The red blood cell depends solely on the anaerobic conversion of glucose by the Embden-Meyerhof pathway for the generation and storage of high-energy phosphates, which is necessary for the maintenance of a number of vital functions. Many red blood cell enzymopathies have been described that disturb the erythrocyte's integrity, shorten its cellular survival, and result in hemolytic anemia. By far the majority of these enzymopathies are hereditary in nature. In this review, we summarize the current knowledge regarding the genetic, biochemical, and structural features of clinically relevant red blood cell enzymopathies involved in the Embden-Meyerhof pathway and the Rapoport-Luebering shunt.
Introduction
The moment the mature red blood cell leaves the bone marrow, it is optimally adapted to perform the binding and transport of oxygen and its delivery to all tissues. This is the most important task of the erythrocyte during its estimated 120-day journey in the blood-stream. The membrane, hemoglobin, and proteins involved in metabolic pathways of the red blood cell interact to modulate oxygen transport, protect hemoglobin from oxidant-induced damage, and maintain the osmotic environment of the cell. The biconcave shape of the red blood cell provides an optimal area for respiratory exchange. The latter requires passage through microcapillaries, which is achieved by a drastic modification of its biconcave shape, made possible only by the loss of the nucleus and cytoplasmic organelles and, consequently, the ability to synthesize proteins.1
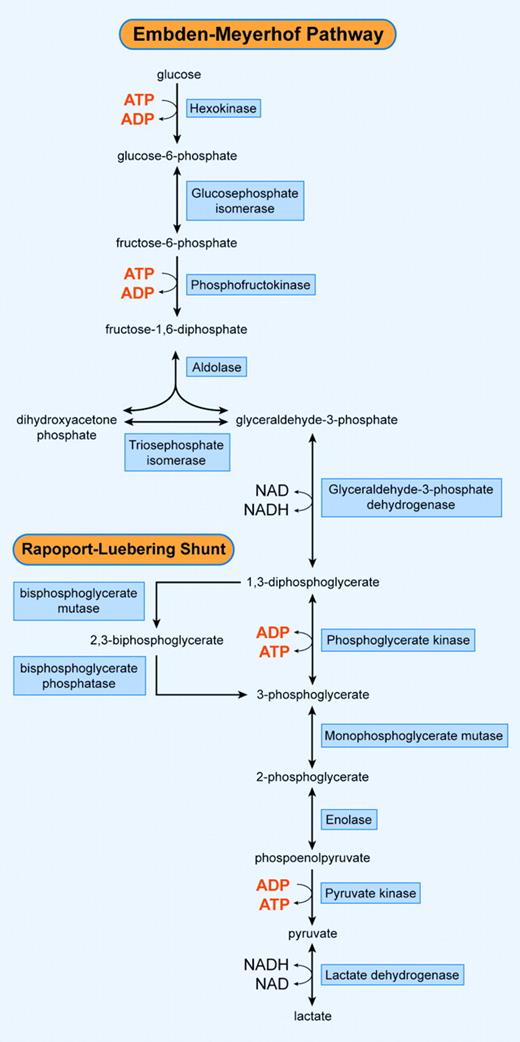
During their intravascular lifespan, erythrocytes require energy to maintain a number of vital cell functions. These include (1) maintenance of glycolysis; (2) maintenance of the electrolyte gradient between plasma and red cell cytoplasm through the activity of adenosine triphosphate (ATP)-driven membrane pumps; (3) synthesis of glutathione and other metabolites; (4) purine and pyrimidine metabolism; (5) maintenance of hemoglobin's iron in its functional, reduced, ferrous state; (6) protection of metabolic enzymes, hemoglobin, and membrane proteins from oxidative denaturation; and (7) preservation of membrane phospholipid asymmetry. Because of the lack of nuclei and mitochondria, mature red blood cells are incapable of generating energy via the (oxidative) Krebs cycle. Instead, erythrocytes depend on the anaerobic conversion of glucose by the Embden-Meyerhof pathway for the generation and storage of high-energy phosphates (Figure 1). Moreover, erythrocytes possess a unique glycolytic bypass for the production of 2,3-bisphosphoglycerate (2,3-DPG), the Rapoport-Luebering shunt. This shunt bypasses the phosphoglycerate kinase (PGK) step and accounts for the synthesis and regulation of 2,3-DPG levels that decrease hemoglobin's affinity for oxygen.2 In addition, 2,3-DPG constitutes an energy buffer.
A number of red blood cell enzymopathies have been described in the Embden-Meyerhof pathway.3-6 The lack of characteristic changes in red blood cell morphology differentiates the glycolytic enzymopathies from erythrocyte membrane defects and most hemoglobinopathies. In general, red blood cell enzymopathies cause chronic nonspherocytic hemolytic anemia (CNSHA), albeit to a variable degree. The continous lack of sufficient energy and other metabolic impairments results in a shortened lifespan of the mature red blood cell.7,8 The degree of hemolysis is dependent on the relative importance of the affected enzyme and the properties of the mutant enzyme with regard to functional abnormalities or instability, or both. The ability to compensate for the enzyme deficiency by overexpressing isozymes or using alternative pathways contributes to the clinical picture of patients with red blood cell enzymopathies. A physiologic response to compensate for anemia caused by hemolysis is increased erythrocyte production: reticulocytosis (reticulocytes normally comprise 0.5% to 2.5% of total erythrocytes). Reticulocytes still preserve cytoplasmic organelles, including ribosomes and mitochondria, and are thus capable of protein synthesis and the production of ATP by oxidative phosphorylation. Several enzymes, including hexokinase (HK), pyruvate kinase (PK), and aldolase, display much higher activity in reticulocytes and are often referred to as the age-related enzymes.9
A number of red blood cell enzymopathies concern enzymes expressed in other tissues as well. In general, the deficiency is more pronounced in red blood cells when compared with other cells, because of the long lifespan of the mature erythrocyte after the loss of protein synthesis. Therefore, once an enzyme in red blood cells is degraded or has otherwise become nonfunctional, it cannot be replaced by newly synthesized proteins. In addition, tissue-to-tissue differences in proteases may play a role in the extent to which various tissues manifest the deficiency state.10 It may well be that those mutant glycolytic enzymes that affect all tissues cause prenatal mortality and are, therefore, never seen by clinicians. The mutant enzymes that come to light are those in whom the distribution of the deficiency is compatible with extrauterine life. By far the majority of red cell enzymopathies are hereditary in nature, although acquired deficiencies have also been described, mainly in malignant hematologic disorders.11
Schematic overview of the Embden-Meyerhof pathway and the Rapoport-Luebering shunt. Illustration enhanced by A. V. Chen.
Schematic overview of the Embden-Meyerhof pathway and the Rapoport-Luebering shunt. Illustration enhanced by A. V. Chen.
In this review, a summary is made of the major features regarding the biochemical, structural, and genetic basis of clinically relevant red blood cell enzymopathies involved in the Embden-Meyerhof pathway and the Rapoport-Luebering shunt.
The Embden-Meyerhof pathway
Glucose is the energy source of the red blood cell. Under normal physiologic circumstances (ie, no excessive oxidative stress), 90% of glucose is catabolized anaerobically to pyruvate or lactate by the Embden-Meyerhof pathway, or glycolysis (Figure 1). Although one mole of ATP is used by HK, and an additional mole of ATP by phosphofructokinase (PFK), the net gain is 2 moles of ATP per mole of glucose, because a total of 4 moles of ATP are generated by PGK and PK. In addition, reductive potential is generated in the form of nicotinamide adenine dinucleotide (NADH) in the step catalyzed by glyceraldehyde-3-phosphate dehydrogenase. This can be used to reduce methemoglobin to hemoglobin by NADH-cytochrome b5 reductase. If this reaction takes place, the end product of the glycolysis is pyruvate. However, if NADH is not reoxidized here, it is used in reducing pyruvate to lactate by lactate dehydrogenase (LDH) in the last step of glycolysis.
The Embden-Meyerhof pathway is subjected to a complex mechanism of inhibiting and stimulating factors. The overall velocity of red blood cell glycolysis is regulated by 3 rate-limiting enzymes, HK, PFK, and PK, and by the availability of NADH and ATP. Some glycolytic enzymes are allosterically stimulated (eg, fructose-1,6-bisphosphate [FBP] for PK) or inhibited (eg, glucose-6-phosphate [G6P] for HK) by intermediate products of the pathway.
Hexokinase
Hexokinase catalyzes the phosphorylation of glucose to G6P, using ATP as a phosphoryl donor (Figure 1). As the initial step of glycolysis, HK is one of the rate-limiting enzymes of this pathway. The activity of hexokinase is significantly higher in reticulocytes compared with mature red cells, in which it is very low. In fact, of all glycolytic enzymes HK has the lowest enzymatic activity in vitro.12
In mammalian tissues, 4 isozymes of HK with different enzymatic properties exist: HK-I to -III, with a molecular mass of 100 kDa, and HK-IV (or glucokinase), with a molecular mass of 50 kDa. HK-I to -III are considered to be evolved from an ancestral 50 kDa HK by gene duplication and fusion.13 Consequently, both the C- and N-terminal halves of HK-I to -III show extensive internal sequence similarity but only in the case of HK-II is catalytic function maintained in both the C- and N-terminal halves. HK-I and HK-III have further evolved into enzymes with catalytic (C-terminal) and regulatory (N-terminal) halves, respectively. HK-I is the predominant isozyme in human tissues that depend strongly on glucose utilization for their physiologic functioning, such as brain, muscle, and erythrocytes. HK-I displays unique regulatory properties in its sensitivity to inhibition by physiologic levels of the product G6P and relief of this inhibition by inorganic phosphate.14,15
The determination of the structures of the human (PDB entry 1DGK) and rat HK-I isozymes has provided substantial insight into ligand binding sites and subsequent modes of interaction of these ligands (Figure 2).16,17 The mode of inhibition by G6P remains subject to debate.
Erythrocytes contain a specific subtype of HK (HK-R)18 that is encoded by the HK-I gene (HK1), localized on chromosome 10q22 and spanning more than 100 kb. The structure of HK1 is complex. It encompasses 25 exons, which, by tissue-specific transcription, generate multiple transcripts by alternative use of the 5′ exons.19 Erythroid-specific transcriptional control results in a unique red blood cell-specific mRNA that differs from HK-I transcripts at the 5′ untranslated region (5′-UTR) and at the first 63 nucleotides of the coding region.20 Consequently, HK-R lacks the porin-binding domain that mediates HK-I binding to mitochondria.21
Hexokinase deficiency (OMIM 235 700) is a rare autosomal, recessively inherited disease with CNSHA as the predominant clinical feature. As with most glycolytic red cell enzyme deficiencies, the severity of hemolysis is variable, ranging from severe neonatal hemolysis and death to a fully compensated chronic hemolytic anemia. Splenectomy is in general beneficial. Seventeen families with hexokinase deficiency have been described to date22 and only 3 patients have been characterized at the molecular level.23-25 One compound heterozygous patient carried a missense mutation that encoded a leucine to serine substitution at residue 529. An as-yet-unidentified mutation on the other allele caused skipping of the sixth exon, as detected in this patient's cDNA.23 One other patient was homozygous for a missense mutation that predicted the substitution of a highly conserved threonine by serine at residue 680 in the enzyme's active site, where it interacted with phosphate moieties of adenosine diphosphate (ADP), ATP, and G6P.25 The last case constituted a lethal out-of-frame deletion of exons 5 to 8 of HK1. This deletion was identified in the homozygous state in a fetus that died in utero.24
Human hexokinase in complex with glucose, phosphate, and ADP. Ribbon representation of human hexokinase I with N-terminal and C-terminal halves depicted in red and blue, respectively. Selected ligands are shown in ball-and-stick representation and colored green (glucose), orange (ADP), and yellow (inorganic phosphate). The figure was generated from the atomic coordinates of the HK-I ADP/glucose complex (protein data bank entry 1DGK) using the program PyMOL. (DeLano WL. The PyMOL Molecular Graphics System [2002], available online at http://www.pymol.org).
Human hexokinase in complex with glucose, phosphate, and ADP. Ribbon representation of human hexokinase I with N-terminal and C-terminal halves depicted in red and blue, respectively. Selected ligands are shown in ball-and-stick representation and colored green (glucose), orange (ADP), and yellow (inorganic phosphate). The figure was generated from the atomic coordinates of the HK-I ADP/glucose complex (protein data bank entry 1DGK) using the program PyMOL. (DeLano WL. The PyMOL Molecular Graphics System [2002], available online at http://www.pymol.org).
In mice, a mutation associated with downeast anemia causes severe hemolytic anemia with extensive tissue iron deposition and marked reticulocytosis, closely resembling human CNSHA. The primary defect is in the murine Hk1 gene and HK activity is markedly decreased in red blood cells, spleen, and kidney. As such, it represents a mouse model of generalized HK deficiency.26
Glucose-6-phosphate isomerase
Glucose 6-phosphate isomerase (GPI) catalyzes the interconversion of G6P into fructose-6-phosphate (F6P) in the second step of the Embden-Meyerhof pathway (Figure 1). As a result of this reversible reaction, products of the hexose-monophosphate shunt can be recycled to G6P. Unlike HK and other age-related enzymes, the GPI activity in reticulocytes is only slightly higher than that of mature erythrocytes. Apart from its role in glycolysis, GPI exerts cytokine properties outside the cell and is involved in several extracellular processes.27 Because GPI knock-out mice die in the embryologic state, GPI is considered to be a crucial enzyme.28
Recently, the crystal structure of human GPI was resolved (PDB entry 1IAT). The enzyme is a homodimer, composed of 2 63-kDa subunits of 558 amino acids each. The active site is composed of polypeptide chains from both subunits. Thus, formation of the dimer is a prerequisite for catalytic activity.29 The structural gene coding for GPI (GPI) is located on chromosome 19q13.1. GPI spans at least 50 kb and consists of 18 exons that are transcribed into a cDNA of 1.9 kb in length.30
GPI deficiency (OMIM 172 400) is an autosomal recessive disease and is second to PK deficiency in frequency, with respect to glycolytic enzymopathies. Approximately 50 families with GPI deficiency have been described worldwide.31 Homozygous or compound heterozygous patients have chronic hemolytic anemia of variable severity and display enzymatic activities of less than 25% of normal. Hemolytic crises may be triggered by viral or bacterial infections. Hydrops fetalis appears more common in GPI deficiency than in other enzyme deficiencies.32 In rare cases, GPI deficiency also affects nonerythroid tissues, causing neurologic symptoms and granulocyte dysfunction.33 Normally, GPI is very stable, but a striking feature of nearly all GPI mutants is their thermolability, whereas kinetic properties are more or less unaffected. Twenty-nine mutations have been detected in GPI, 24 of which were missense mutations, 3 were nonsense mutations, and 2 were mutations that affected splice sites.31 Mapping of these mutations to the crystal structure of human GPI has provided insight into the molecular mechanisms causing hemolytic anemia in this disorder. In accordance with the 3-dimensional structure, mutations can be categorized into 3 distinct groups that affect the overall structure, the dimer interface, and the active site.29
As in humans, homozygous GPI-deficient mice exhibit severe CNSHA, reticulocytosis, and hyperbilirubinemia. The hematologic features in the mouse mutants resemble that of the severe, chronic form of the human enzymopathy with increased G6P levels, and normal or decreased concentrations of glycolytic metabolites following the metabolic block. In addition, other tissues are also affected, indicating a reduced glycolytic capability of the whole organism.34
Phosphofructokinase
Phosphofructokinase catalyzes the rate-limiting, ATP-mediated phosphorylation of F6P to FBP (Figure 1). PFK is a homo- or heterotetramer with a molecular mass of approximately 380 kDa, and the enzyme is allosterically regulated, among other metabolites, by 2,3-DPG.35 Three different subunits have been identified in humans: PFK-M (muscle), PFK-L (liver), and PFK-P (platelet).36 The subunits are expressed in a tissue-specific manner and, in erythrocytes, 5 isoenzymes of varying subunit composition (M4, M3L1, M2L2, ML3, and L4) can be identified.37 The gene encoding the M subunit (PFKM) has been assigned to chromosome 12q13.3 and spans 30 kb. It contains 27 exons and at least 3 promoter regions.38 The L-subunit encoding gene (PFKL) is located on chromosome 21q22.3; it contains 22 exons and spans more than 28 kb.39
PFK deficiency (OMIM 171 850) is a rare autosomal, recessively inherited disorder. Because red blood cells contain both M and L subunits, mutations affecting either gene will affect enzyme activity. Thus, mutations concerning the L subunit will render red blood cells that contain only M4 and they are partially PFK deficient. In such cases, patients display a mild hemolytic disorder without myopathy. Likewise, a deficiency of the M subunit results in the absence of muscle PFK and, in addition, causes PFK deficiency in erythrocytes. Accordingly, deficiency of the M subunit causes myopathy and a mild hemolytic disorder. Erythrocytes that express only the L4 PFK also show a metabolic block at the PFK step in glycolysis and lowered 2,3-DPG levels. To date, 15 PFK-deficient PFKM alleles from more than 30 families have been characterized.40 One-third of the reported patients are of Jewish origin and in this population an intronic splice site mutation, IVS5+1G>A,41 and a single base-pair deletion in exon 22, c.2003delC,42 are among the most frequently encountered mutations.
A naturally occurring animal model of PFK-M deficiency has been described in English springer spaniels. These extensively studied dogs have a chronic compensated hemolytic disorder and exertional myopathy, as is seen in human patients. Molecular analysis identified a nonsense mutation in the penultimate exon of the PFK-M gene, causing the rapid degradation of the truncated PFK-M subunit.43
Aldolase
Aldolase catalyzes the reversible conversion of FBP to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate (DHAP; Figure 1). Aldolase is a tetramer of identical subunits of 40 kDa each, and 3 distinct isoenzymes have been identified: aldolase A, B, and C. The 364-amino-acids-long aldolase A subunits are predominantly expressed in erythrocytes and muscle. The x-ray crystallographic structure of human muscle aldolase A (PDB entries 1ALD, 2ALD, and 4ALD) demonstrates that the homotetrameric enzyme is composed of 4 monomers, each characterized by an 8-membered αβ-barrel structure.44 The active site of each monomer is located at the center of the αβ-barrel. The gene for aldolase A (ALDOA) is located on chromosome 16q22-q24. It spans 7.5 kb and consists of 12 exons. Multiple transcription-initiation sites have been determined and ALDOA pre-mRNA is spliced in a tissue-specific manner.45
Aldolase deficiency (OMIM 103 850) is a very rare disorder and only 6 patients from 5 families have been described.46-50 Patients all displayed moderate chronic hemolytic anemia. Enzyme stability was decreased in a male patient described by Beutler et al.46 In addition to hemolytic anemia, this patient also displayed mental retardation and dysmorphic features.46 The 2 patients reported by Miwa et al47 suffered from severe hemolytic anemia, exacerbated by infection, but none of the features described by Beutler et al. In one of these patients the causative mutation concerned a homozygous substitution of aspartic acid to glycine at residue 128.51 The patient reported by Kreuder et al48 suffered from hemolytic anemia, myopathy, and psychomotor retardation. In this case, aldolase deficiency was caused by a homozygous ALDOA missense mutation that predicted a glutamic acid to lysine substitution at residue 206 at the subunit interface.48 Two additional patients were recently reported; clinical manifestations of the first included transfusion-dependent anemia but normal cognitive function, with death at age 4 associated with rhabdomyolysis. The patient was compound heterozygous for a null mutation, Arg303Stop, and a missense mutation, Cys338Tyr, in ALDOA.49 The second patient was a child with suspected hemolytic anemia and myopathic symptoms at birth in whom a heterozygous Gly346Ser substitution was identified which affected the flexible C-terminal region of the enzyme.50
Triosephosphate isomerase
Triosephosphate isomerase (TPI) is the glycolytic enzyme with the highest activity in vitro.12 TPI catalyzes the interconversion of glyceraldehyde-3-phosphate and DHAP (Figure 1). It consists of a dimer with 2 identical subunits of 248 amino acids (27 kDa).52 No TPI isozymes are known but 3 distinct electrophoretic forms can be distinguished as a result of posttranslational modifications.53 In red blood cells TPI activity is not maturation dependent. TPI is transcribed from a single gene (TPI1) on chromosome 12p13 that consists of 7 exons and spans 3.5 kb.54 Three processed pseudogenes have been identified.54
TPI deficiency (OMIM 190 450) is a rare autosomal recessive disorder, characterized by hemolytic anemia at onset, often accompanied by neonatal hyperbilirubinemia requiring exchange transfusion. In addition, patients display progressive neurologic dysfunction,55 increased susceptibility to infection, and cardiomyopathy.56 Patients show a 20- to 60-fold increased DHAP concentration in their erythrocytes,57 consistent with a metabolic block at the TPI step. Most affected individuals die in childhood before the age of 6 years but there are remarkable exceptions. In a Hungarian family with TPI deficiency, 2 adult germ-line identical compound heterozygous brothers display strikingly different phenotypes. Both have the same severe decrease in TPI activity and congenital hemolytic anemia, but only one suffers from severe neurologic disorder. Studies aimed at the pathogenesis of this differing phenotype point to functional differences in lipid environment influencing the enzyme activities involved in these phenotypic differences.58
Fourteen mutations have been described in TPI1 and the most frequently ocurring mutation (74%) causes a glutamic acid to aspartic acid change at residue 104.59 Knowledge of the crystal structure of the human enzyme (PDB entry 1HTI) has provided insight into the probable effect of this mutation, which likely perturbs the local structure of the active site.52 The enzymatic activity of the Glu104Asp mutant is further reduced by its attachment to erythrocyte inside-out vesicles and to microtubules of brain cells.60 Haplotype analysis strongly suggested a single origin for the Glu104Asp substitution whereby the common ancestor originated from Northern Europe.61 This is in agreement with the European origin of almost all TPI-deficient individuals.
An animal model of TPI deficiency caused by missense and nonsense mutations has been developed in ethylnitrosourea-treated mice. Heterozygous animals display a 50% loss of enzymatic activity, whereas homozygosity for these TPI null alleles is lethal at an early stage of development.62
Phosphoglycerate kinase
Phosphoglycerate kinase generates one molecule of ATP by catalyzing the reversible conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate (Figure 1). This reaction can be bypassed by the Rapoport-Luebering shunt (see “Rapoport-Luebering shunt”), thus preventing the formation of the second ATP molecule. Two isozymes of PGK exist: PGK-1, ubiquitously expressed in all somatic cells, and PGK-2, expressed only in spermatozoa.63 PGK-1 is a 48-kDa monomer consisting of 417 amino acids.64 The 3-dimensional structure of horse muscle PGK, highly homologous to the human enzyme, reveals that PGK consists of 2 domains. These domains are connected by a conserved hinge, allowing for conformational freedom.65 The ADP/ATP binding site is located on the C-terminal domain, whereas phosphoglycerates bind the N-terminal domain. A conformational rearrangement involving bending of the hinge occurs upon binding of both substrates, bringing them in position for phosphate transfer.66
The gene encoding PGK-1 (PGK1) is located on Xq13.3, thereby rendering PGK deficiency an X-linked disorder. PGK1 spans 23 kb and is composed of 11 exons.67
PGK deficiency (OMIM 311 800) is characterized by chronic hemolytic anemia (often fully compensated), dysfunction of the central nervous system, and myopathy. However, the phenotype of PGK-1 deficiency is highly variable because patients usually do not display all 3 clinical features.68 PGK activity varies between 0% and 20% of normal but there is no correlation of residual enzymatic activity with clinical severity. Fourteen different mutations in PGK1 have been described in association with PGK deficiency.40 Most of these mutations are missense mutants of which one, in addition to encoding a nonconserved Glu252Ala substitution, resulted in aberrant splicing with a consequent 90% reduction in mRNA levels.69
Pyruvate kinase
Pyruvate kinase catalyzes the irreversible phosphoryl group transfer from phosphoenolpyruvate to ADP, yielding pyruvate and the second molecule of ATP in glycolysis (Figure 1). PK is a key regulatory enzyme of glycolysis, and pyruvate is crucial for several metabolic pathways. The enzyme is active as a tetramer, and 4 different isozymes are expressed in mammals. R-type PK expression is confined to the red blood cell,70 whereas L-type PK is predominately expressed in the liver. The PK-R and PK-L subunits are both transcribed from a single gene (PKLR), located on chromosome 1q21, by the use of alternative promoters.70,71 PKLR consists of 12 exons and spans 9.5 kb.72,73 Exon 1 is erythroid specific, whereas exon 2 is liver specific. Hence, exons 3 to 12 are included in both liver- and red blood cell-specific mRNAs and encode a PK-R subunit of 574 amino acids, whereas the PK-L subunit comprises 531 amino acids. PK isozymes PK-M1 and PK-M2 are produced from a single gene (PKM) by means of alternative splicing.74 PK-M1 is expressed in skeletal muscle, heart, and brain, and it is the only isozyme that is not subjected to allosteric regulation. The PK-M2 isozyme is expressed in early fetal tissues, but also in most adult tissues, including leucocytes and platelets.
In basophilic erythroblasts, both PK-R and PK-M2 are expressed. During further erythroid differentiation and maturation, a switch in isozymes occurs whereby progressively increased PK-R expression gradually replaces PK-M2.75,76 This is in part due to changes in protein synthesis and degradation rates.77 Human red blood cell PK consists of 2 distinct species, PK-R1 and PK-R2. PK-R1 predominates in reticulocytes and young erythrocytes, whereas mature red blood cells possess mainly PK-R2.78 PK-R1 is a homotetramer composed of 4 PK-R, also called L′, subunits (L′4). Limited proteolytic degradation of this 63-kDa PK-R subunit renders a 57 kDa to 58 kDa PK-L subunit that is incorporated in the heterotetramer PK-R2 (L2L′2).79 The enzymatic activity of PK decreases with increasing cell age of the erythrocyte.
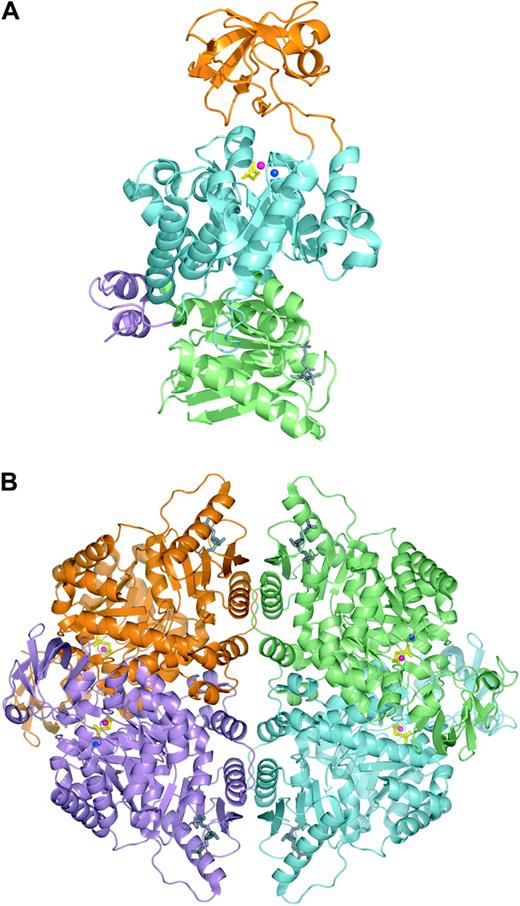
PK is allosterically activated by phosphoenolpyruvate and FBP, and negatively regulated by its product ATP.80 Furthermore, PK has an absolute requirement for cations, normally Mg2+ and K+.81 Recently, the 3-dimensional structure of human erythrocyte PK (PDB entry 1LIU) was elucidated.82 Each PK-R subunit can be divided into 4 domains (Figure 3): the N domain (residues 1-84), A domain (residues 85-159 and 263-431), B domain (residues 160-262), and C domain (residues 432-574).82 Domain A is the most highly conserved, whereas the B and C domain are more variable.83 The active site lies in a cleft between the A domain and the flexible B domain. The B domain is capable of rotating away from the A domain generating either the “open” or “closed” conformation. The C domain contains the binding site for FBP. The allosteric transition from the inactive T state to the active R state involves the simultaneous and concerted rotations of entire domains of each subunit, in such a way that all subunit and domain interfaces are modified.84 The allosteric and catalytic sites are able to communicate with each other across the relatively long distance that separates the FBP binding site from the catalytic center. The A/A′ and C/C′ subunit interface interactions and the A/B interdomain interactions are considered key determinants of the allosteric response.84-86
Human erythrocyte pyruvate kinase in complex with substrate analog phosphoglycolate and the allosteric activator fructose-1,6-diphosphate. Ribbon representation of the human erythrocyte pyruvate kinase monomer (A) and tetramer (B). Phosphoglycolate and fructose-1,6-diphosphate are shown in stick representation and colored yellow and gray, respectively. Metal ions in the active site are shown as blue (potassium) and pink (manganese) spheres. (A) PK monomer with domains N, A, B, and C colored violet, cyan, orange, and lime, respectively. (B) PK tetramer with individual subunits colored lime, cyan, violet, and orange. (The figures were generated from the atomic coordinates of protein data bank entry 1LIU using the program PyMOL.)
Human erythrocyte pyruvate kinase in complex with substrate analog phosphoglycolate and the allosteric activator fructose-1,6-diphosphate. Ribbon representation of the human erythrocyte pyruvate kinase monomer (A) and tetramer (B). Phosphoglycolate and fructose-1,6-diphosphate are shown in stick representation and colored yellow and gray, respectively. Metal ions in the active site are shown as blue (potassium) and pink (manganese) spheres. (A) PK monomer with domains N, A, B, and C colored violet, cyan, orange, and lime, respectively. (B) PK tetramer with individual subunits colored lime, cyan, violet, and orange. (The figures were generated from the atomic coordinates of protein data bank entry 1LIU using the program PyMOL.)
PK deficiency (OMIM 266 200) is the most common cause of nonspherocytic hemolytic anemia due to defective glycolysis and is inherited in an autosomal recessive manner. The estimated prevalence is 51 cases (ie, homozygous or compound heterozygous patients) per million in the white population.87 Two major metabolic abnormalities result from PK deficiency: ATP depletion and increased 2,3-DPG content.7 However, the precise mechanisms that lead to a shortened lifespan of the mature PK-deficient erythrocyte are still unknown. The increased 2,3-DPG levels ameliorate the anemia by lowering the oxygen-affinity of hemoglobin (see “Rapoport-Luebering shunt”).88 Phenotypically, the clinical picture varies from severe hemolysis causing neonatal death to a well-compensated hemolytic anemia. Some PK-deficient patients present with hydrops fetalis.89 Reticulocytosis is almost always observed. Splenectomy often ameliorates the hemolysis, especially in severe cases, and increases reticulocyte counts even further. Persistent expression of the PK-M2 isozyme has been reported in the red blood cells of patients (and animals; see below) with severe PK deficiency.90,91 The survival of these patients, though not in all cases,92 may be enabled by this compensatory increase in PK activity.
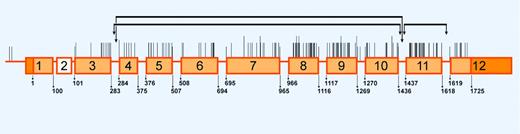
By the year 2005, more than 160 mutations in PKLR have been reported to be associated with pyruvate kinase deficiency.93 A schematic overview is presented in Figure 4. Most mutations (70%) are missense mutations affecting conserved residues in structurally and functionally important domains of PK. In the European and North-American populations, the most frequently detected mutations are missense mutants c.1456C>T (Arg486Trp), c.1529G>A (Arg510Gln), c.994G>A (Gly332Ser), and nonsense mutant c.721G>T (Glu241stop). There appears to be no direct relationship between the nature and location of the substituted amino acid and the type of molecular perturbation.82 As in TPI deficiency, patients with identical genotypes may be differently affected. For example, the clinical phenotype of 12 c.1529G>A homozygotes ranges from a very mild compensated hemolysis to a severe anemia.91 One transcriptional mutant is of particular interest because it silences erythroid-specific PKLR transcription completely. The causative single-base change disrupts a putative binding domain for an as-yet-unidentified trans-acting factor that mediates the effects of factors necessary for regulation of PK gene expression during red cell differentation and maturation.94
PK deficiency has also been recognized in dogs and mice. In both species, the deficiency causes severe anemia and marked reticulocytosis, closely resembling human PK deficiency. Basenji dogs lack PK-R enzymatic activity as a result of a frameshift mutation. Instead, only the PK-M2 isozyme is expressed in their red blood cells.95 PK-deficient mice homozygous for a missense mutation show delayed switching from PK-M2 to PK-R, resulting in delayed onset of the hemolytic anemia.96 Another loss of function mutation in homozygous PK-deficient mice underlies the malaria resistance in these animals and may, similarly, protect humans against malaria.97
Distribution of PKLR mutations associated with PK deficiency. Schematic representation of the PKLR gene and its erythroid-specific promoter. Exons, but not introns, are drawn to scale. Exons are numbered and depicted as orange rectangles with 5′ and 3′ noncoding sequences in dark orange. The white rectangle represents the liver-specific exon 2. Nucleotides are numbered starting from ATG in red blood cell-specific exon 1. The locations of the more than 160 mutations associated with PK deficiency93 are indicated by vertical lines. Larger vertical lines represent multiple base changes at the same nucleotide position. The horizontal lines indicate the extent of the 3 large deletions known to date. Illustration enhanced by A. V. Chen.
Distribution of PKLR mutations associated with PK deficiency. Schematic representation of the PKLR gene and its erythroid-specific promoter. Exons, but not introns, are drawn to scale. Exons are numbered and depicted as orange rectangles with 5′ and 3′ noncoding sequences in dark orange. The white rectangle represents the liver-specific exon 2. Nucleotides are numbered starting from ATG in red blood cell-specific exon 1. The locations of the more than 160 mutations associated with PK deficiency93 are indicated by vertical lines. Larger vertical lines represent multiple base changes at the same nucleotide position. The horizontal lines indicate the extent of the 3 large deletions known to date. Illustration enhanced by A. V. Chen.
Glyceraldehyde-3-phosphate dehydrogenase, monophosphoglycerate mutase, enolase, and lactate dehydrogenase
Red blood cell deficiencies of glyceraldehyde-3-phosphate dehydrogenase98,99 and enolase100,101 have been described in association with hemolytic anemia but a causal relationship was not established.
Monophosphoglycerate mutase (MPGM) in erythrocytes is a homodimer, composed of 2 of the ubiquitously expressed B subunits.102 Only one case of B subunit MPGM deficiency is known to date: a patient with moderate hemolytic anemia due to hereditary spherocytosis displayed, in addition, a 50% reduction in red blood cell MPGM activity caused by a homozygous Met230Ile substitution.103
Rapoport-Luebering shunt
In the erythrocyte, 2,3-DPG is synthesized and dephosphorylated in the Rapoport-Luebering shunt (Figure 1). This glycolytic bypass is unique to mammalian red blood cells and represents an important physiologic means to regulate oxygen affinity of hemoglobin.106 The oxygen affinity of hemoglobin is also influenced by slight changes of blood pH, and a corresponding sensitivity for the pH exists in the Rapoport-Luebering shunt that, again, permits changes in 2,3-DPG contents to fine-tune the oxygen affinity of hemoglobin. Quantitatively, 2,3-DPG is the major glycolytic intermediate in the red blood cell and its levels are about equal to the sum of the other glycolytic intermediates.1 Enzyme deficiencies proximal to the 2,3-DPG step (ie, PGK and PK deficiency) show increased 2,3-DPG levels as a result of the respective metabolic blocks and of a retrograde accumulation of products of glycolysis. The increased 2,3-DPG levels result in a decreased oxygen affinity of hemoglobin so that oxygen is more readily transferred to tissue. Thus, the anemia is ameliorated and exertional tolerance is improved. This beneficial effect is absent in the distal glycolytic enzyme defects HK, GPI, PFK, aldolase, and TPI deficiency that all cause a decrease in 2,3-DPG levels. Several of the distal glycolytic enzymes (ie, HK and PFK) are inhibited by 2,3-DPG.
Both reactions in the Rapoport-Luebering shunt are catalyzed by the erythrocyte-specific multifunctional enzyme bisphosphoglycerate mutase (BPGM), which posesses synthase (formation of 2,3, BPG) and phosphatase (hydrolysis of 2,3-DPG to 3-phosphoglycerate) activity (Figure 1).107 BPGM is a homodimer, with 30-kDa subunits consisting of 258 amino acids. The enzyme is closely related to the glycolytic housekeeping enzyme MPGM.108 Recently, the crystal structure of human BPGM has been determined (PDB entry 1T8P), confirming the concept that BPGM and MPGM are structurally homologous enzymes, and providing a rationale for the specific residues that are crucial for synthase, mutase, and phosphatase activity.109
The gene for BPGM (BPGM) has been mapped to chromosome 7q31-34 and it consists of 3 exons, spanning more than 22 kb.110 BPGM deficiency (OMIM 222 800) is a very rare autosomal recessive disorder and only 2 affected families have been described. In the first family, patients had severely reduced 2,3-DPG levels and increased ATP levels.111 They were clinically normal and displayed no hemolytic anemia. Instead, they presented with erythrocytosis that likely resulted from the reduced 2,3-DPG levels and, consequently, the increased oxygen affinity of hemoglobin. These patients were later found to be compound heterozygous for a single-nucleotide change in BPGM that predicted the replacement of a highly conserved arginine by cysteine at residue 89 (BPGM Créteil I) and a single-nucleotide deletion that introduces a premature stop codon and, consequently, encodes a truncated peptide (BPGM Créteil II).112 In the second BPGM-deficient family, the asymptomatic proband also presented with secondary erythrocytosis. He displayed markedly decreased BPGM enzyme activity levels whereas his consanguineous parents showed normal 2,3-DPG levels but BPGM activity levels of approximately 50% of normal. In addition, all family members had markedly decreased glucose-6-phosphate dehydrogenase activity although there was no laboratory evidence of hemolysis. DNA sequencing of the BPGM gene showed that the propositus was homozygous for a point mutation in exon 2 that predicted the substitution of Arg62 by Gln.113
Concluding remarks
A variety of clinical features are associated with the described hereditary enzymopathies of the red blood cell. This is mainly due to the role of the affected enzyme in glycolysis as well as the underlying molecular alteration responsible for defective enzymatic function. Therefore, a better understanding of the clinical phenotype of defective glycolyis in the red blood cell requires knowledge regarding the genetic, biochemical, and structural consequences of mutations in the genes and the respective enzymes they encode. However, the phenotype is not solely dependent on the molecular properties of mutant proteins but rather reflects a complex interplay between physiologic, environmental, and other (genetic) factors. Putative phenotypic modifiers include differences in genetic background, concomitant functional polymorphisms of other glycolytic enzymes (many enzymes are regulated by their product or other metabolites), posttranslational modification, epigenetic modification, ineffective erythropoiesis, and different splenic function. Also, aberrant enzymatic function in nonerythroid tissues may influence the clinical outcome of hereditary enzymopathies. Hence, future research aimed at the relationship between genotype and phenotype correlation in red blood cell enzymopathies will also have to take these phenotypic modifiers into account.
Prepublished online as Blood First Edition Paper, July 28, 2005; DOI 10.1182/blood-2005-04-1622.
Both authors contributed equally to the writing of the paper.
The authors sincerely wish to thank Gert Rijksen for helpful discussion of the manuscript. We thank Eric Huizinga and Michael Hadders for the preparation of Figures 2 and 3.

![Figure 2. Human hexokinase in complex with glucose, phosphate, and ADP. Ribbon representation of human hexokinase I with N-terminal and C-terminal halves depicted in red and blue, respectively. Selected ligands are shown in ball-and-stick representation and colored green (glucose), orange (ADP), and yellow (inorganic phosphate). The figure was generated from the atomic coordinates of the HK-I ADP/glucose complex (protein data bank entry 1DGK) using the program PyMOL. (DeLano WL. The PyMOL Molecular Graphics System [2002], available online at http://www.pymol.org).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/13/10.1182_blood-2005-04-1622/2/m_zh80240587920002.jpeg?Expires=1769082800&Signature=4fJ-Ubyp-MnYbGZy22nUS24KSFkMvkPpjBMpa6~uCcybeppSKCWFtzxmdZWKLJt0nFHS9X~z53E~SwdJu~D76W69yo3CfEyKJ94JW~9xD~ulE-5e0VCGWvc7SmyJvzf~6Wvd9MrMizE-GiuZUlsjGbYUEfW1toAlFv-OD2HVFRqE-xy0RBWbckZMtNK7ru4nr1xw1-InvAOdyLCJdyCEV7AEyhyLWROtmgC4g8ka2tF8VXoaCbpoU1dsmPwstdcs~M-xXysDsTvG2lrWA5bFQZx1eN1BpgLLzA6pmmAbir1-KNv2aJ9YJe9iNGsdyia5Pa9dW3wWp6QTlSRl0BaFmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal