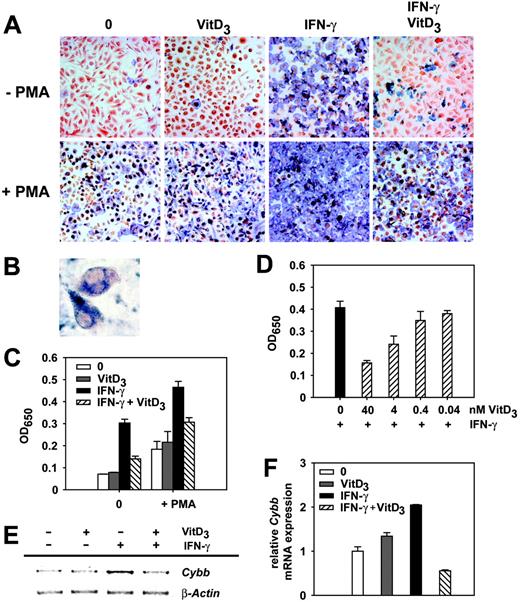
It has been established that the listericidal activity of macrophages depends on the generation of superoxide anion and reactive nitrogen by the phagocyte oxidase (phox) and the inducible nitric oxide synthase (iNOS), respectively. We therefore addressed if 1α,25(OH)2D3 might inhibit these mechanisms of antimicrobial activity in IFN-γ–stimulated macrophages. We first examined the generation of intracellular superoxide anion radicals (O2-·) after treatment of BMDMs with IFN-γ or 1α,25(OH)2D3 using the NBT assay, which is based on the conversion of nitroblue tetrazolium into blue formazan precipitates. Treatment of macrophages with 1α,25(OH)2D3 alone did not influence superoxide anion production by mature macrophages. As expected, stimulation with IFN-γ strongly increased the production of O2-·, whereas simultaneous treatment of BMDMs with IFN-γ and 1α,25(OH)2D3 clearly inhibited the IFN-γ–induced generation of superoxide (Figure 3A-C). The suppression of oxidative burst by 1α,25(OH)2D3 was visible with and without addition of PMA, which is a strong inducer of superoxide anion production.30 As already shown for bacterial killing (Figure 1C), we found a strong correlation between the dose of 1α,25(OH)2D3 and its suppressive effects on O2- · generation (Figure 3D). Therefore, these data suggest that the 1α,25(OH)2D3-mediated inhibition of the oxidative burst is responsible for the diminished killing activity of IFN-γ–activated macrophages. To address this in more detail we quantified the expression levels of the genes for the different key components of phox by RT-PCR. Indeed, we found that Cybb expression (encoding gp91phox) was reduced in IFN-γ– and 1α,25(OH)2D3-treated BMDMs when compared to macrophages treated with IFN-γ alone (Figure 3E-F). Therefore, the inhibition of Cybb expression by 1α,25(OH)2D3 seems to be responsible for the suppression of the IFN-γ–induced oxidative burst. In contrast, we could not detect any influence of 1α,25(OH)2D3 on the generation of nitric oxide in macrophages pretreated with IFN-γ and 1α,25(OH)2D3 and infected with L monocytogenes (data not shown). In conclusion, our results suggest that 1α,25(OH)2D3-mediated inhibition of the Cybb expression in IFN-γ–activated macrophages leads to a suppression of oxidative burst and consequently to a reduced killing of intracellular L monocytogenes.
1α,25(OH)2D3 inhibits the oxidative burst in IFN-γ–activated macrophages. (A) Intracellular production of superoxide anion was measured by the conversion of NBT into formazan. BMDMs grown on coverslips were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3. NBT (0.1%) with or without PMA was added for 1 hour and cells were subsequently fixed. Shown is a representative of 3 independent experiments. Original magnification × 100. (B) Single cells from panel A demonstrate specific intracellular formazan precipitation after cellular activation with PMA or IFN-γ. Original magnification × 200. (C) Quantification of O2- · production. BMDMs were cultured and NBT/PMA was added as described in panel A. The OD650 of cell homogenates was determined photometrically. (D) BMDMs were cultured with IFN-γ and different concentrations of 1α,25(OH)2D3. NBT was added and O2- · production was quantified as described. Data are presented as the mean ± SEM calculated from triplicate wells; experiments were repeated twice with similar results (C-D). (E) Semiquantitative Cybb RT-PCR. BMDMs were incubated with IFN-γ and VitD3 as described. Cybb- and β-actin–specific primers were used for amplification. Shown is a representative of 3 independent experiments. (F) Real-time Cybb RT-PCR. RNA for analysis was prepared from BMDMs described in panel E and mRNA expression was normalized as described (= relative mRNA expression).
1α,25(OH)2D3 inhibits the oxidative burst in IFN-γ–activated macrophages. (A) Intracellular production of superoxide anion was measured by the conversion of NBT into formazan. BMDMs grown on coverslips were cultured in the presence or absence of IFN-γ and 1α,25(OH)2D3. NBT (0.1%) with or without PMA was added for 1 hour and cells were subsequently fixed. Shown is a representative of 3 independent experiments. Original magnification × 100. (B) Single cells from panel A demonstrate specific intracellular formazan precipitation after cellular activation with PMA or IFN-γ. Original magnification × 200. (C) Quantification of O2- · production. BMDMs were cultured and NBT/PMA was added as described in panel A. The OD650 of cell homogenates was determined photometrically. (D) BMDMs were cultured with IFN-γ and different concentrations of 1α,25(OH)2D3. NBT was added and O2- · production was quantified as described. Data are presented as the mean ± SEM calculated from triplicate wells; experiments were repeated twice with similar results (C-D). (E) Semiquantitative Cybb RT-PCR. BMDMs were incubated with IFN-γ and VitD3 as described. Cybb- and β-actin–specific primers were used for amplification. Shown is a representative of 3 independent experiments. (F) Real-time Cybb RT-PCR. RNA for analysis was prepared from BMDMs described in panel E and mRNA expression was normalized as described (= relative mRNA expression).
The suppression of IFN-γ–induced responses depends on Vdr expression, localization, and function but is independent of Stat1
Next, we investigated if the suppressive effects of 1α,25(OH)2D3 on IFN-γ–activated macrophages were dependent on Vdr. We first examined the mRNA expression of the Vdr gene after 48 hours of treatment with IFN-γ and 1α,25(OH)2D3 using real-time RT-PCR. Quantification of Vdr mRNA levels showed that treatment of BMDMs with 1α,25(OH)2D3 but also the activation of macrophages with IFN-γ led to an induction of Vdr expression, which was 135-fold and 27-fold, respectively (Figure 4A). Interestingly, simultaneous treatment of macrophages with IFN-γ and 1α,25(OH)2D3 induced a 735-fold and therefore synergistic expression of Vdr (Figure 4A). This demonstrates that Vdr expression can be induced in IFN-γ–stimulated macrophages and that IFN-γ and 1α,25(OH)2D3 are capable of synergistically inducing Vdr expression after 48 hours. This is in line with our previous observation that the biologic responses of the IFN-γ and 1α,25(OH)2D3 stimulation, such as the inhibition of listericidal activity and suppression of the oxidative burst, were most effective after 48 hours of treatment (Figures 1A-B and 3A-C). To investigate the kinetics of induction of Vdr expression we quantified mRNA levels at earlier time points of stimulation. We found that the Vdr gene was synergistically induced after 12 hours of treatment with IFN-γ and 1α,25(OH)2D3, but not after 6 hours of stimulation (Figure 4B). As a nuclear receptor, the ligand-bound Vdr exerts its effects by translocation to the nucleus. IFN-γ treatment of macrophages showed that most of the detectable Vdr protein was still localized in the cytoplasm (Figure 4C). In contrast, combined treatment of BMDMs with 1α,25(OH)2D3 and IFN-γ resulted in an accumulation of Vdr in the nucleus (Figure 4C).
The roles of the Vdr and Stat1 for the 1α,25(OH)2D3-mediated inhibitory effects. (A) Real-time Vdr RT-PCR. BMDMs were incubated with IFN-γ or 1α,25(OH)2D3 or both for 48 hours, and total RNA was isolated and reverse transcribed. Vdr-specific primers were used for amplification and mRNA expression was normalized as described (= relative mRNA expression). Synergistic induction of Vdr mRNA by IFN-γ and 1α,25(OH)2D3 was confirmed in 3 additional experiments. (B) Time kinetics of Vdr mRNA induction. Vdr transcript levels were determined as described in panel A using real-time RT-PCR. (C) Intracellular localization of Vdr protein. BMDMs were cultured as described in panel A, fixed, and stained with an anti-Vdr antibody (green) and analyzed by confocal microscopy. (D) The inhibition of O2- · production by 1α,25(OH)2D3 is dependent on a functional Vdr. BMDMs isolated from WT and Vdr-KO mice were cultured with IFN-γ and 1α,25(OH)2D3 and intracellular production of O2- · was analyzed. (E) The 1α,25(OH)2D3-mediated inhibition of listericidal activity depends on the Vdr. BMDMs from WT and Vdr-KO mice were cultured with IFN-γ and 1α,25(OH)2D3 and infected with opsonized L monocytogenes for 3 hours as described in Figure 1. Data are presented as the mean ± SEM calculated from triplicate wells (plating was carried out in duplicate). (F) Real-time RT-PCR of Cybb. BMDMs (WT and Vdr-KO) were cultured as described in panel A. Cybb-specific primers were used for amplification and mRNA expression was normalized as described. The experiment was repeated 3 times with similar results. (G) Quantification of activated Stat1 after 1α,25(OH)2D3 and IFN-γ treatment. BMDMs were cultured as described in panel A and the amount of tyrosine 701-phosphorylated Stat1 protein was determined. *P < 0.05; Wilcoxon-signed rank test. Shown is a representative of at least 3 independent experiments (C-G).
The roles of the Vdr and Stat1 for the 1α,25(OH)2D3-mediated inhibitory effects. (A) Real-time Vdr RT-PCR. BMDMs were incubated with IFN-γ or 1α,25(OH)2D3 or both for 48 hours, and total RNA was isolated and reverse transcribed. Vdr-specific primers were used for amplification and mRNA expression was normalized as described (= relative mRNA expression). Synergistic induction of Vdr mRNA by IFN-γ and 1α,25(OH)2D3 was confirmed in 3 additional experiments. (B) Time kinetics of Vdr mRNA induction. Vdr transcript levels were determined as described in panel A using real-time RT-PCR. (C) Intracellular localization of Vdr protein. BMDMs were cultured as described in panel A, fixed, and stained with an anti-Vdr antibody (green) and analyzed by confocal microscopy. (D) The inhibition of O2- · production by 1α,25(OH)2D3 is dependent on a functional Vdr. BMDMs isolated from WT and Vdr-KO mice were cultured with IFN-γ and 1α,25(OH)2D3 and intracellular production of O2- · was analyzed. (E) The 1α,25(OH)2D3-mediated inhibition of listericidal activity depends on the Vdr. BMDMs from WT and Vdr-KO mice were cultured with IFN-γ and 1α,25(OH)2D3 and infected with opsonized L monocytogenes for 3 hours as described in Figure 1. Data are presented as the mean ± SEM calculated from triplicate wells (plating was carried out in duplicate). (F) Real-time RT-PCR of Cybb. BMDMs (WT and Vdr-KO) were cultured as described in panel A. Cybb-specific primers were used for amplification and mRNA expression was normalized as described. The experiment was repeated 3 times with similar results. (G) Quantification of activated Stat1 after 1α,25(OH)2D3 and IFN-γ treatment. BMDMs were cultured as described in panel A and the amount of tyrosine 701-phosphorylated Stat1 protein was determined. *P < 0.05; Wilcoxon-signed rank test. Shown is a representative of at least 3 independent experiments (C-G).
To address if Vdr is indeed functionally involved in the observed inhibitory effects of 1α,25(OH)2D3 on IFN-γ–stimulated macrophages, we investigated the induction of oxidative burst and the listericidal activity in BMDMs obtained from Vdr knockout mice23 (Vdr-KO). We found that macrophages from Vdr-KO mice were not able to suppress superoxide anion generation after treatment with 1α,25(OH)2D3 and IFN-γ (Figure 4D) and were consequently still able to control the intracellular growth of L monocytogenes under this condition (Figure 4E). In line with the previously obtained results, the 1α,25(OH)2D3-mediated down-regulation of Cybb mRNA in IFN-γ/1α,25(OH)2D3-treated macrophages was not detectable in Vdr-KO macrophages using real-time RT-PCR (Figure 4F). These results demonstrate that the observed suppressive effects of 1α,25(OH)2D3 on IFN-γ–stimulated macrophages depend on a functional Vdr.
Because we found that 1α,25(OH)2D3 specifically suppressed IFN-γ–induced effector mechanisms, we tested if this effect was dependent on Stat1, an essential component of the classical IFN-γ signal transduction pathway. Ligation of the IFN-γ receptor triggers activation of Stat1 by phosphorylation on tyrosine 701, which is a prerequisite for Stat1 nuclear translocation and subsequent transcriptional regulation of downstream target genes. We therefore evaluated the influence of 1α,25(OH)2D3 on the amount of tyrosin 701-phosphorylated Stat1 protein in IFN-γ–activated BMDMs. Western blot analysis showed that activation of Stat1 was not influenced by 1α,25(OH)2D3 (Figure 4G).
Stimulation of macrophages with IFN-γ and 1α,25(OH)2D3 leads to a suppression of several important IFN-γ target genes
Macrophage activation by IFN-γ leads to the expression of several cytokines and chemokines that are key mediators of inflammatory responses. We therefore investigated if 1α,25(OH)2D3 might directly influence the expression of IFN-γ target genes in macrophages. We initially screened for genes differentially expressed in BMDMs treated with 1α,25(OH)2D3, IFN-γ, or both using gene expression profiling. We found that the expression of about 130 genes from more than 400 IFN-γ–induced genes was significantly inhibited by simultaneous treatment with 1α,25(OH)2D3 (Figure S1 and data not shown). Among those, some were known to be crucial for IFN-γ–mediated regulation of innate and adaptive immune responses. We further quantified and compared the expression of several identified genes in BMDMs isolated from Vdr-KO and wild-type (WT) mice using real-time RT-PCR to analyze if the observed suppression is dependent on Vdr (Figure 5). IFN-γ treatment of WT and Vdr-KO macrophages strongly induced expression of the Th1 cell regulatory chemokines Ccl5 (RANTES), Cxcl10 (IP-10), Cxcl9 (MIG), and Cxcl16 (SR-PSOX). Double treatment of macrophages clearly suppressed the induction of these proinflammatory chemokines in WT macrophages by a factor of 2 to 3, but not in Vdr-deficient macrophages. The same pattern of suppression was also detectable for additional IFN-γ–inducible genes. Analysis of the interferon regulatory factor 2 (Irf2), interferon-activated gene 203 (Ifi203), and chemokine receptor 5 (Ccr5) showed a specific Vdr-dependent inhibition of their expression. Interestingly, also the IFN-γ–induced expression of the Fc receptors 1 and 3 (Fcgr1 and Fcgr3, respectively), which are key receptors in the development of immune responses and also the Toll-like receptor 2 (Tlr2) gene, which is important for recognition of Gram-positive bacteria including L monocytogenes, were suppressed by 1α,25(OH)2D3, further demonstrating that the host defense program of IFN-γ–activated macrophages can be notably inhibited by 1α,25(OH)2D3 (Figure 5). Taken together, our results clearly demonstrate that 1α,25(OH)2D3 can specifically suppress the IFN-γ activation of macrophages via inhibition of important IFN-γ target genes. This suppression is strictly dependent on Vdr.
1α,25(OH)2D3 inhibits the expression of numerous IFN-γ–induced genes. Real-time quantitative PCR analysis of IFN-γ–induced gene expression. BMDMs isolated from WT or Vdr-KO mice were incubated with IFN-γ or 1α,25(OH)2D3 or both. Gene-specific primers were used for amplification and mRNA expression was normalized as described. Analysis was performed in duplicate and data are presented as the mean ± SEM. Differential gene expression was confirmed at least in 2 independent experiments.
1α,25(OH)2D3 inhibits the expression of numerous IFN-γ–induced genes. Real-time quantitative PCR analysis of IFN-γ–induced gene expression. BMDMs isolated from WT or Vdr-KO mice were incubated with IFN-γ or 1α,25(OH)2D3 or both. Gene-specific primers were used for amplification and mRNA expression was normalized as described. Analysis was performed in duplicate and data are presented as the mean ± SEM. Differential gene expression was confirmed at least in 2 independent experiments.
Discussion
We show here that 1α,25(OH)2D3 can act as a potent suppressor of IFN-γ–induced macrophage activation. Previous studies investigating the influence of 1α,25(OH)2D3 on macrophage function have mainly analyzed the ability of the steroid to induce differentiation of myeloid precursors into macrophages or showed influences of 1α,25(OH)2D3 on myeloid cell lines and monocytes but did not address the effects of 1α,25(OH)2D3 on mature macrophages.15,16,26,31 However, early on it was recognized that monocytes down-regulate the expression of Vdr when they differentiate into mature macrophages while their capability to synthesize 1α,25(OH)2D3 is increased21 . Therefore, it was hypothesized that the high amounts of 1α,25(OH)2D3 that can be released by mature, activated macrophages would act rather in a paracrine fashion but not on the 1α,25(OH)2D3-secreting macrophage itself.22
Using mature macrophages we show that IFN-γ activation together with the simultaneous presence of 1α,25(OH)2D3 leads to a strong synergistic induction of Vdr expression. Under this condition, 1α,25(OH)2D3 is specifically able to suppress major IFN-γ responses of macrophages. Among those are the induction of oxidative burst, microbicidal killing activity, and the expression of important mediators of inflammation and host defense, including Ccl5, Cxcl9, Cxcl10, Cxcl16, Fcgr1, Fcgr3, lrf2, lfi203, Ccr5, and Tlr2.
Based on our data, we are suggesting a new negative feedback loop model on the way by which 1α,25(OH)2D3 might regulate IFN-γ responses in mature macrophages in an autocrine, dose-dependent fashion. (1) IFN-γ stimulation of macrophages induces the release of 1α,25(OH)2D3; however, at these low doses the steroid has no effect on macrophages because the Vdr is expressed at low levels. (2) IFN-γ induces expression of the Vdr, but at low concentrations of 1α,25(OH)2D3 the Vdr protein is predominantly present in the cytoplasm. (3) Finally, when the 1α,25(OH)2D3 accumulates and its concentration passes a certain threshold, Vdr expression is synergistically induced and the Vdr protein is able to translocate to the nucleus. This leads to the suppression of IFN-γ–induced gene expression in macrophages.
We also found that 1α,25(OH)2D3/IFN-γ treatment decreased the listericidal activity of macrophages. This correlated with a strong 1α,25(OH)2D3-mediated suppression of oxidative burst, which was most likely caused by transcriptional inhibition of the gp91phox (Cybb) component of the phox complex. In addition, other factors that are affected by 1α,25(OH)2D3 might also contribute together with gp91phox to the decreased listericidal activity. In contrast to the results presented here, it has been reported for human fibrosarcoma cell lines and monocytes, that Stat1/Vdr protein-protein interactions lead to a synergistic effect on IFN-γ–mediated transcription mediated by a prolonged phosphorylation of Stat1.31 In mature murine macrophages, however, we found that 1α,25(OH)2D3 had no influence on the amount of tyrosin 701-phophorylated Stat1 and that 1α,25(OH)2D3 could lead to the inhibition of the listericidal activity independently on Stat1. Therefore, it is very likely that the underlying mechanisms are distinct. It is possible that the effect of 1α,25(OH)2D3 on IFN-γ responses is dependent on the differentiation status of the cell and therefore markedly differs in myeloid precursors/monocytes and mature macrophages. Interestingly, similar opposing effects on monocytes and macrophages have been reported for the “macrophage-deactivating” cytokine transforming growth factor β 1 (TGF-β1). Depending on the state of cellular differentiation and activation TGF-β1 stimulation leads to proinflammatory or anti-inflammatory responses. During early stages of inflammation TGF-β1 acts as a proinflammatory agent on monocytes, whereas the cytokine has potent immunosuppressive effects on mature macrophages to mediate the resolution of inflammation (for a review, see Ashcroft32 ).
We hypothesize that the transcriptional inhibition of IFN-γ–induced genes in mature macrophages is mediated by transcriptional crosstalk. Nuclear receptors can act on other transcriptional pathways either by direct interaction with other transcription factors or through competition for common coactivators that may be present in limiting amounts in the cell. It is well established that the regulation of IFN-γ responses is dependent on the recruitment of the coactivators CREB-binding protein (CBP) and p300, which both interact with Stat1.33 CBP also binds to PU.1, another transcription factor known to be essential for IFN-γ–induced gene transcription.34 Importantly, also Vdr has been reported to interact with CBP.35 Because we found the Vdr expression to be synergistically induced by IFN-γ and 1α,25(OH)2D3, it is possible that increased amounts of Vdr protein preferentially bind to limiting amounts of CBP or other coactivators, which are essential for IFN-γ–induced gene transcription. Inhibition of gene expression within the immune system by Vdr-mediated transcriptional crosstalk has been observed in several other studies. In monocytes, DCs, and T cells it has been demonstrated that 1α,25(OH)2D3 can repress the expression of immunoregulatory genes due to the interaction with the nuclear factor κB and activator protein 1 (AP1) signal transduction pathways.36-38 Because those pathways are particularly important for the activation of macrophages via Toll-like receptor signaling, 1α,25(OH)2D3 might also interfere with macrophage activation by microbial stimuli such as LPS, as already demonstrated for other nuclear receptors.39,40 We show here that for IFN-γ–mediated macrophage activation, expression of the Vdr gene is synergistically induced by the simultaneous presence of 1α,25(OH)2D3. In contrast, when we activated macrophages with LPS in the presence or absence of 1α,25(OH)2D3, we found no up-regulation of Vdr expression (data not shown). In agreement with this, we also found no influence of 1α,25(OH)2D3 on LPS-induced NO production and expression of the TNF-α gene (Tnfa; data not shown). We therefore conclude that in mature macrophages that express low amounts of Vdr, 1α,25(OH)2D3 specifically suppresses IFN-γ–mediated macrophage activation and this is mediated by up-regulation of Vdr expression. It is well established that IFN-γ–induced macrophage activation does not only lead to enhanced antibacterial activity and enhancement of inflammatory responses, but also to up-regulation of major histocompatibility complex (MHC) class II molecule expression.41 Although we found that listericidal activity and inflammatory gene expression were suppressed in IFN-γ–activated macrophages by 1α,25(OH)2D3, the expression of MHC class II molecules was found to be not affected (data not shown).
The suppression of IFN-γ activation of macrophages by 1α,25(OH)2D3 might be an important mechanism to prevent uncontrolled and excessive reactions in local inflammatory environments. A tight control of IFN-γ responses is especially crucial for the outcome of granulomatous diseases such as tuberculosis and sarcoidosis. Here, unbalanced IFN-γ responses can lead to granulomatous necrosis and to severe tissue destruction. In addition, it is known that excessive oxidative burst can lead to DNA damage and post-granuloma tumor development.42,43 Thus, the synthesis of 1α,25(OH)2D3 by granuloma-associated macrophages might be an important autoregulatory feedback mechanism to prevent excessive inflammation. The suppression of Th1-dominated responses by 1α,25(OH)2D3 during autoimmune diseases in vivo is one of the well-established features of steroids. Based on our data, we can extend this general concept of immunosuppression from Th1 cells and DCs to mature, Th1-activated macrophages. Because macrophages also play important roles in several autoimmune diseases, this may be of special clinical importance.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-03-1029.
Supported by the National German Genome Network (NGFN; 01GR0439), EU FP5 project EUMORPHIIA (QLG2-CT-2002-00930), and Volkswagenstiftung. L.H. performed research and wrote the paper; J.B., J.E., and S.S. performed research; T.F. contributed reagents and analytical tools; R.G. and M.P.K analyzed data; R.B. initiated the study; and A.L. designed research and wrote the paper.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Bastian Pasche and Eva Medina for helpful comments on the manuscripts, Reinhold Erben for Vdr-KO mice, and Jürgen Wehland for providing Listeria monocytogenes EGD and the del hly-L monocytogenes mutant.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal