Angiogenesis plays an important role in solid tumors and hematologic malignancies. The prognostic significance of angiogenic factors in adult acute lymphoblastic leukemia (ALL) remains ambiguous. We therefore analyzed the impact of angiogenic factor levels on overall survival of newly diagnosed adult ALL patients. Plasma levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin-1 receptor alpha (IL-1Rα), IL-6, IL-8, VEGF receptors VEGFR1 and VEGFR2, and thrombopoietin (TPO) were measured in plasma samples of 95 patients by enzyme-linked immunosorbent assay (ELISA). In a univariate Cox proportional hazards model, higher levels of IL-1Rα, IL-8, VEGFR1, and VEGFR2 were predictive of poor survival. In contrast, higher levels of VEGF were predictive of longer survival, and higher levels of bFGF suggested a similar trend (P = .09). The multivariate model simultaneously included VEGF (relative risk [RR] for death, 8.01; P = .001 for levels less than or equal to 19.5 pg/mL), IL-1Rα (RR, 5.12; P = .007 for levels greater than 373 pg/mL), and VEGFR2 (RR, 4.01; P = .04 for levels greater than 8222 pg/mL) as independent factors for survival. Of interest is the association of high levels of VEGF with good prognosis and higher levels of VEGF receptors with poor outcome. These data reflect the complexity by which angiogenic factors may affect the clinical behavior of patients with ALL, and this complexity should be considered in any therapeutic strategy incorporating antiangiogenic agents.
Introduction
Angiogenesis is the formation of new vessels from an existing network of vasculature, and it plays a significant role in a variety of physiologic and pathophysiologic processes.1 Recognized as a crucial component in the growth and metastatic spread of solid tumors, angiogenesis is a complicated development in the disease process that involves the degradation of extracellular matrix proteins and the activation, proliferation, and migration of endothelial cells and pericytes in a multistep manner.2 This process of neovascularization is regulated by the impact of competing influences between inhibitors and activators of angiogenesis.3 From the more than 20 proangiogenic and antiangiogenic agents identified, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) have been established as the 2 most potent positive regulators of angiogenesis.4-6 However, other cytokines—interleukin-1 receptor α (IL-1Rα), IL-6, and IL-8, tumor growth factor-α (TGF-α), TGF-β, tumor necrosis factor-α (TNF-α), hepatocyte growth factor (HGF), leptin, platelet-derived growth factor (PDGF), and others—have been reported to be involved as well, though their roles have not always been clearly defined.7-12
Recently, it has also been recognized that dysregulation of angiogenesis constitutes an important step in the development and progression of hematologic malignancies and that leukemias may invade and proliferate in the bone marrow and other organs by mechanisms similar to those reported in solid tumors.13 Measurement of microvessel density by immunohistochemistry and quantitation of vascular endothelial cells have been widely used for assessing the extent of angiogenic activity. In addition, the interaction of endothelial cells and the marrow microenvironment with hematopoietic progenitors and leukemia cells plays a role in the development of the leukemic phenotype by virtue of the secretion of proangiogenic and antiangiogenic cytokines and the expression of receptors for these cytokines by some or all of the leukemia cells. Elevated plasma levels of VEGF, bFGF, and HGF have been reported in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) and have been correlated with worse survival in some studies.14 Levels of HGF, TNF-α, and bFGF, but not of VEGF, were found to be elevated in blood samples of patients with acute lymphoblastic leukemia (ALL).15 However, almost all studies of the role of angiogenic factors in ALL have been conducted in children, and data are limited in adults. Perez-Atayde et al16 showed significantly higher microvessel density in the marrow of 40 children with ALL than in control marrow. Similarly, urinary bFGF levels were significantly elevated in all 22 children with newly diagnosed leukemia compared with 39 healthy, age-matched controls and showed decreases at the time of complete response. However, other studies either reported increasing levels of angiogenic factors, such as VEGF and bFGF, in patients in remission or failed to detect significant differences in microvessel density at presentation or remission in children with ALL.17,18 Thus, many questions remain regarding the origin and prognostic significance of angiogenic cytokines in patients with ALL.
In the current study, we evaluate the prognostic significance of angiogenic factors VEGF, bFGF, IL-1Rα, IL-6, and IL-8; serum VEGF receptors VEGFR1 and VEGFR2; and thrombopoietin (TPO) in 95 adult patients with ALL treated with a uniform induction regimen (hyper-cyclophosphamide [Cytoxan], vincristine, doxorubicin [Adriamycin], dexamethasone [hyper-CVAD] based).
Patients, materials, and methods
Patient samples
Pretreatment concentrations of VEGF, bFGF, IL-1Rα, IL-6, IL-8, VEGFR1, VEGFR2, and TPO were measured in the plasma of peripheral-blood samples obtained from 95 patients with newly diagnosed ALL treated at the MD Anderson Cancer Center. Blood plasma samples were obtained at presentation, stored at -70°C, and analyzed at a later stage. Consent forms were obtained according to institutional guidelines, and samples were collected and stored according to approved protocols. Levels of VEGF, bFGF, and IL-8 were measured in the samples of all 95 patients. IL-6 levels were measured in samples of 93 patients, levels of VEGFR2 in samples of 89, VEGFR1 in samples of 84, IL-1Rα in samples of 78, and TPO in samples of 72 patients. Table 1 summarizes the patient characteristics. Patients were uniformly treated with hyper-CVAD–based regimens, as described elsewhere with or without the anti-CD20 monoclonal antibody rituximab, depending on the presence or absence of expression of CD20 on the leukemia blasts as determined by immunophenotyping or imatinib (Gleevec) in some patients with Philadelphia chromosome–positive ALL.19-21
Patient characteristics
Characteristic . | Patients, no. (%) . |
|---|---|
| Diagnosis | |
| Precursor B-ALL | 85 (89) |
| Mature B-ALL | 10 (11) |
| Treatment regimen | |
| Hyper-CVAD | 45 (47) |
| Hyper-CVAD plus rituximab | 38 (40) |
| Hyper-CVAD plus imatinib | 12 (13) |
| Karyotype | |
| Diploid | 28 (35) |
| Insufficient metaphases | 16 (20) |
| t(9;22) | 17 (21) |
| t(8;14), t(2;8), t(8;22) | 6 (7) |
| Hypodiploid | 8 (10) |
| Other | 6 (7) |
| Data not available | 14 |
| Antecedent hematologic abnormality | |
| Absent | 86 (93) |
| Present | 6 (7) |
| Data not available | 3 |
| Response to therapy | |
| Complete response | 91 (96) |
| No response | 4 (4) |
| ECOG performance status | |
| 2 or lower | 87 (97) |
| Greater than 2 | 3 (3) |
| Data not available | 5 |
Characteristic . | Patients, no. (%) . |
|---|---|
| Diagnosis | |
| Precursor B-ALL | 85 (89) |
| Mature B-ALL | 10 (11) |
| Treatment regimen | |
| Hyper-CVAD | 45 (47) |
| Hyper-CVAD plus rituximab | 38 (40) |
| Hyper-CVAD plus imatinib | 12 (13) |
| Karyotype | |
| Diploid | 28 (35) |
| Insufficient metaphases | 16 (20) |
| t(9;22) | 17 (21) |
| t(8;14), t(2;8), t(8;22) | 6 (7) |
| Hypodiploid | 8 (10) |
| Other | 6 (7) |
| Data not available | 14 |
| Antecedent hematologic abnormality | |
| Absent | 86 (93) |
| Present | 6 (7) |
| Data not available | 3 |
| Response to therapy | |
| Complete response | 91 (96) |
| No response | 4 (4) |
| ECOG performance status | |
| 2 or lower | 87 (97) |
| Greater than 2 | 3 (3) |
| Data not available | 5 |
N equals 95 patients. The mean age of patients was 46 years; ages ranged from 17 to 83 years.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was performed using commercially available kits from R&D Systems (Minneapolis, MN). Protocols recommended by the manufacturer were used. Briefly, plasma was collected using EDTA (ethylenediaminetetraacetic acid) as an anticoagulant and was stored at -82°C. Patient samples were added to separate microplates, each containing a specific monoclonal antibody. Mixtures were incubated at room temperature for 2 hours. Plates were washed 8 times to remove any unbound substances. Enzyme-linked polyclonal antibodies specific for each protein were added to the wells, and the mixtures were incubated at room temperature for 2 hours; this was followed by another washing to remove any unbound antibody or enzyme reagent. A substrate solution was added to the wells, and the mixture turned blue. The intensity of the blue was proportionate to the amount of cytokine bound in the initial step. The color development was stopped, and the intensity of the color was measured and compared with a standard curve. Readings were made at wavelengths according to the manufacturer's recommendations.
Statistical methods
Associations between patient characteristics and among levels of angiogenic factors (covariates) were assessed for continuous variables by Mann-Whitney and Kruskal-Wallis tests and for categoric variables by Fisher exact or χ2 tests. In addition, Spearman rank correlation coefficients were computed for pairs of angiogenic factors.
Overall survival (OS) was the primary outcome studied. The Kaplan-Meier method was used to compute survival, and log-rank tests were used to compare survival between groups. Survival was defined as the number of weeks from the date treatment was initiated until the date of death if the patient died or until the date of last follow-up for patients still alive. Patients still alive at last follow-up were considered censored.
Furthermore, the prognostic effects of treatment (hyper-CVAD vs hyper-CVAD plus rituximab vs hyper-CVAD plus imatinib), age, and levels of VEGF, bFGF, IL-1Rα, IL-6, IL-8, VEGFR1, VEGFR2, and TPO on OS were evaluated using univariate and multivariate Cox proportional hazards regression models. Martingale residual plots were used to assess the appropriate functional form of the predictors and the goodness of fit. RPART, a recursive partitioning procedure or tree classification algorithm in S-PLUS (Insightful, Seattle, WA), was used to identify optimal cut points for each marker. This analysis was implemented using a method suggested by Therneau et al.22 In this method, the censored survival data are transformed into a single uncensored data value (the so-called null martingale residual), which is used as input into a standard regression tree algorithm.23 This ad hoc method has been shown to perform reasonably well for censored time-to-event data.24 The size of the trees was determined based on built-in 10-fold cross-validation.23 In addition to the default tree generated by the RPART algorithm, we examined initial splits using systematic inspection.25 Bootstrap simulations were also computed to assess the frequency of alternative splits.26 The following dichotomous cut points were thus obtained for further analyses of angiogenic factors: 19.1 pg/mL (VEGF), 9.707 pg/mL (bFGF), 4.1 pg/mL (IL-6), 36.1 pg/mL (IL-8), 373 pg/mL (IL-1Rα), 482 pg/mL (VEGFR1), 8222 pg/mL (VEGFR2), and 275 pg/mL (TPO). Initially, univariate Cox models were fit to evaluate the predictive effect of each factor alone on survival. Finally, factors statistically significant in the univariate models at a 5% level were included in the multivariate models. Multivariate models were reduced one factor at a time such that all factors remaining in the model were statistically significant at a 5% significance level. All reported P values are 2-sided. All analyses were performed using the SAS/STAT User's Guide (SAS Institute, Cary, NC) and S-PLUS (MathSoft).27,28
Results
Plasma levels and correlations of angiogenic factors
Levels of angiogenic factors VEGF, IL-1Rα, IL-6, IL-8, VEGFR1, VEGFR2, and TPO in plasma samples of patients with precursor B-cell ALL (B-ALL) and mature B-ALL were evaluated before therapy (Table 2).
Levels of angiogenic factors
Factor . | No. patients . | Median (range), pg/mL . |
|---|---|---|
| VEGF | 95 | 47.03 (15.42-867.41) |
| bFGF | 95 | 11.01 (5.34-214.68) |
| IL-8 | 95 | 23.49 (9.41-956.01) |
| IL-6 | 93 | 6.31 (1.98-327.54) |
| VEGFR2 | 89 | 8276.5 (1903.3-16533.00) |
| VEGFR1 | 84 | 98.5 (18.55-1290.6) |
| IL-1Rα | 78 | 299.2 (41.94-2647.4) |
| TPO | 72 | 428.07 (25.87-2584.00) |
Factor . | No. patients . | Median (range), pg/mL . |
|---|---|---|
| VEGF | 95 | 47.03 (15.42-867.41) |
| bFGF | 95 | 11.01 (5.34-214.68) |
| IL-8 | 95 | 23.49 (9.41-956.01) |
| IL-6 | 93 | 6.31 (1.98-327.54) |
| VEGFR2 | 89 | 8276.5 (1903.3-16533.00) |
| VEGFR1 | 84 | 98.5 (18.55-1290.6) |
| IL-1Rα | 78 | 299.2 (41.94-2647.4) |
| TPO | 72 | 428.07 (25.87-2584.00) |
N indicates number of patients.
Levels of all factors were elevated in ALL patients compared with healthy controls (P < .01). Spearman correlation coefficients have been calculated between pairs of angiogenic factors (Table 3). VEGF was positively correlated with bFGF (r = 0.61; P < .0001) yet negatively correlated with VEGFR1 (r = -0.34; P = .0013) and TPO (r = -0.44; P = .0001). In addition to VEGF, bFGF levels also negatively correlated with TPO (r = -0.31; P = .0082). Levels of IL-6 positively correlated with IL-8 (r = 0.60; P < .0001), IL-1Rα (r = 0.37; P = .0008), and VEGFR1 (r = 0.22; P = .04). IL-8 in turn also demonstrated positive correlation with VEGFR1 (r = 0.26; P = .01) and with IL-1Rα (r = 0.23; P = .05).
Correlation coefficients among angiogenic factors
Spearman correlation . | bFGF . | IL-6 . | IL-8 . | IL-1Rα . | VEGFR1 . | VEGFR2 . | TPO . |
|---|---|---|---|---|---|---|---|
| VEGF | 0.61 | –0.05 | –0.18 | 0.13 | –0.34 | 0.09 | –0.44 |
| bFGF | — | –0.08 | –0.10 | 0.21 | –0.06 | 0.08 | –0.31 |
| IL-6 | — | — | 0.60 | 0.37 | 0.22 | 0.03 | 0.17 |
| IL-8 | — | — | — | 0.23 | 0.26 | –0.07 | 0.20 |
| IL-1Rα | — | — | — | — | 0.12 | 0.16 | 0.05 |
| VEGFR1 | — | — | — | — | — | 0.06 | 0.06 |
| VEGFR2 | — | — | — | — | — | — | –0.06 |
Spearman correlation . | bFGF . | IL-6 . | IL-8 . | IL-1Rα . | VEGFR1 . | VEGFR2 . | TPO . |
|---|---|---|---|---|---|---|---|
| VEGF | 0.61 | –0.05 | –0.18 | 0.13 | –0.34 | 0.09 | –0.44 |
| bFGF | — | –0.08 | –0.10 | 0.21 | –0.06 | 0.08 | –0.31 |
| IL-6 | — | — | 0.60 | 0.37 | 0.22 | 0.03 | 0.17 |
| IL-8 | — | — | — | 0.23 | 0.26 | –0.07 | 0.20 |
| IL-1Rα | — | — | — | — | 0.12 | 0.16 | 0.05 |
| VEGFR1 | — | — | — | — | — | 0.06 | 0.06 |
| VEGFR2 | — | — | — | — | — | — | –0.06 |
— indicates not applicable.
Although only 3 patients had an Eastern Cooperative Oncology Group (ECOG) performance status greater than 2, a statistically significant difference in the levels of IL-6 (P = .05) was found between patients with a performance score of 2 or less (median, 4.8 pg/mL) compared with those whose performance score exceeded 2 (median, 79.5 pg/mL). No statistically significant differences were found between median angiogenic factor levels when comparing patients with or without an antecedent hematologic disorder.
Levels of angiogenic factors and outcome
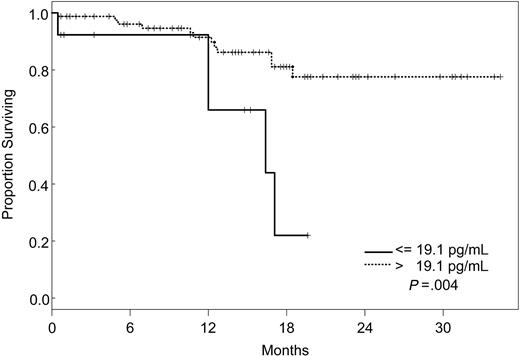
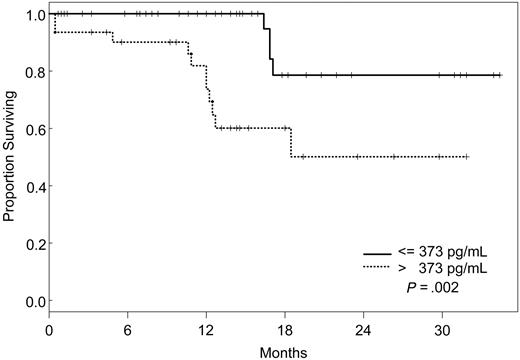
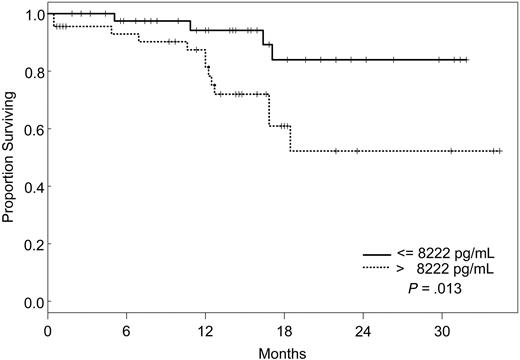
Seventeen (17.9%) of the 95 patients died, but the median OS was not attained. Univariate Cox proportional hazards models were then fit to evaluate the predictive effect of each angiogenic factor alone on survival (Table 4). Predictive effects were found for VEGF, IL-8, IL-1Rα, VEGFR1, and VEGFR2 using the dichotomous cut points determined from recursive partitioning, but not as continuous variables (Table 4). The relative risk for death was significantly higher for patients with pretreatment levels of VEGF less than or equal to 19.1 pg/mL (RR, 4.16 [95% confidence interval (CI), 1.45-12.0]; P = .008), IL-8 greater than 36.1 pg/mL (RR, 2.65 [95% CI, 1.02-6.91]; P = .05), IL-1Rα greater than 373 pg/mL (RR, 4.97 [95% CI, 1.57-15.7]; P = .0063), VEGFR1 greater than 482 pg/mL (RR, 7.69 [95% CI, 1.88-31.4]; P = .0045), and VEGFR2 greater than 8222 pg/mL (RR, 3.78 [95% CI, 1.23-11.6]; P = .021).
Univariate Cox proportional hazards models for survival
Variable . | No. patients . | Relative risk . | 95% CI . | P . |
|---|---|---|---|---|
| Diagnosis | ||||
| Precursor B-ALL | 85 | 1.00 | — | — |
| Mature B-ALL | 10 | 1.57 | 0.36-6.88 | .55 |
| Treatment | ||||
| Hyper-CVAD | 45 | 1.00 | — | — |
| Hyper-CVAD + rituximab | 38 | 0.81 | 0.27-2.43 | .71 |
| Hyper-CVAD + imatinib | 12 | 0.87 | 0.23-3.22 | .83 |
| White blood cell count | ||||
| 30 × 109/L or less | 66 | 1.00 | — | — |
| More than 30 × 109/L | 27 | 3.65 | 1.40-9.55 | .008 |
| Karyotype | ||||
| No Ph chromosome | 70 | 1.00 | — | — |
| Ph chromosome-positive | 17 | 1.37 | 0.48-3.97 | .56 |
| Log | ||||
| Age | 95 | 1.62 | 0.53-4.94 | .39 |
| VEGF | 95 | 0.82 | 0.50-1.33 | .42 |
| Greater than 19.1 pg/mL | 82 | 1.00 | — | — |
| 19.1 pg/mL or less | 13 | 4.16 | 1.45-12.00 | .008 |
| bFGF | 95 | 0.80 | 0.47-1.38 | .43 |
| Greater than 9.707 pg/mL | 52 | 1.00 | — | — |
| 9.707 pg/mL or less | 43 | 2.39 | 0.88-6.48 | .086 |
| IL-6 | 93 | 0.84 | 0.55-1.30 | .44 |
| Greater than 4.1 pg/mL | 53 | 1.00 | — | — |
| 4.1 pg/mL or less | 40 | 2.29 | 0.83-6.31 | .11 |
| IL-8 | 95 | 1.15 | 0.73-1.81 | .54 |
| 36.1 pg/mL or less | 63 | 1.00 | — | — |
| Greater than 36.1 pg/mL | 32 | 2.65 | 1.02-6.91 | .046 |
| IL-1Rα | 78 | 1.53 | 0.90-2.59 | .11 |
| 373 pg/mL or less | 47 | 1.00 | — | — |
| Greater than 373 pg/mL | 31 | 4.97 | 1.57-15.70 | .0063 |
| VEGFR1 | 84 | 1.16 | 0.69-1.93 | .58 |
| 482 pg/mL or less | 76 | 1.00 | — | — |
| Greater than 482 pg/mL | 8 | 7.69 | 1.88-31.4 | .0045 |
| VEGFR2 | 89 | 3.31 | 0.65-16.80 | .15 |
| 8222 pg/mL or less | 44 | 1.00 | — | — |
| Greater than 8222 pg/mL | 45 | 3.78 | 1.23-11.60 | .021 |
| TPO | 72 | 1.18 | 0.72-1.94 | .52 |
| 275 pg/mL or less | 24 | 1.00 | — | — |
| Greater than 275 pg/mL | 48 | 3.01 | 0.65-13.90 | .16 |
Variable . | No. patients . | Relative risk . | 95% CI . | P . |
|---|---|---|---|---|
| Diagnosis | ||||
| Precursor B-ALL | 85 | 1.00 | — | — |
| Mature B-ALL | 10 | 1.57 | 0.36-6.88 | .55 |
| Treatment | ||||
| Hyper-CVAD | 45 | 1.00 | — | — |
| Hyper-CVAD + rituximab | 38 | 0.81 | 0.27-2.43 | .71 |
| Hyper-CVAD + imatinib | 12 | 0.87 | 0.23-3.22 | .83 |
| White blood cell count | ||||
| 30 × 109/L or less | 66 | 1.00 | — | — |
| More than 30 × 109/L | 27 | 3.65 | 1.40-9.55 | .008 |
| Karyotype | ||||
| No Ph chromosome | 70 | 1.00 | — | — |
| Ph chromosome-positive | 17 | 1.37 | 0.48-3.97 | .56 |
| Log | ||||
| Age | 95 | 1.62 | 0.53-4.94 | .39 |
| VEGF | 95 | 0.82 | 0.50-1.33 | .42 |
| Greater than 19.1 pg/mL | 82 | 1.00 | — | — |
| 19.1 pg/mL or less | 13 | 4.16 | 1.45-12.00 | .008 |
| bFGF | 95 | 0.80 | 0.47-1.38 | .43 |
| Greater than 9.707 pg/mL | 52 | 1.00 | — | — |
| 9.707 pg/mL or less | 43 | 2.39 | 0.88-6.48 | .086 |
| IL-6 | 93 | 0.84 | 0.55-1.30 | .44 |
| Greater than 4.1 pg/mL | 53 | 1.00 | — | — |
| 4.1 pg/mL or less | 40 | 2.29 | 0.83-6.31 | .11 |
| IL-8 | 95 | 1.15 | 0.73-1.81 | .54 |
| 36.1 pg/mL or less | 63 | 1.00 | — | — |
| Greater than 36.1 pg/mL | 32 | 2.65 | 1.02-6.91 | .046 |
| IL-1Rα | 78 | 1.53 | 0.90-2.59 | .11 |
| 373 pg/mL or less | 47 | 1.00 | — | — |
| Greater than 373 pg/mL | 31 | 4.97 | 1.57-15.70 | .0063 |
| VEGFR1 | 84 | 1.16 | 0.69-1.93 | .58 |
| 482 pg/mL or less | 76 | 1.00 | — | — |
| Greater than 482 pg/mL | 8 | 7.69 | 1.88-31.4 | .0045 |
| VEGFR2 | 89 | 3.31 | 0.65-16.80 | .15 |
| 8222 pg/mL or less | 44 | 1.00 | — | — |
| Greater than 8222 pg/mL | 45 | 3.78 | 1.23-11.60 | .021 |
| TPO | 72 | 1.18 | 0.72-1.94 | .52 |
| 275 pg/mL or less | 24 | 1.00 | — | — |
| Greater than 275 pg/mL | 48 | 3.01 | 0.65-13.90 | .16 |
Ph indicates Philadelphia chromosome; —, not applicable.
Factors that had a statistically significant effect on the univariate model were included in the multivariate model and were reduced one factor at a time such that all factors remaining in the model were statistically significant at P ≤ .05. The final model simultaneously included VEGF, IL-1Rα, and VEGFR2 (Table 5). According to our model, the likelihood of death was 8 times higher for patients with VEGF levels less than or equal to 19.1 pg/mL (95% CI, 2.28-28.1), 5 times increased for those with IL-1Rα levels greater than 373 pg/mL (95% CI, 1.55-17), and 4 times increased for patients with VEGFR2 levels greater than 8222 pg/mL (95% CI, 1.05-15.3), independently. Kaplan-Meier survival plots for VEGF, IL-1Rα, and VEGFR2 are shown in Figures 1, 2, and 3.
Multivariate Cox proportional hazards model for survival
Variable, pg/mL . | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| VEGF | .001 | ||
| Greater than 19.1 | 1.00 | — | — |
| 19.1 or less | 8.01 | 2.28-28.10 | — |
| IL-1R | .008 | ||
| 373 or less | 1.00 | — | — |
| Greater than 373 | 5.12 | 1.55-17.00 | — |
| VEGFR2 | .04 | ||
| 8222 or less | 1.00 | — | — |
| Greater than 8222 | 4.01 | 1.05-15.30 | — |
Variable, pg/mL . | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| VEGF | .001 | ||
| Greater than 19.1 | 1.00 | — | — |
| 19.1 or less | 8.01 | 2.28-28.10 | — |
| IL-1R | .008 | ||
| 373 or less | 1.00 | — | — |
| Greater than 373 | 5.12 | 1.55-17.00 | — |
| VEGFR2 | .04 | ||
| 8222 or less | 1.00 | — | — |
| Greater than 8222 | 4.01 | 1.05-15.30 | — |
— indicates not applicable.
Discussion
Angiogenesis is a pivotal process during normal embryogenesis and physiologic processes in later life. That it also plays a significant role in tumor development was established in the 1970s.1,2 Angiogenesis correlates with metastatic potential, recurrence, and progression in numerous solid tumors and has been recognized as an independent prognostic factor for survival in tumors of the lung, breast, esophagus, and prostate.29-32 Dysregulation of angiogenesis was also found to have a major impact on leukemic growth and to constitute an important step in the development and progression of hematologic malignancies, including leukemias.13 Increases in microvessel density and elevated levels of numerous proangiogenic factors have been reported in acute and chronic leukemias. Higher levels of VEGF, VEGFR1, VEGFR2, and plasma endostatin have been demonstrated in AML and MDS samples compared with healthy controls and occasionally (VEGF, plasma endostatin) have been correlated with worse survival.14,15
Increasing evidence indicates that angiogenesis markers play a role in the pathogenesis of patients with ALL. Most data are derived from studies of angiogenesis in children, and the role of angiogenic factors in adult ALL is poorly studied. We investigated the plasma levels of VEGF, VEGFR1, VEGFR2, bFGF, IL-1Rα, IL-6, IL-8, and TPO in 95 adults with ALL treated with the hyper-CVAD regimen except for the addition of rituximab or imatinib for patients whose leukemic blasts expressed CD20 or harbored the Philadelphia chromosome abnormality, respectively. In a multivariate model, we identified levels of VEGF, IL-1Rα, and VEGFR2 as independent predictors of survival. Interestingly, patients with high levels of VEGF (higher than 19.1 pg/mL) had a significantly better survival than those with low levels (median not attained vs median of 71 weeks). On the other hand, survival was worse in patients with high levels of IL-1Rα (greater than 373 pg/mL) and VEGFR2 (greater than 8222 pg/mL). Although the follow-up was short (maximum, 2.5. years), these findings indicated that angiogenic factors have different prognostic significance, depending on the biologic background in which they are analyzed. These findings are in contrast to what has been described in other hematologic malignancies, and they suggest significant biologic differences between ALL and AML and between adult and childhood ALL itself. Numerous studies in AML have indicated that higher levels of VEGF correlate with more aggressive disease.14,33,34 In a study of 31 children with newly diagnosed ALL and 22 with recurrent ALL, VEGF levels were found to be significantly higher in patients with recurrent disease than in those with newly diagnosed ALL.35 In addition, relapse-free survival and OS were significantly longer in patients with low VEGF levels. Schneider et al36 analyzed urinary bFGF and plasma VEGF levels in 39 children with newly diagnosed B-cell ALL. Levels of bFGF were higher in patients than in control subjects. Among the patient group, the bFGF levels were significantly lower in resistant patients than in complete responders. Median VEGF levels, on the other hand, were similar in patients and control subjects, and higher VEGF levels appeared to be correlated with a higher risk for relapse. Yetgin et al17 analyzed serum levels of bFGF and VEGF in 10 healthy controls and 31 children with ALL at the time of diagnosis and again during remission. VEGF levels in that study were lower at diagnosis than at the time of remission and in the control group. Interestingly, 26 of 31 patients showed a trend of increase in VEGF levels in remission. No difference was observed with regard to bFGF levels in the healthy controls and the patient group, although among patients, levels at diagnosis were significantly lower than those in remission. Although our data demonstrate a distinctly different association between VEGF levels and prognosis in adult ALL, some similarities nevertheless exist. At least one study associated higher levels of VEGF with remission,17 and differences in association between high and low levels of different angiogenic factors have been described consistently in other studies.36
The driving forces behind the dysregulation of angiogenesis in leukemia thus remain insufficiently characterized. The results from our analysis and other studies highlight the prognostic value of angiogenic factors, but their complicated net of interactions between host- and disease-related factors remains largely unknown. Angiogenic factors can be produced by normal, nonclonal cells of hematopoietic lineage, and it may be difficult to distinguish to what extent the production and excretion of angiogenic factors is reactive versus a true reflection of the pathophysiologic process.37,38 Yetgin and al17 have suggested patients at diagnosis might have higher levels of cellular VEGF, correlating with the level of leukemic blasts and possibly having a stronger influence on the behavior of the imminent microenvironment (or even representing stromal abnormalities), whereas in remission marrow angiogenesis becomes reactivated with the excretion of VEGF or other proangiogenic factors into the serum.17 This issue also raises the question of the influence of the source of the sample. Cellular levels of angiogenic factors may not adequately reflect their levels in serum or urine. Biologic differences exist between adult and childhood ALL, making any attempt at the extrapolation of childhood ALL data into adult ALL even more complicated. Another important aspect remains the connection between receptor (eg, VEGF receptors) and ligand (eg, VEGF).39 It is also known from other examples (eg, chronic lymphocytic leukemia and non-Hodgkin lymphomas) that receptors may exist in soluble form in the plasma, binding the ligand before it can bind to the actual cell-bound receptor and thus decreasing the activity of angiogenic factors such as VEGF.40,41
In summary, our data suggest that the biology of angiogenic factors in adult ALL appears different from that in children with ALL, AML, and other hematologic malignancies. Further investigations of the role of angiogenesis and angiogenic factors in ALL may provide additional information in understanding the complex interaction between angiogenesis and the biologic behavior of the leukemic cells. The goal of these endeavors should remain to establish as individual-specific a therapeutic approach to patients as possible based on a more thorough understanding of disease-relevant pathophysiologic pathways.
Prepublished online as Blood First Edition Paper, August 25, 2005; DOI 10.1182/blood-2005-03-1010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal