It has been suggested that marrow stromal cells (MSCs) may be immunoprivileged and can engraft in allogeneic recipients with intact immune systems. We determined if the implantation of murine MSCs engineered to release erythropoietin (Epo) would be feasible in major histocompatibility complex (MHC)-mismatched allogeneic mice without immunosuppression, and we monitored hematocrit (Hct) as a reporter of MSC graft survival. MSCs from C57Bl/6 mice were engineered to release murine Epo (Epo+ MSCs) and implanted subcutaneously in either syngeneic C57Bl/6 mice or MHC-mismatched Balb/c mice. In syngeneic recipients, the Hct rapidly rose from baseline level and remained higher than .88 (88%) for more than 200 days. However, in MHC-mismatched recipient Balb/c mice, the Hct rose transiently and rapidly declined to baseline values. Repeat implantations in these same mice were associated with an acquired refractoriness in the Hct response consistent with alloimmunization to donor Epo+ MSCs. Allogeneic MSC implants had an increased proportion of host-derived lymphoid CD8+, natural killer T (NKT), and NK infiltrating cells compared with syngeneic controls, and splenocytes isolated from Balb/c mice that had received implants also displayed a significant interferon-gamma (IFNγ) response to C57Bl/6 MSCs in vitro. These results strongly suggest that MSCs are not intrinsically immunoprivileged and cannot serve as a “universal donor” in immunocompetent MHC-mismatched recipients.
Introduction
Bone marrow stromal cells (MSCs),1 also sometimes referred to as mesenchymal stem cells, are promising for regenerative medicine due to their innate ability to differentiate into various cell types.2-5 Thus, autologous MSCs and their genetically engineered progeny have potential use in several preclinical regenerative medicine scenarios, such as cardiovascular regeneration,6-14 brain and spinal cord regeneration,15,16 as well as bone and cartilage repair.17-23 Furthermore, genetically engineered MSCs may operate as cellular vehicles for the delivery of therapeutic proteins in hereditary and acquired metabolic, endocrine, and malignant diseases.4,24-30
In vitro studies on human, baboon, and murine MSCs have revealed that MSCs are immunosuppressive.31-34 Depending upon the experimental circumstances, suppression of mixed lymphocyte reaction (MLR) in vitro between major histocompatibility complex (MHC)-mismatched stimulator and responder cells by MSCs appears to arise from both contact-dependent35 and soluble factors including, but not limited to,36-38 hepatocyte growth factor (HGF) and transforming growth factor β1 (TGF-β1).32 However, alternative experimental settings suggest that MSCs may also behave as nonprofessional antigen-presenting cells (APCs).39,40 In support of their in vivo immunosuppressive features are the observations that allogeneic MSCs may prolong skin allograft survival in immunocompetent baboons,31 prevent the rejection of allogeneic B16 mouse melanoma cells in immunocompetent C3H mice,34 and attenuate graft-versus-host disease in mice and humans.38,41
The sum of these observations supports the notion that MSCs may also be immunoprivileged as well as immunosuppressive and that MHC-mismatched MSCs may be exploited as “universal donor” cells in an array of clinical settings where the pharmaceutical effect of MSCs is dependent upon biologically meaningful engraftment to allow for a clinical benefit. It is therefore possible that universal donor MSCs may also be developed for cell and gene therapy applications where they are exploited as protein-producing cells. Indeed, we have previously published the utility of genetically engineered MSCs for the secretion of erythropoietin (Epo) in normal syngeneic mice.28 In a follow-up study, we demonstrated that embedding gene-modified MSCs within a human-compatible collagen-based subcutaneous organoid led to enhancement and prolongation of transgene effect.29 Our objective in this study was to determine if implantation of murine MSCs derived from C57Bl/6 and gene-modified to secrete Epo is achievable in class I and II MHC-mismatched immunocompetent Balb/c recipients. To our disappointment, we show that MHC-mismatched murine MSCs led to a robust and specific cellular immune response in nonimmunosuppressed allogeneic mice, markedly limiting their use as a universal cellular platform for delivery of therapeutic proteins in vivo.
Materials and methods
Generation of retroviral vector and of retrovirus-producing cells
The retroviral plasmid vector pEpo was produced in our laboratory as previously described.29 Retrovirus-producing cells GP+E86-Epo were generated, as also earlier reported,29 and maintained in complete media— that is, Dulbecco modified Eagle medium (DMEM) with high glucose (Wisent Technologies, St Bruno, QC), supplemented with 10% heat-inactivated fetal bovine serum (FBS; Wisent Technologies) and 50 units/mL penicillin, 50 μg/mL streptomycin (Pen/Strep; Wisent Technologies)—in a 37°C incubator with 5% CO2.
Harvest and gene modification of primary murine marrow stroma
A female C57Bl/6 mouse (Charles River, Laprairie, QC) weighing 15 to 20 g was killed, and whole bone marrow harvested from femurs and tibias was placed in culture with complete media at 37°C with 5% CO2. Five days later, the nonadherent hematopoietic cells were discarded and the adherent marrow stromal cells (MSCs) cultured for approximately 12 to 17 passages in complete media, to generate the polyclonal population of “null” MSCs. The generation of Epo gene-modified MSCs was conducted by transduction of MSCs twice per day for 3 successive days per each of 4 consecutive weeks. More specifically, for each round of transduction, 0.45 μm filtered retroviral supernatant from virus producers GP+E86-Epo was placed on approximately 60% confluent MSCs with 6 μg/mL lipofectamine reagent (Invitrogen/Life Technologies, Frederick, MD). The monoclonal population of Epo gene-modified MSCs used in the present study (ie, Epo+ MSCs) was created through selection and expansion of single clones that arose following plating of the polyclonal population at limiting dilutions. Supernatant was collected from Epo+ MSCs, and enzyme-linked immunosorbent assay (ELISA) specific for human Epo (Roche Diagnostics, Indianapolis, IN) conducted and revealed the secretion of 3 to 4 units of Epo/million cells per 24 hours. Animals were handled under the guidelines promulgated by the Canadian Council on Animal Care and with the Animal Welfare Act Regulations and other federal statutes relating to animals and experiments involving animals, and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, US National Research Council, 1996.42
Murine marrow stroma phenotypic analysis
Prior to implantation in mice, Epo+ MSCs were analyzed by flow cytometry for expression of cell surface antigens CD31, CD34, CD44, CD45, CD73, CD90, CD105, and Mac1. Cells were incubated with the following monoclonal antibodies (Abs) after Fc receptor blocking using anti-mouse CD16/CD32 Ab (clone 24-G2): biotin-conjugated rat anti-mouse CD31 (clone 30-F11), CD34 (clone RAM 34), phycoerythrin (PE)-labeled rat anti-mouse CD44 (clone IM7), CD45 (clone 30-F11), CD73 (clone TY/23), and Mac1 (clone M1/70) (all from BD Biosciences, San Diego, CA); fluorescein isothiocyanate (FITC)-labeled rat anti-mouse CD90 (clone G7; Southern Biotechnology Associates, Birmingham, AL); and biotin-conjugated rat anti-mouse CD105 (clone MJ7/18; eBioscience, San Diego, CA); isotypic controls included the following: PE-labeled rat immunoglobulin G2a (IgG2a), rat IgG2b, and biotin-conjugated rat IgG2a (all from BD Biosciences). Biotinylated Abs were revealed by Tri-Color (TC)-streptavidin (Caltag Laboratories, Burlingame, CA).
Epo+ MSCs and polyclonal null MSCs were plated and maintained in culture in absence or presence of 50 ng/mL interferon-gamma (IFNγ; Biosource International, Camarillo, CA) for 24 hours, and flow cytometry analysis was conducted for expression of MHC class I, MHC class II, CD80, and CD86. Specifically, cells were incubated with the following monoclonal Abs after Fc receptor blocking: R-PE-conjugated mouse anti-mouse H-2Kb (clone AF6-88.5), R-PE-conjugated mouse anti-mouse I-Ab (clone AF6-120.1), biotin-conjugated hamster anti-mouse CD80 (clone 16-10A1), and biotin-conjugated rat anti-mouse CD86 (clone PO3); isotypic controls included the following: R-PE-conjugated mouse IgG2a, biotin-conjugated hamster IgG2, and biotin-conjugated rat IgG2b (all from BD Biosciences). Biotinylated Abs were revealed by PE-streptavidin (BD Biosciences).
Cells were washed and acquired using a fluorescence-activated cell sorter (FACS) Calibur flow cytometer (BD Immunocytometry systems, San Jose, CA), and data analysis was conducted with Cellquest Pro software (BD Immunocytometry Systems, Mississauga, ON, Canada).
Differentiation of marrow stromal cells
Epo+ MSCs were exposed to specific media to induce their differentiation. For osteogenic differentiation, Epo+ MSCs (at 70%-80% confluency) were cultured in complete media supplemented with β-glycerol phosphate (10 mM), dexamethasone (10-8 M), and ascorbic acid 2-phosphate (5 μg/mL) (Sigma-Aldrich Canada, Oakville, ON) for 4 weeks, changing the media every 2 to 3 days.43,44 Alizarin Red S was then used to stain calcium in the mineralized extracellular matrix. More specifically, these adherent cells were rinsed with phosphate-buffered saline (PBS), then exposed to a 2% preparation of Alizarin Red S (pH 4.1 using ammonium hydroxide) for 5 minutes and rinsed with distilled H2O.44 To induce adipogenic differentiation, Epo+ MSCs (at 50%-60% confluency) were cultured in complete media supplemented with indomethacin (46 μM), 3-isobutyl-methylxanthine (0.5 mM), dexamethasone (1 μM), and insulin (10 μg/mL) (Sigma-Aldrich Canada)40 for 7 days, changing the media twice in that interval. Oil Red O (Sigma-Aldrich Canada) was used for lipid droplet staining. More specifically, cells were fixed with paraformaldehyde (4% in PBS at room temperature for 1 hour) and then exposed for 10 minutes to a preparation of Oil Red O consisting of 3 parts Oil Red O (3.75% in isopropanol) and 2 parts distilled H2O at room temperature, and thereafter rinsed with distilled H2O.43 Photographs of cells were taken under light microscopy using an Axiovert25 Zeiss microscope (Carl Zeiss, Heidelberg, Germany) attached to a Contax167MT camera with 400 ISO film (Kyocera, Tokyo, Japan).
Mixed lymphocyte culture
C57Bl/6 and Balb/c splenocytes were isolated by mechanical dissociation using microscope slides followed by red blood cell lysis with ammonium chloride (8.3 g/mL; Sigma-Aldrich, St-Louis, MO). In quadruplicates, 105 C57Bl/6 splenocytes and 105 Balb/c splenocytes per well were cocultured in a 96-well plate with or without 105 C57Bl/6-derived MSCs in 200 μL media (RPMI, 10% FBS, 1% Pen/Strep). Prior to coculture, MSCs were pretreated or not for 20 hours with 50 ng/mL recombinant mouse IFNγ (Biosource International), followed by extensive washing with PBS. After 3 days, the mixed lymphocytes cultures were centrifuged and the supernatant was tested for the presence of mouse IFNγ using a commercial ELISA kit (R and D Systems, Minneapolis, MN).
Implantation of marrow stroma and long-term blood collection and analysis
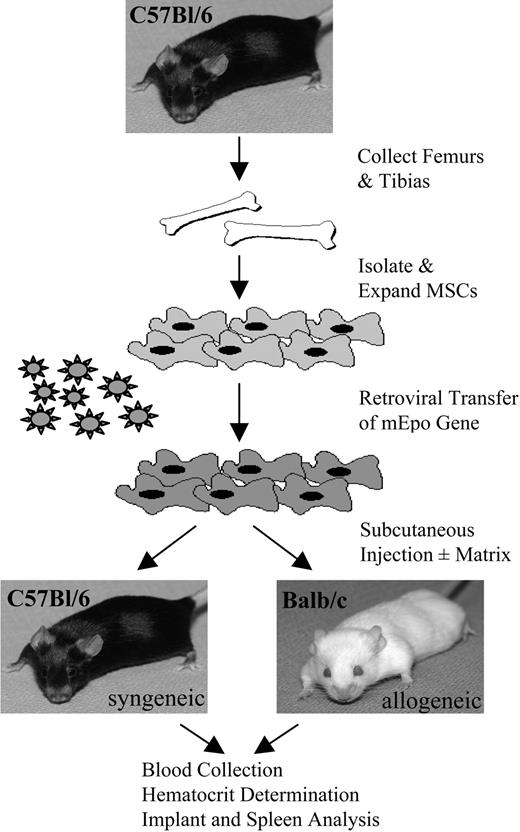
For implantation of Epo+ MSCs “with a matrix,” Epo+ MSCs were concentrated by centrifugation, and 107 cells were resuspended in approximately 100 μL serum-free RPMI1640 media (Wisent Technologies), mixed with 500 to 600 μL of a viscous type I collagen-based, FDA-approved, “human-compatible” material Contigen (C. R. Bard, Covington, GA), and injected subcutaneously in the right flank of each of 5 syngeneic C57Bl/6 mice and 5 allogeneic Balb/c mice. For implantation of Epo+ MSCs “without a matrix,” Epo+ MSCs, at 107 cells, were resuspended in approximately 500 to 600 μL serum-free RPMI media, and administered by subcutaneous injection in the right flank of each of 4 syngeneic C57Bl/6 mice and 5 allogeneic Balb/c mice. Allogeneic Balb/c mice similarly received 107 Epo+ MSCs a second and third time 107 and 170 days later, respectively, in the group “with a matrix,” and 69 and 132 days later, respectively, in the group “without a matrix.” Blood was collected from the saphenous vein of mice before implantation and once per week or more after implantation, using heparinized microhematocrit tubes (Fisher Scientific, Pittsburgh, PA), and hematocrit (Hct) was determined over time by standard microhematocrit method. Mice were followed for more than 140 to 190 days. Figure 1 shows the outline of implantations.
Anti-Epo antibodies detection
For antibody titering, plasma samples from C57Bl/6 mice and Balb/c mice, both implanted with Epo+ MSCs with and without Contigen matrix, were diluted in PBS supplemented with 10% FBS, incubated for 2 hours at 37°C onto 96-well plates previously coated overnight at 4°C with mouse recombinant Epo (0.1 mL of 0.125 μg/mL; Roche), blocked 2 hours with 1% gelatin type A (Sigma-Aldrich) in PBS, and revealed using anti-mouse Ig-horseradish peroxidase (HRP) antibody (1:1000 in 10% FBS in PBS; BD Pharmingen, San Diego, CA) and 3,3′,5,5′-tetramethyl-benzidine (TMB) substrate (eBioscience).
Marrow stroma implantation for implant and spleen retrieval
In a distinct experiment, 107 Epo+ MSCs mixed in Contigen were implanted as described above in additional syngeneic C57Bl/6 mice and allogeneic Balb/c mice. Also, null MSCs (that is MSCs that were not gene-modified and thus not secreting Epo, but also derived from C57Bl/6 donor) were likewise implanted embedded in Contigen in syngeneic C57Bl/6 and allogeneic Balb/c mice, at 107 cells per mouse. At day 15 after implantation, mice were killed, and implants removed and digested with collagenase type IV (Sigma-Aldrich Canada) 1.6 mg/mL and DNAse I (Sigma-Aldrich Canada) 200 μg/mL, in 1X PBS at 37°C for 2 hours. Cells were incubated with the following monoclonal Abs after Fc receptor blocking (clone 2-4G2): FITC-labeled rat anti-mouse CD8 (clone 53-6.7), PE-labeled rat anti-mouse CD4 (clone RM4-5), PE-labeled rat anti-mouse natural killer (NK)/NKT (clone 45A2-13), mouse anti-mouse NK1.1 (PK136), and APC-labeled rat anti-mouse CD19 (clone1D3); isotypic controls included the following: PE-labeled rat IgG2b, PE-labeled mouse IgG2a, and FITC-, PE-, or APC-labeled rat IgG2a (all from BD Biosciences). Cells were washed and acquired using a FACS Calibur flow cytometer (BD Immunocytometry systems) and data analysis was conducted with Cellquest software. In addition, spleens were recovered and mechanically dissociated, and red blood cells were lysed by resuspending the cells in 1 mL of 8.3 g/L ammonium chloride red blood cell lysing buffer (Sigma-Aldrich Canada), followed by extensive washing in PBS (Wisent Technologies). For the IFNγ release assay, splenocytes were incubated in 96-well U-bottom culture plates, at 106 cells/well, with syngeneic or allogeneic null MSCs or Epo+ MSCs, at 5 × 104 cells/well, in 0.2 mL RPMI 1640 media (Wisent Technologies) containing 10% FBS and 50 μM 2-mercaptoethanol. Following 24 hours, 0.1 mL supernatant was collected and assayed for the presence of mouse IFNγ by ELISA (R and D Systems).
Human marrow stroma collection and phenotypic analysis
Human bone marrow aspirates were obtained from 2 individuals, 63 and 84 years of age, by Dr John Antoniou (Jewish General Hospital, Montreal, QC) and under the guidelines approved by the Jewish General Hospital Ethics committee. After removal of fat layer, mononuclear cells were isolated by Ficoll-Paque (Amersham, Piscataway, NJ) density gradient and cultured in DMEM with high glucose (Wisent Technologies) supplemented with 10% heat-inactivated FBS (Wisent Technologies) and 50 units/mL Pen/Strep (Wisent Technologies), in a 37°C incubator with 5% CO2. Media was changed once to twice per week and adherent cells were passaged at 1 in 3 dilutions until passage 6 when resulting human MSCs were used as follows. Briefly, the MSCs prepared from both donors were cultured in the absence and presence of IFNγ, at 100 units/mL, for 48 hours. Cells were then prepared for flow cytometric analysis. Cells were recovered and stained with anti-human PE-labeled human leukocyte antigen-DR (HLA-DR, cloneG46.6), PE-labeled CD80 (clone L307.4), and FITC-labeled β2-microglobulin (clone TU99); mouse isotypic controls included the following: FITC-labeled IgG, PE-labeled IgG2a, and PE-labeled IgG1 (all from BD Biosciences). Cells were washed and acquired using a FACS Calibur flow cytometer (BD Immunocytometry Systems), and data analysis was performed with Cellquest software.
Human marrow stroma implantation in mice and spleen retrieval
Human MSCs obtained from a healthy volunteer/donor were implanted subcutaneously without a matrix in each of 3 Balb/c mice, at 106 cells/mouse. At 15 days after implantation, mice were killed; splenocytes were isolated and dissociated mechanically; and red blood cells were lysed by suspension in 1 mL of 8.3 g/L ammonium chloride red blood cell lysing buffer (Sigma-Aldrich Canada), and splenocytes were subsequently washed with PBS (Wisent Technologies). For the IFNγ release assay, splenocytes were incubated in 96-well U-bottom culture plates, at 106 cells/well, with syngeneic Balb/c MSCs or xenogeneic human MSCs, at 5 × 104 cells/well, in 0.2 mL RPMI 1640 media (Wisent Technologies) supplemented with 10% FBS and 50 μM 2-mercaptoethanol. After 24 hours, 0.1 mL cell supernatant was harvested and tested for the existence of mouse IFNγ by ELISA (R and D Systems).
Results
Phenotypic analysis of erythropoietin-secreting murine marrow stromal cells
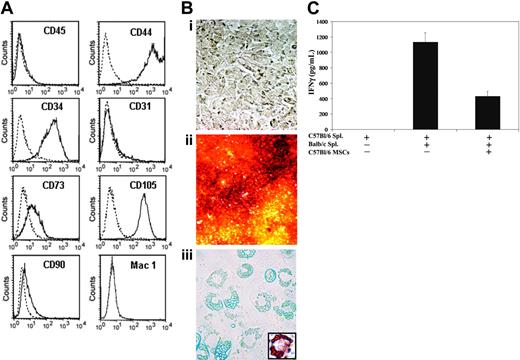
We retrovirally engineered C57Bl/6 MSCs to express murine Epo (mEpo). The vector construct used led to the expression of Epo solely without coexpression of potentially immunogenic reporter or selection genes.29 Single MSC clones were isolated by limiting dilution, and a clonal subset secreting 3 to 4 units of Epo/million cells per 24 hours was used for all further experiments in vivo. Flow cytometric analysis of this clonal subset was performed. As shown in Figure 2A, Epo+ MSCs were negative for CD31, CD45, and Mac1, and expressed CD34, CD44, CD73, CD90, and CD105.
Experimental outline of implantations. As described in “Materials and methods,” C57Bl/6 and Balb/c mice received implants of C57Bl/6-derived MSCs retrovirally engineered to secrete Epo. Blood was collected over time for Hct measurements, and MSC implants and spleens were retrieved for analysis.
Experimental outline of implantations. As described in “Materials and methods,” C57Bl/6 and Balb/c mice received implants of C57Bl/6-derived MSCs retrovirally engineered to secrete Epo. Blood was collected over time for Hct measurements, and MSC implants and spleens were retrieved for analysis.
Characterization of murine MSCs. Flow cytometry analysis was conducted on in vitro-cultured Epo+ MSCs to determine cell surface antigen expression of CD31, CD34, CD44, CD45, CD73, CD90, CD105, and Mac1, as described in “Materials and methods” (A). The dashed line represents the isotype control, and the solid line represents the specific antibody. Epo+ MSCs, undifferentiated (Bi, × 200 magnification; objective, ×20/0.4 NA), were cultured in conditions inductive of osteogenic or adipogenic differentiation. Staining with Alizarin Red S following osteogenic differentiation revealed the mineralization of the extracellular matrix (Bii, × 50; objective, ×5/0.12 NA). Adipogenic differentiation was visualized with light microscopy (Biii, × 400; objective, ×40/0.55 NA) as well as by Oil Red O staining of lipid droplets (Biii inset, × 400; objective, ×40/0.55 NA). The immunosuppressive effects of C57Bl/6-derived MSCs against allogeneic mixed lymphocyte cultures were determined as described in “Materials and methods” (C). C57Bl/6 and Balb/c splenocytes were cocultured with or without C57Bl/6-derived MSCs, and IFNγ production was assessed by ELISA after 3 days. MSCs were pretreated with recombinant mouse IFNγ and extensively washed in PBS prior to addition to the mixed lymphocytes cultures (n = 4 per group, mean ± SEM).
Characterization of murine MSCs. Flow cytometry analysis was conducted on in vitro-cultured Epo+ MSCs to determine cell surface antigen expression of CD31, CD34, CD44, CD45, CD73, CD90, CD105, and Mac1, as described in “Materials and methods” (A). The dashed line represents the isotype control, and the solid line represents the specific antibody. Epo+ MSCs, undifferentiated (Bi, × 200 magnification; objective, ×20/0.4 NA), were cultured in conditions inductive of osteogenic or adipogenic differentiation. Staining with Alizarin Red S following osteogenic differentiation revealed the mineralization of the extracellular matrix (Bii, × 50; objective, ×5/0.12 NA). Adipogenic differentiation was visualized with light microscopy (Biii, × 400; objective, ×40/0.55 NA) as well as by Oil Red O staining of lipid droplets (Biii inset, × 400; objective, ×40/0.55 NA). The immunosuppressive effects of C57Bl/6-derived MSCs against allogeneic mixed lymphocyte cultures were determined as described in “Materials and methods” (C). C57Bl/6 and Balb/c splenocytes were cocultured with or without C57Bl/6-derived MSCs, and IFNγ production was assessed by ELISA after 3 days. MSCs were pretreated with recombinant mouse IFNγ and extensively washed in PBS prior to addition to the mixed lymphocytes cultures (n = 4 per group, mean ± SEM).
Differentiation of marrow stromal cells
In order to demonstrate the osteogenic and adipogenic differentiation ability of our primary marrow stromal cells genetically engineered to secrete Epo, Epo+ MSCs were exposed to specific differentiation-inducing agents added to the culture media. Epo+ MSCs acquired an osteogenic phenotype when cultured in the presence of β-glycerol phosphate, dexamethasone, and ascorbic acid 2-phosphate, visualized following staining with Alizarin Red S of calcium in the mineralized extracellular matrix (Figure 2Bii). Moreover, Epo+ MSCs acquired an adipogenic phenotype when cultured in the presence of indomethacin, 3-isobutyl-methylxanthine, dexamethasone, and insulin (Figure 2Biii), which was further evidenced following lipid droplet staining with Oil Red O.
Mixed lymphocyte culture
In order to characterize the in vitro immunomodulating phenotype of the C57Bl/6-derived MSCs, we performed allogeneic mixed lymphocyte cultures in the presence of MSCs and assessed their immunosuppressive effects. We observed that C57Bl/6-derived MSCs added to cocultures of C57Bl/6 and Balb/c splenocytes at a ratio of 1:1:1 for 3 days induced a 62% inhibition in the production of IFNγ compared with cocultures without MSCs (P < .005 by t test; Figure 2C). The pretreatment of MSCs with recombinant IFNγ did not modulate their immunosuppressive effects (P > .05 by t test).
Hematocrit of syngeneic and allogeneic mice that received implants of Epo-secreting MSCs
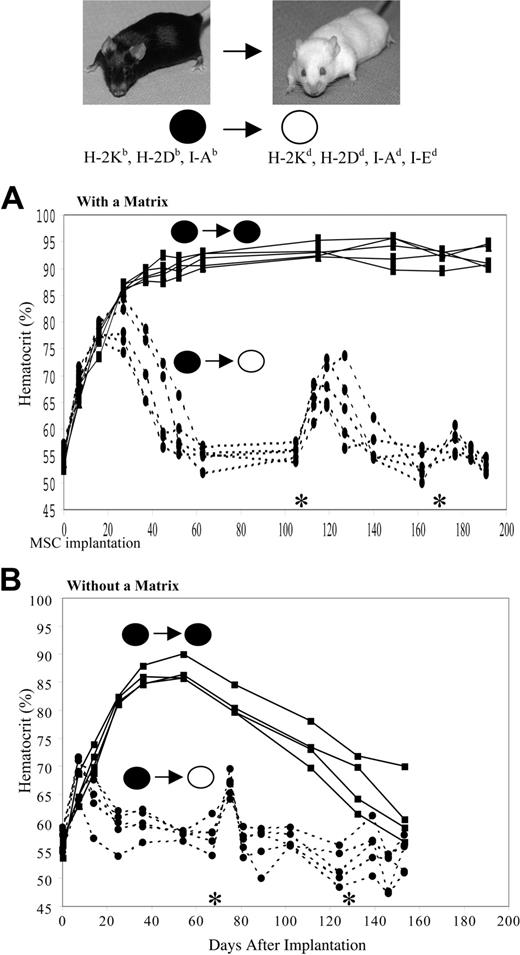
We determined whether the implantation of primary murine MSCs retrovirally engineered to secrete mEpo would be effective in MHC-mismatched allogeneic mice without immunosuppression and compared the effect with that achieved in syngeneic recipients. C57Bl/6 (H-2Kb, H-2Db, I-Ab) mice and Balb/c (H-2Kd, H-2Dd, I-Ad, I-Ed) mice were implanted with a clonal subset of mEpo MSCs (Epo+ MSCs) derived from a donor C57Bl/6 mouse. Balb/c mice are class I and II MHC mismatched relative to C57Bl/6 donors. Epo+ MSCs were implanted subcutaneously with and without a collagen-based, Food and Drug Administration (FDA)-approved matrix at 20 million cells/mL. Balb/c mice (n = 5) were injected subcutaneously with 0.5 mL Epo+ MSCs/matrix. In parallel, 5 recipient syngeneic C57Bl/6 mice likewise received implants of Epo+ MSCs. As seen in Figure 3A, in these latter syngeneic recipients, the Hct increased from a baseline value of .54 ± .0006 (54% ± 0.6%) (mean ± SEM) to .86 ± .0003 (86% ± 0.3%) at 27 days after implantation and further increased, maintaining Hct levels surpassing .88 (88%) for more than 200 days. However, in MHC-mismatched recipients, the Hct increased from a basal .56 ± .0006 (56% ± 0.6%) to a peak value of .79 ± .0019 (79% ± 1.9%) at 27 days after implantation and then decreased to baseline levels by day 52. Moreover, when these allogeneic mice received a second implant on day 107 of the same Epo+ MSCs, the Hct rise was of significantly lower intensity and shorter duration, specifically rising to a maximum value of .68 ± .0018 (68% ± 1.8%) at 12 days after second implantation and returning to a baseline value 21 days later. A third identical implant at day 170 in these same Balb/c mice led to no significant effect on Hct (Figure 3A).
The collagen matrix used to embed the MSCs is bovine in origin. Although we have not observed any significant immune reaction to the matrix alone,29 it is conceivable that the matrix acts as an “adjuvant” and promotes the observed acquired refractoriness to allogeneic MSCs. To address this possibility, repeat experiments were performed in the absence of collagen matrix. As shown in Figure 3B, an acquired refractoriness to allogeneic MSCs was again observed. In more detail, in syngeneic recipients, Epo+ MSCs caused a Hct rise from basal .56 ± .0006 (56% ± 0.6%) to .82 ± .0003 (82 ± 0.3%) at 25 days following implantation, which remained at levels of .81 (81%) to .86 (86%) until day 77, thereafter gradually dropping to .62 (62%) by day 153 after implantation. In MHC-mismatched recipient mice, the Hct augmented from a baseline value of .57 ± .0009 (57% ± 0.9%) to a peak of .69 ± .0014 (69% ± 1.4%) at 7 days after implantation and thereafter dropped and returned to baseline by day 54. A second identical implant of Epo+ MSCs on day 69 in these Balb/c mice led to a brief Hct increase to .67 ± .0009 (67% ± 0.9%) 6 days later, which then following 6 more days decreased back to a basal .57 ± .0009 (57% ± 0.9%). A third implant at day 132 of these same MSCs in these allogeneic mice led to no significant effect on Hct (Figure 3B). Control C57Bl/6 mice, as well as control Balb/c mice that did not receive implants of Epo+ MSCs, maintained Hct values of approximately .56 (56%) over time (data not shown). In addition, to ascertain that the immune rejection of MHC-mismatched MSCs is not dependent on the mode of administration, Epo+ MSCs were likewise implanted at 107 cells/mouse by intraperitoneal injection in C57Bl/6 and Balb/c mice. A rapid loss of hematocrit effect occurred within 3 weeks in allogeneic Balb/c recipients, whereas a substantial hematocrit rise persisted in syngeneic C57Bl/6 hosts (data not shown).
Hematocrit of C57Bl/6 and Balb/c mice implanted with Epo-secreting C57Bl/6-derived MSCs. As detailed in “Materials and methods,” 107 Epo+ C57Bl/6 MSCs (black dot) were implanted subcutaneously at day 0 in C57Bl/6 mice (black dot) and Balb/c mice (white dot) embedded either in a collagen-based matrix (A) or without a matrix (B), and the Hct of recipient mice was determined over time (n = 4-5 per group). Additional subcutaneous implantations of 107 Epo+ C57Bl/6 MSCs were conducted in Balb/c mice only, at days 107 and 170 (A) or at days 69 and 132 (B), as indicated by asterisk (*).
Hematocrit of C57Bl/6 and Balb/c mice implanted with Epo-secreting C57Bl/6-derived MSCs. As detailed in “Materials and methods,” 107 Epo+ C57Bl/6 MSCs (black dot) were implanted subcutaneously at day 0 in C57Bl/6 mice (black dot) and Balb/c mice (white dot) embedded either in a collagen-based matrix (A) or without a matrix (B), and the Hct of recipient mice was determined over time (n = 4-5 per group). Additional subcutaneous implantations of 107 Epo+ C57Bl/6 MSCs were conducted in Balb/c mice only, at days 107 and 170 (A) or at days 69 and 132 (B), as indicated by asterisk (*).
Anti-Epo antibody detection in plasma
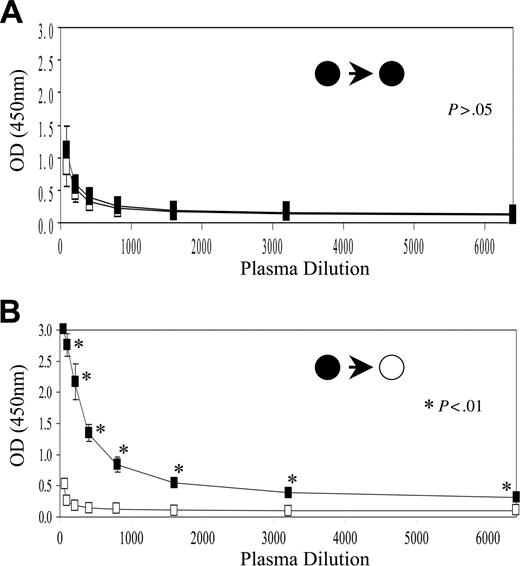
To determine the presence or absence of a humoral response against the Epo protein, plasma from C57Bl/6 and Balb/c mice at days 100 to 150 after implantation with Epo+ MSCs with and without a matrix were assayed. As shown in Figure 4B, significant levels of anti-Epo antibodies were detected in plasma of allogeneic Balb/c mice that had received implants of C57Bl/6-derived Epo+ MSCs embedded in Contigen. No anti-Epo antibodies were seen in plasma of syngeneic C57Bl/6 host mice (Figure 4A). Similarly, anti-Epo antibodies were detected in plasma of allogeneic mice that had received implants of the Epo+ MSCs without a matrix, but not in samples of syngeneic recipients (data not shown).
Flow cytometry analysis of host-derived lymphoid cells retrieved from implants
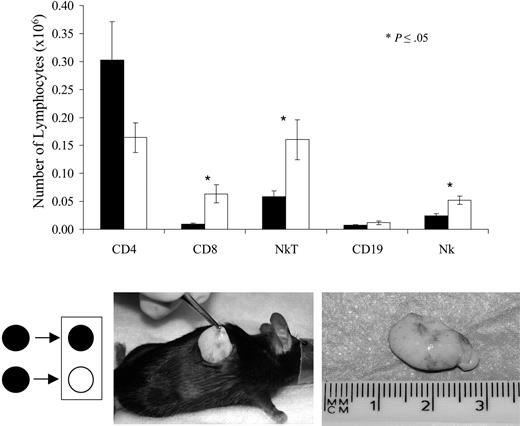
In order to characterize the host-derived immune response to the MHC-mismatched Epo+MSC implants, allogeneic Balb/c and syngeneic C57Bl/6 mice received identical implants of C57Bl/6-derived Epo+ MSCs and killed at day 15. Implants were recovered, dissociated with collagenase, and analyzed. Flow cytometric analysis conducted on host-derived lymphoid cells revealed, as illustrated in Figure 5, that allogeneic implants compared with syngeneic comprised significantly greater amounts of CD8+ cells (6.0 × 104 vs 1.0 × 104, P ≤ .05), NKT cells (16 × 104 vs 5.9 × 104, P ≤ .05), and NK cells (5.2 × 104 vs 2.5 × 104, P ≤ .05), results consistent with a host cellular immune response to donor allogeneic Epo+ MSCs. Matrix-embedded null MSCs from C57Bl/6 mice were likewise implanted in C57Bl/6 and Balb/c mice, and similarly revealed significantly greater numbers of CD8+ cells in implants retrieved from allogenic recipients (data not shown).
Analysis of murine MSCs before and after IFNγ exposure in vitro
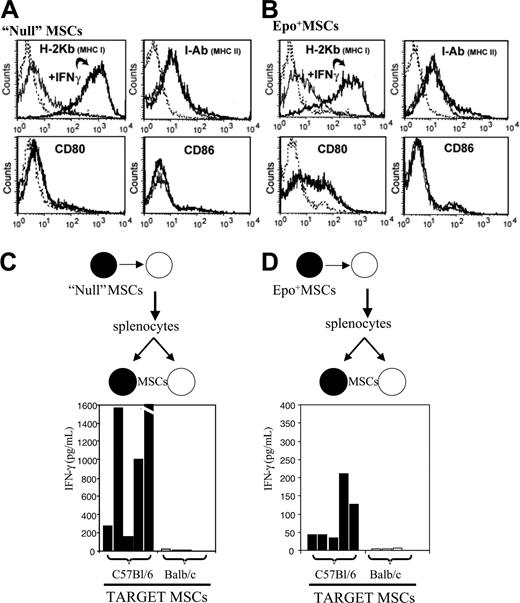
We determined whether MSCs used in our experiments expressed MHC class I and II by flow cytometry and whether this expression could be modulated by IFNγ. As indicated in Figure 6A-B, we found that polyclonal C57Bl/6 null MSCs as well as the Epo+MSC clonal subset expressed MHC class I constitutively and that the expression level could be robustly up-regulated by IFNγ. MHC class II and the CD80 costimulatory molecule were also expressed at baseline, though IFNγ exposure did not up-regulate surface expression of these antigens.
Anti-Epo antibodies detection. As described in “Materials and methods,” plasma samples from C57Bl/6 mice (A) and from Balb/c mice (B) that had received implants of matrix-embedded Epo+ MSCs derived from C57Bl/6 mice were diluted and incubated onto mouse recombinant Epo-coated 96-well plates, and following blocking, anti-Epo antibodies were revealed using anti-mouse Ig-HRP antibody and TMB substrate (n = 4-5, mean ± SEM). OD indicates optical density. The filled circle on the left side of the arrow indicates C57Bl/6-derived MSCs as donor; the filled circle on the right side of the arrow indicates C57Bl/6 mouse as recipient, whereas the open circle on the right side of the arrow indicates Balb/c mouse as recipient.
Anti-Epo antibodies detection. As described in “Materials and methods,” plasma samples from C57Bl/6 mice (A) and from Balb/c mice (B) that had received implants of matrix-embedded Epo+ MSCs derived from C57Bl/6 mice were diluted and incubated onto mouse recombinant Epo-coated 96-well plates, and following blocking, anti-Epo antibodies were revealed using anti-mouse Ig-HRP antibody and TMB substrate (n = 4-5, mean ± SEM). OD indicates optical density. The filled circle on the left side of the arrow indicates C57Bl/6-derived MSCs as donor; the filled circle on the right side of the arrow indicates C57Bl/6 mouse as recipient, whereas the open circle on the right side of the arrow indicates Balb/c mouse as recipient.
Analysis of cells isolated from retrieved implants. Implants of C57Bl/6 Epo+ MSCs in collagen recovered surgically (bottom picture) 15 days following subcutaneous implantation were dissociated with collagenase, and single-cell suspensions were analyzed, as indicated in “Materials and methods,” by flow cytometry, gating on lymphoid subsets on forward and side scatter. The amount of CD4, CD8, NKT, CD19, and NK cells among the host-derived lymphocytes in implants was compared between C57Bl/6 (▪) and Balb/c (□) recipient mice (average of n = 5 per group ± SEM). Filled circle on the right side of arrow indicates C57Bl/6 mouse as recipient, whereas open circle indicates Balb/c mouse as recipient of Epo+ C57Bl/6-derived MSCs.
Analysis of cells isolated from retrieved implants. Implants of C57Bl/6 Epo+ MSCs in collagen recovered surgically (bottom picture) 15 days following subcutaneous implantation were dissociated with collagenase, and single-cell suspensions were analyzed, as indicated in “Materials and methods,” by flow cytometry, gating on lymphoid subsets on forward and side scatter. The amount of CD4, CD8, NKT, CD19, and NK cells among the host-derived lymphocytes in implants was compared between C57Bl/6 (▪) and Balb/c (□) recipient mice (average of n = 5 per group ± SEM). Filled circle on the right side of arrow indicates C57Bl/6 mouse as recipient, whereas open circle indicates Balb/c mouse as recipient of Epo+ C57Bl/6-derived MSCs.
Cell-mediated immunity and murine MSCs
Epo has been invoked as a potential stimulator of the immune system.45 Therefore, we tested whether splenocytes derived from allogeneic MSC recipients would become activated following implantation of mismatched polyclonal MSCs bereft of genetic engineering. As described in “Materials and methods,” null polyclonal C57Bl/6 MSCs (ie, not genetically engineered and thus lacking Epo production) were implanted in Balb/c mice, and splenocytes from these recipients were isolated 15 days later and tested for their IFNγ activation by C57Bl/6 MSCs in vitro. As illustrated in Figure 6C, splenocytes from these test Balb/c mice displayed a robust, specific, and significant IFNγ response to null C57Bl/6 MSCs with IFNγ levels of 162 to 2199 pg/mL (average: 1042 pg/mL, n = 5), in contrast to controls where 0 to 22 pg/mL (average: 9.4 pg/mL, n = 5) IFNγ production was detected (P < .05). Similar results, albeit of lower magnitude, were obtained testing splenocytes from Balb/c mice that received implants of Epo-secreting C57Bl/6 MSCs (Figure 6D). Specifically, significantly high IFNγ levels, average 91.8 pg/mL (n = 5), were obtained in response to Epo+ C57Bl/6 MSCs in vitro versus average 3.2 pg/mL (n = 5) in the control group (P < .05). Moreover, no IFNγ production was detected when splenocytes from untreated naive Balb/c mice (ie, null) were cocultured with allogeneic C57Bl/6 MSCs (data not shown).
Analysis of human MSCs before and after IFNγ exposure in vitro
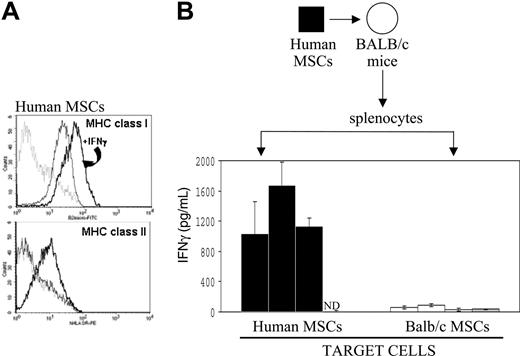
Human MSCs have been previously shown to express MHC class I, and MHC class II expression can be up-regulated with IFNγ.39 We tested whether the cell culture conditions we used for murine MSCs, once applied to human MSCs, would lead to a similar pattern of MHC class I and II expression. MSCs harvested from a healthy human volunteer were culture-expanded in vitro for 6 passages, and flow cytometric analysis was conducted before and after exposure to IFNγ. As shown in Figure 7A, human MSCs constitutively expressed MHC class I, which was markedly up-regulated with IFNγ. MHC class II expression was absent in resting human MSCs, however its expression was up-regulated following culture with IFNγ. Similar results were obtained with MSCs from a second volunteer donor (data not shown).
Human MSCs and cell-mediated immunity
To determine whether splenocytes isolated from mice would become activated subsequent to implantation of xenogeneic human MSCs, we assayed splenocytes from Balb/c mice that had received implants of human MSCs 15 days earlier for their IFNγ activation by human MSCs in vitro. As seen in Figure 7B, splenocytes from these Balb/c mice showed a strong and specific IFNγ response to human MSCs, with IFNγ values ranging from 1034 to 1670 pg/mL, in contrast to control Balb/c MSCs, where IFNγ levels were 27.87 to 91.28 pg/mL. In addition, no IFNγ release was detected when splenocytes from untreated naive Balb/c mice were cocultured with xenogeneic human MSCs.
Discussion
The potential clinical use of MSCs, and their engineered derivatives, would be greatly enhanced if use of universal donor allogeneic MSCs was feasible in normal nonimmunosuppressed recipients. Nonautologous masterbanked MSCs would be particularly valuable in settings where there is a limited access to Good Manufacturing Practice (GMP) facilities required for production of autologous MSCs or where time delays associated with culture expansion of autologous MSC preparation from individual patients are not compatible with the clinical indication addressed. One could envisage off-the-shelf convenience of large amounts of validated cellular pharmaceuticals. From a practical perspective, an MSC-derived cellular biopharmaceutical could be mass-produced, stored, and distributed in a manner similar to more classic biologicals.
Immunophenotypic analysis of murine MSCs and immune effects in mice. As detailed in “Materials and methods,” flow cytometry analysis was conducted to determine expression of MHC class I, MHC class II, CD80, and CD86 on cultured murine polyclonal null MSCs (A) and clonal Epo+ MSCs (B), prior to and following in vitro exposure to IFNγ. The dashed line represents the isotype control, the solid line represents the specific antibody, and the bolder line represents the specific antibody after IFNγ. As described in “Materials and methods,” polyclonal null C57Bl/6 MSCs (black dot) were implanted in Balb/c (white dot) recipients; splenocytes were recovered 15 days later and incubated in vitro for 24 hours with C57Bl/6 (▪) or Balb/c (□) MSCs; and supernatants were assayed for mouse IFNγ by ELISA (n = 5 per group) (C). Likewise, clonal Epo+ C57Bl/6 MSCs (black dot) were implanted in Balb/c (white dot) recipients; splenocytes were recovered 15 days later and incubated in vitro for 24 hours with C57Bl/6 (▪) or Balb/c (□) MSCs; and supernatants were assayed for mouse IFNγ by ELISA (n = 5 per group) (D).
Immunophenotypic analysis of murine MSCs and immune effects in mice. As detailed in “Materials and methods,” flow cytometry analysis was conducted to determine expression of MHC class I, MHC class II, CD80, and CD86 on cultured murine polyclonal null MSCs (A) and clonal Epo+ MSCs (B), prior to and following in vitro exposure to IFNγ. The dashed line represents the isotype control, the solid line represents the specific antibody, and the bolder line represents the specific antibody after IFNγ. As described in “Materials and methods,” polyclonal null C57Bl/6 MSCs (black dot) were implanted in Balb/c (white dot) recipients; splenocytes were recovered 15 days later and incubated in vitro for 24 hours with C57Bl/6 (▪) or Balb/c (□) MSCs; and supernatants were assayed for mouse IFNγ by ELISA (n = 5 per group) (C). Likewise, clonal Epo+ C57Bl/6 MSCs (black dot) were implanted in Balb/c (white dot) recipients; splenocytes were recovered 15 days later and incubated in vitro for 24 hours with C57Bl/6 (▪) or Balb/c (□) MSCs; and supernatants were assayed for mouse IFNγ by ELISA (n = 5 per group) (D).
Analysis of human MSCs following IFNγ exposure and immune effects in mice. (A) Flow cytometry analysis on MHC class I and MHC class II was carried out on cultured primary human MSCs before and after in vitro exposure to IFNγ, as indicated in “Materials and methods”. The dashed line represents the isotype control, the solid line represents the specific antibody, and the bolder line represents the specific antibody after IFNγ. (B) As also detailed in “Materials and methods,” human MSCs (black box) were implanted in Balb/c (white dot) recipients; splenocytes were recovered 15 days later and cultured in vitro for 24 hours with human MSCs (▪) or Balb/c MSCs (□); and supernatants were tested for mouse IFNγ by ELISA (n = 3 per group). No IFNγ release was detected when splenocytes from untreated naive Balb/c mice (▦) were cocultured with xenogeneic human MSCs (ND indicates nondetected). Data are shown as average ± SD.
Analysis of human MSCs following IFNγ exposure and immune effects in mice. (A) Flow cytometry analysis on MHC class I and MHC class II was carried out on cultured primary human MSCs before and after in vitro exposure to IFNγ, as indicated in “Materials and methods”. The dashed line represents the isotype control, the solid line represents the specific antibody, and the bolder line represents the specific antibody after IFNγ. (B) As also detailed in “Materials and methods,” human MSCs (black box) were implanted in Balb/c (white dot) recipients; splenocytes were recovered 15 days later and cultured in vitro for 24 hours with human MSCs (▪) or Balb/c MSCs (□); and supernatants were tested for mouse IFNγ by ELISA (n = 3 per group). No IFNγ release was detected when splenocytes from untreated naive Balb/c mice (▦) were cocultured with xenogeneic human MSCs (ND indicates nondetected). Data are shown as average ± SD.
Phase 1 and 2 studies in humans have clearly established that intravenous infusion of autologous MSCs in the setting of high-dose chemotherapy and autologous peripheral blood stem cell rescue is safe.46 Allogeneic MSCs can be safely and effectively administered in humans in utero47 as well as intravenously in immunosuppressed patients, especially in the setting of allogeneic cord blood48 or allogeneic marrow transplantation, as in the treatment of osteogenesis imperfecta, metachromatic leukodystrophy, and Hurler syndrome.46,49 Similarly, allogeneic MSCs may attenuate graft-versus-host disease complicating allogeneic marrow transplantation.31,39,50 Although a substantive body of literature buttresses the notion that MSCs are immunosuppressive,31-34 as we have also here demonstrated in vitro (Figure 2C), it is not yet clearly proven that MHC-mismatched MSCs are universally immunoprivileged.
In this study, we confirmed our previously published findings28,29 that primary murine MSCs retrovirally gene-modified to deliver Epo engender a significant and prolonged rise in Hct when implanted in syngeneic mice. Of greater note, however, are our results indicating that MHC class I- and class II-mismatched murine MSCs are not immunoprivileged, as the implantation of C57Bl/6-derived Epo+ MSCs in allogeneic Balb/c recipient mice led to their in vivo rejection. This apparent rejection of the MHC-mismatched MSCs was amplified by repeated challenge, as a second and later third implantation of the Epo+ MSCs in the allogeneic recipients provoked a lower and then negligible MSC effect, respectively. This pattern of acquired refractoriness is one familiarly seen in the setting of alloimmunization to foreign antigens. Moreover, we performed implant analysis and revealed that host-derived lymphoid cells in allogeneic implants consisted of significantly more CD8+, NKT, and NK cells, observations in accordance with a host cellular immune response to the Epo+ MSCs from MHC-mismatched donor. Furthermore, we showed an immune response evoked by the implantation of xenogeneic human MSCs in immunocompetent mice, as we observed considerably elevated IFNγ production by splenocytes from Balb/c mice that had received implants of human MSCs when these splenocytes were activated in vitro with the human MSCs (Figure 7B).
A possible influence of FBS presence in culture media and acquired immunogenicity has been described.18,51 Syngeneic C57Bl/6 lymphoblasts ex vivo expanded in media containing FBS have been shown to elicit lymphocytotoxic antibodies in vivo specific for bovine β2 microglobulin.51 A significant humoral response against MSCs was also recently shown to occur in rats when autologous MSCs were expanded in media containing 20% fetal calf serum. In contrast, MSCs cultured by a protocol that almost completely eliminated internalized antigens and that comprised a 6-day treatment with adult rat serum and growth factors did not lead to a neutralizing humoral response.52 The Epo+ MSCs used in the present investigation were cultured in cell culture media with 10% FBS. However, if FBS was solely responsible for the observed immune rejection, we expect that an immune response would also have arisen against MSCs in the syngeneic C57Bl/6 mice as well. Indeed, in Balb/c mice that had received implants of C57Bl/6 MSCs, we were not able to demonstrate splenocyte activation despite using FBS-exposed Balb/c stimulator MSCs in vitro. Hence, the FBS is likely not the primary cause of the immune response and in vivo rejection of the MHC-mismatched MSCs. However, due to the suggestion that autologous MSCs cultured with media containing FBS will lead to an immune response in autologous hosts, we tested the use of species-specific serum. We demonstrated that substitution of FBS by mouse serum did not prevent the rejection of allogeneic MSCs (data not shown).
In addition, we show that the Epo secreted was not the cause of the immune response, as we detected significantly elevated IFNγ release by splenocytes from allogeneic Balb/c mice that had received implants of null C57Bl/6 MSCs when these splenocytes were restimulated with these C57Bl/6 MSCs in vitro (Figure 6C). We detected, however, the presence of anti-Epo antibodies in the plasma of allogeneic but not in the plasma of syngeneic recipients, both when the Epo+ MSCs were delivered subcutaneously embedded in a matrix (Figure 4), as well as when these cells were injected without any matrix support, emphasizing that allogeneic MSCs cannot be used as “universal” cellular vehicles for long-term delivery of plasma-soluble proteins.
Our immunophenotypic analysis of culture-expanded primary human MSCs revealed them to constitutively express MHC class I, which was up-regulated with IFNγ, and to express MHC class II only upon induction with IFNγ. These findings are similar to those of other investigators.39 Of interest, freshly isolated human MSCs robustly express MHC class II and appear to lose its expression after culture expansion in vitro.53 Indeed, it has also been reported that MHC class II-positive human MSCs are able to elicit an allogeneic response in a one-way MLR, and that the soluble factors secreted by these cells did not appear fundamental in mediating the allogeneic response.54 These data suggest that human MSCs may elicit a rejection immune response as we have observed in mice. Although we show that IFNγ-stimulated human MSCs can express MHC class I and II in vitro, the functional clinical significance of this observation is unclear and what we observe in mice may be species specific and not necessarily applicable to humans, as has been proposed by others,55 especially in light of our observation that the response to IFNγ is apparently different between mouse and human MSCs at least for MHC II expression. Human MSCs have been observed to engraft in utero in sheep56 and in brains of albino rats.57 However, the lack of an anti-MSC immune response may be due to implantations in immune-privileged locations, as suggested by others.34 Allogeneic rat MSCs gene-modified with bone morphogenetic protein 2 (BMP-2) can repair femoral segmental damage similarly to autologous MSCs, although the former recipients were immunosuppressant-drug treated.58 Similarly, allogeneic simian MSCs will be detectable by polymerase chain reaction (PCR) in the bone marrow of recipient monkeys if they received hemi or total body irradiation as a conditioning regimen.59-61 These reports as an aggregate support the notion that allogeneic MSCs will engraft in conditioned, immunocompromised hosts or in immunoprivileged sites. However, in support of our observations detailed in this study, there are published reports that give credence to the hypothesis that allogeneic and xenogenic MSCs may be immunogenic in animals with normal immune systems. For instance, human MSCs can engraft in infarcted rat myocardium, but this is associated with a significant immune infiltration at the injection site.62 More specifically, there was significant myocardial infiltration of mainly macrophages at the MSC injection site in normal rats, whereas there was persistent engraftment of these human MSCs in immunologically incompetent rats.62 In a similar vein, murine MSCs gene-modified to express BMP-2 showed persistence and differentiation in bone and cartilage in allogeneic hosts.63 Nonetheless, an inflammatory immune infiltrate was noted by histologic evaluation.
In summary, we have demonstrated that class I and II MHC-mismatched MSCs elicit a robust and specific cellular immune response in allogeneic hosts with normal immune systems and that rejection of the MHC-mismatched MSCs is amplified by repeated challenge. Our results thus indicate that murine MSCs, even though possessing immunosuppressive properties in vitro, are not immunoprivileged in vivo, at least not when there is complete MHC class I and II mismatch. The immunosuppressive ability of these MSCs was not sufficient to prevent their own rejection. Immunoprivilege is an indispensable feature if aspiring to use universal nonautologous MSCs for a long-lived effect, such as is often hoped for in clinical applications. Accordingly, the use of allogeneic MSCs gene-modified for disease treatment would be futile, as immune rejection by the host would prevent their persistence for an enduring beneficial effect. In conclusion, our results strongly suggest that MSCs, at least in the murine system, cannot serve as a universal donor in cell and gene therapy applications. It is unknown whether administration of MHC-mismatched MSCs in immunocompetent postnatal humans is safe or useful. The possibility of immune rejection—and its clinical consequences—must be considered as part of the panel of potential adverse events to be examined in early-phase clinical trials.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-03-1004.
Supported by the US Army Medical Research Acquisition Activity, Fort Detrick, MD (US Army Medical Research and Material Command [USAMRMC] Breast Cancer Research program, award no. DAMD17-02-1-0447) (N.E.), the Anemia Institute for Research and Education (AIRE) of Canada, and the Fonds de la Recherche en Santé au Québec (FRSQ) Programme Hémovigilance (J.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The content of the information in this article does not necessarily reflect the position or the policy of the US government.
We thank Moira Francois for her help with the animal work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal