Proinflammatory T helper 1 (Th1) cells express high levels of carbohydrate ligands for the endothelial selectins, but the molecular basis for this phenotype is incompletely understood. We document here a significant role in selectin ligand formation for the recently described Th1 transcription factor T-bet. Th1 cells generated from T-bet-/- mice showed significantly lower levels of ligands for both E-selectin and P-selectin, compared with wild-type (WT) Th1 cells. Enforced expression of T-bet in WT Th0 cells only modestly up-regulated P-selectin ligands and had no effect on E-selectin ligands. To define a mechanism for the defects observed in T-bet-/- mice, we examined expression of glycosyltransferases involved in selectin ligand biosynthesis. T-bet-/- Th1 cells expressed significantly lower levels of core 2 β1,6 N-acetylglucosaminyltransferase I (C2GlcNAcT-I), but no differences in levels of α 2,3-sialyltransferase IV (ST3Gal-IV). Further, we show that T-bet is responsible for the signal transducer and activator of transcription 4 (Stat4)–independent increase in Th1 cells of fucosyltransferase VII (FucT-VII). We also identify ST3Gal-VI, which is thought to play an important role in E- and P-selectin ligand formation, as an interleukin 12 (IL-12)–regulated, T-bet–dependent gene. These data show that T-bet controls selectin ligand formation in Th1 cells via control of expression of multiple key enzymes in response to IL-12 signaling and establishes an independent transcriptional pathway for control of Th1 cell traffic.
Introduction
Proinflammatory T helper 1 (Th1) cells are essential for resolution of infections by numerous intracellular pathogens and are also responsible for an array of organ-specific autoimmune diseases. Although Th1 cells are defined and identified by their expression of the signature Th1 cytokine interferon-γ (IFN-γ), these cells also characteristically express a number of other molecules critically involved in their differentiation and/or function. Among these molecules are certain cytokine receptors, transcription factors, and cell-surface molecules, including those responsible for their ability to migrate to inflammatory sites. The latter include both chemokine receptors, such as CXC chemokine receptor 3 (CXCR3), and carbohydrate ligands for the endothelial selectins.1,2
Selectins are carbohydrate-binding adhesion receptors of the vascular system that play key roles in directing leukocytes to various target organs, both during normal homeostasis and in settings of inflammation. It is well established that Th1 cells express high levels of selectin ligands, whereas naive CD4 cells express no detectable selectin ligands, and Th2 cells, at least in vitro, express low levels.2-4 This pattern of expression of selectin ligands parallels closely the expression of fucosyltransferase VII (FucT-VII), a key glycosyltransferase that is essential for the formation of selectin ligands, particularly in T lymphocytes.3,5,6 Recent work indicates that induction of FucT-VII is controlled primarily via the T-cell receptor (TCR) by strong Harvey-ras (H-Ras) signaling,7,8 consistent with the association between strong TCR signaling and Th1 cell fate. However, Th1 cells express higher levels of FucT-VII mRNA than do Th0 cells, and this elevated level does not require signal transducer and activator of transcription 4 (Stat4).9 How interleukin 12 (IL-12) augments FucT-VII levels in activated T cells is not known.
A number of other enzymes also play essential roles in selectin ligand biosynthesis. These include the O-linked branching enzyme core 2 β1,6 N-acetylglucosaminyltransferase I (C2GlcNAcT-I), which is essential for functional modification of P-selectin glycoprotein ligand 1 (PSGL-1), the leukocyte ligand for P-selectin, to bind P-selectin.10-13 Importantly, C2GlcNAcT-I is not essential for E-selectin ligand formation.11 In addition, binding of PSGL-1 to P-selectin requires tyrosine sulfation by at least 1 of 2 tyrosine sulfotransferases.14-16 Finally, binding to both selectins requires the action of α 2,3-sialyltransferase IV (ST3Gal-IV) and at least 1 additional sialyltransferase of the ST3GalT family.17 How expression of these various enzymes is controlled in T cells is not well understood.
We previously showed that CD4 cells from mice deficient in Stat4 (Stat4-/-) displayed no IL-12–inducible C2GlcNAcT-I or P-selectin ligands and reduced IL-12–inducible E-selectin ligands and that the absence of Stat4 did not impair induction or up-regulation of FucT-VII.9 We have now analyzed the effect of targeted mutations in T-bet, a transcription factor independently essential for Th1 development,18-20 on induction of selectin ligands and relevant glycosyltransferases in Th1 cells.
Materials and methods
Mice
Mice heterozygous for a null mutation in T-bet19 (T-bet+/-) backcrossed 7 generations to Balb/C (N7) were generously supplied by Dr Laurie Glimcher, Harvard School of Public Health. These mice were mated once with wild-type (WT) Balb/C and then intercrossed to produce N8 T-bet-/- mice. WT Balb/C mice were obtained originally from Jackson Laboratories (Bar Harbor, ME) and were bred and maintained in our own colony. Mice with a homozygous null mutation in Stat421 were generously supplied by Dr Mark Kaplan, Indiana University School of Medicine, and were maintained in our own colony. Mice with a homozygous null mutation in ST3Gal-IV backcrossed at least 6 generations to C57BL6 have been described.17 WT C57BL6 mice were obtained originally from Jackson Laboratories and were bred and maintained in our own colony. All mice were used at 4 to 12 weeks of age. All procedures were reviewed and approved by the Northwestern University Animal Care and Use Committee.
T-cell activation
Purification of CD4+ T cells from spleens of mice by positive selection using magnetic beads and activation by plate bound CD3/CD28 under Th0 (IL-2 only) or Th1 (IL-12 + anti–IL-4 + IL-2) conditions was exactly as previously described.9,11 Media and cytokines were replaced every 2 days. Activated T cells were removed at the indicated time points for fluorescence-activated cell sorting (FACS) analysis, preparation of RNA, preparation of whole-cell lysates for Western blotting, or rolling assays.
Flow cytometry
FACS analysis for expression of the epitope defined by the glycosylation-dependent anti-CD43 monoclonal antibody (mAb) 1B11 (Pharmingen, San Diego, CA) or with selectin chimeras6 (E- or P-selectin receptor immunoglobulin M [E/P-RIgM]) was performed on a FACScan or FASCsort, using CellQuest software (BD Biosciences, San Jose, CA), exactly as previously described.9,11
Rolling assays
Interaction of activated T cells with Chinese hamster ovary (CHO) cells stably expressing E-selectin or P-selectin under defined flow conditions of 1.5 dynes/cm2 in a parallel plate flow chamber was performed, and data were analyzed by the CellTrak system (Compix, Cranberry Township, PA), all exactly as previously described.9,11,22 Data are depicted as mean plus or minus SEM of the number of rolling events obtained for duplicate runs of each cell type for each experiment. All rolling experiments are from cells harvested at day 8 of culture. In some experiments, T cells were treated for 2 hours at 37°C with 0.2 U/mL neuraminidase (Clostridium perfringens; Sigma, St Louis, MO), washed extensively, and used immediately for rolling assays.
Quantitative RT-PCR
Total RNA was isolated from cells harvested at day 6 and immediately suspended in Trizol. Quantitative reverse transcription–polymerase chain reaction (qRT-PCR) was done exactly as described,23 using primers and probes for murine FucT-VII, ST3Gal-IV, ST3Gal-VI, and phosphoglycerate kinase (pgk). Primer and probe sequences are available on request. Levels of FucT-VII, ST3Gal-IV, and ST3Gal-VI mRNA were normalized to that of pgk, based on the minimum cycle number required to cross the threshold (Ct), and are expressed as mean normalized fold increase over the WT Th0 levels.
Retroviral infections
Preparation of retrovirus-containing supernatant was exactly as previously described,7,8 except that we used Phoenix-Eco cells, generously supplied by Dr Gary Nolan, Stanford University, as the packaging cell line to generate ecotropic virus. T-bet cDNA, kindly supplied by Dr Laurie Glimcher, was subcloned upstream of the internal ribosome entry site (IRES) in the murine stem cell virus (MSCV)–IRES–green fluorescent protein (GFP) vector. Empty vector containing no cDNA was used as a negative control. CD4+ T cells activated for 2 days on plate-bound anti-CD3/CD28 as described under “T-cell activation” were infected with 1 ml fresh retroviral supernatant and subsequently cultured under Th0 conditions as described under “T-cell activation.”
Western blotting
Activated CD4 cells were harvested on day 6 of Th1 cultures, washed, rested for 4 hours, and then stimulated with IL-12 for the indicated periods of time. Cells were then lysed in cell lysis buffer (1% Triton X-100 in 10 mM Tris [tris(hydroxymethyl)aminomethane] HCl pH 7.4, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.5 mM EDTA [ethylenediaminetetraacetic acid], plus protease inhibitors), the lysates were clarified by centrifugation, and whole-cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and Western blotted with rabbit polyclonal Ab to tyr-phospho-Stat4 (Invitrogen, Carlsbad, CA). Blots were then stripped and blotted with rabbit polyclonal Ab to total Stat4 (Biosource, Camarillo, CA) and developed by enhanced chemiluminescence (ECL).
Statistical analysis
Pairwise comparisons between groups were carried out with the Student t test, with P less than .05 being considered significant.
Results
Absence of T-bet sharply impairs IL-12–induced selectin ligands in developing Th1 cells
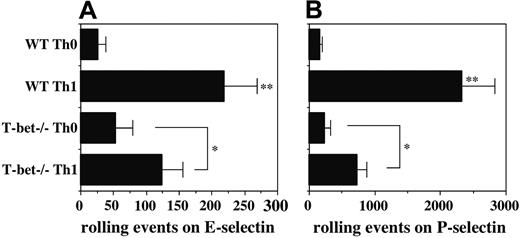
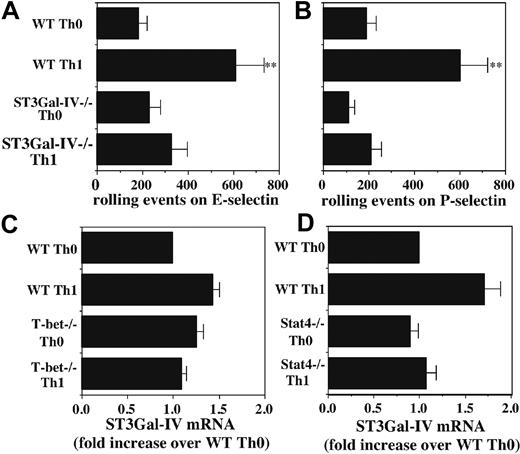
Although both Stat4 and T-bet are clearly essential for Th1 differentiation, the precise functions of these transcription factors in various facets of Th1 differentiation are still not completely understood. Our previous work showing an essential role for Stat4 in induction of selectin ligands on developing Th1 cells9 prompted us to examine a potential role for T-bet in this process. Naive CD4+ T cells were isolated from WT and T-bet-/- mice, activated with plate-bound anti-CD3/CD28, and cultured under Th0 or Th1 conditions. Comparison of rolling by T cells activated under these 2 conditions allows direct assessment of the IL-12–inducible component of rolling, represented by the difference between the Th0 and Th1 groups.9,11 Cell yields and expression of activation markers were not significantly different between WT and T-bet-/- Th0 or between WT and T-bet-/- Th1 cells (data not shown), indicating equivalent activation. As in previous studies, WT Th1 cells rolled 5- to 8-fold better than WT Th0 cells on both endothelial selectins. In contrast, T-bet-/- Th1 cells showed substantially reduced rolling compared with WT Th1 cells, with levels of rolling on E-selectin reduced approximately 50% (Figure 1A), and levels of rolling on P-selectin reduced approximately 65% (Figure 1B). No differences were observed between the low levels of E- and P-selectin ligands expressed on WT versus T-bet-/- Th0 cells, indicating that the low level of selectin ligands associated with T-cell activation, independent of IL-12, is preserved in T-bet-/- CD4 cells, consistent with equivalent activation. These data show that, in the absence of T-bet, induction of selectin ligands by IL-12 is significantly reduced but not eliminated.
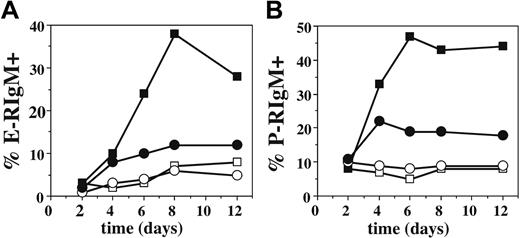
To ensure that the weak induction of selectin ligands on T-bet-/- Th1 cells by IL-12 was not due to delayed or altered kinetics of expression, we examined cells from these cultures by FACS using E- and P-selectin chimeras,6 which faithfully report the distribution of selectin ligands on activated T cells.5,9 These data (Figure 2) showed that maximal selectin ligand expression on WT Th1 cells is not achieved until days 6 to 8, consistent with previous data.9 Furthermore, these data revealed no alteration in the kinetics of expression of ligands for E- or P-selectin on T-bet-/- Th1 cells, confirming the impaired induction of selectin ligands on these cells by IL-12.
Defective induction of selectin ligands in T-bet-/- Th1 cells. Purified CD4+ T cells were cultured under either Th1 (IL-2 plus IL-12 plus anti–IL-4) or Th0 (IL-2 only) conditions and analyzed on day 8 for rolling on (A) E-selectin and (B) P-selectin. One representative of at least 2 experiments. **Significantly different (P < .05) from all other groups; *significantly different (P < .05).
Defective induction of selectin ligands in T-bet-/- Th1 cells. Purified CD4+ T cells were cultured under either Th1 (IL-2 plus IL-12 plus anti–IL-4) or Th0 (IL-2 only) conditions and analyzed on day 8 for rolling on (A) E-selectin and (B) P-selectin. One representative of at least 2 experiments. **Significantly different (P < .05) from all other groups; *significantly different (P < .05).
Direct and indirect control of expression of selectin ligands by T-bet
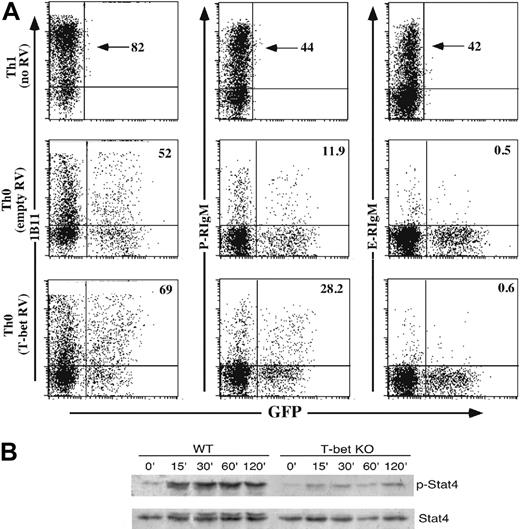
T-bet might exert control of selectin ligand formation directly, by direct transcriptional induction of involved glycosyltransferases, and/or indirectly, via control of expression of the IL12Rβ2,20,24 which is essential for Stat4 activation. To approach this question, we first used retroviruses expressing T-bet to infect WT Th0 cells, which under these in vitro conditions express very low levels of T-bet, C2GlcNAcT-I, and selectin ligands.9 Retroviral expression of T-bet in WT Th0 cells had no effect on the low level of E-selectin ligands expressed on Th0 cells (Figure 3). However, expression of T-bet modestly up-regulated C2GlcNAcT-I and P-selectin ligands, although the levels of both remained well below that of WT Th1 cells (Figure 3A). These findings show that overexpression of T-bet leads to an increase in P-selectin ligands due to an increase in C2GlcNAcT-I levels but fails to autonomously induce maximal levels of selectin ligands in the absence of IL-12 signaling.
In addition to the modest inductive effect of T-bet on C2GlcNAcT-I expression, T-bet could more globally affect IL-12–driven induction of selectin ligands via its role in expression of the IL12R, specifically the IL12Rβ2.20,24 To address this question, we examined the degree of Stat4 tyrosine phosphorylation in WT and T-bet-/- CD4 cells activated for 6 days under Th1 conditions and then restimulated with IL-12. The results (Figure 3B) show that a significant increase in Stat4 tyrosine phosphorylation was observed in WT CD4 cells, as expected. In sharp contrast, no increase in Stat4 tyrosine phosphorylation was observed in response to IL-12 in T-bet-/- CD4 cells. These results demonstrate that T-bet deficiency can lead to severely impaired IL-12 signaling, thereby impairing Stat4-dependent IL-12–induced expression of selectin ligands.
Kinetics of expression of selectin ligands is not altered by the absence of T-bet. Cells from T-cell activation cultures as in Figure 1 were also analyzed over time by FACS with selectin chimeras to determine the fraction which expressed ligands for (A) E-selectin or (B) P-selectin. Filled symbols, Th1; open symbols, Th0; squares, WT; circles, T-bet-/-. One of at least 5 experiments.
Kinetics of expression of selectin ligands is not altered by the absence of T-bet. Cells from T-cell activation cultures as in Figure 1 were also analyzed over time by FACS with selectin chimeras to determine the fraction which expressed ligands for (A) E-selectin or (B) P-selectin. Filled symbols, Th1; open symbols, Th0; squares, WT; circles, T-bet-/-. One of at least 5 experiments.
Direct and indirect effects of T-bet on selectin ligand induction. (A) Enforced overexpression of T-bet in WT Th0 cells fails to induce maximal levels of selectin ligands. WT CD4+ T cells were activated under Th0 conditions, infected with retroviruses (RVs) expressing no cDNA (empty) or T-bet, and cultured under Th0 conditions. Cells were then stained with P- or E-selectin chimeras or 1B11. Uninfected WT Th1 cells were included side-by-side for comparison. Numbers given for retrovirally transduced cells are the percentage of the GFP+ cells which stain for either 1B11 (first column), P-selectin ligands (second column), or E-selectin ligands (third column). One representative of 3 similar experiments. (B) T-bet deficiency inhibits functional expression of the IL12R. WT and T-bet-/- CD4 cells were cultured under Th1 conditions for 6 days, washed, and restimulated with IL-12. At the indicated time points, cells were harvested, lysed, and subjected to Western blotting for tyrosine phosphorylated, and total Stat4. Results are representative of 2 experiments. KO indicates knock-out.
Direct and indirect effects of T-bet on selectin ligand induction. (A) Enforced overexpression of T-bet in WT Th0 cells fails to induce maximal levels of selectin ligands. WT CD4+ T cells were activated under Th0 conditions, infected with retroviruses (RVs) expressing no cDNA (empty) or T-bet, and cultured under Th0 conditions. Cells were then stained with P- or E-selectin chimeras or 1B11. Uninfected WT Th1 cells were included side-by-side for comparison. Numbers given for retrovirally transduced cells are the percentage of the GFP+ cells which stain for either 1B11 (first column), P-selectin ligands (second column), or E-selectin ligands (third column). One representative of 3 similar experiments. (B) T-bet deficiency inhibits functional expression of the IL12R. WT and T-bet-/- CD4 cells were cultured under Th1 conditions for 6 days, washed, and restimulated with IL-12. At the indicated time points, cells were harvested, lysed, and subjected to Western blotting for tyrosine phosphorylated, and total Stat4. Results are representative of 2 experiments. KO indicates knock-out.
Role of T-bet in induction of enzymes required for selectin ligand formation
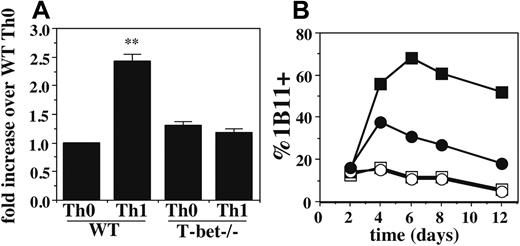
To identify a molecular basis for the defects in selectin ligand formation in T-bet-/- Th1 cells, we first examined the expression of 2 key enzymes: FucT-VII, which is essential for both P- and E-selectin ligands, and C2GlcNAcT-I, which is essential for P-selectin ligands but not E-selectin ligands.10-13 Expression of FucT-VII was determined by qRT-PCR. As shown in Figure 4A, there was a slight (∼ 2-fold) difference between WT Th1 cells and WT Th0 or T-bet-/- Th1 or Th0 cells. Although small, this difference was reproducible and significant, and it is reflective of differences previously observed for Th0 versus Th1 cells.9 Moreover, small differences in the levels of glycosyltransferases can have disproportionately large effects on the glycosylation of their substrates, suggesting that these small differences in FucT-VII mRNA levels may be biologically meaningful.
To evaluate expression of C2GlcNAcT-I, we used staining with the 1B11 mAb, which on murine CD4 cells detects a C2GlcNAcT-I–dependent epitope on CD43 that serves as a reporter for the activity of this enzyme.10,11 Th0 cells typically show low levels of 1B11 staining, whereas Th1 cells show much higher levels. Th1 cells from T-bet-/- mice showed significantly reduced levels of 1B11 staining compared with WT Th1 cells (Figure 4B). As expected, Th0 cells from both genotypes showed low levels of 1B11 staining. These data show that expression of T-bet is required for maximal induction of C2GlcNAcT-I in response to IL-12 in developing Th1 cells.
T-bet is required for up-regulation of C2GlcNAcT-I and FucT-VII in Th1 cells. (A) qRT-PCR of FucT-VII mRNA levels of WT and T-bet-/- Th0 and Th1 cells. Levels are normalized to pgk mRNA levels and expressed as fold induction over WT Th0 cells. Error bars indicate SD. (B) Expression of the C2GlcNAcT-I reporter epitope defined by the 1B11 mAb on WT and T-bet-/- Th0 and Th1 cells over time. Results are representative of at least 3 experiments. Filled symbols, Th1; open symbols, Th0; squares, WT; circles, T-bet-/-, as in Figure 2. **Significantly different from all other groups.
T-bet is required for up-regulation of C2GlcNAcT-I and FucT-VII in Th1 cells. (A) qRT-PCR of FucT-VII mRNA levels of WT and T-bet-/- Th0 and Th1 cells. Levels are normalized to pgk mRNA levels and expressed as fold induction over WT Th0 cells. Error bars indicate SD. (B) Expression of the C2GlcNAcT-I reporter epitope defined by the 1B11 mAb on WT and T-bet-/- Th0 and Th1 cells over time. Results are representative of at least 3 experiments. Filled symbols, Th1; open symbols, Th0; squares, WT; circles, T-bet-/-, as in Figure 2. **Significantly different from all other groups.
Because the defects in E-selectin ligands cannot be due to the deficiency in IL-12–induced C2GlcNAcT-I,10,11 we considered the possibility that these defects in E-selectin ligands could be due to impaired induction of 1 or more sialyltransferases. We have previously shown that neutrophils isolated from ST3Gal-IV-/- mice show modestly reduced levels of ligands for both E- and P-selectin.17 We therefore examined CD4+ T cells from WT and ST3Gal-IV-/- mice, activated and cultured as before, to determine whether ST3Gal-IV also makes significant contributions to expression of selectin ligands on activated T cells. These data disclosed that ST3Gal-IV-/- Th1 cells exhibit substantially reduced rolling on both E-selectin (Figure 5A) and P-selectin (Figure 5B) compared with WT Th1 cells. These data confirm that ST3Gal-IV is important for selectin ligand formation in activated T cells.
This defect in rolling on E- and P-selectin by ST3Gal-IV-/- Th1 cells was similar to the data described in Figure 1 with T-bet-/- Th1 cells, suggesting that IL-12 may induce ST3Gal-IV in a T-bet–dependent fashion. We therefore examined the levels of ST3Gal-IV mRNA by qRT-PCR. Surprisingly, levels of ST3Gal-IV mRNA were not significantly different between WT or T-bet-/- Th0 or Th1 cells (Figure 5C) or between WT or Stat4-/- Th0 or Th1 cells (Figure 5D). Taken together, these finding show that ST3Gal-IV makes an important contribution to selectin ligand formation in activated T cells, but that expression of ST3Gal-IV is independent of IL-12 signaling.
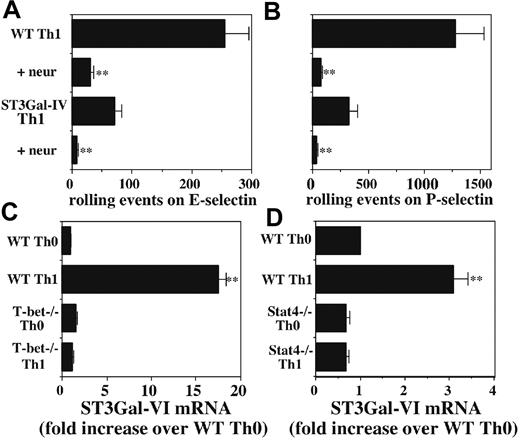
ST3Gal-I, -II, and -III do not apparently contribute to selectin ligand biosynthesis, at least in neutrophils,17 and ST3Gal-V is primarily involved in synthesis of the ganglioside M3 (GM3) ganglioside.25,26 We therefore first confirmed that, as expected, rolling of ST3Gal-IV-/- T cells on both E-selectin (Figure 6A) and P-selectin (Figure 6B) was sensitive to neuraminidase, indicating the activity of a second sialyltransferase. We therefore examined the mRNA levels of ST3Gal-VI, a cloned member of the ST3Gal family whose enzymatic properties makes it a good candidate to play an important role in selectin ligand biosynthesis.27 Strikingly, ST3Gal-VI mRNA levels were substantially higher in WT Th1 cells than in either WT Th0 cells or T-bet-/- Th1 or Th0 cells (Figure 6C). These results prompted us to examine the levels of ST3Gal-VI in WT and Stat4-/- Th0 and Th1 cells. Similar to the results with T-bet-/- CD4 cells, Stat4 deficiency reduced the induction of ST3Gal-VI in Th1 cells in response to IL-12, although the magnitude of the difference was substantially smaller than with T-bet (Figure 6D). These data identify ST3Gal-VI as an additional glycosyltransferase whose expression is regulated by IL-12 signaling in a T-bet- and Stat4-dependent fashion and strongly suggest that ST3Gal-IV and ST3Gal-VI collaborate in selectin ligand formation in activated T cells.
Role of ST3Gal-IV in selectin ligand formation in Th1 cells. Rolling of WT and ST3Gal-IV-/- Th0 and Th1 cells on (A) E-selectin and (B) P-selectin reveals significantly decreased rolling of ST3Gal-IV-/- Th1 cells, compared with WT Th1 cells. (C) qRT-PCR shows no significant difference in ST3Gal-IV-/- mRNA levels between WT or T-bet-/- Th0 or Th1 cells. (D) qRT-PCR also shows no significant difference in ST3Gal-IV mRNA levels between WT and Stat4-/- Th0 or Th1 cells. Data are depicted as in Figure 4A. Results are representative of at least 3 experiments. **Significantly different from all other groups.
Role of ST3Gal-IV in selectin ligand formation in Th1 cells. Rolling of WT and ST3Gal-IV-/- Th0 and Th1 cells on (A) E-selectin and (B) P-selectin reveals significantly decreased rolling of ST3Gal-IV-/- Th1 cells, compared with WT Th1 cells. (C) qRT-PCR shows no significant difference in ST3Gal-IV-/- mRNA levels between WT or T-bet-/- Th0 or Th1 cells. (D) qRT-PCR also shows no significant difference in ST3Gal-IV mRNA levels between WT and Stat4-/- Th0 or Th1 cells. Data are depicted as in Figure 4A. Results are representative of at least 3 experiments. **Significantly different from all other groups.
Possible role of ST3Gal-VI in selectin ligand formation. WT and ST3Gal-IV-/- Th1 cells were either untreated or treated with neuraminidase, and rolling on (A) E-selectin or (B) P-selectin was measured. Neuraminidase blocked rolling of cells of both genotypes on both selectins. (A-B) **Significantly different from the corresponding group without neuraminidase treatment. (C) qRT-PCR reveals significant induction of ST3Gal-VI mRNA by IL-12 in WT cells but virtually no effect of IL-12 in T-bet-/- cells. (D) A similar effect on ST3Gal-VI induction is observed with CD4 cells from Stat4-/- mice, although the magnitude of the effect of Stat4 deficiency is smaller than that for T-bet (note different scales). (C-D) **Significantly different from all other groups.
Possible role of ST3Gal-VI in selectin ligand formation. WT and ST3Gal-IV-/- Th1 cells were either untreated or treated with neuraminidase, and rolling on (A) E-selectin or (B) P-selectin was measured. Neuraminidase blocked rolling of cells of both genotypes on both selectins. (A-B) **Significantly different from the corresponding group without neuraminidase treatment. (C) qRT-PCR reveals significant induction of ST3Gal-VI mRNA by IL-12 in WT cells but virtually no effect of IL-12 in T-bet-/- cells. (D) A similar effect on ST3Gal-VI induction is observed with CD4 cells from Stat4-/- mice, although the magnitude of the effect of Stat4 deficiency is smaller than that for T-bet (note different scales). (C-D) **Significantly different from all other groups.
Discussion
The ability of T cells to home to sites of inflammation is essential for effective immunity, and the expression of ligands for selectins is a critical component of the ability of T cells to enter sites of inflammation. Unlike myeloid leukocytes, which constitutively express high levels of selectin ligands, T cells do so only inducibly and in a regulated fashion. The finding that Th1 cells but not Th2 cells express high levels of selectin ligands specifically implicated IL-12 signaling in induction of selectin ligands.2,3 We previously showed that Stat4 plays a crucial role in this process by controlling the up-regulation of C2GlcNAcT-I, which is selectively essential for formation of functional P-selectin ligands.10,11,13 In contrast, induction of FucT-VII, which is essential for biosynthesis of ligands for both P- and E-selectin, appears to be controlled primarily via the TCR through H-Ras, and is enhanced by IL-12, independent of Stat4.9 Despite these findings, signaling and transcriptional pathways responsible for induction of selectin ligands in activated T cells remain incompletely defined.
In addition to Stat4, differentiation of naive CD4 T cells to Th1 cells requires the transcription factor T-bet.18,20 CD4 T cells from mice deficient in T-bet are severely crippled in their ability to produce IFN-γ, as well as their ability to mediate several Th1-like autoimmune diseases.19,28-30 However, the precise functions of Stat4 and T-bet in Th1 differentiation are not yet fully understood, and a role for T-bet in selectin ligand formation in Th1 cells has not previously been examined. We found that CD4 T cells from T-bet-/- mice exhibited significant defects in IL-12–induced formation of ligands for both E- and P-selectin, with no effect on the low levels of selectin ligand induction in Th0 cells. These results establish an important role for T-bet in IL-12–driven induction of selectin ligands in developing Th1 cells.
T-bet could control expression of selectin ligands directly, via direct transcriptional induction of genes encoding specific glycosyltransferases, or indirectly, via control of expression of the IL12Rβ2.20,24 To approach this question, we expressed T-bet in Th0 cells in the absence of IL-12 and examined levels of selectin ligands and C2GlcNACT-I by FACS. We found that retrovirally mediated overexpression of T-bet produced a modest up-regulation of C2GlcNACT-I and P-selectin ligands but had no effect on E-selectin ligands. The up-regulation of P-selectin ligands is consistent with previous findings,18 and the concordant up-regulation of C2GlcNAcT-I and P-selectin ligands in the absence of any IL-12 suggests that one mechanism by which T-bet contributes to selectin ligand formation may be via direct transcriptional activation of C2GlcNAcT-I. T-bet and Stat4 may therefore synergize to initiate transcriptional activation of the C2GlcNAcT-I gene in developing Th1 cells.
However, the inability of enforced T-bet expression in WT Th0 cells to induce levels of P-selectin ligands equivalent to WT Th1 cells, or to have any effect on E-selectin ligand levels, demonstrates that direct transactivation of genes encoding essential glycosyltransferases is not the only mechanism underlying the requirement for T-bet in selectin ligand formation in Th1 cells. Rather, these data collectively indicate that a second important mechanism by which T-bet contributes to selectin ligand formation is via regulation of IL-12 signaling through control of expression of the IL-12R, specifically the IL12Rβ2 chain.20,24 We found that T-bet deficiency led to nearly absent levels of a functional IL12R, which virtually eliminated activation of Stat4, producing a phenotype overall similar to the Stat4-/- mice. This is consistent with the slightly higher levels of C2GlcNAcT-I and P-selectin ligands in the T-bet-/- mice compared with the Stat4-/- mice, and with an overall reduction, but not elimination, in the ability of IL-12 to induce selectin ligands.
To begin to unravel the molecular basis for selectin ligand defects in T-bet-/- mice, we examined levels of expression of several glycosyltransferases known or thought to be involved in selectin ligand formation. We found small but significant decreases in IL-12–induced FucT-VII mRNA levels between WT and T-bet-/- Th1 cells. These differences parallel those previously found for differences in FucT-VII in Th0 versus Th1 cells,9 suggesting that T-bet controls the IL-12–dependent, Stat4-independent enhancement of FucT-VII observed in Th1 cells. T-bet could mediate this enhancement of FucT-VII mRNA via its effect on IL-12 receptor expression through a Stat4-independent pathway, through direct up-regulation of transcription, or conceivably through a posttranscriptional mechanism such as stabilization of the FucT-VII mRNA. Regardless of the precise mechanism, because FucT-VII appears to be the limiting enzyme in biosynthesis of E-selectin ligands, and because small changes in glycosyltransferase levels can result in significantly higher levels of glycosyltransferase activity, it is possible that this small difference contributes to defects in IL-12–induced E-selectin ligands.
We also found significant and much greater defects in induction of C2GlcNAcT-I activity in T-bet-/- Th1 cells. Previous studies have established that, whereas FucT-VII is essential for formation of ligands for both E- and P-selectin, C2GlcNAcT-I is essential only for P-selectin ligands.5,6,11,31 Defective up-regulation of C2GlcNAcT-I is therefore sufficient to explain defects in IL-12–induced P-selectin ligands in T-bet-/- Th1 cells, particularly because this enzyme appears to be the limiting factor for P-selectin ligand biosynthesis.11 However, because C2GlcNAcT-I-/- CD4 cells display no defects in rolling on E-selectin under these conditions,11 reduced C2GlcNAcT-I expression does not explain defective IL-12–induced E-selectin ligands in these cells.
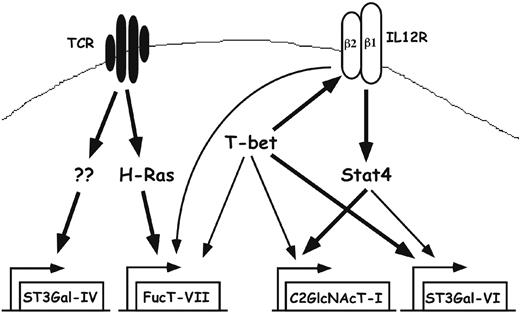
Pathways controlling expression of glycosyltransferases in Th1 cells. Naive T cells express little or no FucT-VII, C2GlcNAcT-I, or ST3Gal-VI. T-cell activation through the TCR leads to activation of H-Ras and induction of FucT-VII, as well as induction or maintenance of constitutive levels of ST3Gal-IV. Whether ST3Gal-IV expression requires H-Ras is unknown. In addition, T-cell activation is associated with induction of the IL12R, particularly the IL12Rβ2 chain, and the IL12R is essential for activation of Stat4, which plays a major role in induction of C2GlcNAcT-I and a minor role in induction of ST3Gal-VI. T-bet plays a role through induction/maintenance of the IL12Rβ2, allowing up-regulation of C2GlcNAcT-I by Stat4; through direct up-regulation of C2GlcNAcT-I and ST3Gal-VI; and through up-regulation of FucT-VII by an as yet undetermined mechanism. Bold lines represent strong, presumably or known to be direct, effects; thin lines represent weak effects.
Pathways controlling expression of glycosyltransferases in Th1 cells. Naive T cells express little or no FucT-VII, C2GlcNAcT-I, or ST3Gal-VI. T-cell activation through the TCR leads to activation of H-Ras and induction of FucT-VII, as well as induction or maintenance of constitutive levels of ST3Gal-IV. Whether ST3Gal-IV expression requires H-Ras is unknown. In addition, T-cell activation is associated with induction of the IL12R, particularly the IL12Rβ2 chain, and the IL12R is essential for activation of Stat4, which plays a major role in induction of C2GlcNAcT-I and a minor role in induction of ST3Gal-VI. T-bet plays a role through induction/maintenance of the IL12Rβ2, allowing up-regulation of C2GlcNAcT-I by Stat4; through direct up-regulation of C2GlcNAcT-I and ST3Gal-VI; and through up-regulation of FucT-VII by an as yet undetermined mechanism. Bold lines represent strong, presumably or known to be direct, effects; thin lines represent weak effects.
Because ST3Gal-IV mRNA levels were not significantly reduced in the absence of T-bet, and FucT-VII levels were only slightly reduced, we sought to determine whether induction of any other key glycosyltransferase was affected. In this regard, a good candidate was ST3Gal-VI, because the other members of the ST3GalT family do not appear to play any role in selectin ligand formation.17 We found that ST3Gal-VI mRNA levels were sharply up-regulated by IL-12, and that this up-regulation was severely blunted in T-bet-/- Th1 cells. We observed the same pattern of expression of ST3Gal-VI when comparing WT and Stat4-/- CD4 cells, although the magnitude of the difference was much smaller. These results suggest that T-bet is quantitatively more important than Stat4 for induction or up-regulation of ST3Gal-VI, and they also imply direct transcriptional control of ST3Gal-VI by T-bet, because Stat4-/- mice displayed defects in ST3Gal-VI induction that were not nearly as severe. These results therefore identify ST3Gal-VI as a second IL-12–inducible, T-bet–dependent, Stat4-dependent enzyme, along with C2GlcNAcT-I. Previous results with Stat4-/- mice9 had indicated the existence of another IL-12–induced enzyme responsible for E-selectin ligands, and our current data suggest that this enzyme is likely to be ST3Gal-VI. Direct proof that ST3Gal-VI is involved in selectin ligand biosynthesis awaits production and characterization of mice deficient in this enzyme. Collectively, these results indicate that ST3Gal-IV and ST3Gal-VI collaborate in biosynthesis of selectin ligands and that sufficient levels of both enzymes are required for maximal selectin ligand expression in Th1 cells. In addition, because levels of ST3Gal-IV are equivalent in activated T cells regardless of the presence or absence of IL-12, T-bet, or Stat4, this implies that the up-regulation of selectin ligands in Th1 cells is due primarily to up-regulation of ST3Gal-VI (along with FucT-VII and C2GlcNAcT-I).
Recently, Lord et al32 also examined CD4 T cells from T-bet-/- mice for their ability to express selectin ligands. In contrast to our results, this group failed to detect any differences in levels of FucT-VII, C2GlcNAcT-I, or E-selectin ligands and attributed differences in P-selectin ligands to a defect in tyrosine sulfation of PSGL-1. However, Lord et al32 used CD4 cells activated for only 4 to 5 days, a time point at which selectin ligands and expression of these glycosyltransferases have not yet reached maximal levels (Figure 2 and White et al9 ). It is highly likely that their failure to detect the differences we observe, particularly in levels of C2GlcNAcT-I or E-selectin ligands, is attributable to the premature time point used for analysis, because other aspects of the experiments were similar or identical to ours.
Our results extend and refine a general model in which induction of glycosyltransferases required for selectin ligand biosynthesis in activated T cells is controlled through pathways that initiate through 2 distinct cell surface receptors, the TCR and IL12R (Figure 7). Thus, induction of FucT-VII is triggered by strong TCR engagement, possibly via H-Ras,8 but is augmented by T-bet by a yet to be determined mechanism. Similarly, ST3Gal-IV in CD4+ T cells is uniformly expressed in activated T cells, independent of cytokine signaling. In contrast, induction of both C2GlcNAcT-I and ST3Gal-VI is triggered by IL-12, through pathways that require both T-bet and Stat4. Our findings indicate that the roles of T-bet and Stat4 in IL-12–induced expression of these 2 genes is distinct. Stat4 appears to play the major role in induction of C2GlcNAcT-I, because Stat4-/- mice display no IL-12–induced up-regulation of C2GlcNAcT-I, with T-bet required primarily to maintain expression of the IL12Rβ2, allowing activation of Stat4. In contrast, T-bet appears to play a direct role in induction of ST3Gal-VI, with Stat4 playing a quantitatively minor role, because deletion of T-bet had a much greater effect than Stat4 deletion on IL-12–induced ST3Gal-VI levels. In combination with our previous results on defects in Stat4-/- mice, the current results fortify the relationship between proinflammatory Th1 cells and selectin ligand expression, offer insight into the transcriptional basis for T-cell migration, and advance our understanding of transcriptional control of cell-surface glycosylation in the immune system.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2005-03-0984.
Supported by the National Institutes of Health (NIH) (grant AI50837) (G.S.K.), an American Heart Association predoctoral award (0410069Z) (D.G.Z.), NIH (P01-HL57345) (J.D.M.), and NIH postdoctoral award (F32CA79130) (L.G.E.). G.H.U. and D.G.Z. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Laurie Glimcher for generously providing T-bet cDNA and T-bet-/- mice.
J.D.M. is supported as an Investigator of the Howard Hughes Medical Institute.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal