The human plasma protein β2-glycoprotein I (β2-GPI) is the most common target for antiphospholipid antibodies associated with thrombotic events in chronic disorders related to endothelial cell dysfunction. Crucial information is needed to clarify why this self-abundant protein is targeted by autoimmune responses. In this study, we investigated whether oxidative modification of β2-GPI, either spontaneous in culture wells or induced by treatment with H2O2, renders this self-protein able to activate immature monocyte-derived dendritic cells (DCs) from healthy human donors. Oxidized β2-GPI caused DCs to mature so that CD83 appeared and CD80, CD86, human leukocyte antigen-D region related (HLA-DR), and CD40 increased. The interaction between oxidized β2-GPI and DCs specifically stimulated these cells to secrete interleukin 12 (IL-12), IL-1β, IL-6, IL-8, tumor necrosis factor α (TNF-α), and IL-10. Oxidized β2-GPI-stimulated DCs had increased allostimulatory ability and primed naive T lymphocytes, thus inducing T helper 1 (Th1) polarization. The interaction between oxidized β2-GPI and DCs involved interleukin-1 receptor associated kinase (IRAK) phosphorylation and nuclear factor κB (NFκB) activation. Pretreatment of β2-GPI with the antioxidant α-tocopherol prevented DC maturation. These findings show that human oxidized β2-GPI, probably by interacting with a member of the Toll-like receptor (TLR) family, causes DCs to mature. Because this key β2-GPI function requires oxidative modification, in several chronic disorders related to endothelial cell dysfunction oxidative stress might trigger the “autoimmune spiral.”
Introduction
β2-glycoprotein I (β2-GPI), a human plasma protein that binds to negatively charged phospholipids, is the most common target for antiphospholipid antibodies (aPLs). These autoantibodies are associated with thrombotic events in systemic lupus erythematosus1 and primary antiphospholipid antibody syndrome2,3 and are proatherogenic.4 β2-GPI also activates lipoprotein lipase,5 lowers the triglyceride level,6 and binds to oxidized low-density lipoprotein (LDL)7 and to nonself particles or apoptotic bodies to allow their clearance.8-10
Another property of β2-GPI is its ability to bind at the monocyte surface, thus promoting tissue factor and thereby increasing the risk of thrombotic events.11 β2-GPI can also be expressed on the endothelial cell membrane12 and on macrophages.13 Among the several candidate β2-GPI cell receptors, annexin II, megaline, and apolipoprotein E receptor 2′ (apoER2′) are involved in activating endothelial cells and platelets.14-16 Recent findings demonstrate that anti-β2-GPI antibodies react with their antigen probably in association with a member of the Toll-like receptor (TLR)/interleukin 1 (IL-1) receptor family on the endothelial cell surface and directly induce activation.17,18 Once bound to endothelial cells, β2-GPI offers suitable epitopes targeting circulating anti-β2-GPI antibodies that affect cell functions and induce a proinflammatory and procoagulant phenotype.19
The molecular structure and location of the major epitopic region(s) on the β2-GPI molecule are controversial. Numerous studies have investigated whether the immune response is directed to native β2-GPI20-22 or to cryptic or neoepitopes.23,24 Decisive events generating cryptic or neoepitopes include β2-GPI binding to anionic surfaces such as phospholipids, the adsorption of β2-GPI onto γ-irradiated polystyrene surface, and oxidative modification that alters phospholipid binding.25-30
Among plasma proteins involved in the hemostatic system, thrombin, coagulation factors V, VIII, X, and fibrinogen have been reported to undergo major structural and functional changes upon exposure to oxidative stress.31 Oxidative damage is increasingly reported in patients with primary antiphospholipid antibody syndrome, systemic lupus erythematosus, and atherosclerosis.32-34 In these chronic disorders related to endothelial cell dysfunction, β2-GPI plays a role as a target antigen for an immune-mediated attack, possibly influencing the progression of disease.35,36 β2-GPI stimulates a vigorous adaptive cellular and humoral immune response.28,37,38 Recent evidence, in patients with antiphospholipid antibody syndrome and healthy subjects, describes autoreactive CD4+ T cells to β2-GPI. These cells promote production of pathogenic aPL antibodies and preferentially recognize cryptic peptides of β2-GPI derived from efficient processing and presentation of β2-GPI complexed to anionic surfaces by mature dendritic cells (DCs).39 Other evidence shows that β2-GPI resides in the subendothelial regions in human atherosclerotic plaques and colocalizes with immune system cells.4
To prevent induction of autoimmunity, the immune system uses a highly effective control mechanism that efficiently discriminates between self and nonself.40 Peripheral tolerance to self-antigens is maintained by immature DCs, whereas mature DCs can activate autoreactive T cells.41 A fundamental question related to the immune response to β2-GPI is the event that triggers the “autoimmune spiral.” An attractive possibility is that the autoimmune response and the response to foreign antigens follow the same rules for induction, namely presentation on DCs.42 DCs might therefore represent the link between innate and adaptive immunity. The function of DCs is to capture antigens at peripheral sites and to migrate to T-cell areas in lymphoid organs, where they elicit a specific T-cell response by presenting antigen.43 During this process, activated DCs undergo distinct changes in phenotype and function, termed DC maturation.44
Although much is known about β2-GPI as a cofactor in autoimmune diseases, crucial information is still lacking to clarify why this abundant self-plasma protein is the target of autoimmune responses.
Prompted by evidence that oxidative stress mediates structural and functional changes in β2-GPI and is involved in several chronic disorders related to endothelial cell dysfunction, our objective in this study was to explore the possible interaction between oxidized β2-GPI and DCs. Using immunochemical and cytofluorimetric analysis, we investigated whether oxidative modification of β2-GPI renders this self-molecule able to activate immature monocyte-derived DCs from healthy human donors, thus inducing phenotypic and functional characteristics typical of mature DCs.
Materials and methods
Characterization of β2-glycoprotein I
Human native β2-GPI prepared from human plasma by barium citrate adsorption, ion exchange, and heparin agarose chromatography and with a purity more than 98% as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was purchased from Calbiochem (La Jolla, CA). Supernatants of β2-GPI-treated DCs were collected after 48 hours of culture to test the spontaneous oxidation of β2-GPI (ox-β2-GPI). Control oxidized β2-GPI was prepared by treating the protein (1 mg/mL in phosphate-buffered saline [PBS]) with 10 μL of 0.25% hydrogen peroxide solution for 1 hour at room temperature. l-methionine (5 mg) was added and allowed to stand at room temperature for 1 hour (H2O2-β2-GPI). To avoid protein oxidation in culture medium control, native β2-GPI was pretreated for 2 hours at 37°C with the antioxidant α-tocopherol (5 μM) (Sigma-Aldrich, Milan, Italy) (αTOC-β2-GPI). To rule out enzymatic modifications of β2-GPI by proteases, we added to the DC culture medium a protease inhibitor cocktail consisting of a mixture of water-soluble protease inhibitors with broad specificity for the inhibition of serine, cysteine, aspartic acid, and metalloproteases (Sigma-Aldrich) (β2-GPI + PI). To investigate whether metals catalyzed oxidative modification of β2-GPI, we added to the DC culture medium metal chelators consisting of diethylenetriaminepentaacetic acid (DTPA) (β2-GPI + DTPA). Endotoxin contamination in β2-GPI, as determined by the quantitative chromogenic limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, MD), was less than 0.03 EU/μg protein.
The degree of protein modification in our samples was evaluated with 2-dimensional electrophoresis (2-DE) and immunoblotting (IB). The samples were dialyzed against water, lyophilized, and dissolved in the appropriate volume of rehydration solution (7 M urea, 2 M thiourea, 4% wt/vol chaps [3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid], 1% Triton X-100, 20 mM Tris [tris(hydroxymethyl)aminomethane], and traces of bromophenol blue; all reagents from Sigma-Aldrich; 0.2% Bio-Lyte ampholytes from Bio-Rad, Hercules, CA). After active rehydration at 50 V for 12 hours, 5 μg of the protein was run in isoelectrofocusing (IEF) onto a linear wide range immobilized pH gradient (IPG) (pH 3-10, 7-cm long IPG strips from Bio-Rad) for 1 hour at 250 V and 14 hours at 4000 V using the Protean IEF Cell (Bio-Rad). After IEF, strips were equilibrated twice for 15 minutes in equilibration buffer containing 6 M urea, 30% vol/vol glycerol (Sigma-Aldrich), 2% wt/vol SDS (Bio-Rad) in 50 mM Tris-HCl buffer (pH 8.9) supplemented with 5 mg/mL dithiothreitol (DTT) for the first treatment and 45 mg/mL iodoacetamide (Sigma-Aldrich) and a trace of bromophenol blue for the second treatment. After equilibration, the IPG gels were transferred for the second dimension into vertical 10% (wt/vol) PAGE apparatus (9 cm × 7 cm × 1.5 mm) and run with the Laemmli SDS-discontinuous system at a maximum of 30 mA per gel until the dye front reached the bottom of the gel. The proteins were electrotransferred from the gels to nitrocellulose for immunoblotting analysis. The nitrocellulose filters were incubated overnight with a polyclonal goat serum specific to the human β2-GPI (GAB2G-AP; Affinity Biologicals, Ancaster, ON, Canada) and revealed with horseradish peroxidase (HRP)-conjugated rabbit anti-goat immunoglobulin G (IgG) antibody (Sigma-Aldrich). The filters were processed and analyzed by GS-700 imaging densitometer (Bio-Rad) and molecular analysis software.
DC differentiation
Peripheral blood mononuclear cells (PBMCs) from 11 healthy human donors were isolated by density gradient separation (Lympholite; Cedarlane, Hornby, ON). Informed consent was provided for the use of blood samples according to the Declaration of Helsinki. The study was approved by the University “La Sapienza” of Rome. CD14+ monocytes were purified by incubation with anti-CD14-coated microbeads (Miltenyi Biotec, Bergish Gladbach, Germany), followed by sorting with a magnetic device (MiniMacs Separation Unit; Miltenyi Biotec), according to the manufacturer's instructions. Monocytes were induced to differentiate to DCs in 5- to 6-day culture in 100 ng/mL recombinant human granulocyte-macrophage colony-stimulating factors (GM-CSFs; R&D Systems, Abingdon, United Kingdom) and 1000 U/mL recombinant human interleukin 4 (rhIL-4; R&D Systems) in RPMI 1640 supplemented with 1% nonessential amino acids, 1% sodium pyruvate, 50 U/mL penicillin, 50 μg/mL streptomycin (Gibco, Grand Island, NY), 5 × 10-5 M 2-mercaptoethanol (Merck, West Point, PA), and 10% fetal calf serum (Hyclone Laboratories, Logan, UT). DC purity and maturation were routinely checked by flow cytometric analysis. Typically, the cultures contained more than 90% CD1a+CD14- cells.
Purification of T cells
CD4+ T cells were purified from PBMCs by negative selection using the untouched CD4+ T-cell Isolation Kit (Miltenyi Biotec) and by positive selection using anti-CD4+ Microbeads (Miltenyi Biotec), according to the manufacturer's instructions. Negatively and positively selected CD4+ T cells were depleted of CD45RO+ cells using anti-CD45RO-coupled magnetic beads and LD-negative selection columns (Miltenyi Biotec) to obtain negatively selected CD45RA+ T cells. The purity of negatively and positively selected CD4+CD45RA+ cells was analyzed by direct staining for membrane expression of CD45RA and of CD4 using phycoerythrin (PE)-conjugated monoclonal antibody (mAb) to CD45RA and fluorescein isothiocyanate (FITC)-conjugated mAb to CD4 (Becton Dickinson-Pharmingen Biosciences, San Jose, CA). A portion of the negatively selected CD4+CD45RA+ T cells was cryopreserved for later use in T-cell priming experiments.
Flow cytometric analysis of phenotypic DC maturation
Preliminary dose-response experiments (2-20 μg/mL) established that β2-GPI effects were dose dependent and we chose 10 μg/mL as the optimal reagent concentration of β2-GPI for DC stimulation. Five-day human DCs were stimulated with or without ox-β2-GPI (10 μg/mL), H2O2-β2-GPI (10 μg/mL), αTOC-β2-GPI (10 μg/mL), β2-GPI + PI (10 μg/mL), β2-GPI + DTPA (10 μg/mL), and lipopolysaccharide (LPS; 100 ng/mL; Escherichia coli strain 0111:B4; Sigma-Aldrich) for 18 and 48 hours, then collected and washed. As control protein, we used human serum albumin (HSA), oxidized as for β2-GPI by treatment with hydrogen peroxide (H2O2-HSA, 10 μg/mL). To rule out endotoxin contamination of ox-β2-GPI and of H2O2-β2-GPI, the same experiments were run in the presence of polymyxin B (10 μg/mL; Sigma-Aldrich). For phenotypic analysis, DCs were stained with PE-conjugated mAbs to CD1a, CD80, and human leukocyte antigen-D region related (HLA-DR), and FITC-conjugated mAbs, to CD83, CD86, and CD40 (Becton Dickinson-Pharmingen Biosciences) for 30 minutes at 4°C, before analysis by flow cytometry on a FACScan using CellQuest software (Becton Dickinson-Pharmingen Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
Because phenotypic DC maturation increases production of proinflammatory cytokines, we determined by ELISA the cytokine content in stimulated and unstimulated DC culture supernatants collected at 18 and 48 hours. Levels of IL-12p70, IL-10, tumor necrosis factor α (TNF-α), IL-6, IL-8, and IL-1β were determined by ELISA (OptEIA sets; Becton Dickinson-PharMingen Biosciences) following the manufacturer's instructions. Interferon-γ (IFN-γ), interleukin 4 (IL-4), and IL-10 production by naive T cells was tested after 3 days of culture in complete medium (OptEIA kits; Becton Dickinson-PharMingen Biosciences). Preliminary experiments were designed to determine the detection limits as well as the linearity and range of the ELISAs, essentially in accordance with the ICH Q2A and Q2B guidelines (International Conference on Harmonization, CPMP, European Medicines Agency). The intra-assay variation ranged from 3% to 6% and the interassay variation, from 4% to 9%.
The limits of detection were as follows: IL-10, TNF-α, and IL-1β: 16 pg/mL; IL-12p70 and IL-4: 7.8 pg/mL; IL-6 and IL-8: 2.2 pg/mL; and IFN-γ: 4.7 pg/mL.
Mixed lymphocyte reaction
Because critical aspects of DC function in vivo are antigen presentation and T-cell activation, we evaluated the ability of stimulated and unstimulated DCs to activate alloreactive T cells in a standard mixed lymphocyte reaction (MLR). This assay was chosen because, although it is not specific for a given antigen, it provides adequate information on the overall antigen-presenting function of DCs. The ability of DCs to stimulate allogeneic T cells was assessed, and irradiated DCs (30 Gy) were used as stimulator cells. Allogeneic T lymphocytes (1 × 105 cells/well) were incubated with the irradiated DCs for 3 days at a different responder-stimulator ratio (1:6 to 1:256 DC/T) in a 96-well round-bottom plate. On day 2, 0.5 μCi (0.0185 MBq)/well 3H-methyl-thymidine (Amersham, Life Science, Buckinghamshire, United Kingdom) was added to each well. After a further 18 hours at 37°C, cells were harvested on glass fiber filter paper (Wallac, EG&G, Turku, Finland), using an automatic cell harvester (Harvester 96, MACH III M; TOMTEC, Orange, CT). 3H-methyl-thymidine uptake into cell DNA was measured by reading samples in a β counter (1450 Microbeta Plus; Wallac, EG&G). Net counts per minute (cpm) of triplicate cultures were measured.
T-cell priming
To find out whether ox-β2-GPI-stimulated DCs primed naive T lymphocytes and induced T helper 1 (Th1) cell polarization, negatively selected naive allogeneic T cells were cultured with ox-β2-GPI-stimulated DCs at a ratio of 20:1. Activated T cells were expanded for 10 days with recombinant IL-2 (30 U/mL; Roche Molecular Biochemicals, Mannheim, Germany), added on day 5, in a 24-well plate in complete medium to obtain polyclonal T-cell lines to be analyzed for IL-4, IFN-γ, and IL-10 expression, by flow cytometry. In brief, 106 cells were stimulated with 10-7 M phorbol 12-myristate 13-acetate (PMA) plus 1 μg/mL ionomycin for 4 hours in the presence of 10 μg/mL brefeldin A (all reagents from Sigma-Aldrich). Cells were labeled with anti-CD3 peridinin-chlorophyll-protein (PerCP) (Becton Dickinson-Pharmingen Biosciences) (5 μL/104 cells, 30 minutes on ice); cells were then fixed with fluorescence-activated cell sorter (FACS) lysing solution, treated with FACS permeabilizing solution (Becton Dickinson-Pharmingen Biosciences), stained with a predetermined optimal concentration of anticytokine mAb or appropriate isotype mAb control (Becton Dickinson-Pharmingen Biosciences), and analyzed on a FACScan. Variables evaluated were the pattern of cytokine expression on the CD3+ population. Cells were gated according to light scatter properties to exclude cell debris. A minimum of 10 000 viable cells was analyzed for each sample. Results were processed using CellQuest software (Becton Dickinson-Pharmingen Biosciences).
IRAK phosphorylation assay
Cell-free lysates from unstimulated DCs or DCs stimulated with ox-β2-GPI, H2O2-β2-GPI, αTOC-β2-GPI, and LPS for 45 minutes at 37°C in 5% CO2 were immunoprecipitated with a polyclonal anti-interleukin-1 receptor associated kinase (IRAK) antibody (MBL, Woburn, MA). In brief, cells were lysed in lysis buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.2], 1% nonidet P-40, 10% glycerol, 50 mM NaF, including protease inhibitors). To preclear nonspecific binding, cell-free lysates were mixed with protein A-acrylic beads (Bio-Rad) and stirred in a rotary shaker for 1 hour at 4°C. After centrifugation (at 500g for 1 minute), the supernatant was immunoprecipitated with the anti-IRAK antibody (4 μg) plus protein A-acrylic beads. The immunoprecipitates were subjected to 7.5% SDS-PAGE. The proteins were electrophoretically transferred to nitrocellulose membrane (Bio-Rad) and then, after blocking with PBS, containing 1% albumin, probed with antiphosphoserine mAb (Sigma-Aldrich). Bound antibodies were visualized with HRP-conjugated anti-mouse IgG (Sigma-Aldrich), and immunoreactivity was assessed by the chemiluminescence reaction using the enhanced chemoluminescence (ECL) Western blotting system (Amersham Life Science). To confirm the positive band as IRAK, the antiphosphoserine antibody was stripped from the nitrocellulose, and the membrane was then reprobed with polyclonal anti-IRAK antibody. As a control for nonspecific reactivity, parallel SDS-PAGE gels were blotted as described, using an anti-mouse IgG (Sigma-Aldrich).
Nuclear factor κB (NFκB) assay
The NFκB p65/p50 transcription factor assay kit (Alexis Biochemicals, San Diego, CA) was used to monitor NFκB activation. Unstimulated DCs and DCs stimulated for 45 minutes at 37°C in 5% CO2 with ox-β2-GPI, H2O2-β2-GPI, and LPS were lysed, protein was quantified, and equal amounts of lysates were used to test activated levels of the p50 and p65 subunits with the antibodies directed against the subunits bound to the oligonucleotide containing the NFκB consensus binding site. An HeLa cell extract was used as a positive control alone or in the presence of wild-type or mutated consensus oligonucleotide.
Statistical analysis
Statistically significant differences were tested with the Student paired t test. Wilcoxon nonparametric test for paired data was used to determine the significance of the time-effect curve. P values less than .05 were considered significant. All cultures were performed in triplicate.
Results
Oxidative modification of β2-GPI
The 2-DE and IB analysis of β2-GPI preparations showed that native β2-GPI and β2-GPI oxidized either spontaneously in the culture medium (ox-β2-GPI) or after treatment with H2O2 (H2O2-β2-GPI) yielded different protein profiles. Native β2-GPI contained a train spot representing the 7 isoforms with isoelectric points (pIs) ranging from 5.0 to 7.0 and with molecular weights ranging from 75 to 70 kDa (Figure 1). Conversely, human β2-GPI, oxidized either spontaneously in the culture medium (ox-β2-GPI) or after treatment with H2O2 (H2O2-β2-GPI), gave a different train spot, and fragments (ranging from 49.6 to 41.6 kDa) and aggregates (114 and 119 kDa), including dimers, appeared. In the H2O2-β2-GPI preparation, additional fragments at 32.6 kDa appeared. After addition to the DC culture medium of a protease inhibitor cocktail, we did not observe any change.
Oxidative modification of human β2-GPI. Structural modifications in human β2-GPI were demonstrated by 2-DE and IB with a polyclonal goat serum specific to the human β2-GPI. Molecular weights are shown on the left (kDa). Native β2-GPI contained a train spot representing the 7 isoforms with pIs ranging from 5.0 to 7.0 and with molecular weights ranging from 75 to 70 kDa (A). Human β2-GPI, oxidized either after treatment with H2O2 (B) or spontaneously in the culture medium (C) gave a main train spot, fragments, and aggregates. In the H2O2-β2-GPI preparation, additional fragments at 32.6 kDa appeared. The addition of a protease inhibitor cocktail to the DC culture medium left the 2-DE pattern unchanged (D).
Oxidative modification of human β2-GPI. Structural modifications in human β2-GPI were demonstrated by 2-DE and IB with a polyclonal goat serum specific to the human β2-GPI. Molecular weights are shown on the left (kDa). Native β2-GPI contained a train spot representing the 7 isoforms with pIs ranging from 5.0 to 7.0 and with molecular weights ranging from 75 to 70 kDa (A). Human β2-GPI, oxidized either after treatment with H2O2 (B) or spontaneously in the culture medium (C) gave a main train spot, fragments, and aggregates. In the H2O2-β2-GPI preparation, additional fragments at 32.6 kDa appeared. The addition of a protease inhibitor cocktail to the DC culture medium left the 2-DE pattern unchanged (D).
Oxidized β2-GPI-induced DC maturation and cytokine secretion
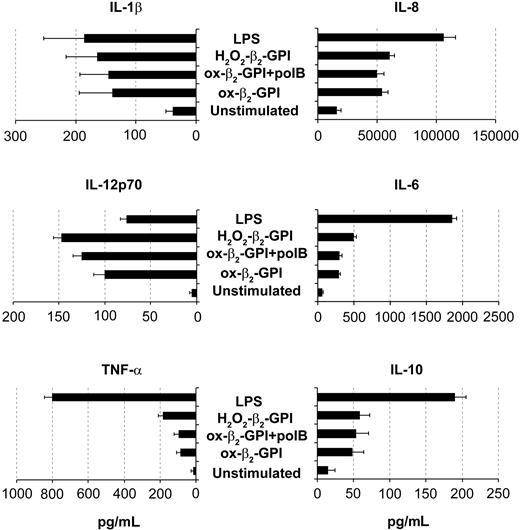
In an extensive phenotypic study, unstimulated DCs showed an immature phenotype (CD1a+ major histocompatibility complex class IIlow [MHC IIlow] CD83-) and were weakly immunoreactive for CD80, CD86, and CD40. As expected, LPS caused DCs to mature, so that CD83 appeared (Figure 2A, left) and CD80, CD86, HLA-DR, and CD40 increased (Figure 2B). Ox-β2-GPI and H2O2-β2-GPI induced almost similar DC maturation, although the degree of maturation varied among individual experiments. Pretreatment of β2-GPI with the antioxidant α-tocopherol prevented the phenotypic maturation of DCs, whereas pretreatment with polymyxin B did not. Pretreatment of LPS with the antioxidant α-tocopherol left LPS-induced phenotypic maturation unchanged. Adding metal chelators (DTPA) also inhibited DC maturation (the appearance of CD83), suggesting that metals probably catalyze β2-GPI oxidative modification (Figure 2A, right). In contrast, protease inhibitor cocktail failed to inhibit β2-GPI-induced DC maturation. Control protein H2O2-HSA failed to induce the DC phenotypic maturation (Figure 2A, right). At 48 hours, ox-β2-GPI and H2O2-β2-GPI triggered statistically significant up-regulation of IL-1β, IL-8, IL-6, IL12p70, TNF-α, and IL-10 secretion (P < .001) (Figure 3). H2O2-HSA left up-regulation of cytokine secretion statistically unchanged (data not shown). The time courses of ox-β2-GPI- and LPS-induced IL-12p70 differed: at 18 hours LPS induced significant secretion of this cytokine, whereas ox-β2-GPI did not (data not shown).
Oxidized β2-GPI induced maturation of human monocyte-derived DCs. Five-day human DCs were stimulated with or without ox-β2-GPI (10 μg/mL), H2O2-β2-GPI (10 μg/mL), αTOC-β2-GPI (10 μg/mL), and LPS (100 ng/mL). Expression of surface molecules was analyzed by flow cytometric analysis as described in “Materials and methods.” Phenotypic maturation of DCs was detected by the appearance of CD83 and by the expression of costimulatory molecules. (A) H2O2-β2-GPI and ox-β2-GPI induced the appearance of CD83 after 18 hours of culture (□) and (B) induced a significant up-regulation of CD80 (□), CD86 (▪), HLA-DR (▦), and CD40 (▧) expression at 48 hours of culture. Pretreatment of β2-GPI with the antioxidant α-tocopherol (5 μM) prevented the phenotypic maturation of DCs, whereas pretreatment with polymyxin B (pol B) did not. Pretreatment of LPS with the antioxidant α-tocopherol left LPS-induced phenotypic maturation unchanged. Adding metal chelators (DTPA) also inhibited DC maturation (the appearance of CD83), suggesting that metals probably catalyze β2-GPI oxidative modification (A, right). In contrast, protease inhibitors failed to inhibit β2-GPI-induced DC maturation. Control protein H2O2-HSA failed to induce DC phenotypic maturation (A, right). Results are expressed as mean ± SD of the positive cell percentages (A) and of the mean fluorescence intensity (B) of 11 independent experiments. Significant differences are indicated (*P < .001; **P = .043 by Student t test; ***P < .002 by Wilcoxon nonparametric test). Samples were analyzed on a FACScan cytofluorimeter using CELLQuest software (Becton Dickinson, Pharmingen Biosciences).
Oxidized β2-GPI induced maturation of human monocyte-derived DCs. Five-day human DCs were stimulated with or without ox-β2-GPI (10 μg/mL), H2O2-β2-GPI (10 μg/mL), αTOC-β2-GPI (10 μg/mL), and LPS (100 ng/mL). Expression of surface molecules was analyzed by flow cytometric analysis as described in “Materials and methods.” Phenotypic maturation of DCs was detected by the appearance of CD83 and by the expression of costimulatory molecules. (A) H2O2-β2-GPI and ox-β2-GPI induced the appearance of CD83 after 18 hours of culture (□) and (B) induced a significant up-regulation of CD80 (□), CD86 (▪), HLA-DR (▦), and CD40 (▧) expression at 48 hours of culture. Pretreatment of β2-GPI with the antioxidant α-tocopherol (5 μM) prevented the phenotypic maturation of DCs, whereas pretreatment with polymyxin B (pol B) did not. Pretreatment of LPS with the antioxidant α-tocopherol left LPS-induced phenotypic maturation unchanged. Adding metal chelators (DTPA) also inhibited DC maturation (the appearance of CD83), suggesting that metals probably catalyze β2-GPI oxidative modification (A, right). In contrast, protease inhibitors failed to inhibit β2-GPI-induced DC maturation. Control protein H2O2-HSA failed to induce DC phenotypic maturation (A, right). Results are expressed as mean ± SD of the positive cell percentages (A) and of the mean fluorescence intensity (B) of 11 independent experiments. Significant differences are indicated (*P < .001; **P = .043 by Student t test; ***P < .002 by Wilcoxon nonparametric test). Samples were analyzed on a FACScan cytofluorimeter using CELLQuest software (Becton Dickinson, Pharmingen Biosciences).
Oxidized β2-GPI-induced increase in the allostimulatory ability of DCs
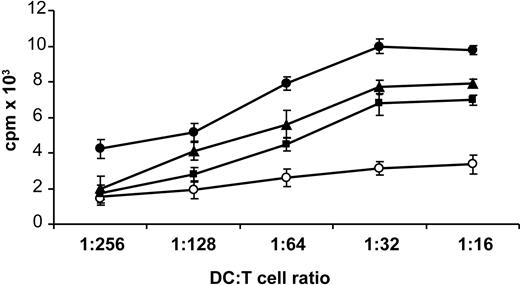
When irradiated DCs, prestimulated with H2O2-β2-GPI, ox-β2-GPI, and LPS, were tested in an MLR, the relatively low proliferative ability (mean cpm) of resting allogeneic T cells achievable with unstimulated DCs significantly increased, starting from a DC/T-cell ratio of 1:128 (DC/T-cell ratio of 1:128: unstimulated = 1256 cpm, ox-β2-GPI = 5139 cpm, P = .004; H2O2-β2-GPI = 3584 cpm, P = .04; LPS = 6594 cpm, P < .001; by Student t test, n = 11 experiments) (Figure 4).
Oxidized β2-GPI induced release of cytokines by DCs. Five-day human DCs were stimulated with or without ox-β2-GPI (10 μg/mL), H2O2-β2-GPI (10 μg/mL), and LPS (100 ng/mL). Supernatants were collected after 48 hours to measure IL-1β, IL-8, IL-12p70, IL-6, TNF-α, and IL-10 by specific ELISA experiments. Ox-β2-GPI and H2O2-β2-GPI triggered statistically significant up-regulation of all cytokine secretion (P < .001). Results are expressed as mean value ± SD of 11 independent experiments.
Oxidized β2-GPI induced release of cytokines by DCs. Five-day human DCs were stimulated with or without ox-β2-GPI (10 μg/mL), H2O2-β2-GPI (10 μg/mL), and LPS (100 ng/mL). Supernatants were collected after 48 hours to measure IL-1β, IL-8, IL-12p70, IL-6, TNF-α, and IL-10 by specific ELISA experiments. Ox-β2-GPI and H2O2-β2-GPI triggered statistically significant up-regulation of all cytokine secretion (P < .001). Results are expressed as mean value ± SD of 11 independent experiments.
T-cell priming induced by oxidized β2-GPI-stimulated DCs
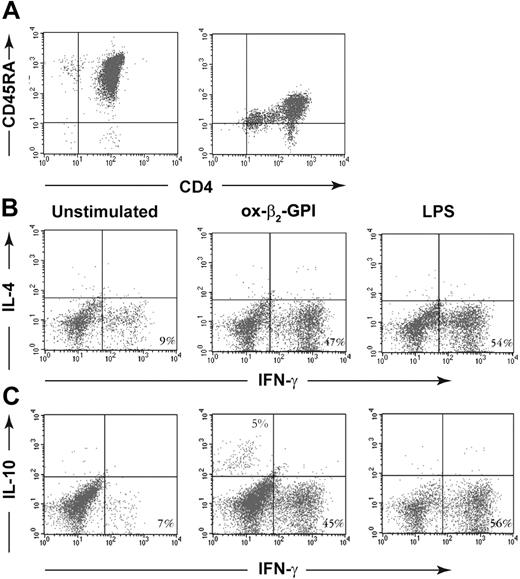
Negatively and positively selected CD4+CD45RA+ T cells produced similar low quantities of IFN-γ (110 vs 150 pg/mL) and no IL-4 and IL-10. In cell cultures and cytofluorimetric experiments designed to find out whether ox-β2-GPI-stimulated DCs primed and polarized allogeneic naive negatively selected CD4+CD45RA+ T lymphocytes, most T cells (47%) primed by ox-β2-GPI-stimulated DCs differentiated into IFN-γ-producing cells, only a small percentage (5%) differentiated into IL-10-producing cells, and none differentiated into IL-4-producing cells (Figure 5).
Oxidized β2-GPI increases allostimulatory ability of DCs. Five-day human DCs were stimulated with ox-β2-GPI (10 μg/mL) (▪), H2O2-β2-GPI (10 μg/mL) (▴), and LPS (100 ng/mL) (•), or left unstimulated (○). After 48 hours, DCs were extensively washed and cultured with allogeneic T lymphocytes (1 × 105 cells/well) for 3 days at a different stimulator-responder ratio (1:16 to 1:256 DC/T). Proliferation of allogenic T cells was measured by 3H-methyl-thymidine incorporation. Data are presented as mean cpm ± SD of triplicate cultures. Results are representative of 11 independent experiments.
Oxidized β2-GPI increases allostimulatory ability of DCs. Five-day human DCs were stimulated with ox-β2-GPI (10 μg/mL) (▪), H2O2-β2-GPI (10 μg/mL) (▴), and LPS (100 ng/mL) (•), or left unstimulated (○). After 48 hours, DCs were extensively washed and cultured with allogeneic T lymphocytes (1 × 105 cells/well) for 3 days at a different stimulator-responder ratio (1:16 to 1:256 DC/T). Proliferation of allogenic T cells was measured by 3H-methyl-thymidine incorporation. Data are presented as mean cpm ± SD of triplicate cultures. Results are representative of 11 independent experiments.
Ox-β2-GPI-treated DCs stimulated allogeneic naive human T cells to produce IFN-γ and IL-10 but not IL-4. Negatively and positively selected CD4+CD45RA+ T cells (A). Five-day human DCs were stimulated with or without ox-β2-GPI (10 μg/mL) and LPS (100 ng/mL) for 18 hours. A total of 5 × 104 DCs were used to stimulate 1 × 106 allogeneic naive negatively selected CD4+CD45RA+ T cells. Activated T cells were expanded with rhIL-2 (30 U/mL) added on day 5. On day 10, polyclonal T-cell lines were stimulated with PMA and ionomycin for 4 hours in the presence of brefeldin A. Cells were stained with anti-hu-CD3PerCP and processed for intracellular labeling with anti-hu-IFN-γ-FITC and anti-hu-IL-4-PE (B) or anti-hu-IL-10-PE (C). The numbers show the percentage of activated CD3+ cells producing the cytokine. Samples were analyzed on a FACScan cytofluorimeter using CELLQuest software (Becton Dickinson, Pharmingen Biosciences). The figure shows a representative experiment from 6 with similar results.
Ox-β2-GPI-treated DCs stimulated allogeneic naive human T cells to produce IFN-γ and IL-10 but not IL-4. Negatively and positively selected CD4+CD45RA+ T cells (A). Five-day human DCs were stimulated with or without ox-β2-GPI (10 μg/mL) and LPS (100 ng/mL) for 18 hours. A total of 5 × 104 DCs were used to stimulate 1 × 106 allogeneic naive negatively selected CD4+CD45RA+ T cells. Activated T cells were expanded with rhIL-2 (30 U/mL) added on day 5. On day 10, polyclonal T-cell lines were stimulated with PMA and ionomycin for 4 hours in the presence of brefeldin A. Cells were stained with anti-hu-CD3PerCP and processed for intracellular labeling with anti-hu-IFN-γ-FITC and anti-hu-IL-4-PE (B) or anti-hu-IL-10-PE (C). The numbers show the percentage of activated CD3+ cells producing the cytokine. Samples were analyzed on a FACScan cytofluorimeter using CELLQuest software (Becton Dickinson, Pharmingen Biosciences). The figure shows a representative experiment from 6 with similar results.
Oxidized β2-GPI-induced IRAK phosphorylation
Western blot analysis of immunoprecipitates from ox-β2-GPI-, H2O2-β2-GPI-, αTOC-β2-GPI-, and LPS-stimulated and unstimulated DCs showed that ox-β2-GPI and H2O2-β2-GPI, as well as LPS, induced IRAK phosphorylation. Immunoprecipitates obtained using non-IRAK-specific IgG, unstimulated cells, and αTOC-β2-GPI-stimulated cells yielded only a weak band in IB (Figure 6A).
Oxidized β2-GPI-induced NFκB activation
In ox-β2-GPI- and H2O2-β2-GPI-stimulated DCs, active p50 and p65 levels significantly increased (2.9-fold and 2.6-fold for p50; and 19.1-fold and 16.3-fold for p65 compared with unstimulated DCs). These levels were only slightly lower than those obtained with LPS stimulation (3.3-fold increase for p50 and 24-fold for p65). The assay was specific, because incubation of an HeLa extract in the presence of a nonbound wild-type consensus oligonucleotide abolished binding of both subunits, whereas the incubation of the HeLa extract with mutated consensus oligonucleotide left NFκB binding unchanged (Figure 6B-C).
Discussion
Our in vitro study provides new information showing an interaction between oxidized β2-GPI and DCs. Our findings strongly suggest that oxidative modification of β2-GPI is an event that renders this self-molecule able to activate immature monocyte-derived DCs from healthy human donors, underlining the role of oxidative stress in mediating structural and functional changes in plasma proteins,31 including β2-GPI.
Oxidized β2-GPI induces IRAK phosphorylation and activates NFκB. DCs were stimulated with ox-β2-GPI, H2O2-β2-GPI, αTOC-β2-GPI, or LPS, or left unstimulated for 45 minutes. Cellular extracts were obtained. (A) Phosphorylated levels of IRAK (p-IRAK) were analyzed by Western blotting with antiphosphoserine mAb. Bound antibodies were visualized with HRP-conjugated anti-mouse IgG and immunoreactivity was assessed by ECL. Immunoprecipitates obtained using unstimulated cells, non-IRAK-specific IgG (irrelevant), and αTOC-β2-GPI-stimulated cells yielded only a weak band. The figure shows a representative experiment from 3 with similar results. (B-C) After cell lysing, protein was quantified, and equal amounts of lysates were used to test activated levels of the p50 and p65 subunits with antibodies directed against the subunits bound to the oligonucleotide containing the NFκB consensus binding site. An HeLa cell extract was used as a positive control alone or in the presence of wild-type or mutated consensus oligonucleotide. Results are expressed as mean ± SD from 3 different experiments. Significant differences between unstimulated samples and samples stimulated with LPS, H2O2-β2-GPI, and ox-β2-GPI are indicated with asterisks (p50: *P = .013, **P = .065, and ***P = .05; p65: *P < .001, **P = .006, and ***P = .007).
Oxidized β2-GPI induces IRAK phosphorylation and activates NFκB. DCs were stimulated with ox-β2-GPI, H2O2-β2-GPI, αTOC-β2-GPI, or LPS, or left unstimulated for 45 minutes. Cellular extracts were obtained. (A) Phosphorylated levels of IRAK (p-IRAK) were analyzed by Western blotting with antiphosphoserine mAb. Bound antibodies were visualized with HRP-conjugated anti-mouse IgG and immunoreactivity was assessed by ECL. Immunoprecipitates obtained using unstimulated cells, non-IRAK-specific IgG (irrelevant), and αTOC-β2-GPI-stimulated cells yielded only a weak band. The figure shows a representative experiment from 3 with similar results. (B-C) After cell lysing, protein was quantified, and equal amounts of lysates were used to test activated levels of the p50 and p65 subunits with antibodies directed against the subunits bound to the oligonucleotide containing the NFκB consensus binding site. An HeLa cell extract was used as a positive control alone or in the presence of wild-type or mutated consensus oligonucleotide. Results are expressed as mean ± SD from 3 different experiments. Significant differences between unstimulated samples and samples stimulated with LPS, H2O2-β2-GPI, and ox-β2-GPI are indicated with asterisks (p50: *P = .013, **P = .065, and ***P = .05; p65: *P < .001, **P = .006, and ***P = .007).
Under our experimental conditions, human β2-GPI—oxidized either spontaneously in the culture medium45,46 or when treated with the physiologic oxidant H2O2—activated immature DCs from healthy donors, thus inducing the phenotypic and functional characteristics typical of mature DCs. We confirmed spontaneous oxidative protein modification in culture medium by measuring the β2-GPI carbonyl group content, the most general indicator and commonly used marker of protein oxidation (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Further evidence that oxidation is a critical event in activating DCs came from our experiment showing that pretreatment of β2-GPI with the antioxidant α-tocopherol prevented β2-GPI-induced DC activation and maturation. Our experiment showing that metal chelators added to the culture medium inhibited DC maturation suggests that modification of β2-GPI is a metal-catalyzed reaction. Our experiments using protease inhibitors rule out the possibility that β2-GPI-induced DC maturation reflects eventual structural enzymatic changes in β2-GPI by proteases.
When we analyzed the phenotypic characteristics of oxidized β2-GPI-stimulated DCs we found that the mature DC-restricted marker CD83 appeared. In parallel, oxidized β2-GPI up-regulated the surface molecules CD80, HLA-DR, CD86, and CD40, indicating that oxidized β2-GPI induces a mature DC phenotype. Our finding that oxidized β2-GPI increased CD40 on DCs is of particular interest, because the CD40 molecule expressed on DCs may be important in regulating T-cell responses.40,47-49
Further information on DC activation induced by oxidized β2-GPI came from our experiments investigating functional changes in DCs. Oxidized β2-GPI specifically stimulated DCs to secrete IL-12, IL-1β, IL-6, IL-8, and TNF-α, the cytokines that support T-cell responses.50 IL-12, a proinflammatory cytokine that induces the production of IFN-γ, favors differentiation of Th1 cells51 and forms a link between innate and adaptive immunity. Oxidized β2-GPI stimulated DCs to secrete also the regulatory cytokine IL-10. Our data indicate that oxidized β2-GPI-stimulated DCs activate a Th1-type response by allogeneic naive T cells, characterized by production of IFN-γ. This finding accords with the Th1 profile of β2-GPI autoreactive T cells determined in the peripheral blood of patients with primary antiphospholipid antibody syndrome.52-54 Notably, T-DC interaction also activated a low percentage of IL-10-secreting T cells. IL-10 is recognized for its immune-suppressive qualities.55 Whether its production reflects efforts by the immune system to control inflammation remains unclear. Further characterization of these IL-10-secreting T cells will establish whether they are a regulatory population able to counteract pathogenic autoreactive Th1 cells.
Our findings also help to explain how β2-GPI interacts with and activates DCs. Although native and oxidized β2-GPI both bind to DCs, the oxidized form binds more extensively, implicating differences in the binding of one or more receptors (Figure S2). Some studies suggest that β2-GPI activates distinct cell types and does so only by receptor binding.13-16 Receptor binding receives further support from our preliminary experiments showing that changes in the percentage of phenotypically mature DCs induced by oxidized β2-GPI are dose dependent. The slower time course of IL-12p70 secretion induced by oxidized β2-GPI than by LPS indicates differences in the binding of one or more receptors (data not shown). The cell membrane receptor(s) for β2-GPI adhesion is still under investigation. In an in vitro study, Raschi et al showed that anti-β2-GPI antibodies, by reacting with their antigen, which is probably associated with a member of the Toll-like/IL-1 receptor family on the endothelial cell surface, activate endothelial cells directly via the myeloid differentiation factor 88 (MyD88)-dependent pathway.17 Under our experimental conditions, DC activation invariably led to NFκB translocation and to a signaling cascade resembling that triggered by TLRs.56-58 Oxidized β2-GPI activated serine phosphorylation of IRAK, the first kinase recruited by the Toll-like/IL-1 receptor family in the MyD88 pathway.59 A previous study demonstrated that β2-GPI dimers bind to endothelial cells, and mimic the effects of β2-GPI/anti-β2-GPI antibody complexes.15 Our data here raise the possibility that dimers of β2-GPI caused by oxidative modification stimulate DC maturation by interacting with a member of the TLR family. The interaction of oxidized β2-GPI with a member of the TLR family is also supported by the molecular mimicry of β2-GPI with common bacteria and viruses.60 Conformational oxidative modifications of β2-GPI might also disclose an amino acid sequence shared with common microbial structures, the natural ligands for TLRs. The major questions for DC binding studies to investigate include the putative TLR involved, how β2-GPI and TLRs interact, and the minimal molecular structure of β2-GPI required for binding.
Another matter our in vitro findings help to explain is why the abundant plasma protein β2-GPI becomes immunogenic in vivo. Immunocryptic epitopes in self-antigens may initiate the autoimmune response because self-tolerance is induced only to efficiently presented, dominant epitopes, not to cryptic epitopes. Because determinant dominance is influenced by protein structure, circumstances that change the molecular context of epitopes (for example, novel cleavage, altered conformation, or tertiary structure) may permit the efficient presentation of previously cryptic determinants.61 In their study investigating mechanisms that reveal cryptic self-determinants thus eliciting autoimmunity, Kuwana et al reported that presentation of the disease-relevant cryptic T-cell determinant in β2-GPI is induced as a direct consequence of antigen processing from β2-GPI bound to anionic phospholipids in functional antigen-presenting cells (APCs).39 Excessive exposure to anionic surfaces, such as microorganisms and apoptotic cells, may induce the formation of a large quantity of bound β2-GPI to anionic surface in vivo. Many other mechanisms may be responsible for generating cryptic structures, and multiple mechanisms receive support from the heterogeneous antigenic specificities in β2-GPI-specific T cells. A possible mechanism is autoantigen cleavage induced by reactive oxygen species. DCs accumulate within the tunica intima in the site of physical stress within blood vessels.62 One possibility is that a microenvironment predisposes local β2-GPI to oxidation thereby initiating a local autoimmune process. The generalized oxidative stress described in patients with autoimmune and cardiovascular diseases mediates an apoptotic program.63 Cells undergoing apoptosis are under oxidative stress and have an increased content of biologically active oxphospholipids.64 Apoptosis may be an alternative pathophysiologic mechanism involved in the oxidation of β2-GPI bound to anionic phospholipids normally expressed on the surface of apoptotic cells.65 The oxidized β2-GPI-induced maturation we observed in DCs accords with evidence that late apoptotic cells are able to activate DCs.66
Our in vitro findings now call for in vivo studies in animals and in patients with chronic disorders related to endothelial cell dysfunction to verify the pathogenetic role of β2-GPI modified by oxidative stress.
Our study takes research into why the abundant self-plasma protein β2-GPI is the target of autoimmune responses a small step ahead. Overall, our findings, along with current knowledge, show that human oxidized β2-GPI, probably by interacting with a member of the TLR family, causes DCs to mature and in parallel to release Th1-promoting cytokines. By contributing to inflammation, oxidative modification of β2-GPI might be an initiating event that triggers the autoimmune spiral. Further studies will need to verify whether oxidized β2-GPI-stimulated DCs effectively present β2-GPI to T cells, thus triggering the immune response.
Our in vitro finding that oxidized β2-GPI promotes the development of mature DCs thus probably contributing in vivo to inflammation again underlines the usefulness of antioxidants as adjunctive drugs in the therapy of human chronic disorders related to endothelial cell dysfunction.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2005-03-1201.
Supported by a grant from the Italian Ministry of Health (no. 4AM/F6/03-05).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Federica Delunardo for help in 2-dimensional electrophoresis and immunoblotting analysis.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal